Abstract
Thermovibrio ammonificans type strain HB-1T is a thermophilic (Topt: 75°C), strictly anaerobic, chemolithoautotrophic bacterium that was isolated from an active, high temperature deep-sea hydrothermal vent on the East Pacific Rise. This organism grows on mineral salts medium in the presence of CO2/H2, using NO3- or S0 as electron acceptors, which are reduced to ammonium or hydrogen sulfide, respectively. T. ammonificans is one of only three species within the genus Thermovibrio, a member of the family Desulfurobacteriaceae, and it forms a deep branch within the phylum Aquificae. Here we report the main features of the genome of T. ammonificans strain HB-1T (DSM 15698T).
Keywords: Aquificae, Desulfurobacteriaceae, thermophilic, anaerobic, chemolithoautotrophic, hydrothermal vent
Introduction
The genus Thermovibrio consists of three validly published, named species: T. ammonificans strain HB-1T [1], T. ruber strain ED11/3LLK T [2] and T. guaymasensis strain SL19T [3]. All three Thermovibrio spp. are anaerobic, chemolithoautotrophic bacteria that grow on mineral salts in the presence of carbon dioxide and hydrogen, reducing nitrate or sulfur to ammonium or hydrogen sulfide, respectively. T. ammonificans was isolated from an active high-temperature deep-sea hydrothermal vent located on the East Pacific Rise at 9° North, while T. ruber was isolated from shallow water hydrothermal vent sediments in Papua New Guinea and T. guaymasensis from a deep-sea hydrothermal vent chimney in the Guaymas Basin [1-3]. Anaerobic chemolithoautotrophic bacteria mediate the transfer of energy and carbon from a geothermal source to the higher trophic levels. These anaerobic primary producers, which depend on inorganic chemical species of geothermal origin (i.e., carbon dioxide, hydrogen and sulfur), are completely independent from photosynthetic processes and represent an important component of the deep-sea hydrothermal vent ecosystem. Furthermore, microorganisms such as T. ammonificans, which also couple autotrophic carbon dioxide fixation with nitrate respiration, are of particular interest, as they link the carbon and nitrogen cycle, the latter of which has been under-studied at deep-sea hydrothermal vents. Here we present a summary of the features of T. ammonificans strain HB-1T and a description of its genome.
Classification and features
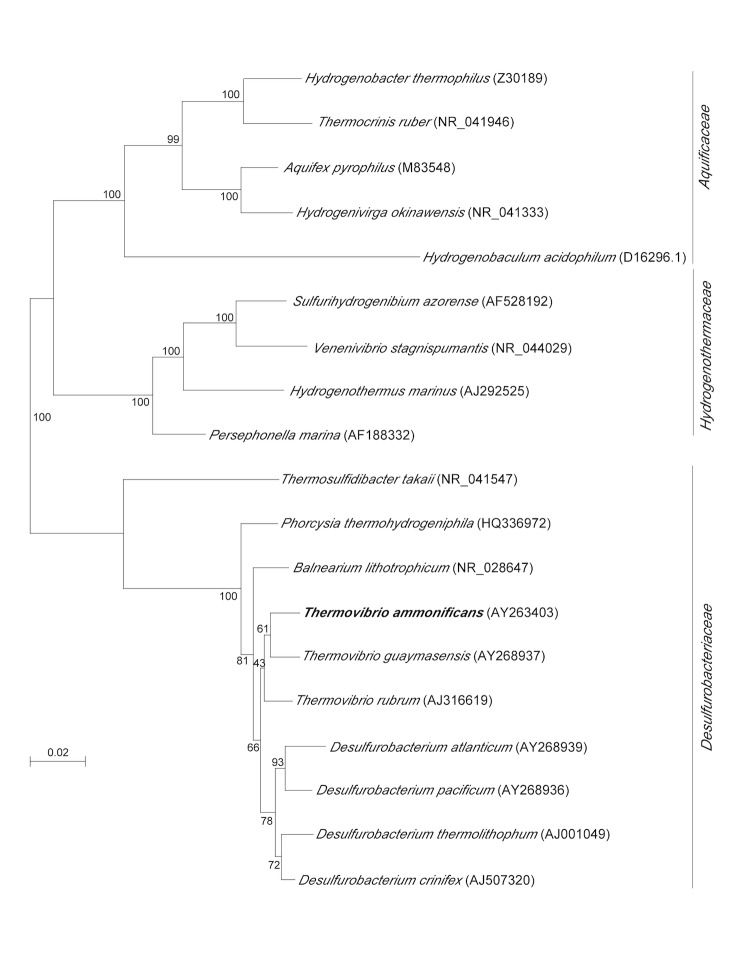
Thermovibrio ammonificans strain HB-1T (=DSM 15698T =JCM 12110T) is a member of the phylum Aquificae, a group of thermophilic, deeply branching bacteria thought to be among the oldest on Earth. The phylum Aquificae consists of a single order, the Aquificales, which is composed of three families, Aquificaceae, Hydrogenothermaceae and Desulfurobacteriaceae (Figure 1). The genus Thermovibrio belongs to the family Desulfurobacteriaceae, which also includes the genera Desulfurobacterium, Balnearium and the newly described Phorcysia [6-8]. While the genomes of several members of the families Aquificaceae and Hydrogenothermaceae have been sequenced, the only genome sequences publicly available for the Desulfurobacteriaceae are those of T. ammonificans and Desulfurobacterium thermolithotrophum [9].
Figure 1.
Phylogenetic position of Thermovibrio ammonificans HB-1T relative to other type strains within the Aquificae. Sequences were aligned automatically using CLUSTAL X and the alignment was manually refined using SEAVIEW [4,5]. The neighbor-joining tree was constructed with Phylo_Win, using the Jukes-Cantor correction [4]. Bootstrap values based on 100 replications. Bar, 0.02 substitutions per nucleotide position.
Table 1 summarizes the classification and general features of Thermovibrio ammonificans HB-1T. Cells of T. ammonificans are Gram-negative, motile rods of about 1.0 µm in length and 0.6 µm in width (Figure 2). Growth occurs between 60 and 80 °C (optimum at 75 °C), 0.5 and 4.5% (w/v) sodium chloride (optimum at 2%) and pH 5 and 7 (optimum at 5.5). Generation time under optimal conditions is 1.5 h. Growth occurs under chemolithoautotrophic conditions in the presence of hydrogen and carbon dioxide, with nitrate or sulfur as the electron acceptor and with concomitant formation of ammonium or hydrogen sulfide, respectively. Thiosulfate, sulfite and oxygen are not used as electron acceptors. Acetate, formate, lactate and yeast extract inhibits growth. No chemoorganoheterotrophic growth was observed on peptone, tryptone or Casamino acids. The genomic DNA G+C content is 52.1 mol% [1].
Table 1. Classification and general features of Thermovibrio ammonificans HB-1T.
| MIGS ID | Property | Term | Evidence code |
|---|---|---|---|
| Current classification | Domain Bacteria | TAS [10] | |
| Phylum ‘Aquificae’ | TAS [11] | ||
| Class Aquificae | TAS [12,13] | ||
| Order Aquificales | TAS [12,14,15] | ||
| Family Desulfurobacteriaceae | TAS [15] | ||
| Genus Thermovibrio | TAS [2] | ||
| Species Thermovibrio ammonificans | TAS [1] | ||
| Type strain HB-1T | |||
| Gram stain | Negative | TAS [1] | |
| Cell shape | Short rod | TAS [1] | |
| Motility | motile | TAS [1] | |
| Sporulation | non-sporulating | TAS [1] | |
| Temperature range | 60-80 | TAS [1] | |
| Optimum temperature | 75 | TAS [1] | |
| Carbon source | CO2 | TAS [1] | |
| Energy source | H2 | TAS [1] | |
| Terminal electron acceptor | NO3-, S0 | TAS [1] | |
| MIGS-6 | Habitat | Marine, deep-sea hydrothermal vent | TAS [1] |
| MIGS-6.3 | Salinity | 20 g NaCl l-1 (range 5 – 45 g NaCl l-1) | TAS [1] |
| MIGS-22 | Oxygen | Anaerobe | TAS [1] |
| MIGS-15 | Biotic relationship | free-living | TAS [1] |
| MIGS-14 | Pathogenicity | Not pathogenic | NAS |
| MIGS-4 | Geographic location | East Pacific Rise | TAS [1] |
| MIGS-5 | Sample collection time | April 2000 | TAS [1] |
| MIGS-4.1 | Latitude – | 9° 50' N | TAS [1] |
| MIGS-4.2 | Longitude | 104° 18' W | TAS [1] |
| MIGS-4.3 | Depth | 2500 m | TAS [1] |
| MIGS-4.4 | Altitude | not applicable |
Evidence codes - IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [16].
Figure 2.

Electron micrograph of a platinum shadowed cell of Thermovibrio ammonificans strain HB-1 T showing multiple flagella. Bar, 1 μm.
Chemotaxonomy
None of the classical chemotaxonomic features (peptidoglycan structure, cell wall sugars, cellular fatty acid profile, respiratory quinones, or polar lipids) are known for Thermovibrio ammonificans strain HB-1T.
Genome sequencing information
Genome project history
T. ammonificans was selected for genome sequencing because of its phylogenetic position within the phylum Aquificae and because of its ecological function as a primary producer at deep-sea hydrothermal vents. Sequencing, finishing and annotation were carried out by the US DOE Joint Genome Institute (JGI). Table 2 shows a summary of the project information and its association with MIGS version 2.0 compliance [17].
Table 2. Project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | 454 pyrosequence standard library, 454 Paired End, Illumina |
| MIGS-29 | Sequencing platforms | 454 GS FLX Titanium and Illumina GAii |
| MIGS-31.2 | Fold coverage | 4,325 × |
| MIGS-30 | Assemblers | Newbler 2.3, Velvet 0.7.63 |
| MIGS-32 | Gene calling method | Prodigal 1.4 |
| Genome Database release | January 7, 2011 | |
| Genbank ID | NC_014926 | |
| GOLD ID | Gc01577 | |
| Project relevance | Chemosynthetic ecosystems, CO2 fixation, Thermophiles | |
Growth conditions and DNA isolation
T. ammonificans was grown in two liters of modified SME medium at 75 °C under a H2/CO2 gas phase (80:20; 200 kPa) with CO2 as the carbon source and nitrate as the electron acceptor [1]. Genomic DNA was isolated from 0.5 - 1 g of pelleted cells using a protocol that included a lysozyme/SDS lysis step, followed by two extractions with phenol:chloroform:isoamyl alcohol (50:49:1) and ethanol precipitation. This procedure yielded about 25 μg of genomic DNA, which was submitted to the DOE JGI for sequencing.
Genome sequencing and assembly
The genome of Thermovibrio ammonificans was sequenced at the DOE JGI [18] using a combination of Illumina [19] and 454 platforms [20]. The following libraries were used: 1) An Illumina GAii shotgun library, which generated 10,255,5615 reads totaling 7,794 Mb; 2) A 454 Titanium standard library, which generated 186,945 reads; and 3) A paired end 454 library with an average insert size of 11.895 +/- 2.973 kb, which generated 115,495 reads totaling 104.7 Mb of 454 data. All general aspects of library construction and sequencing performed at the JGI can be found at the JGI website [21]. The initial draft assembly contained 16 contigs in 2 scaffolds. The 454 Titanium standard data and the 454 paired end data were assembled together with Newbler, version 2.3. The Newbler consensus sequences were computationally shredded into 2 kb overlapping fake reads (shreds). Illumina sequencing data was assembled with VELVET, version 0.7.63 [22], and the consensus sequences were computationally shredded into 1.5 kb overlapping fake reads (shreds). The 454 Newbler consensus shreds, the Illumina VELVET consensus shreds and the read pairs in the 454 paired end library were integrated using parallel phrap, version SPS - 4.24 (High Performance Software, LLC). The software Consed [23] was used in the finishing process. Illumina data were used to correct potential base errors and increase consensus quality using the software Polisher developed at JGI (Alla Lapidus, unpublished). Possible mis-assemblies were corrected using gapResolution (Cliff Han, unpublished), Dupfinisher [24], or sequencing cloned bridging PCR fragments with subcloning. Gaps between contigs were closed by editing in Consed, by PCR and by Bubble PCR (J-F Cheng, unpublished) primer walks. A total of 46 additional reactions and 1 shatter library were necessary to close gaps and to raise the quality of the finished sequence. The total size of the genome is 1,759,526 bp (chromosome and plasmid) and the final assembly is based on 67.7 Mb of 454 draft data, which provide an average 40× coverage of the genome, and 7,284 Mb of Illumina draft data, which provide an average 4,285× coverage of the genome.
Genome annotation
Genes were identified using Prodigal [25] as part of the Oak Ridge National Laboratory genome annotation pipeline, followed by a round of manual curation using the JGI GenePRIMP pipeline [26]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) nonredundant database, UniProt, TIGRFam, Pfam, PRIAM, KEGG, COG, and InterPro databases. These data sources were combined to assert a product description for each predicted protein. Non-coding genes and miscellaneous features were predicted using tRNAscan-SE [27], RNAMMer [28], Rfam [29], TMHMM [30], and signalP [31].
Genome properties
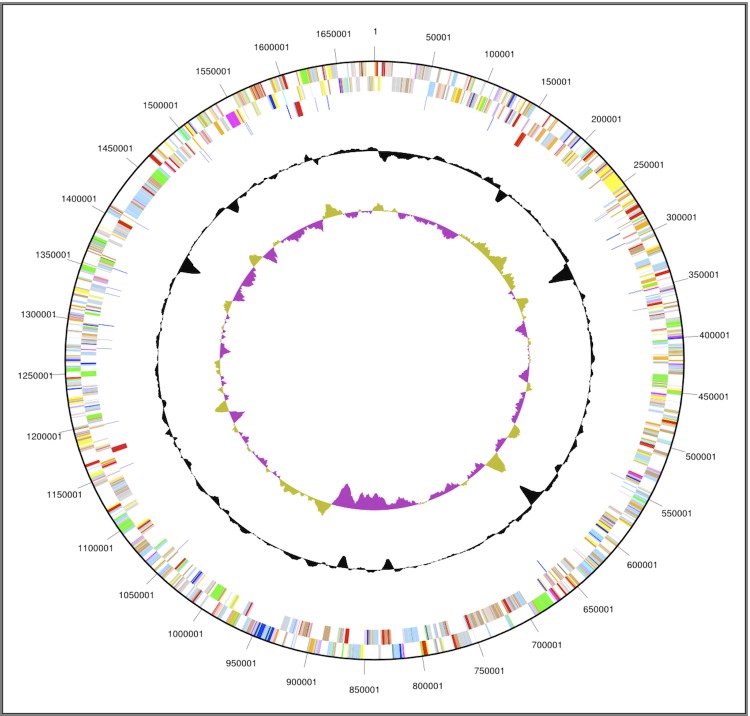
The genome includes one circular chromosome and one plasmid, for a total size of 1,759,526 bp (chromosome size: 1,682,965 bp; GC content: 52.13%). Of the 1,888 genes predicted from the genome, 1,831 are protein-coding genes. Of the protein coding genes, 1,279 were assigned to a putative function, with those remaining annotated as hypothetical proteins. The properties and the statistics of the genome are summarized in Figure 3 and Tables 3 and 4.
Figure 3.
Graphical circular map of the genome. From outside to the center: Genes on forward strand (color by COG categories), Genes on reverse strand (color by COG categories), RNA genes (tRNAs cyan, rRNAs red, other RNAs blue), GC content, GC skew.
Table 3. Genome statistics.
| Attribute | Value | % of totala |
|---|---|---|
| Genome size (bp) | 1,759,526 | |
| DNA Coding region (bp) | 1,674,589 | 95.17% |
| DNA G+C content (bp) | 917,237 | 52.13% |
| Chromosome (bp) | 1,682,965 | |
| Plasmid (bp) | 76,561 | |
| Total genes | 1888 | |
| RNA genes | 57 | 3.02% |
| Protein-coding genes | 1831 | 96.98% |
| Genes in paralog clusters | 2 | 0.11% |
| Genes assigned to COGs | 1419 | 75.16% |
| Genes with signal peptides | 535 | 28.34% |
| Genes with transmembrane helices | 369 | 19.54% |
| Paralogous groups | 1 | 100% |
a The total is based on either the size of the genome in base pairs or the total number of protein coding genes in the annotated genome.
Table 4. Number of genes associated with the 25 general COG functional categories.
| Code | Value | % agea | Description |
|---|---|---|---|
| J | 154 | 8.41 | Translation, ribosomal structure and biogenesis |
| A | - | - | RNA processing and modification |
| K | 70 | 3.82 | Transcription |
| L | 78 | 4.25 | Replication, recombination and repair |
| B | 3 | 0.16 | Chromatin structure and dynamics |
| D | 40 | 2.18 | Cell cycle control, mitosis and meiosis |
| Y | - | - | Nuclear structure |
| V | 33 | 1.80 | Defense mechanisms |
| T | 71 | 3.87 | Signal transduction mechanisms |
| M | 135 | 7.37 | Cell wall/membrane biogenesis |
| N | 70 | 3.82 | Cell motility |
| Z | - | - | Cytoskeleton |
| W | - | - | Extracellular structures |
| U | 67 | 3.66 | Intracellular trafficking and secretion |
| O | 92 | 5.02 | Posttranslational modification, protein turnover, chaperones |
| C | 158 | 8.63 | Energy production and conversion |
| G | 77 | 4.20 | Carbohydrate transport and metabolism |
| E | 155 | 8.46 | Amino acid transport and metabolism |
| F | 72 | 3.93 | Nucleotide transport and metabolism |
| H | 120 | 6.55 | Coenzyme transport and metabolism |
| I | 46 | 2.51 | Lipid transport and metabolism |
| P | 102 | 5.57 | Inorganic ion transport and metabolism |
| Q | 38 | 2.08 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 220 | 12.02 | General function prediction only |
| S | 92 | 5.03 | Function unknown |
| - | 412 | 22.50 | Not in COGs |
a The total is based on the total number of protein coding genes in the annotated genome.
Acknowledgements
The genome of Thermovibrio ammonificans was sequenced under the auspices of the US Department of Energy. Work on T. ammonificans was supported, entirely or in part, by NSF Grants MCB 04-56676, OCE 03-27353, MCB 08-43678, OCE 09-37371 and OCE 11-24141 to CV, and by the New Jersey Agricultural Experiment Station.
References
- 1.Vetriani C, Speck MD, Ellor SV, Lutz R, Starovoytov V. Thermo vibrio ammonificans sp. nov., a thermophilic, themolithotrophic, nitrate-ammonifying bacterium from deep-sea hydrothermal vents. Int J Syst Evol Microbiol 2004; 54:175-181 10.1099/ijs.0.02781-0 [DOI] [PubMed] [Google Scholar]
- 2.Huber H, Diller S, Horn C, Rachel R. Thermovibrio ruber gen. nov., sp. nov., an extremely thermophilic, chemolithoautotrophic, nitrate-reducing bacterium that forms a deep branch within the phylum Aquificae. Int J Syst Evol Microbiol 2002; 52:1859-1865 10.1099/ijs.0.02235-0 [DOI] [PubMed] [Google Scholar]
- 3.L'Haridon S, Reysenbach AL, Tindall BJ, Schonheit P, Banta A, Johnsen U, Schumann P, Gambacorta A, Stackebrandt E, Jeanthon C. Desulfurobacterium atlanticum sp. nov., Desulfurobacterium pacificum sp. nov. and Thermovibrio guaymasensis sp. nov., three thermophilic members of the Desulfurobacteriaceae fam. nov., a deep branching lineage within the Bacteria. Int J Syst Evol Microbiol 2006; 56:2843-2852 10.1099/ijs.0.63994-0 [DOI] [PubMed] [Google Scholar]
- 4.Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO\_WIN: two graphic tools for sequence alignment and molecular phylogeny. Computer applications in the biosciences. CABIOS 1996; 12:543-548 [DOI] [PubMed] [Google Scholar]
- 5.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. [PubMed]. Nucleic Acids Res 1997; 25:4876-4882 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.L'Haridon S, Cilia V, Messner P, Raguenes G, Gambacorta A, Sleytr UB, Prieur D, Jeanthon C. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Bacteriol 1998; 48:701-711 10.1099/00207713-48-3-701 [DOI] [PubMed] [Google Scholar]
- 7.Takai K, Nakagawa S, Sako Y, Horikoshi K. Balnearium lithotrophicum gen. nov., sp. nov., a novel thermophilic, strictly anaerobic, hydrogen-oxidizing chemolithoautotroph isolated from a black smoker chimney in the Suiyo Seamount hydrothermal system. Int J Syst Evol Microbiol 2003; 53:1947-1954 10.1099/ijs.0.02773-0 [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Rodríguez, I., A. Grosche, L. Massenburg, V. Starovoytov, R.A. Lutz and C. Vetriani. Phorcysia thermohydrogeniphila gen. nov. sp. nov., a thermophilic, chemolithoautotrophic, nitrate-ammonifying bacterium from a deep-sea hydrothermal vent on the East Pacific Rise. Int J Syst Evol Microbiol, 2012; 62:2388-2394. [DOI] [PubMed]
- 9.Göker M, Daligault H, Mwirichia R, Lapidus A, Lucas S, Deshpande S, Pagani I, Tapia R, Cheng JF, Goodwin L, et al. Complete genome sequence of the thermophilic sulfur-reducer Desulfurobacterium thermolithotrophum type strain (BSAT) from a deep-sea hydrothermal vent. Stand Genomic Sci 2011; 5:407-415 10.4056/sigs.2465574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reysenbach AL. Phylum BI. Aquificae In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 359-367. [Google Scholar]
- 12.List Editor Validation List no. 85. Validation of publication of new names and new combinations previously effectively published outside the IJSEM. Int J Syst Evol Microbiol 2002; 52:685-690 10.1099/ijs.0.02358-0 [DOI] [PubMed] [Google Scholar]
- 13.Reysenbach AL. Class I. Aquificae class. nov. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 359. [Google Scholar]
- 14.Reysenbach AL. Order I. Aquificales ord. nov. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 359. [Google Scholar]
- 15.L'Haridon S, Reysenbach AL, Tindall BJ, Schönheit P, Banta A, Johnsen U, Schumann P, Gambacorta A, Stackebrandt E, Jeanthon C. Desulfurobacterium atlanticum sp. nov., Desulfurobacterium pacificum sp. nov. and Thermovibrio guaymasensis sp. nov., three thermophilic members of the Desulfurobacteriaceae fam. nov., a deep branching lineage within the Bacteria. Int J Syst Evol Microbiol 2006; 56:2843-2852 10.1099/ijs.0.63994-0 [DOI] [PubMed] [Google Scholar]
- 16.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.JGI website. http://www.jgi.doe.gov/
- 19.Bennett S. Solexa Ltd. Pharmacogenomics 2004; 5:433-438 10.1517/14622416.5.4.433 [DOI] [PubMed] [Google Scholar]
- 20.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 2005; 437:326-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DOE Joint Genome Institute http://www.jgi.doe.gov/
- 22.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008; 18:821-829 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Phred/Phrap/Consed software package. http://www.phrap.com
- 24.Han C, Chain P. Finishing repeat regions auto-matically with Dupfinisher. In: Proceeding of the 2006 international conference on bioinformatics & computational biology. Arabnia HR, Valafar H (eds), CSREA Press. June 26-29, 2006: 141-146. [Google Scholar]
- 25.Hyatt D, Chen GL, LoCascio PF, Land ML, Lari-mer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identifi-cation. BMC Bioinformatics 2010; 11:119 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC. Gene-PRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 2010; 7:455-457 10.1038/nmeth.1457 [DOI] [PubMed] [Google Scholar]
- 27.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 1997; 25:955-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagesen K, Hallin PF, Rødland E, Stærfeldt HH, Rognes T, Ussery DW. RNammer: consistent annotation of rRNA genes in genomic sequences. Nucleic Acids Res 2007; 35:3100-3108 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR. Rfam: an RNA family database. Nucleic Acids Res 2003; 31:439-441 10.1093/nar/gkg006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol 2001; 305:567-580 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 31.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340:783-795 10.1016/j.jmb.2004.05.028 [DOI] [PubMed] [Google Scholar]