Abstract
Dopaminergic and glutamatergic neurotransmissions in the striatum play an essential role in motor- and reward-related behaviors. Dysfunction of these neurotransmitter systems has been found in Parkinson's disease, schizophrenia, and drug addiction. Cyclin-dependent kinase 5 (CDK5) negatively regulates postsynaptic signaling of dopamine in the striatum. This kinase also reduces the behavioral effects of cocaine. Here we demonstrate that, in addition to a postsynaptic role, CDK5 negatively regulates dopamine release in the striatum. Inhibitors of CDK5 increase evoked dopamine release in a way that is additive to that of cocaine. This presynaptic action of CDK5 also regulates glutamatergic transmission. Indeed, inhibition of CDK5 increases the activity and phosphorylation of N-methyl-d-aspartate receptors, and these effects are reduced by a dopamine D1 receptor antagonist. Using mice with a point mutation of the CDK5 site of the postsynaptic protein DARPP-32 (dopamine- and cAMP-regulated phosphoprotein, molecular mass of 32 kDa), in the absence or in the presence of a dopamine D1 receptor antagonist, we provide evidence that CDK5 inhibitors potentiate dopaminergic transmission at both presynaptic and postsynaptic locations. These findings, together with the known ability of CDK5 inhibitors to prevent degeneration of dopaminergic neurons, suggest that this class of compounds could potentially be used as a novel treatment for disorders associated with dopamine deficiency, such as Parkinson's disease.
Keywords: dopamine, glutamate, Parkinson's disease
The striatum plays a crucial role in processing information related to motor function and reward- and goal-oriented behaviors. The striatum integrates dopaminergic inputs from midbrain nuclei and glutamatergic inputs from the neocortex and the thalamus (1). The pathophysiology of several psychiatric and neurological disorders, including Parkinson's disease, schizophrenia, and drug addiction, arises from improper function of the striatum because of a loss of dopamine-producing neurons or an imbalance of dopamine and glutamate transmissions. A more complete understanding of the mechanisms that regulate dopamine/glutamate interactions in the striatum is likely to improve our understanding of the physiology of the striatum under normal and pathological conditions. Recent biochemical evidence showed that the Ser/Thr protein kinase cyclin-dependent kinase 5 (CDK5) inhibits postsynaptic dopaminergic signaling in the striatum. Indeed, by phosphorylating the striatal-enriched postsynaptic protein DARPP-32 (dopamine- and cAMP-regulated phosphoprotein, molecular mass of 32 kDa) at Thr75 and thereby converting this protein into an inhibitor of cAMP-dependant protein kinase A (PKA), CDK5 counteracts dopamine D1 receptor/PKA-mediated signaling (2, 3). In addition, behavioral effects of cocaine are enhanced by striatal infusion of CDK5 inhibitors (4). Thus, CDK5 seems to play a major role in regulating postsynaptic dopamine signaling in the striatum. This study was therefore undertaken to address whether this protein kinase also regulates dopaminergic transmission at a presynaptic location and, moreover, to study the role of CDK5 in glutamatergic synaptic transmission in the striatum.
Methods
All experiments that used animals were approved by the Institutional Animal Care and Use Committee.
Electrophysiology in Brain Slices. Experiments were performed by using methods as described (5, 6). Briefly, coronal brain slices (400 μm thick) containing the striatum and neocortex were prepared with a microslicer (VT1000S; Leica, Deerfield, IL) from C57Bl6 mice aged 22-29 d. Slices were incubated at 32°C in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid containing (in mM) 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.3 MgCl2, 2.4 CaCl2, 10 glucose, and 26 NaHCO3, pH 7.4. Slices were transferred to a recording chamber (Warner Instruments, Hamden, CT) mounted on an upright microscope (Olympus, Melville, NY) and were continuously perfused with oxygenated artificial cerebrospinal fluid at 32°C. Medium spiny neurons in the striatum were visualized within the brain slices with Nomarski-type differential interference contrast optics combined with infrared videomicroscopy. Whole-cell voltage-clamp recordings of medium-sized neurons in the dorsal striatum were made with patch electrodes filled with a solution containing (in mM) 140 CsCl, 2 MgCl2, 1 CaCl2, 10 Hepes, 10 EGTA, 2 MgATP, 0.3 Na3GTP, pH 7.3. Whole-cell membrane currents were recorded with an Axopatch 200B (Axon Instruments, Foster City, CA) driven by a personal computer. Neurons were voltage-clamped at -80 mV. Data were acquired and analyzed with pclamp 8 software (Axon Instruments). Numerical values are expressed as means ± SEM, with n indicating the number of medium spiny striatal neurons tested. Data are expressed as percent of the baseline measured for each cell during the 5 min preceding application of the drug examined. Drugs were applied in the perfusion solution. Excitatory postsynaptic currents (EPSCs) were evoked every 15 sec by electrical stimulation of the slice by using a patch electrode filled with artificial cerebrospinal fluid positioned on the slice surface in the vicinity of the recorded neuron. EPSCs mediated by N-methyl-d-aspartate (NMDA) receptors were recorded in 0.1 mM MgCl2 to reduce magnesium blockade of the receptor and in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM) and bicuculline (10 μM) to inhibit α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-EPSCs and γ-aminobutyric acid type A inhibitory postsynaptic currents, respectively. AMPA-EPSCs were recorded in the presence of dl-2-amino-5-phosphonovaleric acid (100 μM) to inhibit NMDA-EPSCs and bicuculline (10 μM).
Amperometry in Brain Slices. Parasagittal brain slices were prepared as above. Amperometric detection of dopamine release was performed with methods similar to those described (7). Carbon fiber electrodes (WPI, Sarasota, FL; 10-μm diameter) had an active part of 250 μm that was positioned within the striatum in the brain slice. A constant voltage of +500 mV was applied to the carbon fiber through an Axopatch 200B amplifier (Axon Instruments), and currents were recorded with the same amplifier. A stimulating electrode (patch electrode filled with artificial cerebrospinal fluid) was placed on the slice surface in the vicinity of the carbon fiber electrode. Stimulation consisted of a single pulse (0.1 ms, 14-18 μA) applied every minute, which evoked a response corresponding to oxidation of dopamine at the surface of the electrode. When the carbon fiber electrode was held at 0 mV, stimulation of the slice did not produce any current. We performed several tests to determine whether the stimulation-evoked response at +500 mV was due to impulse flow-dependent release of dopamine as described (8, 9). (i) The anatomical specificity was shown by the observation that this response was obtained in the striatum, which is densely innervated by dopamine-containing terminals, and not in the adjacent thalamus, which does not receive a dense innervation by dopaminergic neurons. (ii) This response was blocked by the sodium-channel blocker tetrodotoxin, which is characteristic of action potential-dependent neurotransmitter release. (iii) The response was modulated by compounds known to affect dopamine release, such as cocaine, nomifensine, and D2 receptor agonists and antagonists. (iv) Because monoamine oxidase operates in the minute time scale to generate dopamine degradation products (such as 3,4-dihydroxyphenylacetic acid), it is unlikely that such compounds contributed to the response following stimulation, given its rapid time course (ms time scale) (10).
In Situ Hybridization. Cryostat sections (12 μm) made from frozen C57Bl6 mice brains were hybridized as described (11) with [α-35S]UTP-labeled riboprobes prepared by in vitro transcription from cDNA clones corresponding to mouse CDK5 and p35. Sections were then exposed to Biomax MR films (Kodak) for 2-14 d. For double in situ hybridization experiments, sections were hybridized with a combination of [α-35S]UTP-labeled riboprobes for CDK5 and p35 and digoxigenin-labeled probes for substance P, enkephalin, choline acetyl transferase, or somatostatin. After hybridization and washing, the digoxigenin signal was detected, and the sections were dipped into Ilford K5 emulsion. After 8 weeks, the sections were developed and mounted without counterstaining.
Biochemical Studies on NMDA Receptors and DARPP-32 Phosphorylation. Striatal brain slices (300 μm) were prepared from adult male C57Bl6 mice, D1 receptor knockout mice (12), Thr75Ala-DARPP-32 mice, and their wild-type littermates (13). In some studies, striatal slices were made from each hemisphere of unilaterally 6-hydroxydopamine (6-OHDA)-lesioned rats. The efficacy of the 6-OHDA lesion was verified by Western blotting against tyrosine hydroxylase. In all slices from 6-OHDA lesioned-hemispheres, the levels of tyrosine hydroxylase were <5% of the levels in intact hemispheres (data not shown). For studies on the phosphorylation state of the NR1 subunit of the NMDA receptor and DARPP-32, slices were incubated in Krebs buffer at 30°C under constant oxygenation (95% O2/5% CO2) for 60 min, with a change of buffer after 30 min. The slices were thereafter treated with either roscovitine (10 μM), butyrolactone I (10 μM), or amphetamine (10 μM) for 10 min. In experiments that used SCH23390 (10 μM), this compound was applied 10 min before roscovitine (10 μM) or butyrolactone I (10 μM). At the end of the treatment, the slices were rapidly frozen, sonicated in 1% SDS, resolved by 10% SDS/PAGE, and transferred to a poly(vinylidene difluoride) membrane followed by immunoblot analysis (14). Immunoblot analysis was performed with phospho-specific antibodies against Ser897-NR1, Thr34-DARPP-32, or Thr75-DARPP-32, or with antibodies against total NR1 (Upstate Biotechnology, Lake Placid, NY) or total DARPP-32, and detected by enhanced chemiluminescence (ECL; Amersham Pharmacia). Autoradiograms were quantified with nih image 1.62. Results are given as means ± SEM. The number of slices per group varied from 8 to 17.
Drugs. Drugs were obtained from Sigma (6-cyano-7-nitroquinoxaline-2,3-dione, Bicuculline, dl-2-amino-5-phosphonovaleric acid, cocaine, SCH23390, amphetamine). Roscovitine (Calbiochem) and Butyrolactone I (Biomol, Plymouth Meeting, PA) selectively inhibit CDK5 activity with IC50 values of ≈0.5-2.0 μM (15-17).
Results
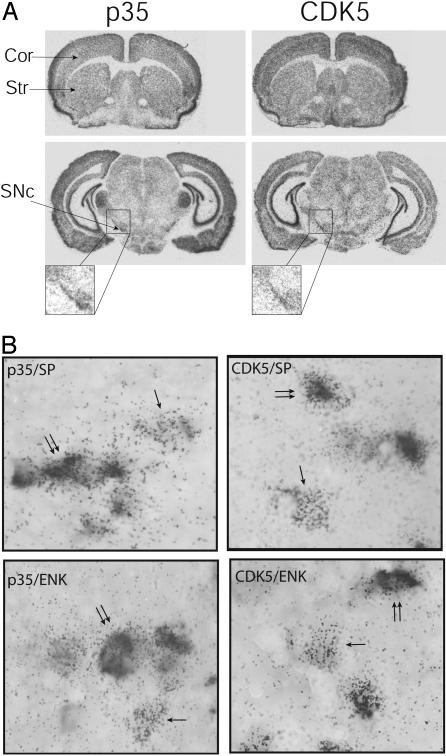
To identify potential sites of regulation of the physiology of the striatum by CDK5, we examined the distribution of this kinase and its coactivator, p35, in the mouse brain. Using in situ hybridization techniques, we found that CDK5 and p35 mRNAs are expressed in the striatum and in the substantia nigra pars compacta and neocortex, which provide the main dopaminergic and glutamatergic inputs to the striatum, respectively (1) (Fig. 1A). Within the striatum, two projection pathways have been identified, one that contains high levels of substance P and directly innervates the substantia nigra pars reticulata (striatonigral pathway) and another that contains enkephalin and indirectly projects to this structure through the globus pallidus and the subthalamic nucleus (striatopallidal pathway) (1). Using double in situ hybridization approaches, we demonstrated the presence of CDK5/p35 mRNAs in substance P- and enkephalin-containing projection neurons (Fig. 1B) and acetylcholine- and somatostatin-containing interneurons (data not shown). Thus, CDK5 and p35 are expressed in the two main inputs to the striatum, the cerebral cortex and the substantia nigra pars compacta, and in the two principal striatal outputs, the striatonigral and striatopallidal pathways. These results raised the possibility that CDK5 and p35 might be able to modulate neurotransmission at multiple locations within the striatum and thus play a major role in the physiology of this nucleus.
Fig. 1.
Expression of CDK5 and p35 in the brain. (A) In situ hybridization of mRNAs coding for CDK5 and its activator p35 in coronal sections of mouse brain. CDK5 and p35 are present in several brain structures, including the neocortex (Cor), striatum (Str), and substantia nigra zona compacta (SNc). (B) Double in situ hybridization showing that CDK5 (silver grains) and p35 (silver grains) are present in the two main populations of projection neurons: those containing substance P (SP, digoxigenin labeling) and those containing the precursor of enkephalin, preproenkephalin A (PPA, digoxigenin labeling). Single arrows point to neurons labeled only with p35 or CDK5. Double arrows point to double-labeled neurons.
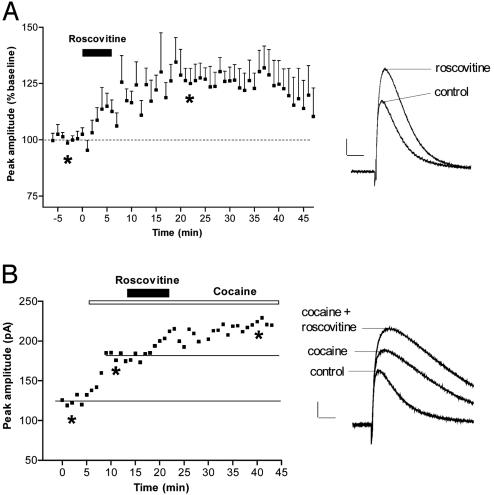
In light of the observations that CDK5/p35 mRNAs are present in dopamine-containing neurons and that intrastriatal infusion of CDK5 inhibitors potentiates the behavioral response to the psychostimulant cocaine (4), we hypothesized that CDK5 could modulate dopamine release in the striatum. To test this possibility, we evaluated the effect of CDK5 inhibition on dopamine release, which we evoked by stimulation of nigrostriatal fibers within the striatum and monitored by using amperometry coupled to carbon-fiber electrodes. We found that the specific CDK5 inhibitor roscovitine (15) applied in the perfusion solution increased evoked dopamine release (Fig. 2A). Given that cocaine raises levels of released dopamine in the striatum by blocking the dopamine transporter in axon terminals and that CDK5 inhibitors potentiate behavioral effects of cocaine (4), we tested the possibility that CDK5 inhibitors could potentiate the increased levels of released dopamine caused by cocaine. Application of cocaine caused the classical prolongation of the decay time of evoked dopamine release (Fig. 2B). Roscovitine applied in conjunction with cocaine further increased this signal (Fig. 2B; 139.3 ± 6.9%, n = 3). This result suggests that the enhancement of the behavioral effect of cocaine by CDK5 inhibitors could result from both postsynaptic (4) and presynaptic (present data) mechanisms.
Fig. 2.
CDK5 inhibition increases dopamine release in striatal slices. (A) Time course of the effect of 2 μM roscovitine (n = 6) on the amplitude of the evoked dopamine release (Left) and example of amperometric recordings in one slice before and after roscovitine. (B) Effect of coapplication of 5 μM cocaine and 2 μM roscovitine on evoked dopamine release in one slice. (Scale bars = 40 pA, 100 ms.) *, time points chosen for illustration with records.
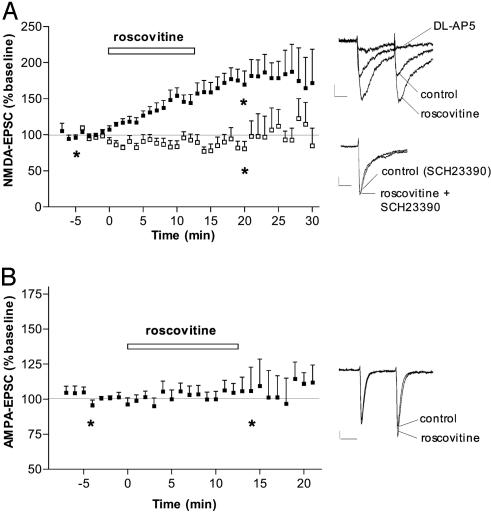
CDK5 and p35 are also expressed in the two main populations of striatal projection neurons and in their main glutamatergic afferent, the neocortex. We therefore evaluated the role of CDK5 in glutamatergic transmission in medium spiny neurons and the potential contribution of dopamine in the observed effects. Pharmacologically isolated AMPA receptor- or NMDA receptor-mediated EPSCs were evoked by electrical stimulation of glutamatergic fibers within the striatum. Roscovitine produced a robust increase in NMDA-EPSC amplitude (Fig. 3A) with a comparable time course to that observed for dopamine release (Fig. 2 A). A second CDK5 inhibitor, butyrolactone I (10 μM) (16), produced a similar effect: NMDA-EPSC amplitude was increased to 145.6 ± 8% relative to baseline (n = 3). By contrast, AMPA-EPSCs were not affected by roscovitine (Fig. 3B) or by butyrolactone I (10 μM, 96.9 ± 12.2%, n = 6), which suggests that inhibition of CDK5 does not directly affect the release of glutamate. A postsynaptic rather than a direct presynaptic effect of CDK5 on glutamatergic transmission in the increased NMDA-EPSC is further supported by the observation that the EPSC2/EPSC1 ratio, which is a good indicator for presynaptic effects, was not affected by roscovitine for either NMDA-EPSCs or AMPA-EPSCs (records in Fig. 3 A and B).
Fig. 3.
CDK5 inhibition increases NMDA receptor but not AMPA receptor activity. (A) (Left) Time course of the effect of the CDK5 inhibitor roscovitine on NMDA-EPSC amplitude (results obtained with 10 μM and 20 μM roscovitine were identical and have been combined) in control conditions (▪, n = 16) and in the presence of the dopamine D1 receptor antagonist SCH23390 (10 μM; □, n = 6). (Right) Superimposed NMDA-EPSCs recorded in representative striatal neurons before (control) and after roscovitine in the absence of SCH23390 (Upper) or in its presence (Lower). The EPSCs are mediated by NMDA receptors as evidenced by their blockade by the NMDA receptor antagonist dl-2-amino-5-phosphonovaleric acid (100 μM). No change in EPSC2/EPSC1 was observed after the addition of roscovitine. (B) Time course of the effect of roscovitine (20 μM; n = 9) on AMPA-EPSC amplitude. (Right) Superimposed AMPA-EPSCs recorded in a representative striatal neuron before (control) and after roscovitine. (Scale bars = 20 pA, 20 ms.) *, Time points chosen for illustration with records.
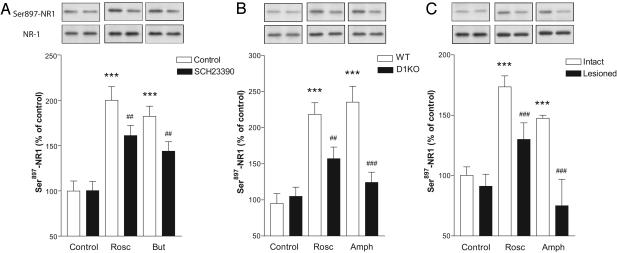
NMDA receptor activity is regulated by several mechanisms, one of which is the phosphorylation of the receptor by protein kinases (18). We therefore examined the phosphorylation level of the NR1 subunit of the NMDA receptor, which is highly expressed in the striatum (19, 20), after the incubation of striatal brain slices with CDK5 inhibitors. We found that roscovitine and butyrolactone I increased the phosphorylation levels of Ser897-NR1 when compared with paired brain slices incubated in control solution (no inhibitor added) (Fig. 4A). Thus, our results show a good correlation between the phosphorylation level of NMDA receptors and their activity after CDK5 inhibition: i.e., increased NMDA receptor activity is accompanied by an increased phosphorylation level of the NR1 subunit.
Fig. 4.
Inhibition of CDK5 increases NR1 phosphorylation levels and involves dopamine D1 receptors. (A) Effect of 10 μM roscovitine (Rosc), 10 μM butyrolactone I (But), and 10 μM SCH23390 on Ser897-NR1 phosphorylation. ***, P < 0.001 compared with control slices without SCH23390; ##, P < 0.01 compared with roscovitine or butyrolactone I in the absence of SCH23390. (B) Effect of 10 μM roscovitine and 10 μM d-amphetamine (Amph) on phosphorylation of Ser897-NR1 in dopamine D1 receptor knockout (D1KO) mice. ***, P < 0.001 compared with slices from wild-type (WT) mice; ##, P < 0.01; ###, P < 0.001 compared with roscovitine or amphetamine in slices from wild-type animals. (C) Effect of 10 μM roscovitine and 10 μM d-amphetamine on phosphorylation of Ser897-NR1 in intact and dopamine-depleted striatal slices from unilaterally 6-OHDA-lesioned rats. ***, P < 0.001 compared with control intact slices; ###, P < 0.001 compared with roscovitine or amphetamine in intact slices. All statistical analyses were made by using one-way ANOVA followed by Newman-Keul's test for pairwise comparisons.
Because CDK5 inhibition increases evoked dopamine release (Fig. 2 A) and dopamine D1 receptor activation increases NMDA receptor activity and phosphorylation state (5, 21-23), we examined a possible involvement of dopamine D1 receptors in the increased NMDA-EPSC amplitude and NMDA receptor phosphorylation after CDK5 inhibition. In the presence of the dopamine D1 receptor antagonist SCH23390, the increase in NMDA-EPSC amplitude produced by roscovitine was reduced (Fig. 3A), and the increases in phosphorylation of Ser897-NR1 after roscovitine or butyrolactone I were significantly attenuated (Fig. 4A). To further ascertain the participation of D1 receptors in the biochemical effect of CDK5 inhibition, we used mice lacking the D1 receptor. We found that the effect of roscovitine on the phosphorylation of Ser897-NR1 was significantly reduced in D1 receptor knockout mice (Fig. 4B). We obtained similar results with butyrolactone I: the phosphorylation level of Ser897-NR1 was increased to 207.8 ± 42.7% in wild-type animals and 149.6 ± 16.6% in D1 receptor knockout mice. Thus, inhibition of CDK5 increases NMDA receptor activity and phosphorylation state in the striatum, partly through activation of dopamine D1 receptors.
To investigate further the importance of dopamine release in the biochemical effect of CDK5 inhibition, we examined whether it depended on intact dopaminergic innervation. Animals were unilaterally treated with 6-OHDA, a toxin that produces a selective degeneration of dopamine-containing neurons. The increase in phosphorylation of Ser897-NR1 produced by roscovitine was significantly reduced in the 6-OHDA-lesioned hemispheres as compared with the intact hemispheres (Fig. 4C). In addition, the psychostimulant d-amphetamine, which releases dopamine from terminals and inhibits its reuptake, increased the phosphorylation level of Ser897-NR1 (Fig. 4 B and C). This effect was reduced in D1 receptor knockout mice (Fig. 4B) and absent in dopamine-denervated striata (Fig. 4C). These results demonstrate that the ability of amphetamine and roscovitine to increase phosphorylation of Ser897-NR1 depends in part (roscovitine) or entirely (d-amphetamine) on dopaminergic innervation and dopamine D1 receptors.
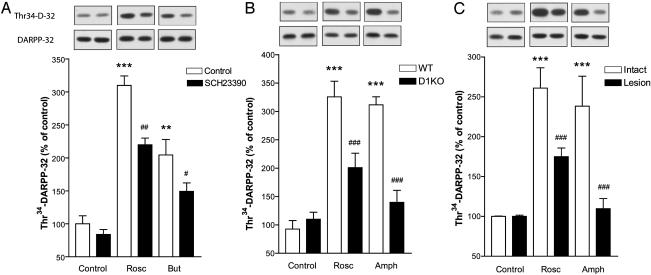
The striatal-enriched postsynaptic protein DARPP-32 plays a central role in dopamine signaling (3). Indeed, activation of D1 receptors increases PKA-mediated phosphorylation of DARPP-32 at Thr34. This effect is counteracted by CDK5-mediated phosphorylation of DARPP-32 at Thr75 (2). In agreement with previous results (2), inhibition of CDK5 by roscovitine or butyrolactone I increased the phosphorylation state of DARPP-32 at Thr34 (Fig. 5A) and decreased phosphorylation at Thr75 (data not shown). Because CDK5 inhibition increases dopamine release, we examined whether phosphorylation of DARPP-32 at Thr34 produced by CDK5 inhibitors involved dopamine D1 receptors. Although SCH23390 did not significantly alter the phosphorylation level of DARPP-32 in control slices, it significantly attenuated the increase produced by roscovitine and butyrolactone I (Fig. 5A). In slices from mice lacking D1 receptors, phosphorylation of Thr34-DARPP-32 produced by roscovitine was significantly reduced (Fig. 5B). We obtained similar results with butyrolactone I: phosphorylation levels of Thr34-DARPP-32 were increased in wild-type animals (179.3 ± 32.9% of control) but not in D1 receptor knockout mice (111.7 ± 8.7% of control). The increase in phosphorylation of Thr34-DARPP-32 produced by roscovitine was significantly reduced in 6-OHDA-lesioned hemispheres as compared with intact hemispheres (Fig. 4C). d-amphetamine also increased the phosphorylation level of Thr34-DARPP-32, and this effect was reduced in D1 receptor knockout mice and in 6-OHDA-lesioned hemispheres (Fig. 5 B and C). Thus, effects of CDK5 inhibition on Thr34-DARPP-32 are partly mediated by dopamine D1 receptor activation and partly depend on intact dopaminergic innervation.
Fig. 5.
Inhibition of CDK5 increases Thr34-DARPP-32 phosphorylation levels partly through dopamine D1 receptors. (A) Effect of 10 μM SCH23390 on the increase of Thr34-DARPP-32 phosphorylation by 10 μM roscovitine (Rosc) and 10 μM butyrolactone I (But). **, P < 0.01; ***, P < 0.001 compared with control slices without SCH23390; #, P < 0.05; ##, P < 0.01 compared with roscovitine or butyrolactone I in the absence of SCH23390. (B) Effect of 10 μM roscovitine and 10 μM d-amphetamine (Amph) on phosphorylation of Thr34-DARPP-32 in dopamine D1 receptor knockout (D1KO) mice. ***, P < 0.001 compared with slices from wild-type (WT) mice; ###, P < 0.001 compared with roscovitine or amphetamine in slices from wild-type animals. (C) Effect of 10 μM roscovitine and 10 μM d-amphetamine on phosphorylation of Thr34-DARPP-32 in intact and dopamine-depleted striatal slices from unilaterally 6-OHDA-lesioned rats. ***, P < 0.001 compared with control intact slices; ###, P < 0.001 compared with roscovitine or amphetamine in intact slices. All statistical analyses were made by using one-way ANOVA followed by Newman-Keul's test for pairwise comparisons.
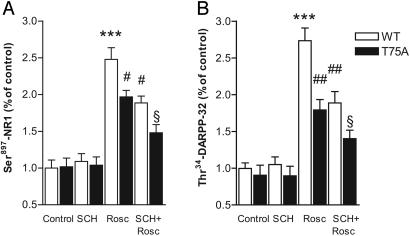
To distinguish between presynaptic and postsynaptic actions of roscovitine in striatal neurons, we performed experiments in mice in which Thr75, the CDK5-site of DARPP-32, had been mutated to an Ala residue. We found that the ability of roscovitine to increase phosphorylation levels of Ser897-NR1 and of Thr34-DARPP-32 was significantly reduced in the phosphomutant mice (Fig. 6). These results indicate a postsynaptic contribution of CDK5 in regulating phosphorylation of NMDA receptors and DARPP-32. In addition to this postsynaptic effect, the observation that SCH23390 further decreases the effect of roscovitine on phosphorylation of Ser897-NR1 and of Thr34-DARPP-32 in Thr75Ala-DARPP-32 phosphomutant mice indicates that inhibition of CDK5 presynaptically regulates dopamine release (Fig. 6). We cannot rule out the possibility that an effect of roscovitine on release of some other neurotransmitters contributed to the observed effects on phosphorylation of Ser897-NR1 and Thr34-DARPP-32.
Fig. 6.
Evidence that CDK5 inhibition increases Ser897-NR1 and Thr34-DARPP-32 phosphorylation levels through a concerted action at presynaptic and postsynaptic loci. (A) Effect of 10 μM SCH23390 (SCH) on 10 μM roscovitine (Rosc)-induced increase in Ser897-NR1 phosphorylation in wild-type (WT) and Thr75Ala-DARPP-32 phosphomutant mice. (B) Effect of 10 μM SCH23390 on the 10 μM roscovitine-induced increase of Thr34-DARPP-32 phosphorylation in wild-type and Thr75Ala-DARPP-32 (T75A) phosphomutant mice. ***, P < 0.01 compared with control in slices from wild-type mice; #, P < 0.05; ##, P < 0.01 compared with roscovitine in slices from wild-type mice. §, P < 0.05 compared with roscovitine in slices from Thr75Ala-DARPP-32 phosphomutant mice or SCH23390 plus roscovitine in slices from wild-type mice. All statistical analyses were made by using one-way ANOVA followed by Newman-Keul's test for pairwise comparisons.
Discussion
Our results demonstrate a functional link between CDK5, dopamine, NMDA receptors, and DARPP-32 in the striatum. CDK5 regulates dopamine release and indirectly modulates the function of NMDA receptors through the activation of dopamine D1 receptors. CDK5 utilizes this presynaptic action in cooperation with a DARPP-32-dependent postsynaptic mechanism to modulate glutamatergic transmission in the striatum.
It appears that CDK5 regulates the release of dopamine but not of glutamate in the striatum. In agreement with our results, roscovitine had no effect on AMPA-mediated excitatory postsynaptic potentials in the CA1 area of the hippocampus (24). However, longer (35 min) application of CDK5 inhibitors produced a delayed increase in AMPA-mediated responses through the modulation of calcium-channel activity (25). Likewise, it has been shown in dissociated neurons that roscovitine, by means of an extracellular mechanism, positively regulates P/Q-type calcium channels and increases neurotransmitter release (26). Our results showed that the increased dopamine release produced by roscovitine is additive to that produced by cocaine, which raises extracellular levels of dopamine by blocking its reuptake. It is conceivable that this presynaptic effect of CDK5 inhibition acts in conjunction with its previously documented (4) postsynaptic action to potentiate the behavioral effects of cocaine. Thus, CDK5 inhibitors would stimulate dopaminergic neurotransmission both by increasing extracellular levels of dopamine, which can activate D1 receptor-mediated PKA signaling, and by relieving the postsynaptic Thr75-DARPP-32-mediated inhibition of PKA. Thus, both pre- and postsynaptic effects of CDK5 inhibitors appear to contribute to changes in the function of postsynaptic proteins, such as DARPP-32. The fact that intact dopaminergic input and functional dopamine D1 receptors are necessary for CDK5 inhibitors to exert maximum increases of phosphorylation of DARPP-32 at Thr34 shows that these compounds exert a presynaptic action. Our finding that the ability of CDK5 inhibitors to increase Thr34-DARPP-32 is reduced in Thr75Ala-DARPP-32 phoshomutant mice provides evidence for a postsynaptic action of CDK5 inhibitors in regulating the function of striatal neurons.
CDK5 also regulates NMDA receptor function as its inhibition increases NMDA-EPSC and phosphorylation of the NR1 subunit. As in the case of DARPP-32 phosphorylation, these effects are partly mediated by D1 receptors and require intact dopaminergic input, which additionally demonstrates an important physiological role of CDK5 in the regulation of dopamine release. The ability of CDK5 to regulate dopamine release may explain the difference in the effect of roscovitine on NMDA currents in the striatum and in the hippocampus, where the dopamine levels are much lower and roscovitine decreases extrasynaptically activated NMDA currents. Such a depressant effect presumably results from inhibition of CDK5-mediated phosphorylation of the NMDA receptor subunit NR2A (24). This observation constitutes an additional difference with the striatum where the NR2A subunit is expressed at low levels (19, 20) and probably does not account for the majority of functional NMDA receptors.
Our results show that CDK5 is an important regulator of both dopaminergic and glutamatergic transmission in the striatum and in the integration of these neurotransmitter systems. Our findings have direct implications for the function of the striatum and the pathophysiology of several psychiatric and neurological disorders. Indeed, dopamine and glutamate interaction provide a major basis for the role of the striatum in motor control and reward- and goal-oriented behaviors. Dysfunction of dopaminergic and glutamatergic transmission is believed to contribute to the development of Parkinson's disease, schizophrenia, and drug addiction; CDK5 inhibitors might therefore be good candidates for the treatment of these diseases. CDK5 inhibitors would seem to be particularly good as possible candidates for use in Parkinson's disease. In the present and previous studies, we have shown that CDK5 inhibitors are able to increase both dopamine release and the postsynaptic effects of dopamine. These observations, together with the recent observation that CDK5 inhibition protects nigral neurons from degeneration and improves motor behavior in the 1-methyl-4-phenyl-1,2,4,6-tetrahydropyridine mouse model of Parkinson's disease (27), strongly suggest that CDK5 inhibitors may have anti-Parkinsonian actions. In fact, to our knowledge, CDK5 inhibitors are unique in their capability of acting at three different levels (presynaptic, postsynaptic, and neuroprotection) to reduce effects of hypodopaminergia.
Acknowledgments
We thank Dr. François Gonon for his invaluable advice on amperometric detection of dopamine release in brain slices, Elisabeth Griggs for excellent graphic artwork, Robert Carruthers for help with some biochemical experiments, and Dr. Zhen Yan for reading the manuscript. This work was supported by grants from the National Institute of Mental Health, the National Institute on Drug Abuse, and the Michael Stern Foundation for Parkinson's research (to P.G.). P.S. was supported by a fellowship from the Stiftelsen för Internationalisering av Högre Utbilding och Forskning. K.C. was an Essel Investigator within the Essel Foundation Young Investigator Award Program from the National Alliance for Research on Schizophrenia and Depression and was supported by a fellowship from the Swedish Society for Medical Research and a travel grant from the Swedish Medical Research Council.
Abbreviations: CDK5, cyclin-dependent kinase 5; DARPP-32, dopamine- and cAMP-regulated phosphoprotein, molecular mass of 32 kDa; PKA, cAMP-dependent protein kinase A; EPSC, excitatory postsynaptic current; NMDA, N-methyl-d-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; 6-OHDA, 6-hyroxydopamine.
References
- 1.Gerfen, C. R. & Wilson, C. J. (1996) in Handbook of Chemical Neuroanatomy: Integrated Systems of the CNS, eds. Swanson, L. W., Björklund, A. & Hökfelt, T. (Elsevier, Amsterdam), Vol. 12.
- 2.Bibb, J. A., Snyder, G. L., Nishi, A., Yan, Z., Meijer, L., Fienberg, A. A., Tsai, L. H., Kwon, Y. T., Girault, J. A., Czernik, A. J., et al. (1999) Nature 402, 669-671. [DOI] [PubMed] [Google Scholar]
- 3.Greengard, P. (2001) Science 294, 1024-1030. [DOI] [PubMed] [Google Scholar]
- 4.Bibb, J. A., Chen, J., Taylor, J. R., Svenningsson, P., Nishi, A., Snyder, G. L., Yan, Z., Sagawa, Z. K., Ouimet, C. C., Nairn, A. C., et al. (2001) Nature 410, 376-380. [DOI] [PubMed] [Google Scholar]
- 5.Chergui, K. & Lacey, M. G. (1999) Neuropharmacology 38, 223-231. [DOI] [PubMed] [Google Scholar]
- 6.Chergui, K., Bouron, A., Normand, E. & Mulle, C. (2000) J. Neurosci. 20, 2175-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz, Y., Lee, C. J., Schmauss, C., Gonon, F. & Sulzer, D. (2001) J. Neurosci. 21, 5916-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dugast, C., Suaud-Chagny, M. F. & Gonon, F. (1994) Neuroscience 62, 647-654. [DOI] [PubMed] [Google Scholar]
- 9.Chergui, K., Suaud-Chagny, M. F. & Gonon, F. (1994) Neuroscience 62, 641-645. [DOI] [PubMed] [Google Scholar]
- 10.Michael, D. J. & Wightman, R. M. (1999) J. Pharm. Biomed. Anal. 19, 33-46. [DOI] [PubMed] [Google Scholar]
- 11.Svenningsson, P., LeMoine, C., Kull, B., Sunahara, R., Bloch, B. & Fredholm, B. B. (1997) Neuroscience 80, 1171-1185. [DOI] [PubMed] [Google Scholar]
- 12.Drago, J., Gerfen, C. R., Lachowicz, J. E., Steiner, H., Hollon, T. R., Love, P. E., Ooi, G. T., Grinberg, A., Lee, E. J. & Huang, S. P. (1994) Proc. Natl. Acad. Sci. USA 91, 12564-12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svenningsson, P., Tzavara, E. T., Carruthers, R., Rachleff, I., Wattler, S., Nehls, M., McKinzie, D. L., Fienberg, A. A., Nomikos, G. G. & Greengard, P. (2003) Science 302, 1412-1416. [DOI] [PubMed] [Google Scholar]
- 14.Svenningsson, P., Lindskog, M., Rognoni, F., Fredholm, B. B., Greengard, P. & Fisone, G. (1998) Neuroscience 84, 223-228. [DOI] [PubMed] [Google Scholar]
- 15.Meijer, L., Borgne, A., Mulner, O., Chong, J. P., Blow, J. J., Inagaki, N., Inagaki, M., Delcros, J. G. & Moulinoux, J. P. (1997) Eur. J. Biochem. 243, 527-536. [DOI] [PubMed] [Google Scholar]
- 16.Gray, N., Detivaud, L., Doerig, C. & Meijer, L. (1999) Curr. Med. Chem. 6, 859-875. [PubMed] [Google Scholar]
- 17.Liu, F., Ma, X. H., Ule, J., Bibb, J. A., Nishi, A., DeMaggio, A. J., Yan, Z., Nairn, A. C. & Greengard, P. (2001) Proc. Natl. Acad. Sci. USA 98, 11062-11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scannevin, R. H. & Huganir, R. L. (2000) Nat. Rev. Neurosci. 1, 133-141. [DOI] [PubMed] [Google Scholar]
- 19.Landwehrmeyer, G. B., Standaert, D. G., Testa, C. M., Penney, J. B., Jr., & Young, A. B. (1995) J. Neurosci. 15, 5297-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Standaert, D. G., Friberg, I. K., Landwehrmeyer, G. B., Young, A. B. & Penney, J. B., Jr. (1999) Brain Res. Mol. Brain Res. 64, 11-23. [DOI] [PubMed] [Google Scholar]
- 21.Snyder, G. L., Fienberg, A. A., Huganir, R. L. & Greengard, P. (1998) J. Neurosci. 18, 10297-10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores-Hernandez, J., Cepeda, C., Hernandez-Echeagaray, E., Calvert, C. R., Jokel, E. S., Fienberg, A. A., Greengard, P. & Levine, M. S. (2002) J. Neurophysiol. 80, 3010-3020. [DOI] [PubMed] [Google Scholar]
- 23.Levine, M. S., Altemus, K. L., Cepeda, C., Cromwell, H. C., Crawford, C., Ariano, M. A., Drago, J., Sibley, D. R. & Westphal, H. (1996) J. Neurosci. 16, 5870-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, B. S., Sun, M. K., Zhang, L., Takahashi, S., Ma, W., Vinade, L., Kulkarni, A. B., Brady, R. O. & Pant, H. C. (2001) Proc. Natl. Acad. Sci. USA 98, 12742-12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomizawa, K., Ohta, J., Matsushita, M., Moriwaki, A., Li, S. T., Takei, K. & Matsui, H. (2002) J. Neurosci. 22, 2590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan, Z., Chi, P., Bibb, J. A., Ryan, T. A. & Greengard, P. (2002) J. Physiol. 540, 761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, P. D., Croker, S. J., Jackeson-Lewis, V., Jordan-Sciutto, K. L., Hayley, S., Mount, M. P., O'Hare, M. J., Callaghan, S., Slack, R. S., Przedborski, S., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 13650-13655. [DOI] [PMC free article] [PubMed] [Google Scholar]