Abstract
IFN-γ is essential for idiopathic and murine mercury-induced systemic autoimmunity (mHgIA), and heterozygous IFN-γ+/− mice also exhibit reduced disease. This suggests that blocking specific IFN-γ-related pathways that may only partially inhibit IFN-γ production or function will also suppress autoimmunity. To test this hypothesis, mice deficient in genes regulating IFN-γ expression (Casp1, Nlrp3, Il12a, Il12b, Stat4) or function (Ifngr1, Irf1) were examined for mHgIA susceptibility. Absence of either Ifngr1 or Irf1 resulted in a striking reduction of disease, while deficiency of genes promoting IFN-γ expression had modest to no effect. Furthermore, both Irf1– and Ifng-deficiency only modestly reduced the expansion of CD44hi and CD44hiCD55lo CD4+ T cells, indicating that they are not absolutely required for T cell activation. Thus, there is substantial redundancy in genes that regulate IFN-γ expression in contrast to those that mediate later signaling events. These findings have implications for the therapeutic targeting of IFN-γ pathways in systemic autoimmunity.
Keywords: Interferon, Animal model, Mercury
1. Introduction
The sole type II interferon, IFN-γ, is secreted predominantly by CD4+ and CD8+ T cells and natural killer (NK) cells [1] and, to a lesser extent, by other cell types such as macrophages, dendritic cells and B cells [2,3]. It exhibits a broad array of effects mediated by cell-specific expression of over 300 IFN-γ-regulated genes [4,5], for which functional classification encompasses inflammatory mediators, signaling molecules, transcriptional activators, mediators of apoptosis and immune modulators [6]. The variety of cell types that possess the IFN-γ receptor (IFN-γR) and the molecular events that constitute IFN-γ-dependent signaling pathways have been described [2,7–9].
Notably, IFN-γ production by the CD4+ T helper 1 (Th1) subset promotes inflammatory responses, clearance of intracellular pathogens and class-switching to IgG2a, IgG2b and IgG3 [2,10], and is one of the main cytokines that distinguishes Th1 from other CD4+ subsets, including Th2, Th17, TFH and Treg cells [11]. Differentiation of CD4+ T cells to the Th1 subset is driven primarily by IL-12 in the absence of IL-4 and TGF-β [11,12]. Subsequently, IFN-γ production in this subset is sustained by many factors, including IL-12, IL-18, IL-27, and Stat4 [2,12,13].
Studies of lupus-prone strains have amply documented the importance of IFN-γ in systemic autoimmunity [14]. RNA and protein measurements have repeatedly demonstrated increased expression of IFN-γ in lupus-prone mice, including NZBWF1, BXSB and MRL-Faslpr mice [15–17]. Furthermore, in NZBWF1 mice, administration of IFN-γ exacerbated lupus, while IFN-γ blockade or deletion of the IFN-γ or IFN-γ receptor genes in lupus-prone strains ameliorated disease, directly demonstrating the dependence of SLE pathogenesis on IFN-γ [18–23]. In addition, knockout of T-box transcription factor TBX21 (T-bet), required for the generation of Th1 cells, abrogated IFN-γ-mediated production of IgG2a as well as both humoral and pathologic manifestations of lupus in MRL-Faslpr mice [24]. Interestingly, hemizygous deletion of the IFN-γ gene in MRL-Faslpr mice was sufficient to significantly reduce glomerular damage and increase survival, indicating a dose-dependent effect of IFN-γ on disease expression [20]. A similar IFN-γ dose effect was also observed in murine mercury-induced autoimmunity (mHgIA) [25].
MHgIA is a T cell-dependent systemic autoimmune disease associated with the production of anti-nuclear autoAbs and immune complex-deposits. It is dependent on IFN-γ and exacerbated by exogenous administration of this cytokine [25–27]. To more fully define the characteristics of IFN-γ dependency in chemically-induced systemic autoimmunity, the present study examined the role of genes that contribute to the production and function of IFN-γ. Consistent with the dependence of mHgIA on IFN-γ, absence of the IFN-γ receptor (Ifngr1) also inhibited mHgIA. Deficiency of genes that promote IFN-γ production, including interleukin-12 (Il12), Stat4, caspase-1 (Casp1) and NLR family, pyrin domain containing 3 (Nlrp3), however, had only minor, if any, effects. In contrast, lack of interferon regulatory factor-1 (Irf1), a critical component in IFN-γ-mediated signaling [28], significantly reduced mHgIA severity to a similar degree as Ifngr1 deficiency. Reduced disease in Ifng– and Irf1-deficient mice, however, only modestly reduced the formation of activated/memory CD4+ T cells, arguing that the requirement for IFN-γ in mHgIA occurs subsequent to T cell activation. These studies demonstrate redundancy in genes involved in the induction of IFN-γ production in chemically-induced systemic autoimmunity, whereas Irf1, involved in the IFN-γ signaling pathway, contributes significantly to all aspects of disease.
2. Material and methods
2.1. Mice
C57BL/6 mice with targeted disruption of Ifng, Ifngr1, Il12a, Il12b, Stat4, and Irf1 were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6-Nlrp3−/− mice were as previously described [29]. Casp1-deficient mice were obtained from Lili Feng (The Scripps Research Institute). C57BL/6 mice were from The Scripps Research Institute Animal Facility. Breeding and maintenance were performed under specific-pathogen-free conditions at The Scripps Research Institute Animal Facility (La Jolla, CA). Gene-deficient mice on the B10.S-H2s/SgMcdJ background were produced as previously described [25,30]. Wildtype and knockout genes were typed by PCR of genomic DNA using protocols described by The Jackson Laboratory. All procedures were approved by The Scripps Research Institute Institutional Animal Care and Use Committee.
2.2. Mercury exposure
Mice were injected subcutaneously twice per week for 4 weeks with 40 ug HgCl2 (Mallinckrodt Baker Inc, Phillipsburg NJ) in PBS as previously described [25], unless noted otherwise. Controls received PBS alone. Use of mercuric chloride was approved by The Scripps Research Institute Department of Environmental Health and Safety.
2.3. Serology
Serum IgG, IgG1, and IgG2a levels were quantified by ELISA as previously described [27,30].
Anti-nuclear antibodies (ANA) were detected as previously described [27,30] using HEp-2 cell slides (Bion Enterprises, Park Ridge, IL or INOVA Diagnostics, San Diego, CA) and goat anti-mouse IgG-FITC (Invitrogen Corporation, Grand Island, NY). Immunofluorescent staining of the nucleus was identified as either nucleolar (ANoA) or other nuclear structures (ANA) and the intensity of fluorescence recorded on a scale of 0–4. Fluorescence intensities of 1, which indicates a clearly discernable staining pattern, or greater were called positive. Sera were scored by an experienced observer (K.M.P.) blinded to the identity of the samples. Anti-chromatin antibodies were detected by ELISA as previously described [27,31] with HRP-conjugated goat anti-mouse IgG (Caltag Laboratories, South San Francisco, CA) diluted 2000 fold. Anti-chromatin monoclonal antibody 1D12 [32] was used as positive control. Serum levels of B cell activating factor (BAFF) were determined by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
2.4. Immunopathology
Sections of kidney were prepared and stained for direct immunofluorescence as previously described [25] using serial dilutions of FITC-conjugated goat antibodies to IgG (gamma chain specific) and C3 (Southern Biotechnology Associates, Birmingham, AL). The end-point titer of the glomerular immune deposits was defined as the highest dilution of antibody at which specific fluorescence could be detected. A score of 0 was recorded when no specific fluorescence was detected at a dilution of 1/40. The slides were examined by an experienced pathologist (P.H.) without knowledge of treatment or other results.
2.5. Flow cytometry
Flow cytometry was performed as previously described [27,33] with the following antibodies: FITC-conjugated anti-CD44 (IM7, Pharmingen, San Diego, CA); PerCP-conjugated anti-CD4 (RM4-5, eBioscience, San Diego, CA); PE-conjugated anti-CD55 (RIKO-5, Pharmingen); APC-conjugated anti-CD3ε (145-2C11, Pharmingen); APC-conjugated anti-CD62L (MEL-14, eBioscience); APC-conjugated anti-CD25 (PC61, Pharmingen); biotinylated anti-CD69 (H1.2F3, Pharmingen) and APC-conjugated streptavidin (eBioscience). Fluorescence analysis was done using a dual laser FACSCalibur flow cytometer with CELLQuest Pro software (BD biosciences, San Jose, CA). Dead cells were excluded on the basis of forward and side light scatter.
2.6. Statistics
Unless otherwise noted, all data is expressed as mean and standard error. Statistical analysis was done using GraphPad Software, San Diego, CA. Mann–Whitney U test was used for comparisons between PBS and HgCl2 exposed groups. Analysis of variance (ANOVA) was used for comparisons between wildtype, heterozygous and homozygous mice. P < 0.05 was considered significant.
3. Results
3.1. Effects of Casp1 deficiency on mHgIA
The previous finding that heterozygous-deficiency of IFN-γ reduced the severity of mHgIA [25] suggested that genes influencing the expression of IFN-γ would also affect the development of mHgIA. We therefore investigated whether deficiency of genes known to promote IFN-γ production, including Casp1, Nlrp3, IL-12a (Il12a, p35), IL-12b (IL-12b, p40) and signal transducer and activator of transcription 4 (Stat4), could reduce susceptibility to mHgIA.
Caspase 1, which cleaves promolecules of IL-1 and IL-18 to their bioactive forms and, through activation of IL-18, promotes IFN-γ production [34] was examined first. In both wildtype (wt) and heterozygous (Casp1+/−) littermates, exposure to mercury induced increases in total IgG with elevations in both IgG1 and IgG2a subclasses (Table 1). In contrast, Casp1−/− mice developed elevations in serum IgG and IgG1, but not IgG2a, following mercury exposure, consistent with reduced IFN-γ. Mercury-induced ANoA and ANA, however, were found in all groups (Table 1), and the staining intensities were similar (Fig. 1). Anti-chromatin antibodies were also substantially elevated in all mercury-exposed groups compared with PBS-treated mice, but were not significantly different among the three genotypes (Table 1).
Table 1.
Features of autoimmunity elicited by exposure to HgCl2 in Casp1, Il12a, Il12b and Stat4 deficient mice.
| Serum immunoglobulin (mg/ml) |
ANoA |
ANA |
Anti-chromatin Abs |
Kidney Glomeruli |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Genotype | N | Treatment | IgG | IgG1 | IgG2a | IgG | IgG | IgG | IgG | C3 |
| Casp1 | +/+ | 8 | PBS | 6.48 ± 0.97 | 0.53 ± 0.06 | 0.28 ± 0.05 | 0/8 | 0/8 | 0.05 ± 0.01 | 10 ± 10 | 10 ± 10 |
| 9 | HgCl2 | 12.25 ± 1.54c | 1.32 ± 0.23b | 0.50 ± 0.05c | 9/9 | 9/9 | 1.13 ± 0.21e | 658 ± 94f | 907 ± 241c | ||
| +/− | 8 | PBS | 7.31 ± 0.62 | 0.47 ± 0.07 | 0.33 ± 0.05 | 0/8 | 0/8 | 0.04 ± 0.01 | 90 ± 28 | 10 ± 10 | |
| 9 | HgCl2 | 12.29 ± 1.75a | 2.06 ± 0.41c | 0.57 ± 0.09a | 9/9 | 9/9 | 2.91 ± 0.92b | 524 ± 77f | 400 ± 136a | ||
| −/− | 8 | PBS | 4.87 ± 0.60 | 0.57 ± 0.06 | 0.35 ± 0.02 | 0/8 | 1/8 | 0.03 ± 0.00 | 0 ± 0 | 10 ± 10 | |
| 8 | HgCl2 | 11.94 ± 1.46e | 1.74 ± 0.25e | 0.67 ± 0.15 | 8/8 | 8/8 | 1.85 ± 0.33f | 280 ± 150a,h | 200 ± 77a,g | ||
| IL-12p35 | +/+ | 8 | PBS | 5.70 ± 0.53 | 0.56 ± 0.05 | 0.22 ± 0.02 | 0/8 | 0/8 | 0.02 ± 0.00 | 0 ± 0 | 10 ± 10 |
| 8 | HgCl2 | 7.67 ± 1.05 | 1.52 ± 0.08e | 0.46 ± 0.06c | 7/8 | 5/8 | 0.87 ± 0.16d | 390 ± 98e | 630 ± 308d | ||
| +/− | 8 | PBS | 6.12 ± 0.29a | 0.46 ± 0.03 | 0.24 ± 0.03 | 0/8 | 0/8 | 0.02 ± 0.00 | 5 ± 5 | 30 ± 21 | |
| 8 | HgCl2 | 8.53 ± 0.73 | 0.95 ± 0.12c | 0.55 ± 0.13a | 7/8 | 3/8 | 0.43 ± 0.10d | 640±0e | 640 ± 201a | ||
| −/− | 8 | PBS | 5.56 ± 1.10 | 0.86 ± 0.13 | 0.22 ± 0.02 | 0/8 | 0/8 | 0.04 ± 0.02 | 30 ± 21 | 425 ± 309 | |
| 8 | HgCl2 | 6.39 ± 1.66 | 0.89 ± 0.11 | 0.30 ± 0.12 | 7/8 | 4/8 | 0.30 ± 0.11g | 280 ± 84b | 700 ± 406 | ||
| IL-12p40 | +/+ | 8 | PBS | 9.20 ± 1.60 | 0.24 ± 0.04 | 0.32 ± 0.11 | 0/8 | 1/8 | 0.02 ± 0.00 | 135 ± 74 | 200 ± 77 |
| 8 | HgCl2 | 7.85 ± 1.07 | 0.59 ± 0.09c | 0.20 ± 0.06 | 8/8 | 3/8 | 0.62 ± 0.20b | 520 ± 59c | 290 ± 68 | ||
| +/− | 8 | PBS | 9.81 ± 1.05 | 0.27 ± 0.03 | 0.28 ± 0.06 | 0/8 | 1/8 | 0.05 ± 0.01 | 55 ± 39 | 220 ± 39 | |
| 8 | HgCl2 | 10.85 ± 0.92 | 0.65 ± 0.10b | 0.36 ± 0.04 | 6/8 | 4/8 | 0.72 ± 0.10e | 545 ± 136c | 650 ± 155 | ||
| −/− | 8 | PBS | 5.90 ± 0.65 | 0.53 ± 0.12 | 0.12 ± 0.03 | 0/8 | 0/8 | 0.02 ± 0.01 | 90 ± 79 | 80 ± 21 | |
| 8 | HgCl2 | 6.67 ± 0.95 | 0.68 ± 0.11 | 0.22 ± 0.05 | 5/8 | 2/8 | 0.74 ± 0.47d | 650 ± 114c | 440 ± 134b | ||
| Stat4 | +/+ | 8 | PBS | 4.50 ± 1.38 | 0.80 ± 0.17 | 0.28 ± 0.09 | 0/8 | 1/8 | 0.05 ± 0.01 | 80 ± 80 | 40 ± 40 |
| 8 | HgCl2 | 6.47 ± 0.83 | 2.06 ± 0.30c | 0.56 ± 0.12a | 8/8 | 7/8 | 3.23 ± 0.85c | 526 ± 71b | 420 ± 151a | ||
| +/− | 8 | PBS | 4.13 ± 0.65 | 1.04 ± 0.22 | 0.30 ± 0.07 | 0/8 | 2/8 | 0.07 ± 0.01 | 120 ± 59 | 25 ± 20 | |
| 8 | HgCl2 | 7.70 ± 1.71a | 2.31 ± 0.24c | 0.76 ± 0.12b | 8/8 | 7/8 | 5.58 ± 0.91c | 640 ± 105c | 560 ± 168c | ||
| −/− | 8 | PBS | 8.38 ± 1.49 | 0.76 ± 0.11 | 0.28 ± 0.14 | 0/8 | 0/8 | 0.05 ± 0.01 | 57 ± 42 | 0 ± 0 | |
| 8 | HgCl2 | 7.84 ± 1.65 | 2.15 ± 0.15d | 0.49 ± 0.10 | 8/8 | 8/8 | 4.07 ± 1.04c | 720 ± 216a | 620 ± 206c | ||
Values are expressed as mean ± SEM. p values were determined for comparisons between PBS and HgCl2 within each group and HgCl2 between groups.
N = number of animals/group.
For comparison of PBS against HgCl2:
p < 0.05;
p < 0.01;
p < 0.005;
p < 0.001;
p < 0.0005;
p < 0.0001.
For comparison of −/− against +/+:
p < 0.05;
p < 0.01;
p < 0.005;
p < 0.001;
p < 0.0005;
p < 0.0001.
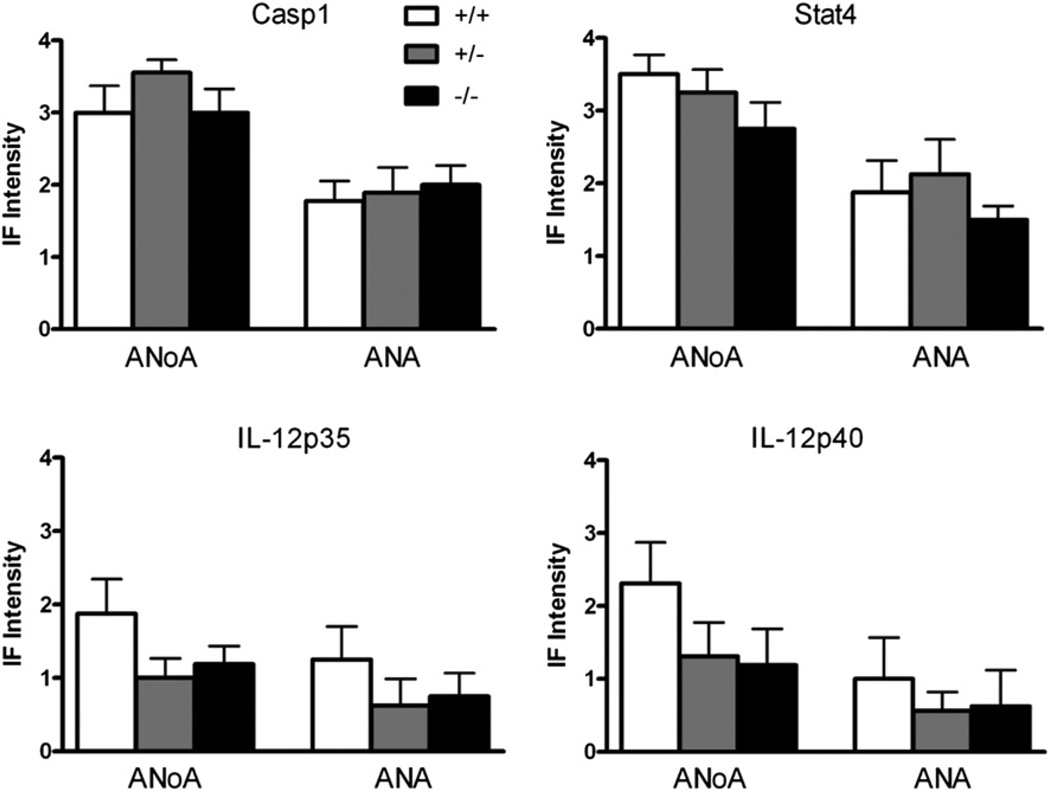
Fig. 1.
Mercury-induced ANoA and ANA are not reduced in Casp1–, Stat4–, Il12a– or Il12b–deficient mice. Three genotypes of mice, wildtype (+/+), heterozygous knockout (+/−), and homozygous knockout (−/−) for each gene, received subcutaneous injections of PBS or HgCl2 (40 µgs) in PBS twice/week for 4 weeks before serum was collected and assayed for autoantibodies as described in Materials and Methods. N = 8–9/genotype. Results are expressed as mean ± 1 SEM of fluorescence intensity at a serum dilution of 1/100. Only results for HgCl2-treated mice are shown. Fluorescence intensity of ANoA for all PBS treated mice was 0. Fluorescence intensity of ANA for PBS-treated Casp1−/− mice was 0.06 ± 0.18. Fluorescence intensity of ANA for all PBS-treated IL-12p35 mice was 0. Fluorescence intensity of ANA for PBS-treated IL-12p40 mice was 0.06 ± 0.18 for wildtype and heterozygous mice. Fluorescence intensity of ANA for PBS-treated Stat4 mice was 0.06 ± 0.18 for wildtype and 0.25 ± 0.46 for heterozygous mice.
Mercury exposure resulted in significant glomerular deposits of IgG and C3 in wt, Casp1+/− and Casp1−/− mice (Table 1). However, heterozygous and homozygous deficiency of Casp1 resulted in a progressive reduction in glomerular deposits of IgG and C3 that were significantly less in Casp1-deficient than in wt mice. Taken together, the absence of Casp1 resulted in a reduction in immune complex deposits, but no detectable effects on autoantibody production.
3.2. Effects of Nlrp3 deficiency on mHgIA
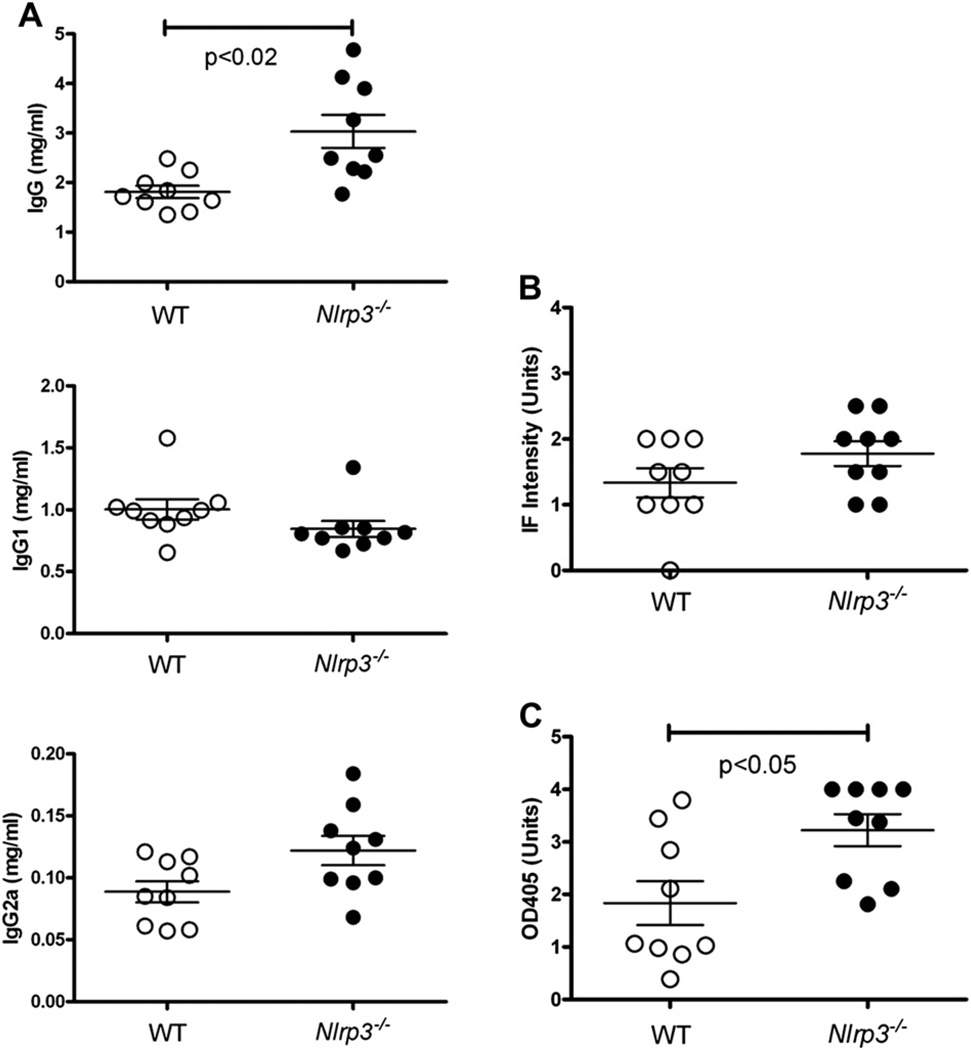
Activation of caspase-1 is achieved by cleavage of its pro-form by the inflammasome, a macromolecular complex that includes NLRP3 [35]. Lack of NLRP3 has been shown to reduce Th1 differentiation and IFN-γ expression in autoimmunity [36,37]. To determine if the NLRP3 inflammasome is required for mHgIA, C57BL/6-Nlrp3−/− mice were exposed to HgCl2 for 4 weeks (Fig. 2). Compared with wildtype C57BL/6 mice, Nlrp3–deficient mice had elevated total IgG (Fig. 2A) and increased anti-chromatin autoantiAbs (Fig. 2C) following HgCl2 exposure. Serum IgG1, IgG2a and ANA in mercury-exposed C57BL/6 and C57BL/6-Nlrp3−/− mice were not different. These observations show that the NLRP3 inflammasome is not required for autoAb production, supporting the findings with Casp1-deficient mice, but also suggest NLRP3 partially suppresses the induction of IgG and anti-chromatin by mercury.
Fig. 2.
Mercury-induced autoimmunity is not reduced in Nlrp3 deficient mice. Mice received subcutaneous injections of HgCl2 (40 µgs) in PBS twice/week for 4 weeks before serum was collected and assayed for immunoglobulins (A), ANA (B) and anti-chromatin antibodies (C) as described in Materials and Methods. N = 9/group. Results are shown for individual mice together with mean ± 1 SEM. Only results for HgCl2-treated mice are shown.
3.3. Effects of IL-12 deficiency on mHgIA
The heterodimeric IL-12p70, consisting of p35 and p40 subunits encoded by Il12a and IL12b, signals through the IL-12R and then Stat4 to induce the production of IFN-γ by NK and T cells, and in CD4+ T cells promote the differentiation of Th1 cells [38,39]. To determine the role of IL-12 in mHgIA, B10.S mice deficient for either Il12a or Il12b genes were exposed to HgCl2 for 4 weeks. Il12a–deficient mice do not express IL-12p70, but can express IL-12p40 and the homodimeric IL-12p80, which may have IL-12 agonist and antagonist activity [40]. When compared with PBS controls, mercury exposure significantly increased total IgG and the IgG2a and IgG1 subclasses in wt and Il12a+/−, but not in Il12a−/− (Table 1). Furthermore, when comparing the mercury-exposed groups, levels of IgG1 in both Il12a+/− and Il12−/− mice were lower than in wt littermates. Nevertheless, Il12a+/− and Il12a−/− mice developed ANoA and ANA with frequencies and elevated levels that were not significantly different from wt mice (Table 1, Fig. 1). Anti-chromatin Abs in homozygous Il12a-knockout mice, however, were elevated above PBS controls, but lower than wt mice. Despite this, significant glomerular IgG and C3 deposits were observed in all groups. However, in contrast to wt and heterozygous mice, which had greater intensity of both IgG and C3 compared to PBS controls, Il12a−/− mice had significant increases in IgG deposits and not C3, but this was because PBS-treated controls developed significant deposits of C3 (Table 1). Thus, the effects of IL-12p35-deficiency on HgIA were limited to partially reducing the severity of antibody production in mHgIA.
Mice deficient in Il12b lack not only IL-12p70, IL-12p40, and IL-12p80, but also IL-23 [40]. When wt, Il12b+/−, and Il12b−/− mice were exposed to mercury, IgG levels among the three groups exhibited substantial variability, however, while the characteristic increase in IgG1 was observed in wt and Il12+/− mice, in Il12b−/− littermates this did not reach statistical significance because of higher levels of IgG1 in the PBS-treated Il12b−/− controls. However, there were no significant differences in the ANoA and ANA or intensity of fluorescence among the three genotypes (Table 1, Fig. 1). All three groups produced levels of anti-chromatin Abs that were significantly higher than corresponding PBS controls. Mercury exposure resulted in significant glomerular deposits of IgG in all groups compared with PBS controls that were similar in severity (Table 1). Collectively, despite the lack of both IL-12 and IL-23, deletion of IL-12p40 had no effect on mHgIA.
3.4. Effects of Stat4 deficiency on mHgIA
Stat4 transduces IL-12 signaling in T cells, leading to Th1 differentiation and IFN-γ production, but can also be activated by IL-23, IL-27, type I IFN and other cytokines [38,41]. Although this suggested that Stat4 deficiency would have a significant effect on HgIA, when Stat4+/− and Stat4−/− mice were exposed to mercury increased IgG1 was observed, and levels of total IgG, IgG1 and IgG2a were similar to those in wt mice given mercury (Table 1). Mercury-induced ANoA, ANA, anti-chromatin Ab levels and glomerular deposits were also not affected by the absence of Stat4 (Table 1, Fig. 1). Collectively, the data indicate that mHgIA is not dependent on Stat4.
3.5. MHgIA is reduced in IFN-γ receptor (Ifngr1) knockout mice
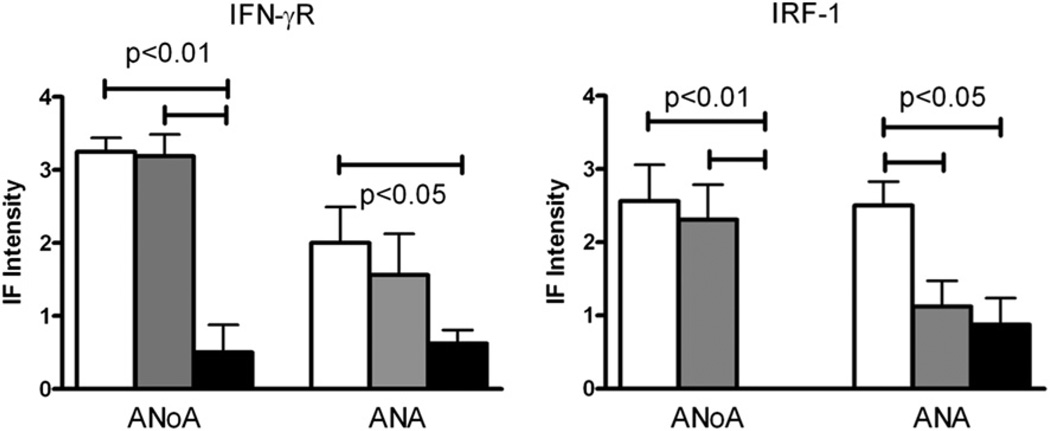
To confirm the dependence of mHgIA on IFN-γ [25], B10.S mice with a targeted disruption of IFN-γR, the sole receptor for IFN-γ, were examined. Consistent with deficiency of IFN-γ signaling, Ifngr1−/− mice exposed to mercury failed to develop elevations in serum IgG or IgG2a, yet exhibited increased levels of IgG1 (Table 2). Importantly, mercury-induced IgG ANoA was present in only two of eight Ifngr1−/− mice in contrast to all of the wt (8/8) and heterozygous (8/8) mice (p = 0.007, Table 2), and the intensity of nucleolar staining was significantly reduced (Fig. 3). Mercury exposure also induced IgG ANA in a similar fraction of mice irrespective of their genotype, but the intensity of ANA fluorescence in Ifngr1−/− mice was significantly reduced compared to wt (Fig. 3). Accordingly, although anti-chromatin Abs were significantly elevated in all three mercury-exposed genotypes compared with corresponding PBS controls, levels were significantly lower in Ifngr1-deficient mice (Table 2). Strikingly, however, despite the production of autoAbs, Ifngr1−/− mice had essentially no glomerular deposits of IgG or C3 after mercury exposure (Table 2). These observations demonstrate a significant, but not absolute, requirement for IFN-γR signaling in the humoral and immunopathological aspects of mHgIA.
Table 2.
Features of autoimmunity elicited by exposure to HgCl2 in Ifngr1 and Irf1 deficient mice.
| Serum immunoglobulin (mg/ml) |
ANoA |
ANA |
Anti-chromatin Abs |
Kidney Glomeruli |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Genotype | N | Treatment | IgG | IgG1 | IgG2a | IgG | IgG | IgG | IgG | C3 |
| IFN-γR | +/+ | 8 | PBS | 7.37 ± 1.14 | 0.30 ± 0.06 | 0.12 ± 0.03 | 0/8 | 0/8 | 0.02 ± 0.01 | 0 ± 0 | 220 ± 52 |
| 8 | HgCl2 | 9.75 ± 0.61a | 1.08 ± 0.11f | 0.20 ± 0.06 | 8/8 | 6/8 | 1.57 ± 0.38c | 640±0e | 800 ± 148c | ||
| +/− | 8 | PBS | 6.16 ± 0.71 | 0.29 ± 0.09 | 0.12 ± 0.05 | 0/8 | 0/8 | 0.03 ± 0.01 | 10 ± 10 | 185 ± 54 | |
| 8 | HgCl2 | 9.25 ± 1.31a | 1.38 ± 0.20e | 0.17 ± 0.06 | 8/8 | 5/8 | 0.85 ± 0.30a | 800 ± 105f | 880 ± 262a | ||
| −/− | 8 | PBS | 6.92 ± 0.52 | 0.61 ± 0.13 | 0.09 ± 0.03 | 0/8 | 1/8 | 0.01 ± 0.01 | 30 ± 15 | 155 ± 52 | |
| 8 | HgCl2 | 7.55 ± 1.04 | 1.58 ± 0.28b | 0.07 ± 0.03 | 2/8 | 5/8 | 0.17 ± 0.07a,i | 155 ± 79k | 340 ± 77g | ||
| IRF-1 | +/+ | 8 | PBS | 7.50 ± 1.05 | 0.46 ± 0.04 | 0.32 ± 0.08 | 0/8 | 1/8 | 0.05 ± 0.02 | 20 ± 20 | 10 ± 10 |
| 8 | HgCl2 | 13.25 ± 1.40a | 1.25 ± 0.11a | 0.69 ± 0.14a | 7/8 | 8/8 | 2.36 ± 0.73d | 709 ± 201a | 360 ± 162a | ||
| +/− | 8 | PBS | 11.03 ± 2.04 | 0.52 ± 0.07 | 0.34 ± 0.06 | 0/8 | 1/8 | 0.04 ± 0.01 | 10 ± 10 | 40 ± 40 | |
| 8 | HgCl2 | 11.30 ± 1.47a | 1.00 ± 0.17a | 0.50 ± 0.10a | 7/8 | 6/8 | 1.18 ± 0.41d | 480 ± 307a | 160 ± 86 | ||
| −/− | 8 | PBS | 4.43 ± 0.69m | 0.54 ± 0.06 | 0.12 ± 0.06m | 0/8 | 1/8 | 0.05 ± 0.01 | 30 ± 21 | 57 ± 27 | |
| 8 | HgCl2 | 3.17 ± 0.51e | 0.80 ± 0.11 | 0.05 ± 0.01 | 0/8n | 5/8 | 0.68 ± 0.28g | 0±0i | 10 ± 10i | ||
Values are expressed as mean ± SEM. p values were determined for comparisons between PBS and HgCl2 within each group and HgCl2 between groups.
N = number of animals/group.
For comparison of PBS against HgCl2:
p < 0.05;
p < 0.01;
p < 0.005;
p < 0.001;
p < 0.0005;
p < 0.0001.
For comparison of −/− against +/+:
p < 0.05;
p < 0.01;
p < 0.005;
p < 0.001;
p < 0.0005;
p < 0.0001.
For comparison against PBS Irf1+/+ and +/−:
p < 0.005.
For comparison against HgCl2 Irf1+/+:
p < 0.001.
Fig. 3.
Mercury-induced ANoA and ANA are reduced in Ifngr1– and Irf1–deficient mice. Three genotypes of mice, wild-type (+/+), heterozygous knockout (+/−), and homozygous knockout (−/−) for each gene, received subcutaneous injections of PBS (open circles) or HgCl2 (40 µgs) in PBS (closed circles) twice/week for 4 weeks before serum was collected and assayed for autoantibodies as described in Materials and Methods. N = 8/genotype. Results are expressed as mean ± 1 SEM of fluorescence intensity at a serum dilution of 1/100. Only results for HgCl2-treated mice are shown. Fluorescence intensity of ANoA for all PBS-treated mice was 0. Fluorescence intensity of ANA for PBS-treated IFN-γR mice was 0 except for Ifnar−/−, which was 0.06 ± 0.18. Fluorescence intensity of ANA for PBS-treated IRF-1 mice was 0.06 ± 0.18 for wildtype, heterozygous and homozygous mice. Columns are as identified in Fig. 1.
3.6. Interferon regulatory factor 1 (Irf1) deficiency dramatically reduces mHgIA
To begin to define the IFN-γ signaling pathways critical for mHgIA, we examined the role of Irf1, which functions as a transcription factor for a variety of immunologically important target genes [28]. Irf1-deficient mice given PBS alone had lower levels of total IgG and IgG2a than wt and Irf1+/− mice and, after exposure to mercury, showed no significant increase in total IgG or IgG1 or IgG2a subclasses (Table 2). In contrast, Irf1+/− littermates had the expected increases in IgG, IgG1 and IgG2a following mercury exposure, similar to wt mice (Table 2). AutoAbs were significantly reduced with Irf1 deficiency, and notably none of the Irf1−/− mice developed ANoA (Table 2). ANAs were also affected, but to a lesser degree, with significantly less intensity of ANA staining in both heterozygous and homozygous Irf1 knockout mice, but with over half of Irf1−/− and Irf1+/− mice developing ANAs (Table 2, Fig. 3). Correspondingly, anti-chromatin Ab levels were elevated in all mercury-treated groups except wt > Irf+/− > Irf1−/− mice, suggestive of a gene dosage effect.
In accordance with the serological findings, mercury exposure resulted in virtually no deposits of IgG or C3 in Irf1−/− kidneys, while heterozygous mice had deposits of IgG, but not C3 (Table 2). Thus, absence of Irf1 significantly reduces major humoral and immunopathological measures of mHgIA to a similar extent as deficiency in IFN-γR.
3.7. Serum BAFF in the absence of IFN-γ and IRF-1
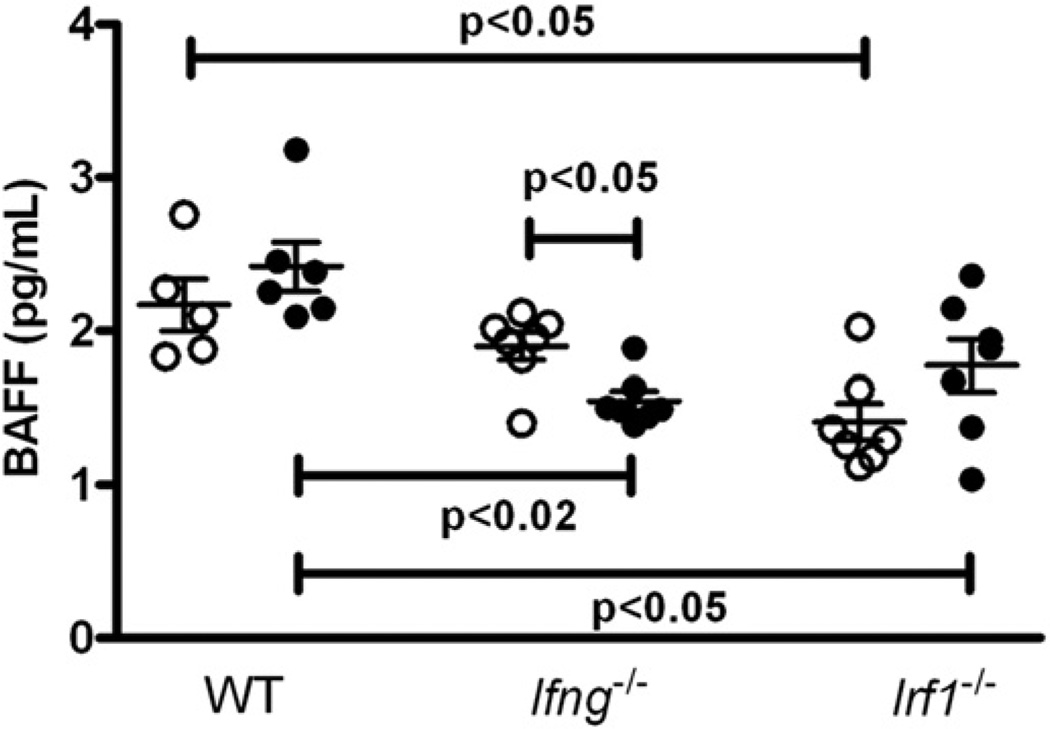
Another gene induced by IFN-γ is B cell activating factor (BAFF) [42]. BAFF plays an essential role in B cell survival, homeostasis and self-tolerance [43] and has been reported to be elevated in after mercury exposure [44] and idiopathic autoimmunity [45]. To determine if BAFF expression in mHgIA is dependent on IFN-γ, BAFF levels were examined in Ifng−/− and Irf1−/− mice exposed to HgCl2. In wt mice, exposure to mercury did not affect BAFF levels but, in sharp contrast, significantly lowered BAFF concentrations in Ifng−/− mice (Fig. 4). Interestingly, serum BAFF levels in Irf1−/− mice were lower than wt when comparing the PBS- and mercury-exposed groups, suggesting a modest role for IRF-1 in BAFF production (Fig. 4). Thus, while absence of IFN-γ or Irf1 had minor effects on BAFF levels, HgCl2 exposure did not increase BAFF expression in the B10.S background.
Fig. 4.
Mercury exposure does not increase serum BAFF in wt, Ifng−/− or Irf1−/− mice. Groups of wt, Ifng−/− and Irf1−/− mice received subcutaneous injections of PBS (open circles) or HgCl2 (40 µgs) in PBS (closed circles) twice/week for 4 weeks before serum was collected and assayed for BAFF as described in Materials and Methods. Results are expressed as mean ± 1 SEM.
3.8. T cell activation in the absence of IFN-γ and IRF-1
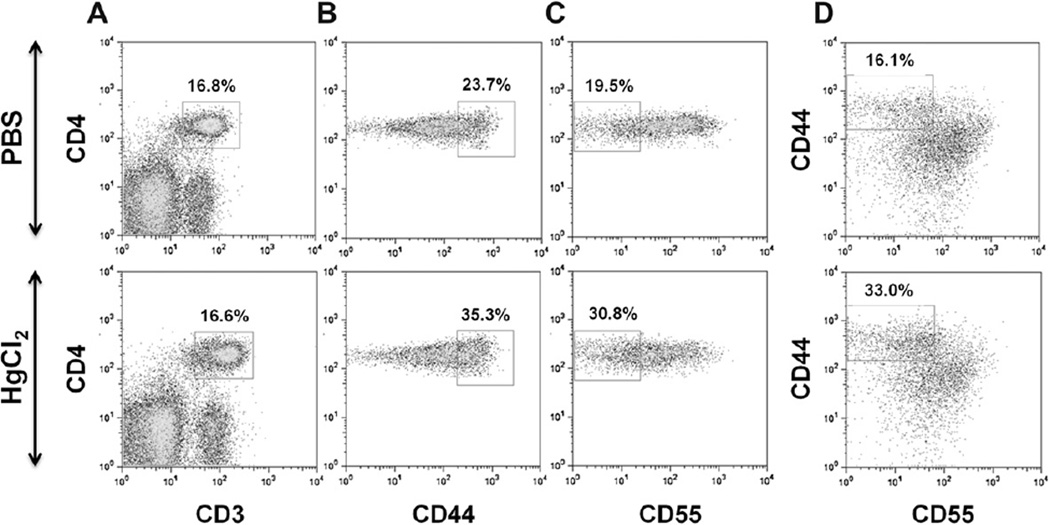
T cell activation is essential for mHgIA [30,33] and is associated with a reduction of CD55 (decay accelerating factor 1), which promotes cytokine production, including IFN-γ [27,33]. To determine if inhibition of mHgIA in IFN-γ- and IRF-1-deficient mice could be caused by impaired mercury-induced T cell activation or CD55 downregulation, the expression levels of CD44 (activation/effector subset marker) and CD55 were assessed on splenic CD4+ T cells from wt, Ifng−/−, and Irf1−/− mice treated with HgCl2 for 4 weeks (Fig. 5). Mercury exposure increased the percentages of CD44hi, CD55lo and CD44hiCD55lo subsets of CD4+ T cells compared with PBS controls in all three genotypes (Table 3). However, the increases of CD44hi and CD44hiCD55lo CD4+ T cells in Irf1−/− and Ifng−/− mice were less than wt. CD55lo CD4+ T cells showed no difference in wt and Irf1−/− and Ifng−/− mice, but were increased in Irf1−/− compared to Ifng−/− mice. These results show that T cell activation is augmented by, but not solely dependent upon, IFN-γ in mHgIA.
Fig. 5.
Representative density plot profiles of CD55 and CD44 expression on CD4± T cells in HgCl2 and PBS treated mice. B10. S mice received twice per week subcutaneous (s.c.) injections of HgCl2 for 4 weeks. Control mice received s.c. injections of PBS. Mice were killed and single-cell suspensions of splenocytes were prepared for flow cytometry analysis as described in material and methods. A) Gated CD3±CD4± cells, B) CD44hi gate on CD3±CD4± cells, C) CD55lo gate on CD3±CD4± cells, and D) CD44hi, CD55lo gate on CD3±CD4± cells.
Table 3.
T cell activation in wt, Ifng−/− and Irf-1−/− mice following exposure to HgCl2.
| CD3+CD4+ |
||||||
|---|---|---|---|---|---|---|
| Mice | Treatment | CD3+ | CD3+CD4+ | CD44h,i | CD44h,i CD55l,o | CD55l,o |
| wt | PBS | 25.8 ± 0.8 | 15.3 ± 0.9 | 21.2 ± 0.9 | 19.5 ± 0.4 | 20.8 ± 0.2 |
| HgCl2 | 26.1 ± 1.3 | 15.9 ± 0.7 | 37.9 ± 1.8b | 33.7 ± 1.1b | 27.5 ± 0.4b | |
| Ifng−/− | PBS | 26.8 ± 0.7 | 15.9 ± 0.5 | 21.2 ± 0.8 | 19.6 ± 0.7 | 17.7 ± 0.3c |
| HgCl2 | 26.2 ± 0.8 | 16.2 ± 0.5 | 30.9 ± 0.1a,b | 28.6 ± 0.8a,b | 23.1 ± 0.6b,c | |
| Irf-1−/− | PBS | 24.2 ± 1.5 | 20.8 ± 1.4 | 19.4 ± 1.5 | 19.5 ± 1.5 | 21.7 ± 0.6 |
| HgCl2 | 21.2 ± 0.9 | 17.9 ± 1.0 | 29.8 ± 0.3a,b | 28.2 ± 0.9a,b | 29.4 ± 1.4b | |
Data is presented as percent of gated cells (mean ± SEM). N = 4 mice/group.
p < 0.05 for comparison of HgCl2 against wt receiving HgCl2.
p < 0.05 for comparison of PBS against HgCl2.
p < 0.05 for comparison of Ifng−/− against Irf-1−/−.
4. Discussion
In this study, we used gene knockout mice to define the role of several IFN-γ pathways in the pathophysiology of mHgIA. First, we found that genes that promote IFN-γ expression, including Casp1, Nlrp3, Il12a, Il12b and Stat4, were not absolutely required for the development of mHgIA, although a modest reduction in certain disease manifestations were evident in Casp1−/− and Il12a−/− mice. Second, mHgIA was dependent on the IFN-γR and this primarily affected the production of ANoAs. Third, mHgIA was dramatically reduced in the absence of one of the main IFN-induced genes, IRF-1. These findings provide new insights into the role of IFN-γ promoting and signaling molecules in mHgIA.
Of the IFN-γ-promoting genes, caspase-1 (also known as IL-1β converting enzyme or ICE) cleaves and activates precursor forms of IL-1β and IL-18 following its activation by inflammasomes, leading to inflammation and enhanced IFN-γ production [35]. In the case of IL-18, induction of IFN-γ production by activated T cells occurs in synergy with IL-12 [46]. Casp1 is not required for type 1 diabetes [47], but its inhibition does attenuate EAE [37]. A role for IL-18 is lupus is unclear, with one study reporting that IL-18 receptor signaling is not required [48] while others report that exogenous IL-18 exacerbates disease [49] and that IL-18 cDNA vaccination reduces IFN-γ and disease severity [50]. The other main target of caspase 1, IL-1β, is elevated in idiopathic murine lupus [17], but it is not known if this plays a significant role in disease. Deficiency of IL-1β does, however, ameloriate the lupus-like disease produced by injection of the anti-DNA idiotype 16/6Id [51]. The requirement for IL-1 or IL-18 in mHgIA has not been directly examined, but HgCl2 does stimulate IL-1 production [52] and IL-1 is required for ex vivo mercury-induced T cell proliferation [53]. In the present study, absence of Casp1 had a moderate impact on the severity of immune deposits, suggesting that caspase-1 cleavage of IL-1β and/or IL-18 might influence pathology, but not humoral autoimmunity. Moreover, the latter is supported by our studies showing that Nlrp3-deficient C57BL/6 mice developed mercury-induced hyper-gammaglobulinemia and autoAbs. Given the importance of Casp1 and Nlrp3 to immune responses [35] it will be important for future studies to determine if either contributes to disease in idiopathic models of lupus.
IL-12, a heterodimeric cytokine composed of two subunits encoded by Il12a (IL-12p35) and Il12b (IL-12p40) [13], signals through Stat4 to induce IFN-γ in NK and T cells [54]. Absence of IL-12p35 has been shown to reduce proteinuria, nephritis and autoAbs to ribonucleoprotein antigens, but not DNA and chromatin in pristane-induced lupus [55], while lack of IL-12p40 delayed nephritis and reduced systemic pathology in lupus-prone MRL-Faslpr mice without effect on autoAbs [56]. Our results showed that lack of either IL-12 subunits had only modest, if any, effects on disease, with reduction of anti-chromatin only with Il12a-, not Il12b, deficiencies. This difference might have been due to the absence of IL-35 in mice lacking Il12a, an essential subunit of this immunoregulatory cytokine [57], or possibly homodimers of IL-12p40 (IL-12p80) that signal through NF-κB/p38 MAPK rather than Stat4 [40]. It should also be noted that the p40 subunit (Il12b) is required not only for IL-12, but also IL-23. Therefore, our finding that Il12b deficiency does not reduce mHgIA indicates that IL-23 is not required.
Stat4 is a transcription factor that transduces IL-12, IL-23 and type 1 interferon cytokine signals in T cells and monocytes, leading to Th1 and Th17 differentiation and monocyte activation [38]. Stat4 is required for several organ-specific autoimmune diseases, including EAE and diabetes [58], however its role is systemic autoimmunity is ambiguous. Absence of Stat4 did not affect disease development in MRL-Faslpr mice [59], but reduced disease in B6. TC mice, a congenic derivative of lupus-prone NZM2410 mice [60]. Other studies using the NZM strains 2410 and 2328 found that Stat4 deficiency resulted in accelerated nephritis and increased mortality [61,62]. These studies suggest that genetic background influences disease phenotype in Stat4- deficient mice and will require further study to identify the controlling factors. In this study the failure of Stat4 deficiency to impact severity of mHgIA further supports our argument that IL-12 and IL-23 are not required for mHgIA.
The requirement for IFN-γ regulated gene expression in the development of mHgIA could involve scores of genes [4,5], which is beyond the scope of the present study. However, one potential candidate is B cell activating factor (BAFF). BAFF is induced by IFN-γ [42] and can itself directly act on T cells to further stimulate IFN-γ production, thereby creating an inflammatory loop shown to exacerbate autoimmunity [63]. Significantly, BAFF was also reported to be elevated in a model of mHgIA using A.SW mice [44], but when we exposed B10.S mice to mercury, we detected no significant increase in BAFF levels. The reason for this discrepancy might be differences in the mouse strain or the HgCl2 exposure protocol (30 µgs on days 0, 2, and 4 versus 40 ug twice weekly for 4 wks), but our finding nevertheless suggests that mHgIA is not dependent on the induction of excess BAFF. We also observed that BAFF levels in IFN-γ-deficient mice were lower after mercury exposure, which could be a consequence of the competing activities of IFN-γ and IL-4 that respectively promote or inhibit BAFF expression [42], while IL-4 induced by HgCl2 [25,64,65] inhibits BAFF expression to a greater degree in the absence of IFN-γ.
Our observation that deficiency of Irf1, which is induced by IFN-γ, leads to reduction in all aspects of disease suggests that it might mediate a significant portion of the mHgIA-promoting activity of IFN-γ. Indeed, Irf1 regulates a large number of genes critical for innate and adaptive immunity, including several involved in antigen presentation and cytokine production [66]. Irf1 was also shown to contribute to spontaneous systemic autoimmunity in lupus-prone MRL-Faslpr mice [67] and several organ-specific autoimmune diseases, including type 1 diabetes mellitus [68], autoimmune prostatitis [69] and experimental autoimmune encephalomyelitis [70].
IFN-γ is produced by cells of the innate and adaptive immune systems [1], but it is unclear which IFN-γ+ cells are essential for mHgIA. Both T cells [71] and T cell costimulation [30] are required for mHgIA. Activated/memory (CD44hi) CD4+ T cells in mHgIA have reduced expression of CD55 [33]. Increased CD44hi, CD55lo and CD44hiCD55lo CD4+ cells were found in both mercury-exposed Irf1−/− and Ifng−/− mice, and any differences between mercury-treated mice were modest. This finding demonstrates that absence of IFN-γ and IFN-γ regulated gene expression does not suppress mercury-induced CD4+ T cell activation and argues that IFN-γ production by innate immune cells is not required for T cell activation in mHgIA.
The reduction in IFN-γ expression necessary to ameliorate mHgIA is unknown. IFN-γ deficiency dramatically reduces mHgIA and heterozygous IFN-γ mice have significantly reduced disease [25] suggesting that IFN-γ needs to be below endogenous levels to impact disease. This is supported by studies with β2-microglobulin deficient mice which lack the increase in IFN-γ necessary for full expression of disease but still have features of disease with levels of IFN-γ no different than PBS controls [65]. Basal IFN-γ may be sufficient for disease expression because IL-1α is known to enhance the expression of IFN-γ dependent genes including Irf1 via NF-κB activation [72]. In vitro studies demonstrate that mercury-induced T cell proliferation requires IL-1α [53] and IL-1α can promote Th1 differentiation [73]. It is therefore possible that mercury-induced IL-1α leads to T cell activation, including Th1 differentiation, IFN-γ production and Ifr1 expression. This can be examined by determining if the presence of IFN-γ+ CD4+ T cells in mHgIA requires IL-1α. This scenario also predicts that IL-1α deficiency should ameliorate mHgIA.
5. Conclusion
In summary, we dissected pathways related to the mHgIA-promoting IFN-γ, a cytokine required for idiopathic lupus in mouse models, and documented that genes that promote IFN-γ are not required for disease development, whereas crucial steps included the interaction of IFN-γ with its receptor, and also IFN-γ-induced Irf1. These results suggest that targeting of the pathways involved in the induction of IFN-γ may be less effective than those mediating function.
Acknowledgments
We acknowledge the gift of caspase-1 deficient mice by Lili Feng, MD (deceased) while she was at the Scripps Research Institute and thank Kat Occhipinti for editorial assistance. This work was supported by NIH grants ES007511 and ES014847 to KMP, AI052430 to HMH, and AR053731 to DHK, and the Swedish Research Council Branch of Medicine, number 09453 to PH. This is manuscript #21691 from The Scripps Research Institute.
Footnotes
Author contributions
K.M.P. and D.H.K. designed research; K.M.P., D.H.K., P.H., C.B.T., D.M.C., H.M.H. and J.C.H. performed research; H.M.H. contributed Nlrp3 deficient mice; K.M.P., D.H.K., P.H., C.B.T., D.M.C. J.C.H. and H.M.H. analyzed data; K.M.P. and D.H.K. wrote the manuscript.
Conflict of interest
None to declare.
References
- 1.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferongamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 2.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho-Pinto CE, Garcia MI, Mellado M, Rodriguez-Frade JM, Martin- Caballero J, Flores J, et al. autocrine production of ifn-gamma by macrophages controls their recruitment to kidney and the development of glomerulonephritis in MRL/lpr mice. J Immunol. 2002;169:1058–1067. doi: 10.4049/jimmunol.169.2.1058. [DOI] [PubMed] [Google Scholar]
- 4.Hertzog P, Forster S, Samarajiwa S. Systems biology of interferon responses. J Interferon Cytokine Res. 2011;31:5–11. doi: 10.1089/jir.2010.0126. [DOI] [PubMed] [Google Scholar]
- 5.Borden EC, Williams BR. Interferon-stimulated genes and their protein products: what and how? J Interferon Cytokine Res. 2011;31:1–4. doi: 10.1089/jir.2010.0129. [DOI] [PubMed] [Google Scholar]
- 6.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 7.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 8.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 9.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baudino L, Azeredo da Silveira S, Nakata M, Izui S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer Semin Immunopathol. 2006;28:175–184. doi: 10.1007/s00281-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paunovic V, Carroll HP, Vandenbroeck K, Gadina M. Signalling, inflammation and arthritis: crossed signals: the role of interleukin (IL)-12, -17, -23 and -27 in autoimmunity. Rheumatology (Oxford) 2008;47:771–776. doi: 10.1093/rheumatology/kem352. [DOI] [PubMed] [Google Scholar]
- 14.Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 2001;3:136–141. doi: 10.1186/ar290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMurray RW, Hoffman RW, Nelson W, Walker SE. Cytokine mRNA expression in the B/W mouse model of systemic lupus erythematosus–analyses of strain, gender, and age effects. Clin Immunol Immunopathol. 1997;84:260–268. doi: 10.1006/clin.1997.4390. [DOI] [PubMed] [Google Scholar]
- 16.Chu EB, Ernst DN, Hobbs MV, Weigle WO. Maturational changes in CD4+ cell subsets and lymphokine production in BXSB mice. J Immunol. 1994;152:4129–4138. [PubMed] [Google Scholar]
- 17.Prud’homme GJ, Kono DH, Theofilopoulos AN. Quantitative polymerase chain reaction analysis reveals marked overexpression of interleukin-1 beta, interleukin-1 and interferon-gamma mRNA in the lymph nodes of lupusprone mice. Mol Immunol. 1995;32:495–503. doi: 10.1016/0161-5890(95)00024-9. [DOI] [PubMed] [Google Scholar]
- 18.Jacob CO, van der Meide PH, McDevitt HO. In vivo treatment of (NZB X NZW) F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng SL, Moslehi J, Craft J. Roles of interferon-gamma and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–1946. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- 22.Haas C, Ryffel B, Le Hir M. IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB x NZW)F1 mice. J Immunol. 1998;160:3713–3718. [PubMed] [Google Scholar]
- 23.Lawson BR, Prud’homme GJ, Chang Y, Gardner HA, Kuan J, Kono DH, et al. Treatment of murine lupus with cDNA encoding IFN-gammaR/Fc. J Clin Invest. 2000;106:207–215. doi: 10.1172/JCI10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kono DH, Balomenos D, Pearson DL, Park MS, Hildebrandt B, Hultman P, et al. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th1/Th2 imbalance. J Immunol. 1998;161:234–240. [PubMed] [Google Scholar]
- 26.Schiraldi M, Monestier M. How can a chemical element elicit complex immunopathology? lessons from mercury-induced autoimmunity. Trends Immunol. 2009;30:502–509. doi: 10.1016/j.it.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Toomey CB, Cauvi DM, Song WC, Pollard KM. Decay-accelerating factor 1 (Daf1) deficiency exacerbates xenobiotic-induced autoimmunity. Immunology. 2010;131:99–106. doi: 10.1111/j.1365-2567.2010.03279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22:5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- 29.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard KM, Arnush M, Hultman P, Kono DH. Costimulation requirements of induced murine systemic autoimmune disease. J Immunol. 2004;173:5880–5887. doi: 10.4049/jimmunol.173.9.5880. [DOI] [PubMed] [Google Scholar]
- 31.Burlingame RW, Rubin RL. Subnucleosome structures as substrates in enzyme-linked immunosorbent assays. J Immunol Methods. 1990;134:187–199. doi: 10.1016/0022-1759(90)90380-e. [DOI] [PubMed] [Google Scholar]
- 32.Kotzin BL, Lafferty JA, Portanova JP, Rubin RL, Tan EM. Monoclonal anti-histone autoantibodies derived from murine models of lupus. J Immunol. 1984;133:2554–2559. [PubMed] [Google Scholar]
- 33.Cauvi DM, Cauvi G, Pollard KM. Reduced expression of decay-accelerating factor 1 on CD4+ T cells in murine systemic autoimmune disease. Arthritis Rheum. 2007;56:1934–1944. doi: 10.1002/art.22639. [DOI] [PubMed] [Google Scholar]
- 34.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 38.Korman BD, Kastner DL, Gregersen PK, Remmers EF. STAT4: genetics, mechanisms, and implications for autoimmunity. Curr Allergy Asthma Rep. 2008;8:398–403. doi: 10.1007/s11882-008-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 40.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 42.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Davidson A. BAFF and selection of autoreactive B cells. Trends Immunol. 2011;32:388–394. doi: 10.1016/j.it.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Y, Gallucci S, Gaughan JP, Gross JA, Monestier M. A role for B cell-activating factor of the TNF family in chemically induced autoimmunity. J Immunol. 2005;175:6163–6168. doi: 10.4049/jimmunol.175.9.6163. [DOI] [PubMed] [Google Scholar]
- 45.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 46.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 47.Schott WH, Haskell BD, Tse HM, Milton MJ, Piganelli JD, Choisy-Rossi CM, et al. Caspase-1 is not required for type 1 diabetes in the NOD mouse. Diabetes. 2004;53:99–104. doi: 10.2337/diabetes.53.1.99. [DOI] [PubMed] [Google Scholar]
- 48.Lin L, Peng SL. Interleukin-18 receptor signaling is not required for autoantibody production and end-organ disease in murine lupus. Arthritis Rheum. 2005;52:984–986. doi: 10.1002/art.20961. [DOI] [PubMed] [Google Scholar]
- 49.Esfandiari E, McInnes IB, Lindop G, Huang FP, Field M, Komai-Koma M, et al. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J Immunol. 2001;167:5338–5347. doi: 10.4049/jimmunol.167.9.5338. [DOI] [PubMed] [Google Scholar]
- 50.Bossu P, Neumann D, Del Giudice E, Ciaramella A, Gloaguen I, Fantuzzi G, et al. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc Natl Acad Sci U S A. 2003;100:14181–14186. doi: 10.1073/pnas.2336094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voronov E, Dayan M, Zinger H, Gayvoronsky L, Lin JP, Iwakura Y, et al. IL-1 beta-deficient mice are resistant to induction of experimental SLE. Eur Cytokine Netw. 2006;17:109–116. [PubMed] [Google Scholar]
- 52.Zdolsek JM, Soder O, Hultman P. Mercury induces in vivo and in vitro secretion of interleukin-1 in mice. Immunopharmacology. 1994;28:201–208. doi: 10.1016/0162-3109(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 53.Pollard KM, Landberg GP. The in vitro proliferation of murine lymphocytes to mercuric chloride is restricted to mature T cells and is interleukin 1 dependent. Int Immunopharmacol. 2001;1:581–593. doi: 10.1016/s1567-5769(00)00034-5. [DOI] [PubMed] [Google Scholar]
- 54.Rosenzweig SD, Holland SM. Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev. 2005;203:38–47. doi: 10.1111/j.0105-2896.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 55.Calvani N, Satoh M, Croker BP, Reeves WH, Richards HB. Nephritogenic autoantibodies but absence of nephritis in Il-12p35-deficient mice with pristane-induced lupus. Kidney Int. 2003;64:897–905. doi: 10.1046/j.1523-1755.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 56.Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. 2003;170:3915–3925. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- 57.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan MH. STAT4: a critical regulator of inflammation in vivo. Immunol Res. 2005;31:231–242. doi: 10.1385/IR:31:3:231. [DOI] [PubMed] [Google Scholar]
- 59.Menke J, Bork T, Kutska B, Byrne KT, Blanfeld M, Relle M, et al. Targeting transcription factor Stat4 uncovers a role for interleukin-18 in the pathogenesis of severe lupus nephritis in mice. Kidney Int. 2011;79:452–463. doi: 10.1038/ki.2010.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Z, Duan B, Croker BP, Morel L. STAT4 deficiency reduces autoantibody production and glomerulonephritis in a mouse model of lupus. Clin Immunol. 2006;120:189–198. doi: 10.1016/j.clim.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Jacob CO, Zang S, Li L, Ciobanu V, Quismorio F, Mizutani A, et al. Pivotal role of Stat4 and Stat6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice. J Immunol. 2003;171:1564–1571. doi: 10.4049/jimmunol.171.3.1564. [DOI] [PubMed] [Google Scholar]
- 62.Singh RR, Saxena V, Zang S, Li L, Finkelman FD, Witte DP, et al. Differential contribution of IL-4 and STAT6 vs STAT4 to the development of lupus nephritis. J Immunol. 2003;170:4818–4825. doi: 10.4049/jimmunol.170.9.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, et al. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pollard KM, Hultman P, Arnush M, Hildebrandt B, Kono DH. Immunology and genetics of xenobiotic-induced autoimmunity. In: Conrad K, Bachmann MP, Chan EK, Fritzler MJ, Humbel RL, Sack U, Shoenfeld Y, editors. From animal models to human genetics: research in the induction and pathogenicity of autoantiS200bodies. Lengerich: Pabst; 2004. pp. 130–144. [Google Scholar]
- 65.Pollard KM, Hultman P, Toomey CB, Cauvi DM, Konoc DH. beta2-microglobulin is required for the full expression of xenobiotic-induced systemic autoimmunity. J Immunotoxicol. 2011;8:228–237. doi: 10.3109/1547691X.2011.583614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi L, Perin JC, Leipzig J, Zhang Z, Sullivan KE. Genome-wide analysis of interferon regulatory factor I binding in primary human monocytes. Gene. 2011;487:21–28. doi: 10.1016/j.gene.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reilly CM, Olgun S, Goodwin D, Gogal RM, Jr, Santo A, Romesburg JW, et al. Interferon regulatory factor-1 gene deletion decreases glomerulonephritis in MRL/lpr mice. Eur J Immunol. 2006;36:1296–1308. doi: 10.1002/eji.200535245. [DOI] [PubMed] [Google Scholar]
- 68.Nakazawa T, Satoh J, Takahashi K, Sakata Y, Ikehata F, Takizawa Y, et al. Complete suppression of insulitis and diabetes in NOD mice lacking interferon regulatory factor-1. J Autoimmun. 2001;17:119–125. doi: 10.1006/jaut.2001.0531. [DOI] [PubMed] [Google Scholar]
- 69.Motrich RD, van Etten E, Baeke F, Riera CM, Mathieu C, Rivero VE. Crucial role of interferon-gamma in experimental autoimmune prostatitis. J Urol. 2010;183:1213–1220. doi: 10.1016/j.juro.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Ren Z, Wang Y, Liebenson D, Liggett T, Goswami R, Stefoski D, et al. IRF-1 signaling in central nervous system glial cells regulates inflammatory demyelination. J Neuroimmunol. 2011;233:147–159. doi: 10.1016/j.jneuroim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Hultman P, Johansson U, Dagnaes-Hansen F. Murine mercury-induced autoimmunity: the role of T-helper cells. J Autoimmun. 1995;8:809–823. doi: 10.1016/s0896-8411(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 72.Hurgin V, Novick D, Werman A, Dinarello CA, Rubinstein M. Antiviral and immunoregulatory activities of IFN-gamma depend on constitutively expressed IL-1alpha. Proc Natl Acad Sci U S A. 2007;104:5044–5049. doi: 10.1073/pnas.0611608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Von Stebut E, Ehrchen JM, Belkaid Y, Kostka SL, Molle K, Knop J, et al. Interleukin 1alpha promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med. 2003;198:191–199. doi: 10.1084/jem.20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]