ABSTRACT
Objective:
The FEBSTAT (Consequences of Prolonged Febrile Seizures) study is prospectively addressing the relationships among serial EEG, MRI, and clinical follow-up in a cohort of children followed from the time of presentation with febrile status epilepticus (FSE).
Methods:
We recruited 199 children with FSE within 72 hours of presentation. Children underwent a detailed history, physical examination, MRI, and EEG within 72 hours. All EEGs were read by 2 teams and then conferenced. Associations with abnormal EEG were determined using logistic regression. Interrater reliability was assessed using the κ statistic.
Results:
Of the 199 EEGs, 90 (45.2%) were abnormal with the most common abnormality being focal slowing (n = 47) or attenuation (n = 25); these were maximal over the temporal areas in almost all cases. Epileptiform abnormalities were present in 13 EEGs (6.5%). In adjusted analysis, the odds of focal slowing were significantly increased by focal FSE (odds ratio [OR] = 5.08) and hippocampal T2 signal abnormality (OR = 3.50) and significantly decreased with high peak temperature (OR = 0.18). Focal EEG attenuation was also associated with hippocampal T2 signal abnormality (OR = 3.3).
Conclusions:
Focal EEG slowing or attenuation are present in EEGs obtained within 72 hours of FSE in a substantial proportion of children and are highly associated with MRI evidence of acute hippocampal injury. These findings may be a sensitive and readily obtainable marker of acute injury associated with FSE.
Febrile status epilepticus (FSE) is an important neurologic emergency and the most severe type of complex febrile seizure. Acute morbidity and mortality are low,1 but the long-term consequences of FSE and risks of specific epilepsies, such as mesial temporal epilepsy, are not well established. Retrospective studies report that approximately 30% of patients with medically refractory temporal lobe epilepsy being evaluated for surgery have a history of prolonged febrile seizures, but prospective studies failed to confirm the association.2–9 The Consequences of Prolonged Febrile Seizures (FEBSTAT) study is designed to prospectively examine the outcome of FSE and predictors of outcome.10 Both MRI and EEG data are collected within 72 hours and then at later intervals to evaluate the possible relationship of FSE to subsequent mesial temporal lobe epilepsy. The methodology of assembling the cohort and a description of the clinical semiology and phenomenology of the first 119 subjects were previously published.10 In this report, we describe the acute EEG findings and their relationship to characteristics of the episode of FSE as well as to the acute imaging findings.
METHODS
Population
Eligible children were ages 1 month through 5 years who presented with FSE at 1 of the 5 recruiting sites (Montefiore Medical Center and Jacobi Hospital in the Bronx, Children's Memorial Hospital in Chicago, Duke University Medical Center in Durham, Virginia Commonwealth University Hospital in Richmond, and Eastern Virginia Medical School in Norfolk). FSE was defined as a single seizure or a series of seizures without full recovery in between lasting ≥30 minutes11,12 that also met the definition of a febrile seizure.11,13 A febrile seizure was defined as a provoked seizure in which the sole acute provocation was fever (temperature >38.4°C, 101.0°F) without history of a febrile seizure and with no evidence of an acute CNS infection or insult.11,13 Children with known severe neurologic disability before entry were excluded from the FEBSTAT study. Subjects were enrolled within 72 hours.
Standard protocol approvals, registrations, and patient consents
The Institutional Review Boards approved the procedures for the Protection of Human Subjects at the participating institutions. Written informed consent was obtained from the parents in all cases.
Procedures
Comprehensive assessments were performed at the time of status including medical and developmental history, physical and neurologic examinations, acute MRI, acute EEG, and human herpesvirus (HHV)-6 and HHV-7 PCR and titers. Three clinical investigators (D.R.N., J.M.P., and S. Shinnar) independently reviewed the clinical features of the FSE blinded to the EEG and MRI findings, and any discrepancies were resolved by consensus.10 In a similar manner, neuroimaging results were analyzed by independent readers blinded to the clinical and EEG findings, compared, and then discussed where they were discordant to reach consensus.14
Electroencephalograms
All EEGs were performed according to the standards of the American Clinical Neurophysiological Society for the recording of pediatric EEGs15 and lasted at least 30 minutes. The goal was to obtain the EEGs within 72 hours of FSE. When this was not feasible, EEGs within 3 months of status epilepticus were also included. Electrodes were applied according to the universal 10-20 system using standard measurements. All EEGs were recorded using digital machines, and the studies were archived onto compact discs for shipment and storage. Whenever possible, the studies were performed with the subjects awake and asleep, but subjects were not sedated for the purpose of the EEG recording. Recordings were interpreted using a time constant of 0.3 and a high-frequency filter of 70 Hz. A notch filter (60 Hz) was used only if line artifact was present. The sensitivity and number of seconds per page were adjusted to optimize the visual display.
To maintain blinding, all EEG studies were initially deidentified and assigned a number that was unique to the EEG database and was not related to the subject ID or the MRI ID numbers. They were then read by 2 teams of board-certified pediatric electroencephalographers (D.R.N. and Y.S. with S.L.M.) who were blinded to any clinical information including patient identity, seizure semiology, reason for the recording (initial EEG vs subsequent EEG), imaging results, and any laboratory findings. The only piece of clinical information provided was the subject's age, which was required for an accurate interpretation of the study. After separate readings, a consensus reading was reached if there was any discrepancy.
The number and type of EEG abnormalities were reported. We examined focal slowing, attenuation, and background rhythms including presence, localization, and degree (mild, moderate, or severe). Given the age of the subjects in this study, we only reported focal slowing in the δ frequency range. Mild slowing was defined as <20% of the record, moderate as 20% to 50%, and severe as >50%. For attenuation, we focused on the faster frequency activity in the α and β frequency ranges. Focal attenuation was present if the voltage of this activity was less than half the contralateral side. We analyzed focal abnormalities using at least 4 montages: longitudinal bipolar, transverse bipolar, average reference, and Laplacian reference. A combination of instrumental phase reversals (bipolar montages) and waveform amplitudes (referential montages) was used to localize any abnormalities. We defined locations using conventional labels including the following for the temporal regions: F7/8 = anterior temporal; T3/4 = midtemporal; and T5/6 = posterior temporal. Epileptiform abnormalities were similarly coded by the location of the discharges (focal with the appropriate localization or generalized), abundance, and morphology (stereotyped vs pleomorphic).
Factors examined as potentially associated with focal slowing or attenuation were clinical features of FSE, including type of status (generalized vs focal and intermittent vs continuous); the laterality of the seizure as inferred from the clinical description; the duration of FSE; the maximal temperature; age at FSE; and gender. After all initial EEGs had been reviewed and conferenced, we then compared focal slowing or attenuation on EEG with acute MRI findings, including the presence of any abnormality, any hippocampal abnormality, or hippocampal T2 signal abnormality. Laterality of focal slowing and focal attenuation was compared with laterality of hippocampal T2 signal abnormality and with lateralization of FSE in those with definite focality determined by consensus.
Statistical analyses
Descriptive analysis was conducted through the determination of a percentage, mean, or median. Standard deviations were presented for means and the interquartile range for the median. Logistic regression was used for bivariate and multivariable analysis of factors associated with focal slowing and attenuation on the acute EEG.16 The κ statistic16 was used to evaluate the agreement between raters for any acute EEG abnormality, and for focal slowing and focal attenuation.
RESULTS
Population characteristics
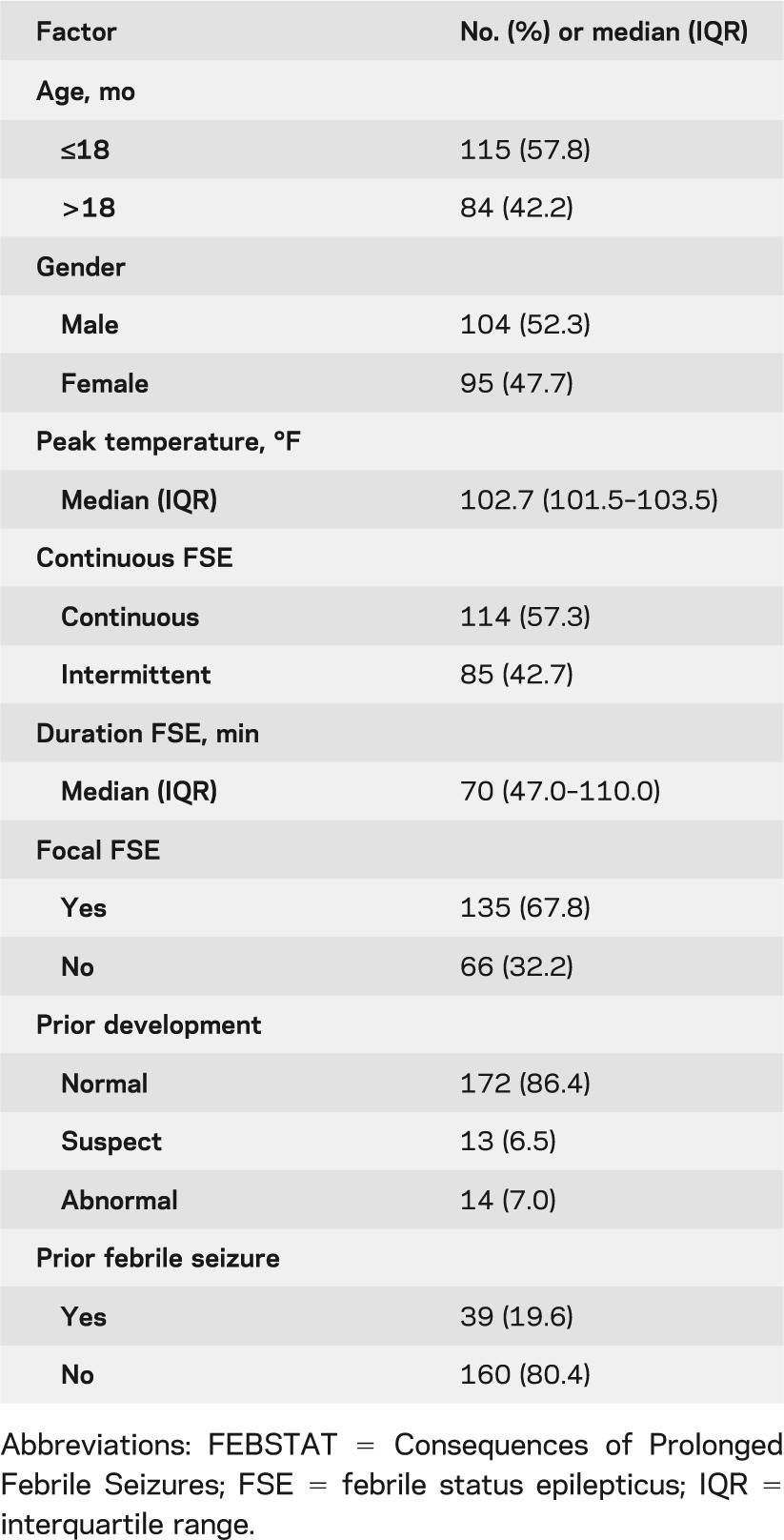
There were 199 children enrolled. The median age was 15.8 months (interquartile range 12.0–24.1 months). Median peak temperature at FSE was 102.7°F and median duration of FSE was 70 minutes. Continuous seizures predominated, as did focal seizures. Prior development was normal in 86.4% of children. The clinical characteristics of the children and of the episode of FSE are summarized in table 1.
Table 1.
Descriptives of the FEBSTAT cohort with acute EEG (N = 191)
Findings on the EEG tracings
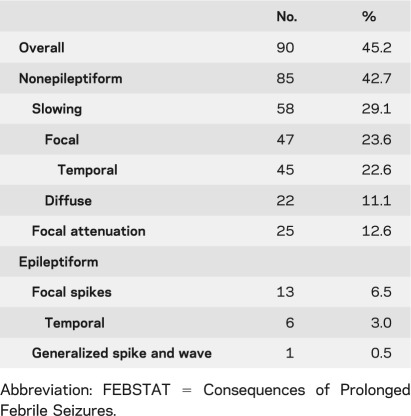
EEGs were available and technically satisfactory in all 199 children in the FEBSTAT cohort. The goal of the study was to obtain the EEGs within 72 hours of status and 96% of the EEGs met this criterion. Of the remaining EEGs, 2.5% were obtained within a week and 1.5% were obtained within a month of the episode of FSE. Of the 199 EEGs reviewed, 90 (45.2%) were abnormal including nonepileptiform abnormalities in 85 (42.7%) and epileptiform abnormalities in 13 (6.5%) (table 2). Specific abnormalities of interest are described below.
Table 2.
Frequency of EEG abnormalities in the FEBSTAT cohort
Focal slowing and attenuation
Overall, significant focal nonepileptiform findings were seen in 60 acute EEGs (30.2%). Focal slowing was seen in 47 cases (figure 1); in 12 cases, focal slowing was associated with focal attenuation. Focal attenuation without slowing was present in 13 EEGs (figure 2); focal slowing was more common on the right (n = 36, 76.6%) than on the left (n = 11, 23.4%). Significant focal slowing (defined as moderate or severe) almost always involved the temporal region (45 of 47 cases) (figure 1); it was maximal in the anterior temporal region in 1, midtemporal in 16, and in the posterior temporal region in 28 cases. Slowing was unilateral in 42 subjects and bilateral in 5. When bilateral, the slowing was more prominent on the right side in 4 of 5 subjects. In one EEG, the slowing involved the entire hemisphere and in another the slowing was maximal in the central region. Focal slowing was associated with diffuse background slowing in 11 cases (23.4%).
Figure 1. EEG of 12-month-old.
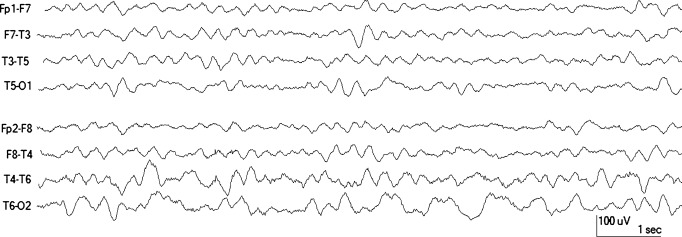
EEG of a 12-month-old who had 1 hour of continuous focal status without clear lateralization. The MRI was normal. The EEG was done 1 day after febrile status epilepticus and shows right temporal slowing. Note that slowing is maximal in the posterior derivation.
Figure 2. EEG of 38-month-old.
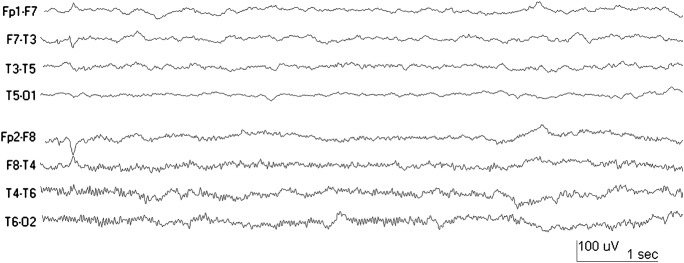
EEG of a 38-month-old who had 1 hour of continuous status with definite clinical lateralization to the left. There was equivocal hippocampal T2 abnormality. The EEG was performed 2 days after febrile status epilepticus and shows left temporal attenuation of faster frequencies.
Focal attenuation was present in 25 cases. It was always unilateral and was more common on the right (15 of 25 cases). Attenuation was most evident in the temporal region in 15. In 9 cases it involved the entire hemisphere and in 1 case it was present only in the central region. Focal slowing in addition to focal attenuation was present in 12 cases (48%), and in 10 of these it was on the same side.
Background slowing
Diffuse background slowing was noted in 22 EEGs, of which 11 also had focal slowing. Among the 109 normal studies, there were 26 tracings that did not contain an awake sample and therefore could not be reliably assessed for diffuse background slowing. These studies were read as being within the broad range of normal, recorded with the subject drowsy and asleep.
Epileptiform EEG abnormalities
Focal sharp waves or spikes were seen in only 13 tracings (6.5%). These were temporal in 6, central in 4, parietooccipital in 1, and frontal in 1. One study had multifocal spikes. Six of these tracings had either focal slowing (n = 3) or attenuation (n = 3). Generalized or diffuse interictal epileptiform discharges were seen in only 1 EEG. In this tracing, the spikes were maximal in the posterior derivation and had a repetition rate faster than 3 Hz.
Interrater reliability
There was excellent interobserver agreement for the overall impression (κ = 0.82; 95% confidence interval [CI] = 0.74–0.89), any focal slowing (κ = 0.75; 95% CI = 0.66–0.85), and any focal attenuation (κ = 0.70; 95% CI = 0.53–0.87)
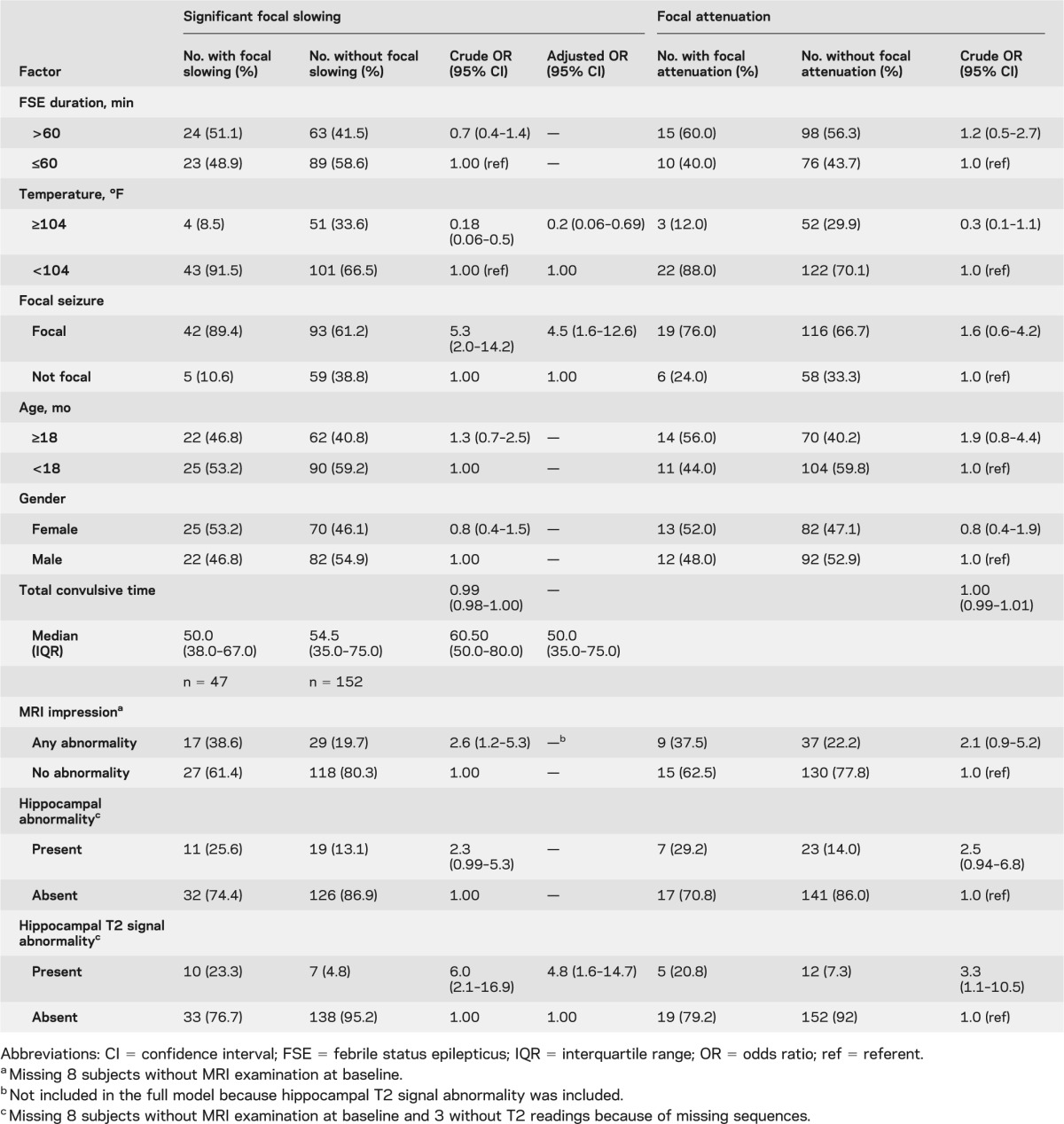
Association between focal slowing and attenuation and clinical and MRI findings
In univariate analysis, the odds of focal slowing were increased by focal FSE, any acute MRI abnormality, and hippocampal T2 signal abnormality. High temperature at the time of FSE was associated with significantly decreased odds of focal slowing (table 3). In adjusted analysis, low peak temperature, focal FSE, and hippocampal T2 signal abnormality increased the odds of focal slowing. Similar patterns were seen for focal attenuation but reached statistical significance only for hippocampal T2 signal abnormality (table 3). Additional analysis of combined focal slowing or attenuation showed similar associations with increased temperature, focal FSE, and hippocampal T2 signal abnormality (data not shown). The results were not different when we excluded the 8 subjects whose EEGs were obtained >72 hours after FSE (data not shown).
Table 3.
Logistic regression for the association with focal slowing on EEG in 199 children with FSE
Among the 19 children with clear lateralization of their seizures and clear lateralization of focal slowing or attenuation, 15 (78.9%) had unilateral slowing or attenuation on the same side of the suspected cortical origin of the seizure, and 4 (21.1%) had unilateral slowing or attenuation on the contralateral side.
DISCUSSION
The acute EEG findings after FSE were remarkable in several respects. Nearly half of the EEGs were abnormal (45.2%). The most significant abnormalities consisted of focal slowing, attenuation, or both, present in 30.2% of all EEGs. There was diffuse background slowing in 11.1% of all acute EEGs. In contrast, few epileptiform abnormalities were found. When present, focal slowing and attenuation more often involved the temporal regions and were usually ipsilateral to the side of seizure lateralization and the side of the hippocampal T2 signal abnormality. Focal slowing and focal attenuation were both associated with hippocampal T2 signal abnormality, but focal slowing was also associated with lower peak temperature and focal FSE, whereas no other factors were associated with focal attenuation. These EEG findings are quite robust given the blinded study design and the high interobserver reliability. The results suggest that EEG may be more sensitive than MRI in detecting children who may be at high risk for subsequent epilepsy. These same children may be candidates for interventions to prevent the development of subsequent epilepsy, such as anti-inflammatory agents, but more work is needed to clarify the role of EEG as a biomarker.
There was a striking predominance of right-sided slowing. This trend is also seen in the acute imaging findings.14 In humans, hippocampal sclerosis after febrile seizures is reported to be more common on the right than the left.17 Interestingly, an animal model of hyperthermic seizures has shown that the hippocampal T2 signal abnormality, although bilateral, was asymmetric and had a right side predominance.18
The preponderance of focal nonepileptiform rather than epileptiform abnormalities makes sense in the clinical context. FSE is not epilepsy but may reflect an acute injury that may lead to subsequent epilepsy. Although we acknowledge the possibility that these EEG abnormalities may have been present before FSE, the combination of hippocampal T2 signal abnormality along with focal EEG slowing or attenuation, most often with the same lateralization, would suggest that these findings are consistent with acute focal cerebral injury. Prior EEG studies obtained shortly after prolonged or severe febrile seizures have documented transient focal slowing, consistent with acute cerebral injury, and these same studies have shown a paucity of interictal epileptiform activity.19–24
Focal slowing or attenuation are frequently seen in EEGs obtained within 72 hours after FSE and may be markers for neuronal injury. Epileptiform abnormalities immediately after prolonged febrile seizures are uncommon. The data from this blinded study underscore the utility of EEG obtained shortly after status epilepticus. The FEBSTAT study with its prospective design, simultaneous imaging, and long-term follow-up will ultimately address the relationship between prolonged febrile seizures and subsequent temporal lobe epilepsy and hippocampal sclerosis. Given the long latency to develop clinical epilepsy after prolonged febrile seizures,5,9,25 it will be some time before the final answer is known. If the acute EEG is confirmed as not only predictive of the acute changes, but also of the long-term outcomes, it offers an inexpensive, noninvasive, readily available bedside tool that does not require sedation that may identify children who may be at high risk of developing epilepsy and who may be suitable for trials of antiepileptogenesis. In addition, the future serial EEGs obtained as part of FEBSTAT along with MRIs and clinical follow-up may serve as biomarkers for the development of epilepsy.26–28
Supplementary Material
ACKNOWLEDGMENT
The authors thank the contributors (listing available on the Neurology® Web site at www.neurology.org).
Glossary
- CI
confidence interval
- FEBSTAT
Consequences of Prolonged Febrile Seizures
- FSE
febrile status epilepticus
- HHV
human herpesvirus
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
All authors participated in the design and execution of the overall FEBSTAT project. Drs. Nordli, Moshé, and Shinnar designed the specific EEG project reported in this article. Drs. Nordli, Moshé, Sogawa, and Shinnar participated in the interpretation of the EEG tracings and their significance. All authors participated in the execution of the work reported in this article. Drs. Hesdorffer, Sun, and Shinnar performed the data analysis and statistical analysis. All authors participated in writing and editing the manuscript.
DISCLOSURE
D. Nordli is funded by the NIH. S. Moshé is funded by the NIH and the Heffer and Segal Family Foundations and received a consultant fee from Eisai and a speaker's fee and travel expenses from GSK. S. Shinnar is funded by the NIH, served on a DSMB for King Pharmaceuticals, has received personal compensation for serving on Scientific Advisory Boards for Questcor and Sunovion, for consulting for Eisai, Questcor, and Neuronex, and speaker honoraria from Eisai, Questcor, and UCB. D. Hesdorffer is funded by the NIH, the CDC, AUCD, Maternal and Child Health Bureau, and grants from the Epilepsy Study Consortium and Epilepsy Foundation of America. She has served on the UCB advisory board. She is a consultant to Mt. Sinai Medical Center, New York, and has a received a travel grant award from GlaxoSmithKline. Y. Sogawa has received support in the form of the Susan Spencer Young Clinical Investigator Award from the American Epilepsy Society. She was an NSADA trainee under K12 NS-48856. J. Pellock is funded by the NIH and the CDC. He has also received research support from Eisai, Marinus, Lundbeck, Pfizer, Questcor, and UCB. He has received compensation for serving on Scientific Advisory Boards for Eisai, Lundbeck, Questcor, Sepracor, and UCB. He has been a consultant for Catalyst, Eisai, GlaxoSmithKline, King, KV Pharmaceuticals, Lundbeck, Marinus, Neuropace, Pfizer, Questcor, Sepracor, Sunovion, UCB, Upsher Smith, and Valeant. All grants, research support, consultant fees, and honoraria are paid to Virginia Commonwealth University or the physician practice plan (MCV Physicians). D. Lewis is funded by the NIH. L.M. Frank is funded by the NIH and has received grant support from Schwarz-UCB, Ovation-Lundbeck, Eisai, Supernus, and AVI BioPharma. He holds or has held stock in AVI Biopharma, Oncothyreon, Neurologix, Peregrine, Positiveid, and Nanoviricides. R. Shinnar is funded by the NIH. She has received compensation for serving on advisory panels for Questcor Pharmaceuticals, Eisai, and Lundbeck and speaker honoraria from Cyberonics and Questcor. S. Sun is funded by the NIH and serves as a consultant for Nestle. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Maytal J, Shinnar S, Moshe SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics 1989;83:323–331 [PubMed] [Google Scholar]
- 2.Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med 1987;316:493–498 [DOI] [PubMed] [Google Scholar]
- 3.Abou-Khalil B, Andermann E, Andermann F, Olivier A, Quesney LF. Temporal lobe epilepsy after prolonged febrile convulsions: excellent outcome after surgical treatment. Epilepsia 1993;34:878–883 [DOI] [PubMed] [Google Scholar]
- 4.Cendes F, Andermann F, Dubeau F, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology 1993;43:1083–1087 [DOI] [PubMed] [Google Scholar]
- 5.French JA, Williamson PD, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol 1993;34:774–780 [DOI] [PubMed] [Google Scholar]
- 6.Camfield P, Camfield C, Gordon K, Dooley J. What types of epilepsy are preceded by febrile seizures? A population-based study of children. Dev Med Child Neurol 1994;36:887–892 [DOI] [PubMed] [Google Scholar]
- 7.Berg AT, Shinnar S, Levy SR, Testa FM. Childhood-onset epilepsy with and without preceding febrile seizures. Neurology 1999;53:1742–1748 [DOI] [PubMed] [Google Scholar]
- 8.Shinnar S. Febrile seizures and mesial temporal sclerosis. Epilepsy Curr 2003;3:115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinnar S. Do febrile seizures lead to temporal lobe epilepsy? Prospective and epidemiological studies. In: Baram TZ, Shinnar S, editors. Febrile Seizures. San Diego: Academic Press; 2002:87–101 [Google Scholar]
- 10.Shinnar S, Hesdorffer DC, Nordli DR, Jr, et al. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology 2008;71:170–176 [DOI] [PubMed] [Google Scholar]
- 11.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 1993;34:592–596 [DOI] [PubMed] [Google Scholar]
- 12.Treatment of convulsive status epilepticus. Recommendations of the Epilepsy Foundation of America's Working Group on Status Epilepticus. JAMA 1993;270:854–859 [PubMed] [Google Scholar]
- 13.National Institutes of Health Febrile Seizures: Consensus Development Conference Summary. Bethesda: National Institutes of Health; 1980 [Google Scholar]
- 14.Shinnar S, Bello JA, Chan S, et al. MRI abnormalities following febrile status epilepticus in children: the FEBSTAT study. Neurology 2012;79:871–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Clinical Neurophysiology Society Guideline 2: minimal technical standards for pediatric electroencephalography. Available at: http://www.acns.org. Accessed February 20, 2006
- 16.Fleiss JL. Statistical Methods for Rates and Proportions. New York: John Wiley & Sons; 1981 [Google Scholar]
- 17.Janszky J, Woermann FG, Barsi P, Schulz R, Halasz P, Ebner A. Right hippocampal sclerosis is more common than left after febrile seizures. Neurology 2003;60:1209–1210 [DOI] [PubMed] [Google Scholar]
- 18.Dube CM, Ravizza T, Hamamura M, et al. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci 2010;30:7484–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lennox MA. Febrile convulsions in childhood, a clinical and electroencephalographic study. Am J Dis Child 1949;78:868–882 [DOI] [PubMed] [Google Scholar]
- 20.Millichap JG, Madsen JA, Aledort LM. Studies in febrile seizures. V. Clinical and electroencephalographic study in unselected patients. Neurology 1960;10:643–653 [DOI] [PubMed] [Google Scholar]
- 21.Lerique-Koechlin A, Misès J, Teyssonière de Gramont M, Losky-Nekhorocheff I. The EEG in febrile seizures [in French]. Rev Neurol (Paris) 1958;99:11–25 [PubMed] [Google Scholar]
- 22.Frantzen E, Lennox-Buchthal M, Nygaard A. Longitudinal EEG and clinical study of children with febrile convulsions. Electroencephalogr Clin Neurophysiol 1968;24:197–212 [DOI] [PubMed] [Google Scholar]
- 23.Doose H, Petersen CE, Volzke E, Herzberger E. Fever cramps and epilepsy. I. Etiology, clinical picture and course of the so-called infection or fever cramps [in German]. Arch Psychiatr Nervenkr 1966;208:400–412 [DOI] [PubMed] [Google Scholar]
- 24.Prichard JS, McGreal DA. Febrile convulsions. Med Clin North Am 1958;42:379–387 [DOI] [PubMed] [Google Scholar]
- 25.Mathern GW, Pretorius JK, Babb TL. Influence of the type of initial precipitating injury and at what age it occurs on course and outcome in patients with temporal lobe seizures. J Neurosurg 1995;82:220–227 [DOI] [PubMed] [Google Scholar]
- 26.Gomes WA, Shinnar S. Prospects for imaging-related biomarkers of human epileptogenesis: a critical review. Biomark Med 2011;5:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel J., Jr Biomarkers in epilepsy: introduction. Biomark Med 2011;5:537–544 [DOI] [PubMed] [Google Scholar]
- 28.Galanopoulou AS, Moshe SL. In search of epilepsy biomarkers in the immature brain: goals, challenges and strategies. Biomark Med 2011;5:615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


