Summary
Arterial PCO2 is tightly regulated via changes in breathing. A rise in PCO2 activates the carotid bodies and exerts additional effects on neurons located within the CNS, causing an increase in lung ventilation. Central respiratory chemoreception refers to the component of this homeostatic reflex that is triggered by activation of receptors located within the brain (central chemoreceptors). Throughout the body, CO2 generally operates via the proxy of pH. Since countless proteins, ion channels and neurons display some degree of pH-sensitivity, the notion that central respiratory chemoreception could rely on a few specialized neurons seems a priori counter-intuitive. Yet, two types of neurons currently stand out as critically important for breathing regulation by CO2: the retrotrapezoid nucleus (RTN) and the raphe. RTN neurons are glutamatergic, strongly activated by hypercapnia in vivo and by CO2 or protons in slices. These neurons target selectively the pontomedullary regions implicated in generating the respiratory rhythm and pattern. Their response to CO2 seems to involve both cell-autonomous and paracrine effects of CO2, the latter presumably mediated by the surrounding glia. The specific connections that these excitatory neurons establish with the rest of the breathing network are likely to be the main explanation of their importance to respiratory chemoreception. Serotonergic neurons have a powerful stimulatory effect on breathing, they facilitate the chemoreflexes and a subset of them likely function as CO2 sensors.
Opto- and pharmacogenetic methods have played an important role in assessing the contribution of RTN and serotonergic neurons as well as glial cells to respiration. These particular experiments are emphasized here for thematic reasons although the current perception of the importance of the RTN and serotonergic cells to respiratory chemoreception also relies on many other types of evidence. A small portion of this evidence is presented as background.
1. The conundrum of central respiratory chemoreception
A conundrum is “a confusing and difficult problem or question”. Central respiratory chemoreception is the extremely sensitive mechanism by which very small changes in brain PCO2 produce very large changes in breathing. The conundrum is that the mammalian brain has a plethora of neurons or channels that individually respond to CO2/pH and, in most cases, it has been very difficult to establish which of these widespread effects of pH are germane to central respiratory chemoreception. The interpretative problems experienced by the field of central chemoreception are in many ways similar to those encountered by investigators who are trying to sort out which of the myriads of glucose- or temperature-sensitive CNS neurons regulate glycemia or body temperature.
The amplitude and frequency of respiratory movements were already measured over a century ago (Haldane and Priestley, 1905). By using a simple procedure for measuring PCO2 in expired alveolar air, Haldane and Priestley established two fundamental aspects of breathing regulation, namely the powerful stimulatory effect of CO2 on lung ventilation and the fact that arterial PCO2 is very tightly regulated regardless of physical activity or altitude. The notion that PCO2 is “the main factor that normally determines lung ventilation” originates from these studies (Haldane and Priestley, 1905). In reality breathing adjusts to any change in behavior and, unless airways are obstructed, these adjustments do not operate in reaction to changes in PCO2 but primarily in a feed-forward mode via multiple CNS inputs to the brainstem respiratory centers. These inputs are related to emotions, exercise, metabolic status, body temperature and the state of vigilance. Although behavior- or emotion-related changes in breathing are not driven by variations in PCO2, PCO2 stability is essential to maintain tissue pH constant and this homeostatic regulation requires a powerful feedback control that operates regardless of the magnitude of lung ventilation. This feedback is driven by CO2 sensors located in the carotid bodies (peripheral chemoreceptors) and within the CNS, the central respiratory chemoreceptors (Smith et al., 2010). The location and cellular nature of the central respiratory chemoreceptors (neurons, glia, vascular cells, pluricellular functional units) is still not definitively established (Nattie and Li, 2009; Gourine et al., 2010; Guyenet et al., 2010). CO2 is thought to work via protons (the “reaction theory” (Loeschcke, 1982)), a plausible theory that has been difficult to put to the test given the plethora of pH-modulated proteins, particularly ion channels, that could serve as “proton receptors”. Molecular CO2 may also exert direct effects on glial cells or neurons, perhaps by changing the properties of connexin hemi-channels (Huckstepp et al., 2010). The identification of central respiratory chemoreceptors has also been hampered by the fact that many neurons are activated or inhibited by acidification in vitro or in culture, regardless of whether their anatomical connections in the intact brain would enable them to exert any influence on the activity of the breathing network (Su et al., 2007). These relatively widespread and sometimes sizeable effects of pH on neurons in vitro (Wang et al., 2001) are not always seen in vivo (Mulkey et al., 2004) and have been variously interpreted.
This review focuses on the contribution to central respiratory chemoreception of the retrotrapezoid nucleus (sections 2-7), the medullary raphe (section 9, for further details on the latter topic see: (Hodges and Richerson, 2010b)) and the glia (section 8). These cells have been selected because they have been subjected to the most exhaustive recent investigations and optogenetics has helped to understand their role in respiration.
This article is not intended as a comprehensive review on chemoreception. The following reviews are recommended for additional information on this topic (Nattie and Li, 2008; Corcoran et al., 2009; Duffin, 2010; Buckler, 2010; Forster and Smith, 2010; Hodges and Richerson, 2010a; Nattie, 2011). Although these authors also highlight the contribution of RTN neurons and the raphe to central chemoreception, their view is that central chemosensitivity is caused by a widespread sensitivity of the lower brainstem respiratory centers to pH/PCO2 combined with a potentially equally widespread pH/PCO2 sensitivity of the many neurons that regulate the activity of this network. These interpretations rely on microdialysis experiments in which focal acidification of various regions of the brain is shown to increase respiration to some degree and on the result of brain lesions or pharmacological interventions.
2. Identification and early evidence for the role of the RTN in respiration
The neurons that generate the respiratory rhythm and the pattern of activity of the various breathing muscles (respiratory pattern generator, RPG) are located within the ventrolateral medulla oblongata and dorsolateral pons (Smith et al., 2009). Experiments conducted in anesthetized cats in the early 1960s suggested that the central respiratory chemoreceptors might be confined to the ventral surface of the medulla oblongata, one of the key regions being located just caudal to the trapezoid bodies (Mitchell et al., 1963). While searching for inputs to the RPG with conventional retrograde labeling methods, Smith et al. (1989) noted the presence of a thin sheet of retrogradely labeled neurons between the facial motor nucleus and the ventral medullary surface. This cell group was soon after also identified in rodents (Ellenberger and Feldman, 1990) and extends rostrally up to the trapezoid body hence its name, the retrotrapezoid nucleus. Smith et al. (1989) speculated that RTN neurons could play a role in respiratory chemosensitivity because their location was roughly in register with a previously identified acid-sensitive zone of the ventral medullary surface (Mitchell et al., 1963). Nattie and colleagues (1999) demonstrated that microinjection of neuronal excitatory substances into the RTN (glutamate, bicuculline) increases breathing, providing a solid clue that this region controls respiration. These authors also demonstrated that controlled acidification of the RTN using dialysis probes increases ventilation, consistent with the involvement of this region in respiratory chemosensitivity (Li and Nattie, 1997). This interpretation is also consistent with evidence that rat RTN neurons express c-Fos following exposure of conscious animals to high levels of CO2 (Sato et al., 1992). CO2 increased the number of Fos-positive neurons in the RTN region even when the animals were given morphine to reduce breathing, suggesting that Fos expression was not a secondary consequence of increased ventilation but more likely a direct local effect of CO2 on these neurons (Sato et al., 1992).
3. Anatomical characterization and identification of cellular markers for RTN neurons
The medullary region identified by Ellenberger & Feldman (1990) was subsequently found to contain CO2-activated glutamatergic neurons that innervate both the ventral respiratory column and the dorsolateral pons (Mulkey et al., 2004; Rosin et al., 2006; Bochorishvili et al., 2011) (represented in Fig. 1A). The most notable feature of CO2-activated RTN neurons in rodents is their extensive superficial dendrites located within 50 microns of the ventral medullary surface in a myelin-free sliver of neuropil called the marginal layer (illustrated in Fig.1C,D). These dendrites seem ideally suited for sampling pH throughout this region of highly vascularized brain tissue (Onimaru et al., 2012).
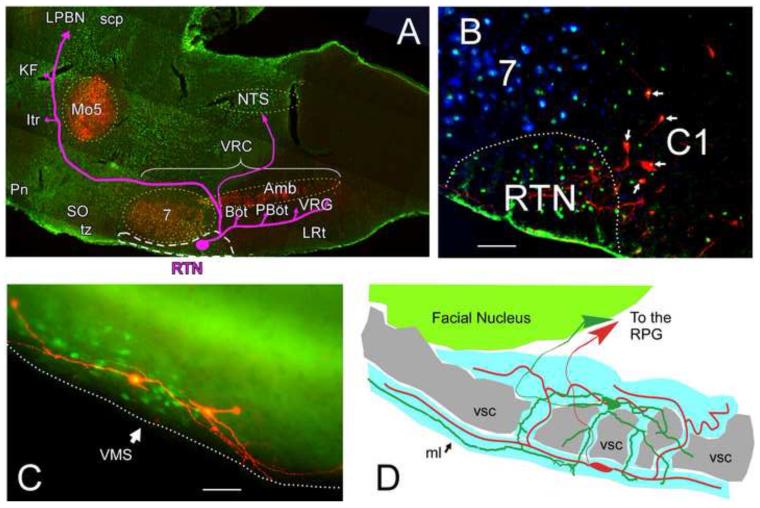
Figure 1.
Location of the Retrotrapezoid nucleus (RTN) and characteristic morphology of RTN neurons
A. Parasagittal section of rat brain. Phox2b–immunoreactive (ir) neuronal nuclei in green and choline acetyltransferase-ir (cholinergic) neurons in red. The RTN is outlined by a white dashed line and its known projections (Bochorishvili et al., 2011) are represented by the pink arrows. Other cell groups are indicated with yellow dashed outlines for anatomical reference. Abbreviations: Amb, nucleus ambiguus; Böt, Bötzinger Complex; Itr, inter trigeminal region; KF, Kölliker-Fuse; LPBN, lateral parabrachial nucleus; LRt, lateral reticular nucleus; Mo5, trigeminal motor nucleus; NTS, nucleus of the solitary tract; PBöt; Pre-Bötzinger Complex; Pn, pontine reticular nucleus; scp, superior cerebellar peduncle; SO, superior olive; tz, trapezoid body; VRG, ventral respiratory group.
B. Transverse section of rat brain through the rostral medulla oblongata showing the ventral region centered about 2 mm lateral to the midline (midline is to the right) with tyrosine hydroxylase-ir neurons in red (C1), facial motor neurons in blue and Phox2b-ir neurons in green (unpublished illustration from (Stornetta et al., 2006)). Note that the C1 neurons also show Phox2b-ir nuclei (appearing yellow, arrows). The RTN is indicated by the dashed white outline. 7, facial motor nucleus. Scale bar: 50 m
C. pH-sensitive RTN neurons filled with biotinamide (red) during whole cell recording. These neurons were recorded in tissue from a Phox2b-EGFP transgenic mouse in which expression of the fluorescent protein (green) was limited to a subset of Phox2b-expressing neurons that included the RTN (modified from (Lazarenko et al., 2009)). Scale bar: 25 m.
D. Schematic drawing of two RTN chemosensitive neurons recorded in a halothane-anesthetized adult Sprague-Dawley rat. These neurons, juxtacellularly labeled with biotinamide and reconstructed through adjacent sections with the aid of a computer driven stage and Neurolucida software (Mulkey et al., 2004), are representative of the population at large. The cell bodies of RTN neurons reside within the region outlined in blue which includes the marginal layer (ml; red cell) and bands of neuropil that are interspersed between the myelinated axon bundles of the ventral cerebellospinal tract (vsc; in gray) and include the more dorsally located green cell. Regardless of the location of their cell bodies, RTN neurons have at least one long dendrite running very close to and parallel to the ventral surface of the brain within the marginal layer. RPG: respiratory rhythm and pattern generator, a network of interconnected neurons located in Böt, pBöt, VRG, KF, Itr and NTS as defined in panel A.
The study of RTN neurons has been greatly accelerated by the discovery that these neurons express high levels of the transcription factor Phox2b throughout life (Stornetta et al., 2006)(Fig. 1B). This finding has provided a histological marker to identify RTN neurons in rodents and, putatively, in man (Rudzinski and Kapur, 2010) and has enabled the design of transgenic mice in which RTN neurons express EGFP (Lazarenko et al., 2009)(Fig. 1C). The finding has also added translational and medical relevance to the field because Phox2b mutations are the cause of the congenital central hypoventilation syndrome (CCHS), a developmental disease whose cardinal signs are sleep apnea and the loss of breathing responses to CO2 (Amiel et al., 2003; Weese-Mayer et al., 2009) and provided the basis for some of the transgenic studies referenced in section 5. Finally, the presence of Phox2b in RTN neurons has enabled us and others to take advantage of an existing Phox2-responsive artificial promoter (PRSx8)(previously developed by Hwang et al. (2001)) to modify the properties of RTN neurons using lentiviral vectors for the pharmaco- and optogenetic studies detailed in sections 6 and 7 (Lonergan et al., 2005; Abbott et al., 2009; Marina et al., 2010).
4. Electrophysiological characterization of RTN neurons in vivo and in vitro
In vivo, RTN neurons are very sensitive to CO2 (0.5 Hz or 1/20th of their dynamic range per 1/100th arterial pH unit, see Fig. 2C). Under anesthesia, their CO2 threshold is below that of the respiratory network and persists even when the activity of the RPG is silenced by morphine (Guyenet et al., 2005). The brainstem contains countless types of CO2-activated neurons that contribute in various ways to the generation of the respiratory rhythm and pattern. RTN neurons are unusual among CO2-activated brainstem neurons because their response to CO2 in vivo is virtually unaffected by pharmacological blockade of glutamatergic transmission, a condition that eliminates the activity of the RPG (Mulkey et al., 2004)(Fig. 2A,C). Their response to CO2 in slices of neonate rodent brain seems mediated primarily by changes in pH because increasing PCO2 is ineffective if pH is maintained constant by the addition of bicarbonate (Mulkey et al., 2004). In vitro the response is temperature-dependent and at near physiological temperature (30 °C), is around 40% of that observed in vivo in adult rats (Guyenet et al., 2005). In voltage clamp (-70 mV) and in the presence of tetrodotoxin, acidification causes an inward current consistent with closure of an as yet unidentified resting potassium conductance (Mulkey et al., 2004) suggesting a cell-autonomous mechanism of pH sensing.
Figure 2.
Response of RTN neurons to CO2 in vivo
A. Single unit recording of an RTN neuron in an anesthetized, paralyzed and ventilated adult rat. Traces show from top to bottom the discharge rate of a single RTN neuron recorded extracellularly (integrated rate histogram in spikes per second), the end-expiratory CO2 (a close approximation of arterial CO2) and the phrenic nerve discharge (multiunit integrated nerve recording expressed in arbitrary units, a.u.). Various concentrations of CO2 were added to the breathing mixture. The broad spectrum glutamate receptor antagonist kynurenic acid (KYN) was administered into the fourth ventricle. Lower traces are excerpts of the integrated phrenic nerve discharge (iPND) and the extracellular action potentials of the recorded neuron. The experiment shows that RTN neurons have a lower CO2 threshold for activation than the respiratory motor outflow. It also shows that the response of RTN neurons is unaffected by blocking the respiratory pattern generator with kynurenic acid, consistent with the notion that RTN neurons are activated by CO2 directly or via a local paracrine effect. A period of asphyxia (as), the most potent stimulus to breathe was imposed to ascertain that the respiratory pattern generator was completely blocked by kynurenic acid. Reproduced from Mulkey et al. (2004).
B. Single unit recording of an RTN neuron in an anesthetized, paralyzed and ventilated adult rat showing the response of a typical RTN neuron to step changes in inspiratory CO2. top: original recording; bottom: graph of steady-state unit discharge vs. end-expiratory CO2. Unpublished example from Guyenet et al. (2005).
C. Average relationship between the discharge rate of RTN neurons in vivo and end-expiratory CO2 (left) or arterial pH (right) after blockade of glutamatergic transmission with kynurenic acid in anesthetized rats. On average the RTN neurons increase their firing by 0.5 Hz (roughly 1/20th of their dynamic range) for every one hundredth unit change in arterial pH. Data from Guyenet et al. (2005).
RTN neurons are also an integrative node for additional respiratory chemoreceptors (for model see Fig. 5) which likely accounts for their greater sensitivity to CO2 in vivo than in slices although these quantitative comparisons are not totally reliable, being made between neonate neurons in vitro and adult neurons in vivo. More importantly, RTN neurons receive polysynaptic excitatory inputs from peripheral chemoreceptors and monosynaptic excitatory inputs from CNS neurons with suspected chemosensitivity such as the medullary raphe serotonin neurons (Wang et al., 2001) and hypothalamic orexin neurons (Takakura et al., 2006; Mulkey et al., 2007; Williams et al., 2007; Lazarenko et al., 2011).
Figure 5.
Presumed role of the RTN in respiratory chemoreception
In short the location, anatomical and electrophysiological properties of RTN neurons are consistent with a long line of prior evidence suggesting that this region contains pH-sensitive neurons that contribute to central respiratory chemoreception.
5. Selective chemical or genetic elimination of the RTN severely attenuates the respiratory chemoreflex
RTN neurons can be destroyed by local injection of saporin conjugated to a substance P analog (Nattie and Li, 2002; Takakura et al., 2008). Their sensitivity to this toxin presumably derives from the fact that they express neurokinin-1 receptors (Mulkey et al., 2007). Receptor internalization following agonist binding (Mantyh et al., 1995) serves as a portal for entry of the ribosomal inactivating protein saporin into the cytoplasm. Chronic bilateral lesions of RTN neurons (70% destruction) with some collateral damage of nearby C1 neurons in rats produced animals that responded very poorly to CO2 under anesthesia, a concentration of 8% CO2 in the inhaled mixture being required to elicit a phrenic nerve discharge instead of the more normal 4.5% (Takakura et al., 2008). Apart from the fact that a higher level of CO2 was required to activate breathing, the rhythm and pattern of the phrenic outflow were unremarkable suggesting that the lesion had little or no effect on the respiratory pattern generator but selectively reduced the ability of CO2 to stimulate the network. In conscious rats, inhibition of the entire RTN region by the GABA-mimetic agent muscimol reduces the respiratory response to hypoxia and hypercapnia, providing further evidence that RTN neurons are important for the chemoreflexes (Takakura et al., 2012).
A series of recent mouse genetic studies have also highlighted the importance of RTN neurons in respiratory chemoreception. These experiments are predicated on the fact that the transcription factor Phox2b is an absolute requirement for RTN neuron development. Transgenic mice carrying the disease-defining mutation for CCHS (Phox2b27ala/+) die shortly after birth of respiratory failure and fail to respond to CO2 (Dubreuil et al., 2008). These mice lack RTN Phox2b-expressing glutamatergic neurons but have no obvious defect in the other Phox2b-expressing neuronal groups suggesting that the loss of the RTN might be the critical factor underlying the respiratory failure. These authors then proceeded to ablate RTN neurons by conditional deletion of Phox2b. Phox2blox/lox mice were crossed with mice expressing Cre recombinase under the control of the Lbx1 or Egr2 promoters (Dubreuil et al., 2009). The resulting mice (Phox2blox/lox;Lbx1cre/+ and Phox2blox/lox;Egr2cre/+) had characteristics similar to the Phox2b27ala/+ mice. They exhibited severely compromised breathing and lack of respiratory chemosensitivity and died shortly after birth. In their most recent experiments, the same authors created a mouse that expresses the PHOX2B CCHS mutation in exon 3 conditionally upon cre recombination (Phox2b27Alacki mice) (Ramanantsoa et al., 2011). By crossing these animals with the above mentioned Egr2cre/+ mice, the Phox2b-27ala mutation could be selectively expressed in neurons of rhombomere 3 and 5 lineage, adding yet another level of specificity since RTN neurons seem much more vulnerable to the cytotoxic effect of the Phox2b mutation than most other Phox2b-containing neurons (Dubreuil et al., 2008). The offspring (Phox2b27Alacki; Egr2cre/+) lacked virtually all RTN Phox2b neurons but unexpectedly survived to adulthood despite a complete loss of respiratory stimulation by CO2 at birth and for approximately 3 weeks thereafter. The unexpected survival of these mice was tentatively attributed to a respiratory compensation via peripheral chemoreceptors (Ramanantsoa et al., 2011). Presumably, the preceding transgenic models (Phox2blox/lox;Lbx1cre/+ and Phox2blox/lox;Egr2cre/+) which died shortly after birth, had additional and unrecognized neuronal abnormalities besides the loss of the RTN. These abnormalities may have been more subtle and therefore remained undetected by histological examination. Taken together, these data suggest that RTN neurons are indispensable for central respiratory chemosensitivity at birth and that, against all expectations, central chemoreception is not absolutely essential to survival, at least in the sheltered environment of a laboratory. Genetic approaches such as these have their own interpretative limitations. For example, an abnormality in RTN development produced by the Phox2b-27ala mutation could produce a cascade of defects that impact on the function of other non-Phox2b chemoreceptive structures. In addition, the Phox2b-27ala mutation could produce as yet undetected but functionally important defects in Phox2b neurons other than those in the RTN.
6. Optogenetic evidence that the RTN activates breathing
We introduced an enhanced channelrhodopsin-2 (ChR2) into RTN neurons using a lentiviral vector that expresses ChR2-mCherry fusion protein under the control of the artificial promoter PRSx8. This promoter is a multimer (eight concatenated repeats) of PRS, a Phox2(a or b)-responsive cis-regulatory element of the human dopamine-hydroxylase gene (Hwang et al., 2001). This relatively strong artificial promoter was originally designed by Hwang et al. (2001) and described by these authors as driving transgene expression specifically in noradrenergic neurons. Based on the fact that PRS is a Phox2 binding site and our observation that RTN neurons express high levels of Phox2b, we injected PRSx8-ChR2- mCherry vector in their midst and were able to achieve a substantial degree of selectivity of ChR2 expression by these neurons (60-70%)(Abbott et al., 2009)(see Fig. 3B). A detailed histological examination of these cases revealed that neither GABAergic nor glycinergic neurons nor nearby facial motor neurons expressed detectable levels of the transgene despite their immediate proximity to RTN neurons. However, consistent with the known efficacy of PRSx8 in driving transgene expression in (nor)- adrenergic neurons, ChR2 was always also expressed by substantial numbers of C1 adrenergic neurons in these experiments (~30-40%)(Abbott et al., 2009). The close proximity of the C1 cells to RTN neurons is illustrated in Figure 1B. In addition, a small minority of cholinergic neurons were also typically positive, consistent with the presence of Phox2b in a subset of these cells. Photostimulation of this heterogeneous population of ChR2-expressing neurons in anesthetized rats produced strong respiratory activation that was monitored by recording the mass discharge of the phrenic nerve (PND) (Abbott et al., 2009)(Fig. 3A,C). Single-unit recordings of RTN neurons provided evidence that these cells were vigorously activated by the light pulses (Fig. 3D). In a subset of rats (N=5), the lentiviral vector was injected into the RTN after treating the animals with a saporin-based toxin that selectively reduced the number of C1 neurons that reside within the RTN region (Abbott et al., 2009). In these animals, 90 % of the ChR2- mCherry-expressing cells were RTN neurons. Photostimulation of these ChR2-positive neurons increased PND to a degree comparable to that observed in the rats in which 30-40% of the ChR2- expressing neurons were C1 cells. Based on this evidence, we concluded that selective activation of RTN neurons is capable of increasing breathing under anesthesia.
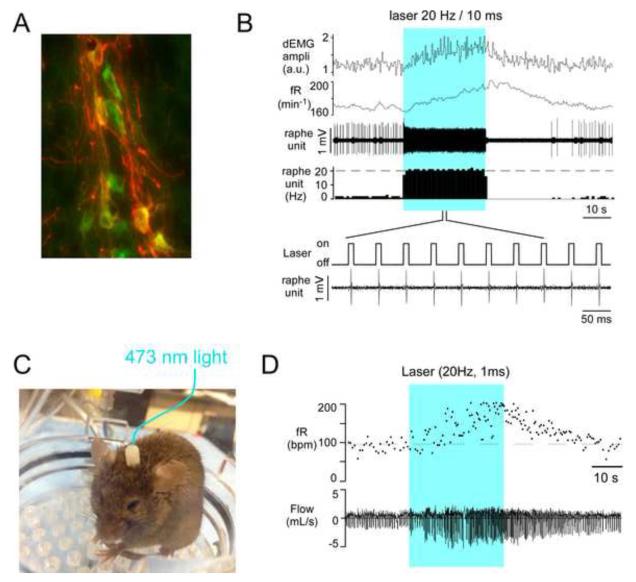
Figure 3.
Photostimulation of channelrhodopsin-2 (ChR2) expressing RTN neurons increases breathing in rats
A. Experimental design. Three weeks following injection of PRSX8-ChR2-mCherry lentivirus into RTN pulses of laser light are applied to the RTN region in anesthetized rats. An RTN unit is recorded extracellularly along with the phrenic nerve discharge (PND). The PND monitors the activity of the respiratory network. B. Example of RTN neurons that express ChR2 (red) 3 weeks after injection of PRSX8-ChR2-mCherry lentivirus. These particular RTN neurons are located within the marginal layer (for definition see “ml” in Fig. 1D) and have Phox2b-ir (green) nuclei. Modified from Abbott et al. (2009). Note that the facial motor nucleus which lies dorsal to the marginal layer does not contain any ChR2-expressing neuron. C. Photostimulation of ChR2-expressing RTN neurons elicits a robust activation of the phrenic nerve activity (PND, integrated, arbitrary units, a.u.) in an anesthetized rat. Prior to photostimulation (30 s, 20 Hz, 10 ms pulses) artificial ventilation was set at a level that lowered arterial pCO2 sufficiently to silence the activity of the respiratory network. Reproduced from Abbott et al. (2009). D. Example of a single RTN neuron that was robustly photoactivated. Activation of this and other RTN neurons also activated the phrenic nerve discharge (iPND, integrated, arbitrary unit). Reproduced from Abbott et al. (2009). A. Four weeks after injecting DIO-Ef1-ChR2-mCherry into raphe obscurus of an ePet-Cre mouse, a large number of serotonergic neurons (tryptophan-hydroxylase-ir, green) contained the ChR2-mCherry fusion protein, here revealed by mCherry-immunoreactivity (red). The transgene was selectively expressed by serotonergic neurons (>98% selectivity). Unpublished photographic example from Depuy et al. (2011). B. Simultaneous recording of a single photoactivated raphe obscurus serotonergic neuron in an anesthetized temperature-controlled (37 °C) freely breathing ePet-Cre mouse. The diaphragmatic electromyogram (dEMG) was simultaneously recorded to monitor the global effect of the laser light on the mouse respiration. From top to bottom, amplitude of dEMG, respiratory frequency (frequency of the diaphragmatic bursts), extracellular action potential of a single serotonergic neuron, integrated rate histogram of the same neuron (spikes/second, Hz). Excerpt shows that each light pulse (473nm, 5 ms) triggered a single action potential. Contrast the instantly ON-OFF response of the unit to the light pulses with the slowly developing increase in the activity of the respiratory network. Reproduced from Depuy et al. (2011). Blue block indicates laser on. C. Photostimulation of raphe obscurus in a conscious ePet-Cre mouse (injected with the same ChR2 vector). The animal is shown at rest in a plethysmography chamber designed to both photostimulate the raphe and simultaneously record breathing rate and amplitude in a non-invasive manner. The white object on the animal’s head is the exteriorized connector of an implantable optical fiber (for details see Sparta et al. (2012). Connection is made with the laser at the time of the experiment (here represented by the blue line) to transmit 473 nm light from the laser. D. Breathing activation elicited by photostimulating raphe obscurus serotonergic neurons in a quietly resting mouse (unpublished results from Depuy, Stornetta and Guyenet). The blue block signifies laser on time. Top trace: respiratory frequency (breaths per min); bottom trace: original plethysmography tracing, inspiration is down. Note the remarkable similarity of the respiratory response to raphe obscurus stimulation under anesthesia (panel B) and in the conscious state (panel D).
In a follow-up study, rats received injections of PRSx8-ChR2-mCherry lentivirus into the RTN and the ChR2-expressing neurons were photoactivated while the animals were conscious (Abbott et al., 2011). Breathing was examined by whole body plethysmography. Photostimulation produced a robust stimulation of breathing consisting of a large increase in rate and tidal volume. Breathing could also be paced by applying the light in short bursts, each burst producing a stereotyped sequence of forced expiration followed by enhanced inspiration. Pagliardini et al. (2011) also used optogenetics to photostimulate the region of the RTN using the non-specific neuronal promoter synapsin to drive ChR2 expression in the region including and surrounding the RTN in anesthetized rats. They were able to elicit increases in both inspiration and abdominal EMG indicative of active expiration during photostimulation. Together, these experiments are consistent with the possibility that RTN activates all aspects of breathing including rate, inspiratory activity and active (abdominal) expiration in both anesthetized and conscious rats.
In summary, within the rostral ventrolateral medulla the artificial promoter PRSx8 drives transgene expression preferentially in Phox2b-expressing neurons and this population consists overwhelmingly of RTN and C1 neurons, the proportion depending on the exact site of injection of the vector. Photostimulation of this mixed population increases breathing robustly under anesthesia and in the conscious state. Under anesthesia, selective activation of RTN neurons is sufficient to drive breathing. Since these neurons respond vigorously to CO2 under the same conditions, this evidence strongly suggests that these CO2-sensitive neurons must be contributing to the breathing stimulation elicited by hypercapnia. However, the above-described experiments do not exclude the possibility that the C1 neurons might also be capable of increasing breathing, especially in conscious animals.
7. Pharmacogenetic evidence that RTN mediates a large fraction of the chemoreflex
To explore the contribution of the Phox2b-expressing neurons of the ventrolateral medulla (VLM) to breathing, Marina et al. (2010) injected into this brain region a PRSx8-based lentiviral vector that expressed the allatostatin receptor (allatR). This Gi/o-linked insect G-protein coupled receptor lacks an endogenous agonist in mammals and is presumed to also lack intrinsic activity when expressed in mammalian neurons (Tan et al., 2006). Intracerebral administration of the agonist, allatostatin, activates the receptors which, in turn, activate inwardly rectifying potassium channels (gKir) and/or inhibit calcium currents (Tan et al., 2006). The discharge rate of the neurons that express the receptor is usually reduced. Presynaptic inhibition of transmitter release by allatR expressing neurons could also be expected if this receptor was present on their axonal varicosities.
In the Marina (2010) study, the transgene was presumably expressed by the same mix of neurons as in our experiments with the ChR2-mCherry lentiviral vector using the same promoter (Abbott et al., 2011), i.e. both RTN and C1 adrenergic neurons. Administration of allatostatin to conscious rats treated with the allatR-expressing lentivirus did not change the basal level of breathing suggesting that the contribution of the targeted Phox2b-expressing neurons to ventilation is low under resting conditions. By contrast, allatostatin reduced the hypercapnic respiratory stimulation by an average of 60%. This figure may well be an underestimation of the contribution of RTN neurons to central respiratory chemoreception because the rats had intact carotid bodies which also contribute notably to the chemoreflex. In addition, it is unlikely that every RTN neuron would have expressed allatRs. In these experiments as in our own study in conscious rats (Abbott et al., 2009), the respiratory effects were interpreted as the consequence of a change (inhibition with allatostatin, excitation with ChR2) in the activity of the RTN neurons and the possible contribution of the nearby C1 catecholaminergic neurons to these respiratory effects was not investigated.
8. Contribution of the glia to the chemosensitivity of RTN neurons: optogenetic and other evidence
The release of ATP contributes to the activation of RTN neurons by CO2 in vivo and in slice cultures from neonate brain (Gourine et al., 2010). In both systems, RTN glial cells, some of which are notably depolarized by acidification (Wenker et al., 2010), are the presumed source of this ATP. Here again the optogenetic methodology has contributed to the development of the theory (Gourine et al., 2010). Using a lentiviral vector that expresses ChR2 under the control of an enhanced glial fibrillary acidic protein promoter, Gourine and coworkers (2010) were able to depolarize glial cells selectively, causing ATP release, downstream activation of RTN neurons and breathing stimulation. ATP also mediates cell to cell communication between glial cells. Whether this molecule is also the gliotransmitter responsible for the activation of RTN neurons by hypercapnia is not definitively established. Application of exogeneous ATP produces mixed and relatively weak effects on RTN neurons in neonatal slices but the effect caused by strong acidification (0.5 pH unit) is attenuated (~30%) by application of ATP receptor antagonists (Mulkey et al., 2006; Mulkey and Wenker, 2011). In summary, the activation of RTN neurons by changes in the surrounding PCO2 is best explained at this time by the combination of a cell-autonomous sensitivity to acid and a paracrine mechanism involving surrounding astrocytes. The relative importance of the two mechanisms is unknown, especially in vivo, and whether ATP is the main signal between glia and RTN neurons is also uncertain.
9. Serotonergic neurons and central chemosensitivity
Serotonergic neurons are powerful regulators of breathing (Hodges and Richerson, 2010b; Ray et al., 2011). There is also considerable evidence that serotonergic neurons could be central respiratory chemoreceptors but this property seems to be restricted to subsets of these neurons whose location and projections are not yet fully defined.
Without a doubt, the serotonergic neurons located in the medulla oblongata (raphe obscurus, RO, particularly) provide a powerful excitatory drive to the RPG. Serotonin application to slices of neonate rat brain increases the frequency of respiratory-like bursts generated by the preserved portion of the RPG, microstimulation of RO produces similar effects which can be antagonized by selected serotonin receptor antagonists and serotonin activates respiratory motoneurons (Bocchiaro and Feldman, 2004; Ptak et al., 2009; Doi and Ramirez, 2010). The optogenetic methodology has also contributed to this interpretation. By injecting a Cre-dependent AAV2 into the RO of ePet-Cre mice, ChR2 can be selectively (> 97%) introduced into serotonergic neurons (Depuy et al., 2011) (Fig. 4A). Photostimulation of these serotonergic neurons produces a slowly developing but robust and protracted increase in breathing rate and amplitude under anesthesia (Depuy et al., 2011) and in conscious mice (Depuy et al. unpublished results (Fig. 4B-D)). In addition, the integrity of CNS serotonergic neurons is clearly required for full expression of the hypercapnic chemoreflex. Mice born without serotonergic neurons exhibit a number of deficits including a considerably attenuated hypercapnic chemoreflex (Hodges et al., 2008). Finally, acute and selective inhibition of serotonergic neurons in adult mice attenuates the chemoreflex substantially. This result was obtained by expressing a synthetic Gi/o protein-coupled receptor (inhibitory DREADD) that can be activated only by injecting an artificial agonist (Ray et al., 2011). Global inhibition of CNS serotonergic neurons by injection of the cognate agonist resulted in a substantial (~50%) decrease in chemoreflex sensitivity in the conscious mouse.
Figure 4.
Photostimulation of ChR2-expressing raphe obscurus serotonergic neurons increases breathing in anesthetized or conscious mice.
CO2 generates protons that activate RTN neurons directly (1) and/or indirectly through a glial mechanism (2). Orexin neurons (hypothalamus) and a subset of serotonergic raphe neurons activate RTN neurons and may be able to detect PCO2 in vivo (3). The RTN neurons also receive inputs from the carotid body via a multi-synaptic pathway that relays in the nucleus of the solitary tract (NTS) (4). The RTN neurons then elicit a respiratory response via the respiratory controller. The respiratory response consists of an increase in rate and in the amplitude of both inspiration and expiration. These three components could conceivably be regulated by distinct subsets of RTN neurons. Other brain nuclei probably contribute to the chemoreflexes along similar lines. The most thoroughly documented case consists of serotonergic neurons. Noradrenergic and orexinergic neurons are also possible candidates.
None of these experiments demonstrate that serotonergic neurons have the ability to detect CO2 in vivo because serotonergic neuron activation or inhibition could merely bias, positively or negatively, the response of the respiratory network to central or even peripheral chemoreceptors. In vivo evidence that serotonergic neurons are capable of responding to changes in CO2 has been difficult to obtain. Cultured serotonergic neurons do respond to acid and a subset of serotonergic neurons recorded in rat slices do likewise (Corcoran et al., 2009). However, these responses are small (<2Hz) and there is no evidence that such responses in vivo would evoke a change in breathing. In fact, optogenetic experiments in vivo suggest that activating RO serotonergic neurons in this frequency range does not stimulate breathing under anesthesia (Depuy et al., 2011) or in conscious mice (Depuy & Guyenet, unpublished data). ChR2- expressing RO serotonergic neurons, identified in vivo by their one-to-one entrainment to short light pulses (0.1-1 ms) were not activated by elevating CO2 despite the fact that CO2 increased breathing substantially (Depuy et al., 2011) and similarly negative results were obtained in the case of untransfected parapyramidal serotonergic neurons in rats (Mulkey et al., 2004). These recordings were made under anesthesia (halothane or isoflurane) which could have interfered with the pH-sensitivity of serotonergic neurons but, under the same conditions, rat RTN neurons respond quite well to CO2 as indicated above. In conscious cats, unit recordings of RO and raphe pallidus neurons uncovered a few putative serotonergic neurons (6 of 27) that responded to hypercapnia (Veasey et al., 1995). These cells (4 out of 4) no longer responded to CO2 when the animals were in slow-wave sleep (Veasey et al., 1995) casting doubts as to whether their excitation by hypercapnia was a cell-autonomous response or was behavior-related.
In sum, the collective evidence demonstrates that medullary serotonergic neurons activate breathing and potentiate the hypercapnic chemoreflex. The medullary raphe is complex and likely to be highly heterogeneous functionally. Although CO2 sensitivity does not appear to be a general attribute of all serotonergic neurons in vivo as originally proposed (Richerson, 2004), a minority of serotonergic cells located in raphe obscurus and pallidus respond to CO2 in vivo, at least during waking (Veasey et al., 1995) and may function as respiratory chemoreceptors. The exact location of these particular serotonergic neurons, their connectivity and the molecular mechanisms that underlie their chemosensitivity remain to be characterized.
10. Conclusions: a solution to the century old central respiratory chemosensitivity conundrum?
Optogenetic technology has contributed to the notion that RTN and serotonergic neurons are powerful regulators of breathing. In addition, it has played a crucial role in suggesting that the glia also contributes to this aspect of brain function. The power of the optogenetic methodology resides in its capacity to selectively target and finely control the activity of small groups of like cells within brain regions that are extremely heterogeneous such as the reticular formation of the brainstem. However, as should be clear from this review, a single technique, even as powerful as optogenetics, is not sufficient to solve an issue of integrative neuroscience as complex as central chemosensitivity.
The contribution of RTN neurons to respiratory chemoreception is supported by convergent neurophysiological, neuroanatomical and genetic studies in vivo and in vitro. Collectively, the data suggest that the specialized connectivity of RTN neurons, their glutamatergic nature and their relatively high sensitivity to CO2 are determinant factors in explaining their large contribution to the chemoreflexes (100% during the first three weeks after birth, at least 60% thereafter in rodents) (Marina et al., 2010; Ramanantsoa et al., 2011). Their CO2 sensitivity in vivo probably relies on three mechanisms whose relative importance is unclear: a cell autonomous sensitivity to acid, a paracrine effect of acid presumably mediated by a subtype of surrounding glial cells and synaptic inputs from the carotid bodies and, possibly, other CO2-sensitive CNS neurons. The molecular mechanisms responsible for the cell-autonomous and paracrine effects of acid on RTN neurons are not understood sufficiently at this time to assess whether they are unique or mundane.
Evidence that serotonergic neurons regulate breathing and the chemoreflexes is equally strong and is also supported by convergent genetic, optogenetic and pharmacological data but the contribution of the glia to the chemosensitivity of the serotonergic neurons has not been explored. Serotonergic neurons have a particularly strong modulatory influence on the chemoreflex, this reflex being reduced by about half when their activity is silenced pharmacogenetically. As indicated above, the only missing piece is the identification of the subset of serotonergic neurons that possess the ability to respond to changes in PCO2 in vivo. Few if any CO2-responsive neurons have so far been detected in vivo within raphe obscurus but the bulk of the CO2-responsive serotonergic neurons could be residing in other portions of the medullary raphe such as raphe magnus. Figure 5 summarizes current ideas regarding the role of the retrotrapezoid nucleus in respiratory control. A similar scheme has been proposed for the medullary serotonergic and other neurons (Ray et al., 2011; Nattie, 2011).
Since RTN and serotonergic neurons seem to be each responsible for at least 50% of central chemosensitivity in adult rodents (Marina et al., 2010; Ramanantsoa et al., 2011; Ray et al., 2011), one might think that the central respiratory conundrum has been solved. However, a number of experimental discrepancies need to be resolved before one could accept such a conclusion. For example, the mild and quasi-ubiquitous respiratory stimulation produced by acidifying discrete subregions of the lower brainstem or hypothalamus using microdialysis is not accounted for. These effects are especially difficult to reconcile with the observation that massive and selective reduction of the chemoreflexes can be produced by lesions of a single small structure like the raphe or the RTN while a normal breathing pattern is apparently retained (Ramanantsoa et al., 2011; Ray et al., 2011).
At the cellular level, one way out of the central respiratory chemosensitivity conundrum is to assume that the combined effect of minor changes in pH on multiple ion channels and other proteins has little overall effect in most neurons in vivo either because these effects cancel one another (neuronal homeostasis) or, more likely, because they concern neurons that lack the proper connections to exert a notable influence on the respiratory centers. Another solution to the conundrum is to postulate that the random effect of acid on the large number of excitatory and inhibitory neurons that contribute to respiratory rhythm and pattern function at cross purposes (network homeostasis) and that sensitive control of the respiratory network by CO2 requires a specialized circuitry that has been shaped by evolution for this purpose. This view is opposed by many respiratory physiologists on the basis of two sets of observations. The first is, once more, that topical acidification of many parts of the brainstem or hypothalamus using microdialysis produces small stimulatory effects on breathing. The second is that lesions of a variety of brain neurons besides RTN or the raphe (e.g. orexin, locus coeruleus) attenuate the whole animal chemoreflexes (Nattie, 2011). The second objection can be provisionally countered by assuming that the neuronal systems that are being destroyed provide facilitatory inputs to the respiratory centers and may not be directly sensitive to CO2 in vivo. This question can only be answered by testing whether these neurons actually respond to a meaningful extent to CO2 in vivo. Optogenetics may be useful to gauge how meaningful their activation by CO2 actually is because this methodology should allow testing whether a respiratory response can be produced by activating a specific cluster of neurons to the same extent as a CO2 challenge. For example, the locus coeruleus is one of many structures that are postulated to be central chemoreceptors (Hartzler et al., 2008; Gargaglioni et al., 2010) but their activation by CO2 in vivo is extremely weak (Elam et al., 1981). Optogenetics might answer whether increasing tonic locus coeruleus discharge rate by ~20%, the effect elicited by adding 5% CO2 to the breathing mixture of a rodent in vivo (Elam et al., 1981), has a detectable effect on breathing. The concept of network homeostasis alluded to above could perhaps account for the effects produced by artificially exposing a single small part of the network to acidification as is done with microdialysis. Another possibility that could explain the small respiratory stimulation caused by acidifying any of the many regions that contain the respiratory network would be that the nerve terminals of the chemoreceptors (RTN, serotonergic neurons, others), which are present throughout these regions are sensitive to acid, just like their cell bodies. Neurons can indeed express the same receptors on their somata and their terminals, for example 2A adrenergic receptors in CNS and autonomic noradrenergic neurons (Trendelenburg et al., 2003).
In conclusion, the chemoreception conundrum is not solved. There is uniform agreement that many types of CNS neurons modulate the breathing response to hypercapnia but limited evidence that these neurons are respiratory chemoreceptors which presupposes that these cells are capable of responding directly to acid and CO2 in vivo and that their response to this variable produces a notable change in breathing. Evidence is most developed in the case of RTN and serotonergic neurons but cannot be viewed as definitive until the molecular mechanisms of pH-sensitivity and the contribution of the glia are fully understood. The advent of ever more numerous transgenic models and viral vectors is ushering in an era of optogenetic and pharmacogenetic experiments that promises to expand our understanding of other neuronal populations such as the locus coeruleus, orexin neurons and many others that control of respiration and may also function as central chemoreceptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J. Neurosci. 2011;31:16410–16422. doi: 10.1523/JNEUROSCI.3280-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J. Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat. Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc. Natl. Acad. Sci. USA. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG. Pre-Botzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J. Comp Neurol. 2011;520:1047–1061. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. Two-pore domain k(+) channels and their role in chemoreception. Adv. Exp Med. Biol. 2010;661:15–30. doi: 10.1007/978-1-60761-500-2_2. [DOI] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir. Physiol. Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J. Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J. Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc. Natl. Acad. Sci. USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in phox2b mutants targeting retrotrapezoid nucleus neurons. J. Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J. The role of the central chemoreceptors: A modeling perspective. Respir Physiol Neurobiol. 2010 doi: 10.1016/j.resp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and Hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic sympathetic nerve. Brain Res. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory repsonse to CO2/H+ J. Appl. Physiol. 2010;108:989–994. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir. Physiol Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J. Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J. Comp. Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane JS, Priestley JG. The regulation of the lung-ventilation. J. Physiol. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv. Exp Med. Biol. 2008;605:333–337. doi: 10.1007/978-0-387-73693-8_58. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir. Physiol Neurobiol. 2010a;173:256–263. doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J. Appl. Physiol. 2010b;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Id BR, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J. Physiol. 2010 doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Carlezon WA, Jr., Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum. Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J. Comp. Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Stornetta RL, Bayliss DA, Guyenet PG. Orexin A activates retrotrapezoid neurons in mice. Respir. Physiol. Neurobiol. 2011;175:283–287. doi: 10.1016/j.resp.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie EE. Focal central chemoreceptor sensitivity in the RTN studied with a CO2 diffusion pipette in vivo. J. Appl. Physiol. 1997;83:420–428. doi: 10.1152/jappl.1997.83.2.420. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J. Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan T, Teschemacher AG, Hwang DY, Kim KS, Pickering AE, Kasparov S. Targeting brain stem centers of cardiovascular control using adenoviral vectors: impact of promoters on transgene expression. Physiol. Genomics. 2005;20:165–172. doi: 10.1152/physiolgenomics.00120.2004. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: Substance P-evoked internalization of its receptor in the rat striatum in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J. Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J. Appl. Physiol. 1963;18:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J. Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J. Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Wenker IC. Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp. Physiol. 2011;96:400–406. doi: 10.1113/expphysiol.2010.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog. Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie E. Julius H. Comroe, Jr., distinguished lecture: central chemoreception: then … and now. J. Appl. Physiol. 2011;110:1–8. doi: 10.1152/japplphysiol.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J. Appl. Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J. Physiol. 2002;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie G, Li A. Multiple central chemoreceptor sites: cell types and function in vivo. Adv. Exp. Med. Biol. 2008;605:343–347. doi: 10.1007/978-0-387-73693-8_60. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Relationship between the distribution of Phox2b expressing cells and blood vessels in the parafacial region of the ventral medulla of neonatal rats 1. Neurosci. 2012 doi: 10.1016/j.neuroscience.2012.03.037. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J. Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J. Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO2 Chemosensitivity in Conditional Phox2b Mutants. J. Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition 1. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J. Comp. Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Rudzinski E, Kapur RP. PHOX2B immunolocalization of the candidate human retrotrapezoid nucleus. Pediatr. Dev. Pathol. 2010;13:291–299. doi: 10.2350/09-07-0682-OA.1. [DOI] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J. Appl. Physiol. 1992;73:96–100. doi: 10.1152/jappl.1992.73.1.96. [DOI] [PubMed] [Google Scholar]
- Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: Evidence and implications for ventilatory control. Respir. Physiol. Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos. Trans. R. Soc Lond B Biol. Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J. Comp. Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van ZR, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 2012;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J. Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J. Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, De Paula PM, Menani JV, Colombari E. Control of breathing and blood pressure by parafacial neurons in conscious rats. Exp. Physiol. 2012 doi: 10.1113/expphysiol.2012.065128. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J. Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M, Albright TD, Callaway EM. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Philipp M, Meyer A, Klebroff W, Hein L, Starke K. All three α2-adrenoceptor types serve as autoreceptors in postganglionic sympathetic neurons. Naunyn Schmiedebergs Arch. Pharmacol. 2003;368:504–512. doi: 10.1007/s00210-003-0829-x. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J. Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WG, Tiwari JK, Bradley SR, Zaykin AV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J. Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Rand CM, Berry-Kravis EM, Jennings LJ, Loghmanee DA, Patwari PP, Ceccherini I. Congenital central hypoventilation syndrome from past to future: model for translational and transitional autonomic medicine. Pediatr. Pulmonol. 2009;44:521–535. doi: 10.1002/ppul.21045. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J. Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc. Natl. Acad. Sci. USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]