Abstract
Objective
To determine whether the temporal onset of visual phenomena distinguishes Lewy body disease (LBD) from Alzheimer’s disease (AD), and to characterize the extent Lewy bodies and neurofibrillary tangles are associated with these clinical features.
Methods
Consecutive cases of autopsy-confirmed LBD (n=41), AD (n=70), and AD with amygdala-predominant Lewy bodies (AD-ALB) (n=14) with a documented clinical history of dementia were included. We mailed questionnaires to next-of-kin asking about symptoms during life. Lewy pathology and neurofibrillary tangle pathology were assessed.
Results
The occurrence of visual hallucinations, misperceptions and family misidentification did not distinguish LBD from AD or AD-ALB, but the onset was earlier in LBD compared to AD and AD-ALB. When visual hallucinations developed within the first 5 years of dementia, the odds were 4 to 5 times greater for autopsy-confirmed LBD (or intermediate/high likelihood DLB) and not AD or AD-ALB. In LBD, limbic but not cortical Lewy body pathology was related to an earlier onset of visual hallucinations, while limbic and cortical Lewy body pathology were associated with visual misperceptions and misidentification. Cortical neurofibrillary tangle burden was associated with an earlier onset of misidentification and misperceptions in LBD and AD, but only with earlier visual hallucinations in AD/AD-ALB.
Conclusion
When visual hallucinations occur within the first 5 years of the dementia, a diagnosis of DLB was more likely than AD. Visual hallucinations in LBD were associated with limbic Lewy body pathology. Visual misperceptions and misidentification delusions were related to cortical Lewy body and neurofibrillary tangle burden in LBD and AD/AD-ALB.
Keywords: Dementia with Lewy bodies, Lewy body disease, Alzheimer’s disease
INTRODUCTION
Visual hallucinations, misperceptions and family misidentification in the context of dementia represent a major treatment challenge for patients, families and care providers. Although visual hallucinations are a core feature in dementia with Lewy bodies (DLB), and occur in 32% to 85% of autopsy-confirmed cases [1-5], this feature also occurs in 11% to 38% of those with Alzheimer’s disease (AD) without concomitant α-synucleinopathy [1-4]. Distinguishing DLB from AD may be difficult due to the overlap of psychiatric features, and deciding on treatment options can be challenging since some pharmacologic interventions for psychosis are poorly tolerated in DLB. When visual hallucinations occur in AD, they tend to be associated with greater cognitive impairment and a more advanced stage of the dementia [6-10]. The purpose of this study was to determine if an early vs. late temporal onset of visual hallucinations distinguishes DLB from AD, and whether a similar distinction is evident with misperceptions and family misidentification phenomena.
Psychosis in AD has been associated with greater neocortical neurofibrillary tangle (NFT) density [11, 12], while the opposite relationship has been shown in DLB [13]. Visual hallucinations in DLB have been associated with greater Lewy body burden in the temporal lobe, amygdala, transentorhinal region and frontal lobe [5, 14]. This suggests that Lewy pathology, and not neurofibrillary tangle pathology may be a vital contributor to psychosis in DLB. A secondary goal of the study was to characterize the contribution of neurofibrillary tangle and Lewy body pathology association with the presentation of visual hallucinations, misperceptions and family visual misidentification in DLB and AD.
METHODS
The sample included consecutive cases from the State of Florida Alzheimer’s Disease Initiative (ADI) Brain Bank [15]. The patients were evaluated during life at Florida ADI memory disorder clinics in Melbourne, Miami Beach, Orlando and Jacksonville, and all had a documented history of dementia using DSM-III-R criteria. The protocols were given ethical approval by the institutional review boards for each institution, and proxies for the subjects provided written informed consent. There was no overlap in patient representation from prior clinicopathologic studies reported from our center [6, 16] .
Since the samples were initially derived from pathology data, the pathologic designation Lewy body disease (LBD) is used. Autopsy groups included LBD (n=41), AD (n=70), and Alzheimer’s disease with amygdala-predominant Lewy bodies (AD-ALB; n=14). The AD group had no concomitant α-synuclein pathology, and the AD-ALB group had Lewy bodies limited to the amygdala [17]. In those with LBD, 16 patients had transitional LBD (TLBD) with Lewy bodies in the brainstem and limbic regions and 25 patients had diffuse LBD (DLBD) with brainstem, limbic and neocortical LB pathology. We compared groups separately, but also did comparisons between the LBD group (n=41) and the AD/AD-ALB combined group (n=84). We also applied the DLB Consortium pathology criteria of no, low, intermediate and high likelihood DLB [18, 19]. Specifically, this pathology criteria allocates those with TLBD and a Braak neurofibrillary tangle stage ≥ 5 into the low likelihood DLB group. With this designation, group membership changed slightly (no/low likelihood DLB group n=93; intermediate/high likelihood DLB n=32).
We mailed questionnaires to the next-of-kin of those who died up to three years earlier. If there was no response after two months of the second mailing, then telephone contact was attempted and if possible, the survey was completed by telephone interview. There was a 71% return rate for the questionnaires. The clinicopathologic analysis is based only on the 71% who responded. Data regarding clinical presentation and onset dates used in the analyses were derived from next-of-kin report.
On the survey, the family member was asked to estimate the age of onset when problems with thinking or forgetfulness began, thereby reflecting the earliest features of the dementia. Estimated dementia duration was calculated by subtracting the death date from the estimated onset of cognitive difficulties. We inquired about visual hallucinations, visual misperceptions and misidentification phenomena (see figure 1). Only images of adults, children, tiny people, objects, insects or animals were considered to be a fully formed visual hallucination. Based on next-of-kin report of their recollection of features during the entire disease course, 73% of the LBD had at least 2 of the 4 DLB clinical features (visual hallucinations, parkinsonism, fluctuations, REM sleep behavior disorder), as did 24% of the AD group and 21% of the AD-ALB group.
Figure 1.
Visual hallucination, Misperception and Misidentification questions
Neuropathologic Methods
Lewy body density was assessed in limbic (amygdala, cingulate gyrus, parahippocampal gyrus) and cortical (frontal, temporal, parietal, occipital) regions. Given the extent of pathology in the LBD brainstems, a rating scale of Lewy body pathology/neuronal loss in the brainstem regions of substantia nigra, locus ceruleus and medulla was applied using a range from 0 (normal) to 3 (severely impaired). NFT density was determined from the limbic regions (subiculum, CA1, CA2/3, endplate) and cortical (frontal, temporal, parietal, occipital) regions. A Braak NFT stage was assigned to each case using distribution of NFT from thioflavin S fluorescent microscopy, as previsously described [20].
RESULTS
Demographics and clinical history
Estimated dementia duration was shortest for the LBD group compared to AD and AD-ALB (see table 1). Estimated age of cognitive onset did not differ between groups. A clinical diagnosis of parkinsonism that was known to the next-of-kin was made during life for 24% LBD, 7% AD, and 7% AD-ALB. Nonetheless, 85% of the LBD patients had either moderate or severe LB pathology and nigral loss in the substantia nigra. None of the patients had a known history of dopamine agonist or amantadine use. Exposure to levodopa-carbidopa was present in 17% of the LBD group, 3% of the AD and 0% of the AD-ALB group. Of the 7 LBD treated with levodopa-carbidopa, four had visual hallucinations during their disease course. The use of anti-parkinsonian medication is not considered a confound for subsequent analyses, since so few patients in this sample used these agents.
Table 1.
Frequency and onset of demographics and clinical features across groups
| LBD | AD | AD-ALB | Χ2 / F | P value | |
|---|---|---|---|---|---|
| Number | 41 | 70 | 14 | - | - |
| Males | 58%a | 49% | 29%b | 3.5 | 0.06 |
| Age at estimated cognitive onset (yrs ± sd) |
70.2 ± 8.8 | 69.1 ± 9.0 | 68.0 ± 8.7 | 0.6 | 0.56 |
| Estimated dementia duration (interval from estimated cognitive onset to death (yrs ± sd) |
8.9 ± 4.1a | 11.2 ± 5.6b | 14.2 ± 4.3c | 5.9 | 0.003 |
| Visual Hallucinations (VH) | 63% | 46% | 44% | 2.8 | 0.25 |
| Estimated onset of VH relative to cognitive onset (yrs ± sd) |
3.1 ± 1.9a | 7.3 ± 3.5b | 7.0 ± 2.2b | 13.4 | 0.000 |
| Misperceptions | 76% | 64% | 50% | 2.6 | 0.27 |
| Estimated onset of misperceptions relative to cognitive onset (yrs ± sd) |
3.2 ± 2.2a | 7.1 ± 3.7b | 5.8 ± 2.9 | 10.1 | 0.000 |
| Misidentification of family | 66%a | 70% | 93%b | 3.8 | 0.05 |
| Estimated onset of family misidentification relative to cognitive onset (yrs ± sd) |
4.5 ± 4.1a | 7.8 ± 4.5b | 7.4 ± 3.8b | 4.8 | 0.01 |
Separate chi-square or Fisher’s least significant differences post-hoc comparisons with different superscript letters indicate significance p ≤ 0.05.
Pathology density and distribution
Nucleus basalis of Meynert, locus coeruleus, medulla and substantia nigra severity ratings of Lewy body burden/neuronal loss were moderate to severe in the LBD group, and did not differentiate DLBD from TLBD. Lewy body density in limbic regions was greater in DLBD than TLBD (t=−4.2, p<0.01). NFT density (cortical or limbic) and Braak NFT stage did not distinguish DLBD from TLBD.
The LBD group had less overall NFT burden and a lower Braak NFT stage compared to AD and AD-ALB. Specifically, a Braak NFT stage > IV was represented in 56% of the LBD group, 95.7% of the AD group, and 100% of the AD-ALB group (LBD versus AD/AD-ALB groups, X2 = 32.1, p<0.01).
In LBD, estimated duration of dementia was not related to NFT (r=0.21, p=0.20) or Lewy body density (r = −0.07, p=0.69). In the AD and AD-ALB groups, longer estimated duration of dementia was related to greater combined limbic and cortical NFT burden (r=0.26, p=0.02).
Visual hallucinations and group differentiation
There was no difference in gender, parkinsonism, age of cognitive onset or estimated duration of dementia between hallucinators and non-hallucinators. The hallucinations included images of fully formed adults or children in 84%, animals or insects in 37%, and objects in 39% of the entire sample. The type of image, frequency, and patient’s state of consciousness at the time of occurrence did not differentiate groups. Unformed images, such as fire, smoke, water and designs occurred in less than 12% of the sample, did not distinguish groups, and were never the sole type of hallucinated image.
There was no difference in the presence/absence of visual hallucinations between the LBD, AD and AD-ALB groups (see table 1), or between LBD subtypes of TLBD and DLBD. Nonetheless, visual hallucinations occurred earlier in the dementia course of LBD than in either AD or AD-ALB (see table 1). This distinction was upheld when groups were classified according to the DLB Consortium pathology criteria (intermediate/high likelihood DLB 2.8 ± 1.9 years vs. no/low likelihood DLB 6.7 ± 3.2 years, t=5.1, p<0.001).
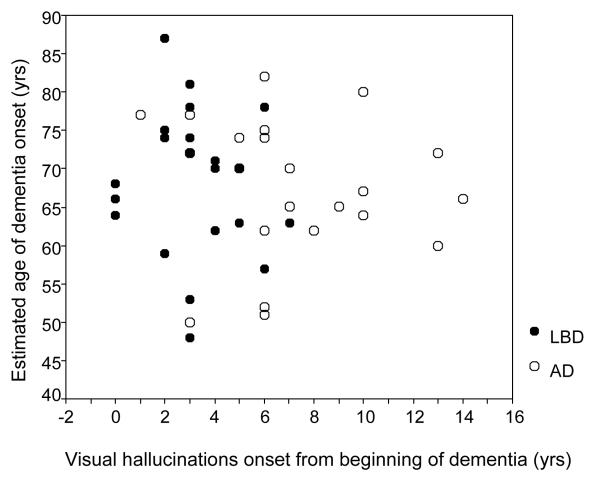
In LBD, visual hallucinations tended to occur within the first 5 years of estimated dementia onset (see figure 2). There was no correlation with age, (LBD r = −0.17, p = 0.22, AD/AD-ALB r = −0.19, p =.17), indicating that hallucinations were not more likely to occur in the older patients.
Figure 2.
When did the visual hallucinations start relative to the estimated onset of dementia?
Logistic regression modeling was carried out to determine whether the onset of visual hallucinations within the first 5 years of estimated cognitive onset improved the likelihood of autopsy-confirmed LBD compared to AD and AD-ALB. This was also done when groups were classified according to the DLB Consortium pathology criteria. Results were consistent across both models and revealed a 1.5 to 2 time greater likelihood of autopsy-confirmed LBD or DLB when visual hallucinations develop at any time during the dementia. In contrast, when visual hallucinations develop within the first 5 years of estimated cognitive onset, the odds are 4 to 5 times greater that the patient has autopsy-confirmed LBD or DLB and not AD or AD-ALB (see table 2).
Table 2.
Logistic regression models for presence of visual hallucinations and the temporal onset of visual hallucinations
| Variable | β Coefficient | Wald | Odds Ratio | P Value |
|---|---|---|---|---|
|
Autopsy-confirmed LBD versus AD/AD-ALB groups
| ||||
| Visual hallucinations | 0.71 | 2.56 | 2.03 | 0.11 |
| Constant | −1.55 | 4.53 | 0.21 | 0.03 |
| Visual hallucinations within the first 5 years of cognitive onset |
1.44 | 13.6 | 4.24 | 0.000 |
| Constant | −1.74 | 7.67 | 0.18 | 0.006 |
|
| ||||
|
Autopsy-confirmed Intermediate/high likelihood DLB versus no/low likelihood DLB
| ||||
| Visual hallucinations | 0.43 | 0.89 | 1.54 | 0.347 |
| Constant | −1.64 | 4.79 | 0.19 | 0.03 |
| Visual hallucinations within the first 5 years of cognitive onset |
1.70 | 9.62 | 5.48 | 0.002 |
| Constant | −3.09 | 9.14 | 0.05 | 0.003 |
Visual hallucinations and pathology
Greater limbic Lewy body density was associated with an earlier onset of visual hallucinations (r = − 0.37, p=0.04), with the anterior cingulate harboring the strongest association (r = − 0.42, p=0.03). The DLBD and TLBD group did not differ in the temporal onset of the visual hallucinations.
In LBD, Braak NFT stage did not distinguish hallucinators from non-hallucinators. The onset of visual hallucinations was also not associated with Braak NFT stage (r=0.05, p=0.42), or total NFT density (r=0.07, p=0.38).
In the AD/AD-ALB group, Braak NFT stage did not differentiate hallucinators from non-hallucinators. An earlier onset of visual hallucinations in AD and AD-ALB was associated with greater combined limbic and cortical NFT density (r =−0.35, p= 0.04). This suggests that visual hallucinations in AD and AD-ALB, may depend on the overall extent of the tangle burden.
Visual misperceptions and Family misidentification
Visual misperceptions occurred across all 3 diagnostic groups. Family misidentification was disproportionately more common in the AD-ALB group (see table 1).
In LBD, there was a significantly earlier onset of misperceptions and family misidentification compared to AD and AD-ALB (see table 1). The DLB Consortium pathology criteria showed the same distinction for misperceptions (intermediate/high likelihood DLB 3.0 ± 2 years vs. no/low likelihood DLB: 6.6 ± 3 years, t=4.1,p<0.001), and misidentification (intermediate/high likelihood DLB 4.5 ± 5 years vs. no/low likelihood DLB 7.4 ± 4 years, t=2.6, p=0.01).
In the LBD group, visual misperceptions and family misidentification were more common in DLBD than in TLBD (misperceptions: 76% DLBD vs. 25% TLBD, χ2= 6.8, p<0.01, misidentification: 77% DLBD vs. 47% TLBD, χ2=3.9, p<0.05). Given this relationship, it is not surprising that greater cortical Lewy body burden was associated with an earlier onset of misperceptions and family misidentification (misperceptions r=−0.40, p<0.03, misidentification r=−0.37, p<0.04). When cortical regions were examined separately, earlier object misperceptions were associated with greater temporal lobe Lewy body burden (r=−0.41, p<0.03), and earlier family misidentification was associated with greater frontal Lewy body burden (r = −0.35, p<0.05).
In LBD, neurofibrillary tangle burden and Braak NFT stage was not related to the presence of visual misperceptions but those with family misidentification did have greater overall NFT density (t=2.86, p=0.01).
In AD and AD-ALB, an earlier temporal onset of visual misperceptions was associated with greater parietal (r=0.33, p=0.04) and temporal (r=−0.34, p=0.04) NFT density. An earlier onset of family misidentification was related to parietal NFT density (r=−0.39, p<0.01).
DISCUSSION
When the entire disease course was considered, visual hallucinations were just as likely to occur in LBD, AD or AD-ALB. There was also no difference in the phenomenology of the visual hallucinations, with people and animals as the most common hallucinated images. When onset is considered, those with LBD developed visual hallucinations significantly earlier than their AD or AD-ALB counterparts. Specifically, when visual hallucinations developed within the first 5 years after estimated dementia/cognitive onset, patients were 4 to 5 times more likely to have autopsy-confirmed LBD and not AD or AD-ALB. This relationship was found when patients were classified by the presence/absence of Lewy body pathology, and by the DLB Consortium pathology criteria that takes NFT distribution into account. This is consistent with studies showing that DLB patients are more likely to have visual hallucinations in the earlier stages of the disease [11, 21], but suggests a possible cut-off that may eventually help improve diagnostic accuracy.
These data are consistent with studies of AD, showing that visual hallucinations tend to occur at a more advanced stage [7]. We extended this further, by revealing that this pattern of a later onset of hallucinations is also true for those with AD with amygdala-predominant Lewy bodies.
We found no relationship between distribution of Lewy body pathology and presence or onset of visual hallucinations. That is, those with Lewy bodies confined to the brainstem and limbic regions (transitional LBD: TLBD) were just as likely to have visual hallucinations and dementia as those with cortical Lewy bodies (diffuse LBD: DLBD). A heavier limbic Lewy body burden was related to the earlier development of visual hallucinations, with the strongest association in the anterior cingulate. This adds to existing evidence that limbic Lewy body deposition may begin earlier in those with visual hallucinations [5, 14, 22]. Harding and colleagues [5] also found increased Lewy body deposition in the inferior temporal cortex. We did not find a temporal lobe relationship, but this may be because our sample was obtained from the superior temporal gyrus. In Braak’s Lewy body staging methodology, the first neocortical area vulnerable to Lewy bodies is the inferior temporal region [23], a region known to subserve visual object discrimination [24]. It is possible that disruption of the limbic and the inferior-posterior temporal regions important for extra-striate visual processing may be a key factor in the development of visual hallucinations in DLB. Other evidence for this includes hypometabolism in the ventral association stream in DLB hallucinators [25], and decreased fractional anisotropy in the inferior longitudinal fasciculus, which projects from the occipital visual association cortex to the temporal lobe [26].
In LBD, visual hallucination history or onset was not associated with Braak NFT stage or NFT density. This is consistent with Tsuang and colleagues who found no difference in tau pathology between hallucinators and non-hallucinators [27]. Other studies with larger samples, go further and reveal that those LBD patients with visual hallucinations have less severe tangle pathology [13, 28]. Our data contributes to the growing evidence that it is the Lewy-related pathology, and not NFT pathology, responsible for the development of visual hallucinations in LBD.
In AD and AD-ALB, there was no difference in Braak NFT stage between hallucinators and non-hallucinators, but earlier visual hallucination onset was associated with greater overall NFT density. This is consistent with findings from Farber and colleagues who found AD patients with psychosis (hallucinations and delusions) had a 2.3 fold greater density of tangle pathology than those without psychosis [11]. Our results are consistent with this and suggest that visual hallucinations in AD and AD-ALB are associated with the widespread and severe NFT pathology of an advanced disease stage.
In LBD, visual misperceptions and family misidentification developed earlier when compared to the onset of these phenomena in AD and AD-ALB. In LBD, the onset of visual misperceptions was related to greater temporal lobe pathology while in AD, it was associated with greater temporal and parietal NFT density. This is consistent with known involvement of the parietal and temporal regions in higher order visual processing [24], and the earlier involvement of the inferior temporal regions in LBD [25].
Family misidentification was common in the AD-ALB group with all 13 of 14 patients experiencing it in the advanced stage of the disease. Moreover, the AD-ALB group had a longer estimated disease duration and a higher limbic NFT count. Thus, it is unclear if the amygdala Lewy bodies have a specific role in this phenomena or if the high occurrence in this subgroup of AD is a reflection of overall advanced pathology. Interestingly, only a small percentage of the AD-ALB group had fully formed visual hallucinations and very few had visual misperceptions. It is not clear how the Lewy bodies in the amygdala contribute to disease presentation in AD.
Family misidentification in LBD was more common in those with cortical Lewy bodies, and those greater frontal Lewy body pathology developed this feature earlier. This is consistent with other studies that demonstrate a relationship between delusional misidentification and frontal dysfunction [29, 30]. In contrast, our analysis of AD and AD-ALB revealed greater parietal NFT density in those with earlier family misidentification. This discrepancy may be because neurochemical alterations (and not just markers of neuronal loss) need to be taken into account. It also possible that subtype of misidentification makes a difference. Specifically, in DLB, when delusions occur, the delusional subtype of Capgras or reduplicative paramnesia (i.e., the belief that a relative is a duplicate impostor) is common, while the family substitution phenomena (i.e., belief that one relative is a different relative, such as an adult child mistaken for a parent) appears to be more frequent in AD. Our survey did not distinguish between delusional subtypes. We do not know if subtypes of delusional misidentification have a different neurologic basis, and further investigation is needed.
There are several limitations to this study, the most important of which involves the utilization of a survey that queries next-of-kin requiring a reliance on their recollection of events that took place from months to years earlier. In addition, despite our reasonably good return rate of the survey, some bias may exist in the subset of those who chose to respond. Since this is an autopsy-only sample, it is not known if there is an inherent bias amongst those who undergo autopsy compared to those who do not. Another major limitation is sample size which may affect results by virtue of restricted range or reduced power. These patients were selected on the basis of pathology and were seen clinically by Memory Disorders Centers, and hence, a recruitment bias is also possible. Clearly, replication of these findings with a larger sample and a prospective design is needed.
Acknowledgments
This study was supported by the Florida AD Initiative, NIA/NIH R01-AG15866, P50-AG16574, The Mangurian Foundation, Robert E. Jacoby Professorship for Alzheimer’s Research and Mayo Foundation for Research and Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Galvin JE, Malcom H, Johnson D, Morris JC. Personality traits distinguishing dementia with Lewy bodies from Alzheimer disease. Neurology. 2007;68:1895–901. doi: 10.1212/01.wnl.0000263131.80945.ad. [DOI] [PubMed] [Google Scholar]
- 2.Klatka LA, Louis ED, Schiffer RB. Psychiatric features in diffuse Lewy body disease: a clinicopathologic study using Alzheimer’s disease and Parkinson’s disease comparison groups. Neurology. 1996;47:1148–52. doi: 10.1212/wnl.47.5.1148. [DOI] [PubMed] [Google Scholar]
- 3.Knuffman J, Mohsin F, Feder J, Grossberg GT. Differentiating between lewy body dementia and Alzheimer’s disease: a retrospective brain bank study. J Am Med Dir Assoc. 2001;2:146–8. [PubMed] [Google Scholar]
- 4.Ballard C, Holmes C, McKeith I, Neill D, O’Brien J, Cairns, et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. American Journal of Psychiatry. 1999;156:1039–45. doi: 10.1176/ajp.156.7.1039. [DOI] [PubMed] [Google Scholar]
- 5.Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125:391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- 6.Ferman TJ, Boeve BF, Smith GE, Lin SC, Silber MH, Pedraza O, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77:875–82. doi: 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hope T, Keene J, Fairburn CG, Jacoby R, McShane R. Natural history of behavioural changes and psychiatric symptoms in Alzheimer’s disease. A longitudinal study. Br J Psychiatry. 1999;174:39–44. doi: 10.1192/bjp.174.1.39. [DOI] [PubMed] [Google Scholar]
- 8.Ala T, Yang K, Sung J, Frey W. Hallucinations and signs of parkinsonism help distinguish patients with dementia and cortical Lewy bodies from patients with Alzheimer’s disease at presentation: a clinicopathological study. Journal of Neurol, Neurosurg Psychiatry. 1997;62:16–21. doi: 10.1136/jnnp.62.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockwell E, Choure J, Galasko D, Olichney J, Jeste DV. Psychopathology at initial diagnosis in dementia with Lewy bodies versus Alzheimer disease: comparison of matched groups with autopsy confirmed diagnoses. Int J Geriatr Psychiatry. 2000;15:819–23. doi: 10.1002/1099-1166(200009)15:9<819::aid-gps206>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–92. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 11.Farber NB, Rubin EH, Newcomer JW, Kinscherf DA, Miller JP, Morris JC, et al. Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch Gen Psychiatry. 2000;57:1165–73. doi: 10.1001/archpsyc.57.12.1165. [DOI] [PubMed] [Google Scholar]
- 12.Zubenko GS, Moossy J, Martinez AJ, Rao G, Claassen D, Rosen J, et al. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. 1991;48:619–24. doi: 10.1001/archneur.1991.00530180075020. [DOI] [PubMed] [Google Scholar]
- 13.Ballard CG, Jacoby R, Del Ser T, Khan MN, Munoz DG, Holmes C, et al. Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with lewy bodies. Am J Psychiatry. 2004;161:843–9. doi: 10.1176/appi.ajp.161.5.843. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher DA, Parkkinen L, O’Sullivan SS, Spratt A, Shah A, Davey CC, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain. 2011;134:3299–309. doi: 10.1093/brain/awr225. [DOI] [PubMed] [Google Scholar]
- 15.Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–12. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Dugger BN, Boeve BF, Murray ME, Parisi JE, Fujishiro H, Dickson DW, et al. Rapid eye movement sleep behavior disorder and subtypes in autopsy-confirmed dementia with Lewy bodies. Mov Disord. 2012;27:72–8. doi: 10.1002/mds.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–97. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 19.Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, et al. Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol. 2008;67:649–56. doi: 10.1097/NEN.0b013e31817d7a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–96. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, et al. Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology. 1998;51:351–7. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- 22.Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RK, Gentleman SM. Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:196–204. doi: 10.1016/j.parkreldis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 24.Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: Two cortical pathways. Trends in Neurosciences. 1983;6:414–7. [Google Scholar]
- 25.Nagahama Y, Okina T, Suzuki N, Matsuda M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. 2010;133:557–67. doi: 10.1093/brain/awp295. [DOI] [PubMed] [Google Scholar]
- 26.Kantarci K, Avula R, Senjem M, Samikoglu A, Zhang B, Weigand S, et al. Dementia with Lewy bodies and Alzheimer disease Neurodegenerative patterns characterized by DTI. Neurology. 2010;74:1814–21. doi: 10.1212/WNL.0b013e3181e0f7cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuang D, Larson EB, Bolen E, Thompson ML, Peskind E, Bowen J, et al. Visual hallucinations in dementia: a prospective community-based study with autopsy. Am J Geriatr Psychiatry. 2009;17:317–23. doi: 10.1097/JGP.0b013e3181953b9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, Ho GJ, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60:1586–90. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- 29.Forstl H, Burns A, Levy R, Cairns N. Neuropathological correlates of psychotic phenomena in confirmed Alzheimer’s disease. Br J Psychiatry. 1994;165:53–9. doi: 10.1192/bjp.165.1.53. [DOI] [PubMed] [Google Scholar]
- 30.Josephs KA. Capgras syndrome and its relationship to neurodegenerative disease. Arch Neurol. 2007;64:1762–6. doi: 10.1001/archneur.64.12.1762. [DOI] [PubMed] [Google Scholar]