Abstract
The trace element selenium is an essential micronutrient that has received considerable attention for its potential use in the prevention of cancer. In spite of this interest, the mechanism(s) by which selenium might function as a chemopreventive remain to be determined. Considerable experimental evidence indicates that one possible mechanism by which selenium supplementation may exert its benefits is by enhancing the DNA damage repair response, and this includes data obtained using cultured cells, animal models as well as in human clinical studies. In these studies, selenium supplementation has been shown to be beneficial in reducing the frequency of DNA adducts and chromosome breaks, consequentially reducing the likelihood of detrimental mutations that ultimately contribute to carcinogenesis. The benefits of selenium can be envisioned as being due, at least in part, to it being a critical constituent of selenoproteins such as glutathione peroxidases and thioredoxin reductases, proteins that play important roles in antioxidant defence and maintaining the cellular reducing environment. Selenium, therefore, may be protective by preventing DNA damage from occurring as well as by increasing the activity of repair enzymes such as DNA glycosylases and DNA damage repair pathways that involve p53, BRCA1 and Gadd45. An improved understanding of the mechanism of selenium’s impact on DNA repair processes may help to resolve the apparently contradicting data obtained from decades of animal work, human epidemiology and more recently, clinical supplementation studies.
Introduction
The daunting challenge of managing cancer morbidity and mortality once diagnosed has focused attention and energy towards the development of chemopreventive compounds that can be administered to reduce overall cancer incidence. Such candidate compounds need to develop for that purpose with extensive pre-clinical evidence of efficacy that can include data from cell culture and animal models, as well as human epidemiology. Most important is the requirement that a chemopreventive strategy not impose any increased risk of disease on the healthy individuals the intervention is designed to benefit, whether this is the population at large or a selected segment of the population who are identified at enhanced cancer risk. One particularly enticing candidate as a useful chemopreventive is the essential trace element selenium, whose benefits have been supported by extensive data obtained from in vitro and animal models of cancer, as well as human studies demonstrating that for at least some types of cancer, there is an inverse association between dietary selenium intake and cancer risk. The enthusiasm for selenium’s use in chemoprevention peaked in 1996 when Larry Clark and colleagues revealed the results of their selenium supplementation trial and offered data that low level, non-toxic supplementation with 200 µg selenium per day in the form of selenised yeast could reduce the incidence of several common cancer types (1). The weight of the evidence eventually led to the investment in the large, well-designed selenium supplementation trial, SELECT, which aimed to determine, among other things, whether providing selenium to men could reduce their risk of prostate cancer. The results of that trial, initially reported in 2009 (2) and updated with additional follow-up in 2011 (3), were disappointing as they indicated that providing selenium in the selected dose and form was ineffective in reducing prostate cancer incidence.
One of the properties of selenium that may impact its ability to modulate cancer risk is its effects on DNA damage accumulation and consequently mutagenesis. DNA lesions in the form of double-strand and single-strand breaks, adducts, as well as aberrations in chromosome structure and number can ultimately lead to mutation and structural rearrangement of the genome. DNA damage response (DDR) pathways constitute a complex network of repair processes whose activation following the sensing of damaged DNA contribute greatly towards generating mutations in the key regulatory genes, driving normal cells towards malignant development and progression. The impact of selenium status on DDR offers one possible mechanism by which the availability of this element can influence cancer risk and perhaps offer a plausible explanation for the apparent contrasting results between promising pre-clinical data and negative results from SELECT. The information relating selenium levels to various DDRs and the implications of these data to strategies aimed at reducing cancer incidence will be the focus of this review.
Selenium and cancer
One of the first studies indicating that selenium may reduce the incidence of cancer in humans came in 1977 when Schrauzer, White and Schneider examined mortality from cancer as a function of selenium intake or selenium levels in whole blood in 27 countries and reported that for several organs, there was indeed an inverse association (4). Subsequently, there have been numerous prospective studies to expand on this observation, with the results being mixed, and these data have been presented in several recent reviews (5, 6). The most promising evidence for a benefit of higher selenium intake has come from meta-analyses that have pooled data from multiple, independent studies. Such efforts have indicated a benefit for a higher selenium status in reducing cancer incidence for the bladder (7) and prostate (8,9). In the case of lung cancer, a comprehensive review of the data from 49 prospective observational studies concluded that there was a reduction in lung cancer with higher selenium (OR = 0.69, 95% CI = 0.53–0.91) as well as mortality (OR = 0.55, 95% CI = 0.36–0.83) (10). Two recent meta-analyses have also concluded that selenium supplementation may be useful in the reduction of several cancer types, with modifying effects such as gender, risk factors and baseline levels of selenium prior to supplementation (11,12).
The possible benefits of selenium in humans is supported by an extensive literature of in vitro and animal model systems indicating that low, non-toxic levels of selenium can protect against cancer. The broad efficacy of selenium in this regard is impressive, and while most of published papers have demonstrated a chemopreventive effect of selenium in breast and colon cancer rodent models, the list of target organs is extensive and includes skin, prostate lung, etc. (13–15). Equally extensive is the list of chemical carcinogens that selenium supplementation has shown to be effective against, including several agents that are carcinogenic due to causing oxidative damage or inducing DNA adducts (see below). Of particular interest were observations that selenium could also protect against both ultraviolet (UV) (14) and ionising radiation (16) insults that require neither transport nor intracellular activation for their pro-carcinogenic effects. Selenium has also been shown to protect mice from prostate cancer induced by organ-specific expression of an oncogene (17).
The historical data indicating that selenium is protective against a wide range of carcinogens and in several different organs indicate that selenium may be working via multiple mechanisms and at different levels of carcinogenesis. There is a growing literature indicating that most of the mechanisms of action of selenium can be generally divided into two broad categories: (i) those that occur due to selenium metabolites and forms that are not associated with specific proteins and (ii) those that include the function of selenoproteins that contain selenium in the form of selenocysteine. In the case of the former, non-protein forms of selenium have been shown to effectively reduce tumour incidence in animal models (18,19), selectively be toxic to tumour cells in vitro and typically require higher concentrations to achieve their benefits (20), being well above the doses required to saturate the production of selenoproteins. Selenoproteins, on the other hand, are a class of proteins that include one or more molecules of selenocysteine and are synthesised using dedicated translational machinery. The mRNAs for these selenoproteins encode selenocysteine as a UGA codon that is deciphered as selenocysteine due to the presence of a specific Selenocysteine-Insertion Sequence (SECIS) located in the 3′-untranslated region of all selenoprotein-encoding mRNA, and the action of selenocysteine tRNA and selenium-specific translation factors (21–23). In humans, there are 25 selenocysteine-containing proteins and the current understanding of their functions has been reviewed elsewhere (5,6,24). The involvement of selenoproteins in determining cancer risk is supported by genetic data indicating that allelic variation in several human selenoproteins are associated with elevated cancer risk as reviewed in References 25 and 26. The involvement of both selenium metabolites and selenoproteins in cancer risk was shown using a mouse animal model of colon cancer (27). In this model, mice were engineered to produce fewer selenoproteins due to the expression of a mutant selenocysteine tRNA (28) and following treatment with the azoxymethane carcinogen, demonstrated increased incidence of aberrant crypt foci (ACF), an established biomarker of colon cancer in rodents as compared with control, wild-type mice. Selenium supplementation of the mutant mice, which did not enhance selenoprotein levels, partially protected the animals from ACF formation (27). More recently, it was shown that the genetic depletion of selenoproteins in the mouse mammary gland increased the susceptibility to tumour formation in that organ when the mice were exposed to the carcinogen 7,12-dimethylbenz[α] anthracene (DMBA) (29). Previously, there were several reports of selenium supplemented to culture media or fed to rodents in a variety of forms being able to reduce the incidence of DMBA-DNA adducts (30–33), but these data may have been accounted for by the effects of selenium on carcinogen metabolism as opposed to the removal of the lesions.
In general, the mechanisms by which selenium prevents tumour development are likely to be complicated and involve multiple mechanisms that impact distinct stages of carcinogenesis. At supranutritional levels well above that required to maximise the expression of selenoproteins, differential toxicity to tumour cells as compared with normal tissues has been documented by several lines of evidence, as well as potential effects of angiogenesis needed to provide nutrients and oxygen to the expanding tumour mass. Such effects of selenium on tumour development and growth are typically supported by the literature using xenograft models to investigate the potential anti-carcinogenic potential of selenium. In contrast, selenium supplementation at or near the nutritional requirement is more likely to impact the levels of individual selenoproteins with protective functions, perhaps doing so in individuals whose selenium intake is insufficient to maximise the expression of those proteins. An additional complication resides in the possibility that selenium may have opposing effects on tumour development depending on the stage of carcinogenesis. This issue has been extensively reviewed with regard to the selenium-dependent glutathione peroxidases (34) and supported by recent data indicating an inverse association between glutathione peroxidase one (GPx-1) and Gleason score, a measure of prostate cancer aggressiveness, among African American men (35). One potential mechanism of action supported by a considerable body of research indicates that selenium protects against cancer, and perhaps promotes the survival of tumours, by minimising/reducing DNA damage that ultimately would result in carcinogenic mutations.
Selenium and DNA damage
A role for selenium in impacting the levels of DNA damage was indicated in 2004 when a cohort of men at elevated risk of prostate cancer in New Zealand were provided a placebo or either a 200 or 400 µg selenium supplement in the form of selenised yeast for 6 months and DNA damage was assessed by the Comet assay (36). The results of this study indicated that for men with initial serum selenium levels below the average of 97.8ng/ml, there was a statistically significant, inverse association between selenium status and accumulated DNA damage. A subsequent 7-month selenium supplementation study in dogs reported a U-shaped dose–response curve, whereas animals with the lowest and highest toenail selenium levels had the higher levels of DNA damage in the prostatic tissue (37). There are numerous complexities in considering a role for selenium in reducing mutagenic DNA damage, including the form of selenium used, the means and timing of delivery and the particular model systems used in the investigations. These issues, as well as a comprehensive review of the literature describing results from a wide variety of assays used to measure the protective effects of selenium in cell culture have been recently discussed (24,38,39).
In vitro effects of selenium on DNA damage repair
While there have been a large number of studies indicating that selenium could reduce DNA damage, many fewer studies have directly assessed selenium’s effect on the repair process. A seminal manuscript was published by Seo et al. (40) in 2002 indicating that pre-treatment of several human, non-tumourigenic fibroblast cell lines enhanced the DNA repair capacity of exposed cells. The authors not only showed that pre-treatment with non-toxic forms of selenium in the form of selenomethionine reduced the amount of DNA damage of UV-exposed cells as measured by the alkaline comet method but also demonstrated that these cells showed the ability to reactivate a reporter gene for chloramphenicol acetyltransferase on a plasmid where that gene was inactivated by cyclobutane pyrimidine dimers prior to transfection into the fibroblasts. The use of this latter approach established that the removal of the dimers was due to enhanced repair as opposed to the prevention of lesion formation (40). In addition, Laffon et al. (41) reported that incubation of human leukocytes with 50 µM selenomethionine was capable of enhancing the repair of bleomycin-induced strand breaks using a modified comet assay, and this was maximised when the cells were pre-incubated with selenium. Recently, De Rosa et al. (42) investigated the potential effects of selenocompounds on DNA repair using an in vitro approach in which cells were pre-incubated with either 30nM sodium selenite or 10 µM selenomethionine, extracts prepared and incubated with DNA substrates that contained oxidative lesions due to exposure to UVA and riboflavin, alkylation caused by exposure to methyl methanesulphonate (MMS) or cyclobutane dimers introduced by exposure to UVC. The repair process was monitored by evaluating the excision capacity of cell extracts, an obligate step in the DNA repair process. Using this approach, it was shown that pre-treatment with both selenium compounds enhanced the excision capacity of the exposed cells, but only for oxidised lesions such as 8-oxoGua (42). It is noteworthy that both selenomethionine and sodium selenite stimulated repair to a similar extent given the possibility that these forms of selenium, as well as others under investigation, may have very different consequences on biological systems, as well as on cancer risk (43–45). These studies are also significant as they distinguish between the reductions in DNA damage that occur by preventing lesions and those occurring from mechanisms that involve the repair of existing damage. Table I presents a summary of the published data specifically indicating the increase in DNA damage repair due to selenium supplementation.
Table I.
Cell culture studies whose results indicated that selenium supplementation can enhance DNA damage repair
| Experimental model | Selenium form and dose | Mode of DNA damage induction | Detection of DNA repair | References |
|---|---|---|---|---|
| LNCaP | Sodium selenite, 30nM; Selenomethionine, 10 µM | UVA, hydrogen peroxide | Alkaline comet assay | (42) |
| MCF-7 breast cancer cells, mouse embryonic fibroblast | Sodium selenite, 30 nM | UV | Micronucleus assay, Big Blue mouse cells (LacI shuttle vector system) | (58) |
| IMR90, GM-08399, or GM-01389 human fibroblast cells | Selenomethionine, 10 µM | UV | Alkaline comet assay | (40) |
| Mouse primary bone marrow cells | Selenomethionine, 15 µM; methylseleninic acid, 1 µM | Carboplatin | Mutation in the reporter gene in Big Blue mouse | (52) |
| Human leukocytes | Selenomethionine, 50 µM | Bleomycin | Alkaline comet assay and 8-OHdG estimation for oxidative DNA damage repair | (41) |
The above-described studies and the majority of the work looking at the protection offered by selenium using cultured cells are conducted in media in which selenium is provided by the 10% serum used in the growth media, typically resulting in the concentration of selenium being in the general range of 15–30nM selenium (46). This level of selenium is below that required to maximise the activity of the selenoproteins, many of which provide antioxidant function; there are numerous examples where providing selenium in the nanomolar range to cultures incubated in 10% serum result in substantial increases in antioxidant selenoprotein levels (47). For example, the levels of glutathione peroxidase 1 (GPx-1), a selenium-containing protein that is often used as a measure of selenium adequacy, can be stimulated in most tissue culture cells several fold by the addition of nanomolar quantities of selenium in either organic (i.e. selenomethionine) or inorganic (sodium selenite) form to the culture media although the amount needed to maximise this enzyme’s activity differs significantly (48). In the work reported by De Rosa et al. (42), incubation of the LNCaP cells used in that study with either 30nM sodium selenite or 10 µM selenomethionine resulted in a 2- to 3-fold increase in GPx-1 and thioredoxin reductase 1 (Trx1) enzyme activities, each with the potential to detoxify reactive oxygen prior to its causing DNA damage. Selenium levels observed in blood are much higher than those used in tissue culture media, for example, the average level of selenium in the serum obtained from participants in SELECT was 135 µg/l or 1.7 µM (2) but the levels in tissues can be much lower. A recent report quantifying the levels of trace elements in human prostates by inductively coupled plasma mass spectrometry along with internal standards of known concentrations obtained post-mortem from 13- to 60-year-old males in Moscow indicated that the selenium in those tissues ranged from 0.216 to 1.3mg/kg dry weight, or 2.7 to 16nM (49), indicating that selenium supplementation may very well stimulate tissue selenoprotein levels. Therefore, many of the studies reporting enhanced genotoxic protection achieved with selenium supplementation can involve the elevation of selenoprotein levels, whereas those studies that have investigated the benefits of selenium using supplements in the micromolar range are likely to be doing so far above the levels needed to achieve maximal selenoprotein induction.
Animal models for the beneficial effects of selenium
As mentioned above, there is a significant amount of data consisting of well over 100 independent publications indicating that selenium supplementation of the diets of laboratory animals can reduce the incidence of cancer in carcinogen-exposed rodents. What is particularly striking about these accumulated data is the efficacy of selenium in multiple organs and against a wide variety of carcinogens that stimulate carcinogenesis via different mechanisms. As in the case of cultured cells, there is insufficient data to indicate whether selenium is protective by reducing DNA lesion formation or by stimulating their repair. There is also conflicting data with regard to whether selenium supplementation can reduce mutation frequencies using the Big Blue rodent model, a transgenic system in which animals contain the Big Blue λLIZ shuttle vector that contains the lacZ and lacI genes, with the latter serving as a target for mutagenesis, integrated into their genome (50). In this model, the animals are exposed to a mutagen, genomic DNA is recovered from tissue, packaged and the resulting phage are used to infect indicator bacteria; a phage containing a wild-type lacI results in a white plaque due to functional repression of the lacZ gene, whereas an inactivating mutation in lacI results in a blue plaque. One study using the Big Blue rat model failed to detect any benefit of higher selenium status in the colon or liver of rats exposed to dimethylhydrazine (51), whereas a subsequent study using a similar reporter construct in mice indicated that selenium in the form of either selenomethionine or methylseleninic acid could protect bone marrow cells from mutations induced by exposure to carboplatin (52). However, the sheer variety of carcinogens that selenium supplementation has been shown to be protective against in animal models argues that the reduction in cancer incidence in exposed animals cannot be simply due to modulation of antioxidant status, as many of the carcinogens that selenium is effective against cause DNA damage by mechanisms other than an increase in reactive oxygen species (ROS). A summary of the types of carcinogens that selenium has been shown to be effective against in animals and the types of lesions they form is presented in Table II.
Table II.
Selenium has been shown to be effective in reducing the incidence of a wide variety of DNA lesions in animal models
| Experimental model | Selenium form and dose | Mode of DNA damage induction | Detection of DNA damage | References |
|---|---|---|---|---|
| Mouse primary bone marrow cells | Selenomethionine, 15 µM; methylseleninic acid, 1 µM | Carboplatin (involves XPC DNA repair protein) | Mutation in the reporter gene in Big Blue mouse | (52) |
| Ovine lymphocyte | Sodium selenite. 1 µg/ml | Carbon tetrachloride | Micronucleus test, | (79) |
| Swiss mice | Selenium ACE, 100 mg | Beryllium chloride | Micronucleus test | (80) |
| Wistar rats | Sodium selenate 2 and 6mg/l drinking water | Methylmercury | Comet assay | (81) |
| Albino rat | Sodium selenite 0.1mg/kg body weight | o-Cresol | mRNA analysis of apoptotic genes | (82) |
| Transgenic mice expressing human mutations in the amyloid precursor protein and human presenilin-1 | Sel-Plex (selenium-enriched diet), 1 µg Sel/g | – | 8-OHdG estimation | (83) |
| Swiss albino mice | Ebselen 2.5–10mg/kg body weight | Cyclophosphamide | Micronucleus test, Comet assay | (84) |
| Rat lymphocytes | Selenium-containing phycocyanin (Se-PC) 2 µM | Hydrogen peroxide | Comet assay | (85) |
| Canine model of prostate cancer | Selenomethionine, 3 µg/kg body weight; high selenium yeast, 6 µg/kg body weight | – | Comet assay | (37) |
Human studies supporting a role for selenium in enhancing DNA damage repair
Selenium supplementation of individuals at elevated risk of DNA damage due to disease or exposure to carcinogens may reduce the levels of DNA damage. Hemodialysed patients are at greater risk of DNA lesions and supplementation of the diets of 42 chronic kidney disease patients with either 200 µg of selenium in the form of selenised yeast or placebo demonstrated a benefit of selenium supplementation as measured by the comet assay (53). A potential benefit of selenium was also implicated by data indicating that higher levels of selenium were associated with reduced DNA lesions in a population environmentally exposed to polychlorinated biphenyls (54). One of the most convincing links between selenium and DNA repair has emerged from studies on women who are at increased risk of breast cancer due to a mutation in the BRCA1 gene. BRCA1 is a protein involved in DNA damage repair and individuals who inherit a defective copy of the BRCA1 gene are at a greatly enhanced risk of developing cancers of the breast, ovary and prostate (55, 56). Evidence for the involvement of BRCA1 with the selenium-mediated stimulation of DNA repair was reported by showing that selenium supplementation increased the association of BRCA1 with p53 (57) and that BRCA1 was required in order for selenium supplementation to provide protection against UV-induced toxicity (57) or DNA damage (58). In humans, selenium status was shown to be inversely associated with the levels of chromosome breaks induced by ex vivo γ-irradiation of lymphocytes as measured by micronuclei formation, but only when those cells were obtained from women who were carriers of one defective copy of BRCA1 (59). Furthermore, the frequency of chromosome breaks observed in cultured lymphocytes from women who were BRCA1 carriers following exposure to bleomycin was significantly higher as compared with non-carrier relatives, but supplementation of BRCA1 carriers with 670 µg of selenium in the form of selenite for 1–3 months returned the levels of breaks to that of the non-carriers (60). Looking at a population of BRCA1 carriers who had undergone adnexectomy and were provided a daily supplement of 300 µg sodium selenite, it was determined that urine obtained from the supplemented group contained more of the product of the base excision repair of oxidative lesions, 8-oxoGua than that obtained from the unsupplemented group (61). These latter data provide direct evidence for the enhanced repair of oxidative lesions as a consequence of the selenium supplement in this cohort. Publications describing human studies including data that support a role for selenium in enhancing DNA damage repair are listed in Table III.
Table III.
Human studies whose results support the ability of selenium supplementation to enhance DNA damage repair
| Experimental model | Selenium form and dose | Mode of DNA damage induction | Detection of DNA damage | References |
|---|---|---|---|---|
| Women BRCA1 mutation carriers | Sodium selenite, 300 µg/day | – | 8-oxodG determination | (61) |
| Lymphocytes isolated from BRCA1 mutation carriers | Mean toenail selenium, 0.97±0.14 µg/g | Ionising radiation | Comet assay, micronucleus test, p-H2AX detection | (59) |
| Lymphocytes isolated from BRCA1 mutation carriers | Sodium selenite, 276 µg/day | Bleomycin | Micronucleus test | (60) |
| Human peripheral blood lymphocytes | Selenomethionine, 0.25–2 µM | Doxorubicin | Comet assay, micronucleus test | (86) |
| Lymphocytes from patients with chronic kidney disease | Selenium-enriched yeast 200 µg/day | Prolonged hemodialysis | Comet assay | (53) |
| Lymphocytes from Inuit people | Serum selenium levels 652±67.5 vs. 694.8±56.3 µg/l | Polychlorinated biphenyls | 8-oxodG determination | (54) |
| Human leukocytes | Selenomethionine, 50 µM | Bleomycin | Comet assay and 8-OHdG estimation for oxidative DNA damage | (41) |
| Lymphocytes from healthy donors | Seleno-yeast, 200 and 400 µg of selenium per day | – | Comet assay | (87) |
| Lymphocytes from human donors | Selenium, 50 µg/day | – | Micronucleus test | (88) |
The ability of selenium to impact the levels of DNA damage in human supplementation studies is likely to be complicated by modifying factors, such as the genotype of selected genes. For example, it was shown that there was lower DNA damage, as determined by the comet assay, in blood obtained from New Zealanders as a function of increasing selenium status, but this relationship only occurred for individuals expressing specific alleles of the GPx-1 (rs1050450 C/C) and GPx-4 (rs713041 T/T) genes (62). A similar result was obtained indicating that the supplementation of a Brazilian population of obese women with selenium, provided by the consumption of Brazil nuts that contain very high selenium levels (one ounce can contain as much as 10 times the US recommended daily allowance), resulted in the reduction of DNA damage in blood, again determined by the comet assay, but only among those expressing the rs1050450 C/C GPx-1 allele (63). As in the case of much of the in vitro data on the protection offered by selenium, it is difficult to know whether these results represent the prevention of damage or its repair.
Possible mechanisms of action
It is apparent that selenium supplementation can reduce the levels of DNA damage by stimulating the production of selenoproteins, several of which are antioxidants and capable of detoxifying ROS prior to their induction of oxidative lesions, this being particularly true if cells, animals or people are initially selenium deficient. How selenium may enhance the repair of damaged DNA is less obvious. It is likely that this may occur by mechanisms that involve elevating the activity of selenoproteins and those that do not. Although none of the activities of the known mammalian selenoproteins are anticipated to alter cellular repair capability directly, it is more likely that these selenoproteins impact signalling pathways, perhaps by the modulation of ROS, resulting in the stimulation of repair.
One possible target of selenium that results in the enhanced repair of the major oxidative DNA lesion 8-hydroxydeoxyguanine (8-oxoGua) is the 8-oxoguanine DNA glycosylase (OGG1). Since OGG1 contains critical redox-sensitive residues whose oxidation results in the attenuation of that enzyme’s activity, the induction of antioxidant selenoproteins by selenium may enhance the repair of oxidised DNA lesions by helping to maintain OGG1 in the reduced, more active state. A polymorphic variant of OGG1, containing a cysteine instead of a serine at position 326, is particularly more sensitive to inactivation following exposure to oxidative stress as compared with the cysteine-containing protein (64) and individuals who express that variant are more susceptible to various forms of cancer (65). Thus, selenium supplementation may enhance antioxidant selenoproteins that in turn helps to maximise OGG1 activity. This scenario is consistent with the observed excision activity for oxidative lesions achieved in selenium-treated cell extracts, but not for DNA alkylation or photoproducts (42).
Selenium provided to mice in the form of selenium-enriched broccoli, which can stimulate the translation of GPx-1, was also shown to stimulate the expression of the Gadd45 protein involved in replication and DNA damage repair (66). Selenium did not induce Gadd45 when provided to MCF-7 human breast cancer cells that do not express GPx-1 but could in the same cells over-expressing GPx-1 (67). The effect of GPx-1 expression on signalling pathways has been recently been reviewed (68).
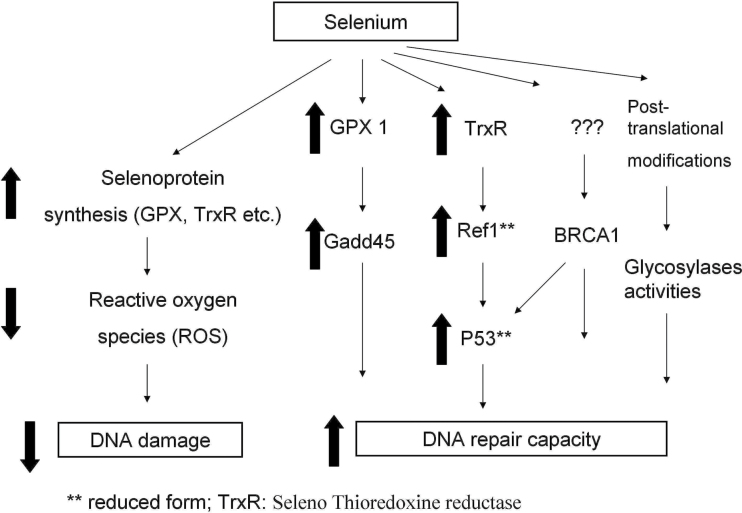
Selenium in the form of selenomethionine was shown to protect mouse embryonic fibroblasts from UV-induced DNA damage in a p53-dependent mechanism that required the Ref-1 protein (69), and since this effect of selenium was achieved when the medium was supplemented with selenium at the same time they were irradiated, it is very unlikely that the induction of any selenoproteins was involved. Selenomethionine modulated p53 activity by redox regulation of key p53 cysteine residues (275/277). The resulting conformational change results in enhanced p53 DNA-binding activity and the subsequent stimulation of DNA repair (57,70). The effect of selenomethionine on p53 requires the cellular protein Ref-1 (or APE1), a protein previously shown to physically interact with p53, a conclusion supported by the demonstration that inactivation of Ref-1 blocks p53 modification by the selenomethionine. Significantly, the redox state of Ref-1, which modulates the reduction of p53, is regulated by thioredoxin (Trx) through its dithiol-reducing activity with the redox activity of Trx being maintained by the selenoprotein thioredoxin reductase (TrxR). Subsequent work supported the involvement of the BRCA1 gene in the in vitro protection of DNA from UV exposure (57,58) Therefore, there would appear to be multiple mechanisms by which selenium could stimulate DNA repair and some of these are presented schematically in Figure 1.
Fig. 1.
Possible mechanisms by which selenium might influence DNA damage repair (arrows indicate up-regulation or down-regulation and asterisk indicates activation of respective proteins).
In addition to the potential actions of selenium on specific proteins and pathways, there may be general effects that ultimately impact DNA repair capacity. For example, selenium may be impacting the activity of repair enzymes by altering post-translational modifications. Several glycosylases are regulated by acetylation and/or phosphorylation and selenium has been shown to alter histone deacetylase and kinase activities (71, 72).
Implications
That selenium can protect against cancer is foregone conclusion, based on a wealth of animal data that has accumulated over decades. In humans, epidemiological trends towards an inverse association between selenium intake and cancer incidence, at least for some organ sites. Although supplementation data from clinical intervention trials may appear conflicting at this time, others have argued that the form of selenium and or the baseline selenium levels of the study population may represent plausible explanations for the discrepancy between data such as has been obtained from the Nutritional Prevention of Cancer (NPC) trial and SELECT (73–75). Indeed, the baseline levels of the subjects in NPC trial were significantly lower than those in SELECT, and participants in NPC that were in the lowest baseline levels of selenium were those who showed the most benefit from the supplement (76,77). These data are, therefore, reminiscent of that reported years ago in which supplementation of the diets of dogs with selenium resulted in a ‘U-shaped curve’ where those with the lowest and highest levels of selenium in their prostatic tissue had the highest levels of DNA damage as compared with those in the mid-range (78). Understanding how selenium may be useful in reducing cancer incidence will continue to be difficult given the large number of physiological changes that will be associated with selenium status. For example, changes in the levels and activities of selenoproteins are likely to result in changes in the reducing state of the cell that would be expected to have profound effects on ROS and the myriad of signalling pathways that respond to changes in ROS. Thus, the biological activity of selenoproteins without apparent antioxidant activity and the effects of non-protein selenium metabolites will make determinations of what specific effects of selenium account for its biological properties a challenge for years to come.
Among the possible consequences of selenium status that might help to explain some of the apparent inconsistencies in the chemoprevention literature, effects on DNA repair is a possible mechanism that deserves careful consideration. Selenium can stimulate DNA damage repair in vitro in addition to preventing oxidative damage and lesions resulting from metabolic activation of carcinogens. The wide range of carcinogens that selenium can protect against in animal models of carcinogenesis also support the concept that the enhancement cellular anti-oxidant capabilities is not the only means by which selenium can be preventive. Furthermore, the interaction between selenium availability and components of the BRCA1 repair pathway observed in humans also support the connection between selenium’s chemopreventive actions and the repair of DNA damage.
A function of selenium in stimulating DNA damage repair also may explain the apparent discrepancy between human epidemiology indicating that dietary selenium is beneficial and the negative results of SELECT. A diet that includes a level of selenium that maximises the DNA repair capacity of susceptible cells will attenuate the accumulation of carcinogenic mutations over the course of a lifetime, and do so for the multiple genes that are required to be altered for prostate cancer to develop. In contrast and as reported in the 2011 update on SELECT data (3), providing selenium to 8737 men over the age of 55 (>50 years old for black men), many of whom are likely to have selenium levels that are already maximising the potential enhancement of DNA damage repair, and observing 575 cases of prostate cancer may be insufficient to achieve a statistically significant effect. Data on the stratification of SELECT participants by selenium status at the time of participation in the trail has not yet been reported, but benefits among those with the lowest levels of selenium, as has been reported in the NPC trial (76), may become apparent. Furthermore, it is of interest to note that the increase in prostate cancer observed among SELECT participants who were in the vitamin E arm may have been reduced by selenium, as the prostate cancer incidence in the arm receiving both vitamin E and selenium appeared to be the same as those receiving only placebo (3), indicating that selenium may have been protecting against the pro-carcinogenic effects of vitamin E. Future efforts to understand the basic biology of selenium compounds and selenoproteins and their impact at physiological conditions may yet lead to a benefit to selenium supplementation, at least among selected populations.
Funding
National Institutes of Health (RO1CA127943) to A.M.D.; Post Doctoral Fellowship from the American Institute for Cancer Research (10A072) to S.B.
References
- 1. Clark L. C., Combs G. F., Jr, Turnbull B. W., et al. (1996). Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA, 276, 1957–1963 [PubMed] [Google Scholar]
- 2. Lippman S. M., Klein E. A., Goodman P. J, et al. (2009). Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 301, 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein E. A., Thompson I. M., Jr, Tangen C. M, et al. (2011). Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 306, 1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schrauzer G. N., White D. A., Schneider C. J. (1977). Cancer mortality correlation studies. III: statistical associations with dietary selenium intakes. Bioinorg. Chem., 7, 23–31 [DOI] [PubMed] [Google Scholar]
- 5. Davis C. D., Tsuji P. A., Milner J. A. (2012). Selenoproteins and cancer prevention. Annu. Rev. Nutr., 32, 73–95 [DOI] [PubMed] [Google Scholar]
- 6. Rayman M. P. (2012). Selenium and human health. Lancet, 379, 1256–1268 [DOI] [PubMed] [Google Scholar]
- 7. Amaral A. F., Cantor K. P., Silverman D. T., Malats N. (2010). Selenium and bladder cancer risk: a meta-analysis. Cancer Epidemiol. Biomarkers Prev., 19, 2407–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Etminan M., FitzGerald J. M., Gleave M., Chambers K. (2005). Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control, 16, 1125–1131 [DOI] [PubMed] [Google Scholar]
- 9. Brinkman M., Reulen R. C., Kellen E., Buntinx F., Zeegers M. P. (2006). Are men with low selenium levels at increased risk of prostate cancer?. Eur. J. Cancer, 42, 2463–2471 [DOI] [PubMed] [Google Scholar]
- 10. Fritz H., Kennedy D., Fergusson D., Fernandes R., Cooley K., Seely A., Sagar S., Wong R., Seely D. (2011). Selenium and lung cancer: a systematic review and meta analysis. PLoS ONE, 6, e26259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dennert G., Zwahlen M., Brinkman M., Vinceti M., Zeegers M. P., Horneber M. (2011). Selenium for preventing cancer. Cochrane Database Syst. Rev., 5, , CD005195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee E. H., Myung S. K., Jeon Y. J., Kim Y., Chang Y. J., Ju W., Seo H. G., Huh B. Y. (2011). Effects of selenium supplements on cancer prevention: meta-analysis of randomized controlled trials. Nutr. Cancer, 63, 1185–1195 [DOI] [PubMed] [Google Scholar]
- 13. Bespalov V. G., Panchenko A. V., Murazov I. a. G., Chepik O. F. (2011). [Influence of sodium selenite on carcinogenesis of the prostate and other organs induced by methylnitrosourea and testosterone in rats]. Vopr. Onkol., 57, 486–492 [PubMed] [Google Scholar]
- 14. Overvad K., Thorling E. B., Bjerring P., Ebbesen P. (1985). Selenium inhibits UV-light-induced skin carcinogenesis in hairless mice. Cancer Lett., 27, 163–170 [DOI] [PubMed] [Google Scholar]
- 15. Li L., Xie Y., El-Sayed W. M., Szakacs J. G., Franklin M. R., Roberts J. C. (2005). Chemopreventive activity of selenocysteine prodrugs against tobacco-derived nitrosamine (NNK) induced lung tumors in the A/J mouse. J. Biochem. Mol. Toxicol., 19, 396–405 [DOI] [PubMed] [Google Scholar]
- 16. Cekan E., Tribukait B., Vokal-Borek H. (1985). Protective effect of selenium against ionizing radiation-induced malformations in mice. Acta Radiol. Oncol., 24, 267–271 [DOI] [PubMed] [Google Scholar]
- 17. Wang L., Bonorden M. J., Li G. X., Lee H. J., Hu H., Zhang Y., Liao J. D., Cleary M. P., Lü J. (2009). Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev. Res., 2, 484–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El-Bayoumy K., Sinha R. (2004). Mechanisms of mammary cancer chemoprevention by organoselenium compounds. Mutat. Res., 551, 181–197 [DOI] [PubMed] [Google Scholar]
- 19. El-Bayoumy K.(ed.) (1991). The Role of Selenium in Cancer Prevention. J.B. Lippincott Co., Philadelphia: [Google Scholar]
- 20. Ip C. (1998). Lessons from basic research in selenium and cancer prevention. J. Nutr., 128, 1845–1854 [DOI] [PubMed] [Google Scholar]
- 21. Amberg R., Mizutani T., Wu X. Q., Gross H. J. (1996). Selenocysteine synthesis in mammalia: an identity switch from tRNA(Ser) to tRNA(Sec). J. Mol. Biol., 263, 8–19 [DOI] [PubMed] [Google Scholar]
- 22. Berry M. J., Banu L., Chen Y. Y., Mandel S. J., Kieffer J. D., Harney J. W., Larsen P. R. (1991). Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3’ untranslated region. Nature, 353, 273–276 [DOI] [PubMed] [Google Scholar]
- 23. Caban K., Copeland P. R. (2006). Size matters: a view of selenocysteine incorporation from the ribosome. Cell Mol. Life Sci., 63, 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferguson L. R., Karunasinghe N., Zhu S., Wang A. H. (2012). Selenium and its’ role in the maintenance of genomic stability. Mutat. Res., 733, 100–110 [DOI] [PubMed] [Google Scholar]
- 25. Rayman M. P. (2009). Selenoproteins and human health: insights from epidemiological data. Biochim. Biophys. Acta, 1790, 1533–1540 [DOI] [PubMed] [Google Scholar]
- 26. Zhuo P., Diamond A. M. (2009). Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochim. Biophys. Acta, 1790, 1546–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Irons R., Carlson B. A., Hatfield D. L., Davis C. D. (2006). Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J. Nutr., 136, 1311–1317 [DOI] [PubMed] [Google Scholar]
- 28. Moustafa M. E., Carlson B. A., El-Saadani M. A, et al. (2001). Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing isopentenyladenosine-deficient selenocysteine tRNA. Mol. Cell Biol., 21, 3840–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hudson T. S., Carlson B. A., Hoeneroff M. J., Young H. A., Sordillo L., Muller W. J., Hatfield D. L., Green J. E. (2012). Selenoproteins reduce susceptibility to DMBA-induced mammary carcinogenesis. Carcinogenesis, 33, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El-Bayoumy K., Das A., Boyiri T., Desai D., Sinha R., Pittman B., Amin S. (2003). Comparative action of 1,4-phenylenebis(methylene)selenocyanate and its metabolites against 7,12-dimethylbenz[a]anthracene-DNA adduct formation in the rat and cell proliferation in rat mammary tumor cells. Chem. Biol. Interact., 146, 179–190 [DOI] [PubMed] [Google Scholar]
- 31. Ejadi S., Bhattacharya I. D., Voss K., Singletary K., Milner J. A. (1989). In vitro and in vivo effects of sodium selenite on 7,12-dimethylbenz[a]anthracene–DNA adduct formation in isolated rat mammary epithelial cells. Carcinogenesis, 10, 823–826 [DOI] [PubMed] [Google Scholar]
- 32. Liu J. Z., Gilbert K., Parker H. M., Haschek W. M., Milner J. A. (1991). Inhibition of 7,12-dimethylbenz(a)anthracene-induced mammary tumors and DNA adducts by dietary selenite. Cancer Res., 51, 4613–4617 [PubMed] [Google Scholar]
- 33. Liu J. Z., Milner J. A. (1992). Age, dietary selenium and quantity of 7,12-dimethylbenz(a)anthracene influence the in vivo occurrence of rat mammary DNA adducts. J. Nutr., 122, 1361–1368 [DOI] [PubMed] [Google Scholar]
- 34. Brigelius-Flohé R., Kipp A. (2009). Glutathione peroxidases in different stages of carcinogenesis. Biochim. Biophys. Acta, 1790, 1555–1568 [DOI] [PubMed] [Google Scholar]
- 35. Jerome-Morais A., Wright M. E., Liu R., Yang W., Jackson M. I., Combs G. F., Jr, Diamond A. M. (2012). Inverse association between glutathione peroxidase activity and both selenium-binding protein 1 levels and Gleason score in human prostate tissue. Prostate, 72, 1006–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karunasinghe N., Ryan J., Tuckey J., Masters J., Jamieson M., Clarke L. C., Marshall J. R., Ferguson L. R. (2004). DNA stability and serum selenium levels in a high-risk group for prostate cancer. Cancer Epidemiol. Biomarkers Prev., 13, 391–397 [PubMed] [Google Scholar]
- 37. Waters D. J., Shen S., Glickman L. T., Cooley D. M., Bostwick D. G., Qian J., Combs G. F., Jr, Morris J. S. (2005). Prostate cancer risk and DNA damage: translational significance of selenium supplementation in a canine model. Carcinogenesis, 26, 1256–1262 [DOI] [PubMed] [Google Scholar]
- 38. Valdiglesias V., Pásaro E., Méndez J., Laffon B. (2010). In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: a review. Arch. Toxicol., 84, 337–351 [DOI] [PubMed] [Google Scholar]
- 39. Brozmanová J., Mániková D., Vlčková V., Chovanec M. (2010). Selenium: a double-edged sword for defense and offence in cancer. Arch. Toxicol., 84, 919–938 [DOI] [PubMed] [Google Scholar]
- 40. Seo Y. R., Sweeney C., Smith M. L. (2002). Selenomethionine induction of DNA repair response in human fibroblasts. Oncogene, 21, 3663–3669 [DOI] [PubMed] [Google Scholar]
- 41. Laffon B., Valdiglesias V., Pásaro E., Méndez J. (2010). The organic selenium compound selenomethionine modulates bleomycin-induced DNA damage and repair in human leukocytes. Biol. Trace Elem. Res., 133, 12–19 [DOI] [PubMed] [Google Scholar]
- 42. de Rosa V., Erkekoğlu P., Forestier A., Favier A., Hincal F., Diamond A. M., Douki T., Rachidi W. (2012). Low doses of selenium specifically stimulate the repair of oxidative DNA damage in LNCaP prostate cancer cells. Free Radic. Res., 46, 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zeng H., Jackson M. I., Cheng W. H., Combs G. F., Jr (2011). Chemical form of selenium affects its uptake, transport, and glutathione peroxidase activity in the human intestinal Caco-2 cell model. Biol. Trace Elem. Res., 143, 1209–1218 [DOI] [PubMed] [Google Scholar]
- 44. Brigelius-Flohé R. (2008). Selenium compounds and selenoproteins in cancer. Chem. Biodivers., 5, 389–395 [DOI] [PubMed] [Google Scholar]
- 45. Gammelgaard B., Jackson M. I., Gabel-Jensen C. (2011). Surveying selenium speciation from soil to cell–forms and transformations. Anal. Bioanal. Chem., 399, 1743–1763 [DOI] [PubMed] [Google Scholar]
- 46. Karlenius T. C., Shah F., Yu W. C., Hawkes H. J., Tinggi U., Clarke F. M., Tonissen K. F. (2011). The selenium content of cell culture serum influences redox-regulated gene expression. Biotechniques, 50, 295–301 [DOI] [PubMed] [Google Scholar]
- 47. Leist M., Raab B., Maurer S., Rösick U., Brigelius-Flohé R. (1996). Conventional cell culture media do not adequately supply cells with antioxidants and thus facilitate peroxide-induced genotoxicity. Free Radic. Biol. Med., 21, 297–306 [DOI] [PubMed] [Google Scholar]
- 48. Zhuo P., Goldberg M., Herman L., Lee B. S., Wang H., Brown R. L., Foster C. B., Peters U., Diamond A. M. (2009). Molecular consequences of genetic variations in the glutathione peroxidase 1 selenoenzyme. Cancer Res., 69, 8183–8190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zaichick S., Zaichick V., Nosenko S., Moskvina I. (2012). Mass fractions of 52 trace elements and zinc/trace element content ratios in intact human prostates investigated by inductively coupled plasma mass spectrometry. Biol. Trace Elem. Res., 149, 171– 83 [DOI] [PubMed] [Google Scholar]
- 50. Kohler S. W., Provost G. S., Fieck A., Kretz P. L., Bullock W. O., Sorge J. A., Putman D. L., Short J. M. (1991). Spectra of spontaneous and mutagen-induced mutations in the lacI gene in transgenic mice. Proc. Natl Acad. Sci. USA, 88, 7958–7962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zeng H., Uthus E. O., Ross S. A., Davis C. D. (2009). High dietary intake of sodium selenite does not affect gene mutation frequency in rat colon and liver. Biol. Trace Elem. Res., 131, 71–80 [DOI] [PubMed] [Google Scholar]
- 52. Kumar M. S., Pollok K. E., Smith M. L. (2010). Selenomethionine or methylseleninic acid inhibits mutagenesis of a reporter gene in mouse bone marrow. Anticancer Res., 30, 291–293 [PubMed] [Google Scholar]
- 53. Zachara B. A., Gromadzinska J., Palus J., Zbrog Z., Swiech R., Twardowska E., Wasowicz W. (2011). The effect of selenium supplementation in the prevention of DNA damage in white blood cells of hemodialyzed patients: a pilot study. Biol. Trace Elem. Res., 142, 274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ravoori S., Srinivasan C., Pereg D., Robertson L. W., Ayotte P., Gupta R. C. (2010). Protective effects of selenium against DNA adduct formation in Inuit environmentally exposed to PCBs. Environ. Int., 36, 980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Venkitaraman A. R. (2002). Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell, 108, 171–182 [DOI] [PubMed] [Google Scholar]
- 56. Hartman A. R., Ford J. M. (2002). BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat. Genet., 32, 180–184 [DOI] [PubMed] [Google Scholar]
- 57. Fischer J. L., Lancia J. K., Mathur A., Smith M. L. (2006). Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer. Res., 26, 899–904 [PubMed] [Google Scholar]
- 58. Baliga M. S., Wang H., Zhuo P., Schwartz J. L., Diamond A. M. (2007). Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biol. Trace Elem. Res., 115, 227–242 [DOI] [PubMed] [Google Scholar]
- 59. Kotsopoulos J., Chen Z., Vallis K. A., Poll A., Ghadirian P., Kennedy G., Ainsworth P., Narod S. A. (2010). Toenail selenium status and DNA repair capacity among female BRCA1 mutation carriers. Cancer Causes Control, 21, 679–687 [DOI] [PubMed] [Google Scholar]
- 60. Kowalska E., Narod S. A., Huzarski T., Zajaczek S., Huzarska J., Gorski B., Lubinski J. (2005). Increased rates of chromosome breakage in BRCA1 carriers are normalized by oral selenium supplementation. Cancer Epidemiol. Biomarkers Prev., 14, 1302–1306 [DOI] [PubMed] [Google Scholar]
- 61. Dziaman T., Huzarski T., Gackowski D, et al. (2009). Selenium supplementation reduced oxidative DNA damage in adnexectomized BRCA1 mutations carriers. Cancer Epidemiol. Biomarkers Prev., 18, 2923–2929 [DOI] [PubMed] [Google Scholar]
- 62. Karunasinghe N., Han D. Y., Zhu S, et al. (2012). Serum selenium and single-nucleotide polymorphisms in genes for selenoproteins: relationship to markers of oxidative stress in men from Auckland, New Zealand. Genes Nutr., 7, 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cominetti C., de Bortoli M. C., Purgatto E., Ong T. P., Moreno F. S., Garrido A. B., Jr, Cozzolino S. M. (2011). Associations between glutathione peroxidase-1 Pro198Leu polymorphism, selenium status, and DNA damage levels in obese women after consumption of Brazil nuts. Nutrition, 27, 891–896 [DOI] [PubMed] [Google Scholar]
- 64. Bravard A., Vacher M., Moritz E., Vaslin L., Hall J., Epe B., Radicella J. P. (2009). Oxidation status of human OGG1-S326C polymorphic variant determines cellular DNA repair capacity. Cancer Res., 69, 3642–3649 [DOI] [PubMed] [Google Scholar]
- 65. Chen L., Elahi A., Pow-Sang J., Lazarus P., Park J. (2003). Association between polymorphism of human oxoguanine glycosylase 1 and risk of prostate cancer. J. Urol., 170, 2471–2474 [DOI] [PubMed] [Google Scholar]
- 66. Zeng H., Davis C. D., Finley J. W. (2003). Effect of selenium-enriched broccoli diet on differential gene expression in min mouse liver(1,2). J. Nutr. Biochem., 14, 227–231 [DOI] [PubMed] [Google Scholar]
- 67. Nasr M., Fedele M. J., Esser K. A., Diamond A. M. (2004). GPx-1 modulates Akt and P70S6K phosphorylation and Gadd45 levels in MCF-7 cells. Free Radic. Biol. Med., 37, 187–195 [DOI] [PubMed] [Google Scholar]
- 68. Lubos E., Loscalzo J., Handy D. E. (2011). Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal., 15, 1957–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Seo Y. R., Kelley M. R., Smith M. L. (2002). Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc. Natl Acad. Sci. USA, 99, 14548–14553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gudkov A. V. (2002). Converting p53 from a killer into a healer. Nat. Med., 8, 1196–1198 [DOI] [PubMed] [Google Scholar]
- 71. Xiang N., Zhao R., Song G., Zhong W. (2008). Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis, 29, 2175–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zeng H. (2002). Selenite and selenomethionine promote HL-60 cell cycle progression. J. Nutr., 132, 674–679 [DOI] [PubMed] [Google Scholar]
- 73. Hatfield D. L., Gladyshev V. N. (2009). The outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol. Interv., 9, 18–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rayman M. P., Combs G. F., Jr, Waters D. J. (2009). Selenium and vitamin E supplementation for cancer prevention. JAMA, 301, 1876; author reply 1877 [DOI] [PubMed] [Google Scholar]
- 75. Jung H. J., Seo Y. R. (2010). Current issues of selenium in cancer chemoprevention. Biofactors, 36, 153–158 [DOI] [PubMed] [Google Scholar]
- 76. Duffield-Lillico A. J., Dalkin B. L., Reid M. E., Turnbull B. W., Slate E. H., Jacobs E. T., Marshall J. R., Clark L. C. (2003). Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int., 91, 608–612 [DOI] [PubMed] [Google Scholar]
- 77. Duffield-Lillico A. J., Slate E. H., Reid M. E, et al. (2003). Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J. Natl Cancer Inst., 95, 1477–1481 [DOI] [PubMed] [Google Scholar]
- 78. Chiang E. C., Shen S., Kengeri S. S., Xu H., Combs G. F., Morris J. S., Bostwick D. G., Waters D. J. (2009). Defining the optimal selenium dose for prostate cancer risk reduction: insights from the U-shaped relationship between selenium status, DNA damage, and apoptosis. Dose Response, 8, 285–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Siviková K., Piesová E., Dianovský J. (2001). The protection of Vitamin E and selenium against carbon tetrachloride-induced genotoxicity in ovine peripheral blood lymphocytes. Mutat. Res., 494, 135–142 [DOI] [PubMed] [Google Scholar]
- 80. Fahmy M. A., Hassan N. H., Farghaly A. A., Hassan E. E. (2008). Studies on the genotoxic effect of beryllium chloride and the possible protective role of selenium/vitamins A, C and E. Mutat. Res., 652, 103–111 [DOI] [PubMed] [Google Scholar]
- 81. Grotto D., Barcelos G. R., Valentini J., Antunes L. M., Angeli J. P., Garcia S. C., Barbosa F., Jr (2009). Low levels of methylmercury induce DNA damage in rats: protective effects of selenium. Arch. Toxicol., 83, 249–254 [DOI] [PubMed] [Google Scholar]
- 82. Khalil W. K., Booles H. F. (2011). Protective role of selenium against over-expression of cancer-related apoptotic genes induced by o-cresol in rats. Arh. Hig. Rada. Toksikol., 62, 121–129 [DOI] [PubMed] [Google Scholar]
- 83. Lovell M. A., Xiong S., Lyubartseva G., Markesbery W. R. (2009). Organoselenium (Sel-Plex diet) decreases amyloid burden and RNA and DNA oxidative damage in APP/PS1 mice. Free Radic. Biol. Med., 46, 1527–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tripathi D. N., Jena G. B. (2008). Ebselen attenuates cyclophosphamide-induced oxidative stress and DNA damage in mice. Free Radic. Res., 42, 966–977 [DOI] [PubMed] [Google Scholar]
- 85. Chen T., Wong Y. S. (2008). In vitro antioxidant and antiproliferative activities of selenium-containing phycocyanin from selenium-enriched Spirulina platensis. J. Agric. Food Chem., 56, 4352–4358 [DOI] [PubMed] [Google Scholar]
- 86. Santos R. A., Takahashi C. S. (2008). Anticlastogenic and antigenotoxic effects of selenomethionine on doxorubicin-induced damage in vitro in human lymphocytes. Food Chem. Toxicol., 46, 671–677 [DOI] [PubMed] [Google Scholar]
- 87. Ferguson L. R., Philpott M., Karunasinghe N. (2004). Dietary cancer and prevention using antimutagens. Toxicology, 198, 147–159 [DOI] [PubMed] [Google Scholar]
- 88. Dusinská M., Kazimírová A., Barancoková M., Beno M., Smolková B., Horská A., Raslová K., Wsólová L., Collins A. R. (2003). Nutritional supplementation with antioxidants decreases chromosomal damage in humans. Mutagenesis, 18, 371–376 [DOI] [PubMed] [Google Scholar]