Abstract
The extinction of conditioned fear memories requires plasticity in the infralimbic medial prefrontal cortex (IL mPFC), but little is known about the molecular mechanisms involved. Brain-derived neurotrophic factor (BDNF) is a key mediator of synaptic plasticity in multiple brain areas. In rats subjected to auditory fear conditioning, BDNF infused into the IL mPFC reduced conditioned fear for up to 48 hours, even in the absence of extinction training, which suggests that BDNF substituted for extinction. Similar to extinction, BDNF-induced reduction in fear required N-methyl-D-aspartate receptors and did not erase the original fear memory. Rats failing to learn extinction showed reduced BDNF in hippocampal inputs to the IL mPFC, and augmenting BDNF in this pathway prevented extinction failure. Hence, boosting BDNF activity in hippocampal-infralimbic circuits may ameliorate disorders of learned fear.
Extinction of conditioned fear forms a new memory in the infralimbic medial prefrontal cortex (IL mPFC) that is critical for the retrieval of extinction (1, 2). IL single-unit responses correlate with the successful retrieval of such extinction memories (3), and IL stimulation strengthens these memories (3). Consolidation of extinction requires plasticity within the IL mPFC, which in turn depends on N-methyl-D-aspartate (NMDA) receptors, mitogen-activated protein kinase, and protein synthesis (2, 4). Understanding the molecular mechanisms that support this extinction-related plasticity could lead to pharmacological approaches for enhancing extinction memory, which might facilitate the treatment of anxiety disorders.
Epigenetic regulation within the IL mPFC of the gene encoding BDNF correlates with fear extinction (5). Because BDNF is a major molecular mediator of memory consolidation (6), we hypothesized that BDNF is responsible for consolidating extinction memory within the IL mPFC. If true, it should be possible to enhance extinction via direct application of BDNF to the IL mPFC. Accordingly, rats were subjected to auditory fear conditioning and, the following day, received bilateral IL mPFC infusion of human recombinant BDNF protein (0.75 μg per side) 60 min before extinction training. Conditioned freezing in BDNF-treated rats was significantly reduced relative to saline-infused rats (main effect of drug F1,14 = 28.359, P < 0.001, Fig. 1A; for suppression of food seeking, see fig. S1). This effect persisted in an extinction test the following day (day 3, main effect of drug F1,14 = 11.029, P = 0.005, Fig. 1A), which indicated that BDNF strengthened extinction memory.
Fig. 1.
BDNF infused into the infralimbic cortex substitutes for behavioral extinction. (A) Rats’ freezing levels in response to tones that were paired with footshocks (Cond) or given alone during extinction (Ext) and Test sessions. BDNF infusions into the IL mPFC before extinction (arrow) reduced freezing on days 2 and 3 relative to saline-infused (SAL) controls (n = 8 per group). (B) A similar effect was observed when BDNF was infused in the absence of training on day 2 (SAL, n = 5; BDNF, n = 7). (C) Infusing BDNF 24 hours before conditioning had no effect (SAL, n = 9; BDNF, n = 7). Trials are shown in blocks of two. **P < 0.01, repeated-measures analysis of variance (ANOVA). Error bars represent SEM.
Freezing was significantly reduced in BDNF rats from the first extinction trial [t(14) = 3.335, P = 0.005], which suggested that BDNF reduced fear independent of extinction training. We therefore repeated the previous experiment but omitted extinction training from day 2. Conditioned rats were infused with BDNF or saline and returned to their home cages. The following day, freezing was again reduced in BDNF-treated rats from the first trial [t(10) = 4.476, P = 0.001, Fig. 1B] and throughout the extinction session (main effect of drug F1,10 = 27.220, P < 0.001). Although the effect of BDNF on fear did not require extinction training, it did require conditioning, because BDNF infused 1 day before conditioning did not significantly reduce freezing (Fig. 1C). BDNF infusions did not alter locomotion, anxiety, or motivation to seek food reward (fig. S2, A to C). The lack of effect on conditioning and open-field anxiety suggests that BDNF infusions did not decrease amygdala activity nonspecifically. Nor could BDNF’s effects be attributed to potentiation of latent inhibition, because removing habituation trials did not prevent the effect (fig. S2D).
There are two interpretations for these results. BDNF could inhibit fear expression (similar to extinction), or it could have degraded the original fear memory. To distinguish between these possibilities, we determined the extent to which freezing could be reinstated after unsignaled footshocks, which can reveal the underlying fear memory (7). One day after infusions, rats were given extinction training followed by two unsignaled shocks. Replicating our previous experiment, BDNF rats showed reduced fear throughout the extinction session (main effect of drug F1,21 = 7.337, P = 0.013, Fig. 2A). On day 4, however, both saline-and BDNF-treated rats froze equivalently to the tone (78% and 80%, respectively; Fig. 2A), indicating that BDNF left the original fear memory intact. The return of freezing on day 4 was not due to BDNF “wearing off” (fig. S3A) or contextual conditioning (fig. S3B).
Fig. 2.
Similar to extinction, the BDNF effect does not degrade the original fear memory and requires NMDA receptors. (A) Conditioned rats received BDNF or saline infusions into the IL mPFC on day 2 (SAL, n = 12; BDNF, n = 11). On day 3, both groups were extinguished, followed by two shocks, resulting in a complete return of freezing in the BDNF group. (B) IL infusion of BDNF was combined with a systemic injection of the NMDA antagonist CPP (CPP + BDNF, n = 8). Controls were infused with BDNF and given a saline injection (SAL + BDNF, n = 10) or were both infused and injected with saline (SAL + SAL, n = 10). On day 3, all groups underwent a single-tone extinction test. *P < 0.05, two-way repeated-measures ANOVA, main effect of drug; *P < 0.05, Student’s t test, SAL + SAL compared to SAL + BDNF.
One hallmark of extinction memory is its dependence on NMDA receptors (4, 8, 9). For example, systemic administration of the NMDA receptor antagonist 3(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) prevents long-term extinction memory (10). The BDNF receptor TrkB interacts with the NMDA receptor in vivo (11), and BDNF enhances NMDA currents in vitro (12). It is possible, therefore, that IL BDNF mediates its extinction-like effects through NMDA receptors. To test this, we conditioned rats as previously on day 1. On day 2, in the absence of training, rats received one of the following treatment combinations: (i) saline injection (intraperitoneally) + saline infusion into IL (SAL + SAL), (ii) saline injection + BDNF infusion (SAL + BDNF), or (iii) CPP injection + BDNF infusion (CPP + BDNF). On day 3, all rats were returned to the chambers for a single-tone test. As before, SAL + BDNF rats showed significantly reduced fear relative to SAL + SAL rats (main effect of drug F2,25 = 4.597, P = 0.020, post hoc P = 0.046; Fig. 2B). However, CPP + BDNF rats were indistinguishable from SAL + SAL rats in their freezing level (post hoc P = 0.828; Fig. 2B), which demonstrated that NMDA receptors are necessary for BDNF-induced reductions in fear.
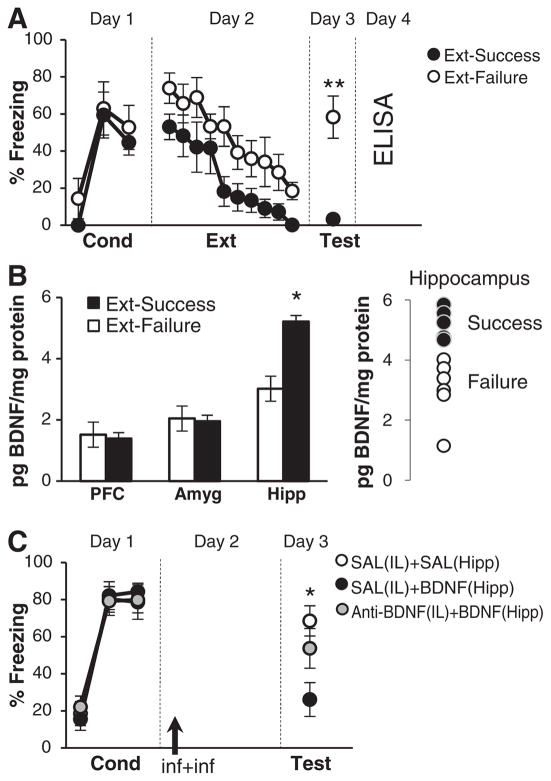
Does extinction depend on endogenous BDNF levels in the IL mPFC or its inputs? We addressed this question by capitalizing on the fact that there can be considerable variability in extinction memory across rats (8, 13). Rats were conditioned and extinguished on days 1 and 2, respectively, as above. We then selected two subgroups on the basis of their ability to successfully recall extinction on day 3. “Extinction Failure” and “Extinction Success” rats had freezing values in the top or bottom 44%, respectively (i.e., the middle 12% was excluded). These two subgroups differed significantly on test day [t(10) = 4.728, P = 0.001] but showed no significant differences during conditioning or extinction training (Fig. 3A). Normal extinction training followed by poor retrieval of extinction is consistent with impaired infralimbic function (1, 2).
Fig. 3.
Hippocampal projections to the infralimbic cortex mediate BDNF-extinction. (A) One day after extinction training, rats were divided into two groups on the basis of their ability to retrieve extinction (Ext-Success, n = 6; Ext-Failure, n = 6; **P < 0.01). The following day, BDNF protein concentrations were determined by enzyme-linked immunosorbent assay (ELISA). (B) Ext-Success rats showed elevated levels of BDNF in the hippocampus but not in the mPFC or amygdala (*P < 0.05, Ext-Success versus Ext-Failure). Hippocampal BDNF levels in individual Success and Failure rats were nonoverlapping. (C) Conditioned rats were divided into three groups. Controls received a saline infusion into the IL mPFC followed by a saline infusion into the hippocampus [SAL(IL) + SAL(Hipp), n = 7]. Another group received a saline infusion into the IL mPFC followed by a BDNF infusion into Hipp [SAL(IL) + BDNF(Hipp), n = 8]. A third group received an infusion of a BDNF-sequestering antibody into the IL mPFC followed by BDNF infusion into the hippocampus [anti-BDNF(IL) + BDNF(Hipp), n = 9]. Infusion of BDNF antibody into the IL mPFC blocked the fear-reducing effects of hippocampal BDNF. *P < 0.05, SAL(IL) + SAL(Hipp) compared to SAL(IL) + BDNF(Hipp).
For each subgroup, brain tissue from the mPFC, amygdala, and hippocampus was dissected 24 hours after the extinction test to determine BDNF levels. The amygdala and hippocampus were chosen as putative BDNF-containing inputs that might be important for supplying BDNF to the IL mPFC to facilitate extinction recall (14–16). Indeed, hippocampal CA1 neurons produce BDNF (16, 17) and project to the IL mPFC (14). BDNF protein levels in the Success group were elevated relative to the Failure group in the hippocampus [t(9) = 4.370, P = 0.002], but not the mPFC or amygdala (Fig. 3B). These data are consistent with previous studies in which genetic knockdown of hippocampal BDNF impaired fear extinction (17).
If the hippocampus is the source of IL BDNF, then increasing the available supply of hippocampal BDNF should have similar effects. We took advantage of the fact that BDNF infusions increase BDNF levels in efferent targets (18). There were three treatment groups in this experiment. After conditioning, one group received a hippocampal infusion of BDNF immediately after a saline infusion into the IL mPFC [SAL(IL) + BDNF(Hipp)]. A second group also received a hippocampal BDNF infusion, but this was preceded by infusion of a BDNF-inactivating antibody into the IL mPFC [anti-BDNF(IL) + BDNF(Hipp)] to test the hypothesis that Hipp-applied BDNF works via the IL mPFC. A control group received SAL infusions into both structures [SAL(IL) + SAL(Hipp)].
Similar to its effect on the IL mPFC, BDNF infused into the hippocampus reduced fear, as measured by both freezing [main effect of drug F2,21 = 4.715, P = 0.020, post hoc P = 0.013 comparing SAL(IL) + SAL(Hipp) to SAL(IL) + BDNF(Hipp)] (Fig. 3C) and conditioned suppression of food seeking (fig. S4). The effect of hippocampal BDNF could be prevented by coadministration of a BDNF-inactivating antibody in the IL mPFC [P = 0.461 comparing SAL(IL) + SAL(Hipp) to Anti-BDNF(IL) + BDNF(Hipp)], which suggests that the IL mPFC is the primary site of action for hippocampal BDNF.
We were able to pharmacologically induce extinction with a single infusion of BDNF into the hippocampal-infralimbic pathway, a key projection for extinction memory. This effect was not a facilitation of extinction, as no extinction training was required. We have adopted the term “BDNF-extinction” to parallel the term “BDNF-LTP” used to describe BDNF induction of hippocampal LTP in the absence of electrical stimulation (19). Extinction potentiates the hippocampal-prefrontal pathway, and disrupting this potentiation disrupts extinction recall (20). Our results provide further support for the importance of this pathway in extinction and extend these findings by identifying BDNF as a key molecular mediator.
In our experiments, BDNF-extinction required NMDA receptors, which are also necessary for extinction-related bursting in IL neurons (8). Because BDNF facilitates NMDA receptor currents (11, 12), exogenously applied BDNF may simulate extinction by inducing bursting in the IL mPFC. Additionally, BDNF-extinction may involve IL targets, such as intercalated (21) or basolateral amygdala (9, 15) neurons, which also participate in extinction.
Because the behavioral effects of BDNF were observed only when BDNF was infused after conditioning, it is possible that BDNF treatment may lead to partial reversal of conditioning-induced changes. Conditioning induces a rapid reduction in hippocampal BDNF, which reverts in 2 days (22). Extinction failure then may arise from a delayed normalization of BDNF levels after conditioning. If so, application of BDNF to the hippocampus (or to the IL mPFC) may work to reduce fear by restoring BDNF to preconditioning levels and/or reversing conditioning-induced reductions in IL excitability (23).
Recall of extinction in healthy human subjects activates the ventromedial PFC and hippocampus (24), both of which are deficient in posttraumatic stress disorder (25). A single-nucleotide polymorphism in the gene encoding human BDNF (Val66 → Met) results in extinction impairment (26) and decreases the release of BDNF from hippocampal neurons (27). Pharmacotherapies that increase hippocampal BDNF may prove to be efficacious treatments for fear disorders characterized by extinction impairments. BDNF-extinction is complementary to reconsolidation blockade, in which pharmacological agents are used to eliminate the original fear memory (7). Both approaches represent potentially powerful strategies to treat anxiety disorders by manipulating traumatic memories within fear circuits.
Supplementary Material
Acknowledgments
We thank V. Garcia, D. Merced, N. Padilla, C. Rodriguez, J. Rodriguez-Romaguera, D. Sierra-Mercado, and A. Torrado for technical assistance and D. Paré and J. F. McGinty for helpful comments. Supported by NIH grants MH058883, MH081975, NS043011, MH083516, MH085383, and RR003051 and by the University of Puerto Rico.
References and Notes
- 1.Sotres-Bayon F, Cain CK, LeDoux JE. Biol Psychiatry. 2006;60:329. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Quirk GJ, Mueller D. Neuropsychopharmacology. 2008;33:56. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad MR, Quirk GJ. Nature. 2002;420:70. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 4.Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Cereb Cortex. 2009;19:474. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredy TW, et al. Learn Mem. 2007;14:268. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramham CR, Messaoudi E. Prog Neurobiol. 2005;76:99. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Duvarci S, Nader K. J Neurosci. 2004;24:9269. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Neuron. 2007;53:871. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Falls WA, Miserendino MJ, Davis M. J Neurosci. 1992;12:854. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santini E, Muller RU, Quirk GJ. J Neurosci. 2001;21:9009. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black IB. J Neurobiol. 1999;41:108. [PubMed] [Google Scholar]
- 12.Levine ES, Crozier RA, Black IB, Plummer MR. Proc Natl Acad Sci USA. 1998;95:10235. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herry C, Garcia R. J Neurosci. 2002;22:577. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoover WB, Vertes RP. Brain Struct Funct. 2007;212:149. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 15.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Nat Neurosci. 2006;9:870. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Science. 1990;250:290. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- 17.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Mol Psychiatry. 2007;12:656. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain Res. 2010;1314:183. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. J Neurosci. 2002;22:7453. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Neurobiol Learn Mem. 2008;89:560. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Nature. 2008;454:642. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmusson AM, Shi L, Duman R. Neuropsychopharmacology. 2002;27:133. doi: 10.1016/S0893-133X(02)00286-5. [DOI] [PubMed] [Google Scholar]
- 23.Santini E, Quirk GJ, Porter JT. J Neurosci. 2008;28:4028. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalisch R, et al. J Neurosci. 2006;26:9503. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milad MR, et al. Biol Psychiatry. 2009;66:1075. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soliman F, et al. Science. 2010;327:863. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen ZY, et al. Science. 2006;314:140. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.