Abstract
The path from hematopoietic stem cells (HSCs) to functional B lymphocytes has long been appreciated as a basic model of differentiation, but much clinically relevant information has also been obtained. It is now possible to conduct single cell studies with increasingly high resolution, revealing that individual stem and progenitor cells differ from each other with respect to differentiation potential and fates. B lymphopoiesis is now seen as a gradual and unsynchronized process where progenitors eventually become B lineage restricted. Major milestones have been identified, but a precise sequence need not be followed and oscillation between states is possible. It is not yet clear if this versatility has survival value, but information is accumulating about infections and age related changes.
Introduction
A series of papers have described surprising diversity in hematopoietic stem cells (HSCs) [1]. While the basis of that variation is unclear, it might confer HSCs with the ability to respond rapidly to changing circumstances. In fact, additional reports describe a much more dynamic process of blood formation than previously imagined. Loss of myeloerythroid differentiation potential is gradual, and the most primitive progenitors of lymphocytes are also heterogeneous [2]. The asynchronous nature of stem/progenitor events continues through these compartments, such that the order of gene expression is not rigid. In addition to that complexity, technical and nomenclature issues have complicated the goal of charting major and alternative differentiation routes. We believe solid progress has been made, and recent reviews detail remarkable advances in understanding how transcription factors work in concert with epigenetic changes to direct progression of cells in the B lineage. There are also age-related changes in immune system replenishment, and they partially resemble ones that occur during infection. The underlying mechanisms for B lymphopoiesis are being dissected in a number of laboratories, and interesting findings relate to stem/progenitor events as well as microenvironmental cues that control them.
Remarkable Heterogeneity of Hematopoietic Stem Cells (HSCs)
Tremendous progress has been made in developing methods for isolating HSCs, such that at least one out of three in a sorted population can reconstitute lethally irradiated mice [3]. One might expect skillfully selected sort parameters to yield relatively homogeneous HSCs. However, Muller-Sieburg and colleagues found evidence for at least three classes of functionally restricted HSC subsets; myeloid biased, lymphoid biased and balanced [4]. More surprisingly, this differentiation bias was stable though multiple cycles of transplantation [4]. Extending those findings, Eaves and colleagues transplanted individual HSCs and observed similar specialization, with different kinetic patterns of differentiation [5]. Notably, lymphoid biased HSCs were less robust than other subsets and produced few cells in secondary transplants [6••].
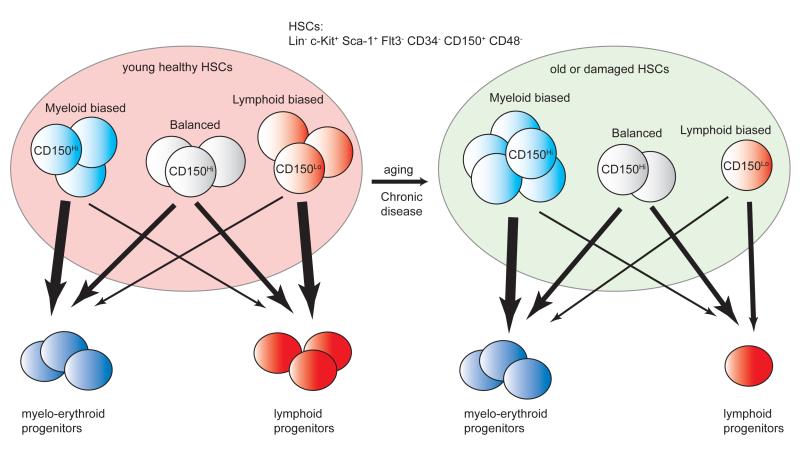
Subsequent studies revealed that cell surface and cytoplasmic staining patterns can be used to partially resolve such HSC subsets (Figure 1). For example, Goodell used Hoechst dye exclusion to identify upper and lower side population (SP) HSCs. The former subset was prone to generate lymphocytes while lower SP cells tended to be myeloid biased [7••]. As another perspective, CD49blowRhodaminelowCD150Hi HSCs had long-term potential for blood cell production and could be distinguished from CD49bHi HSCs that produced red blood cells for shorter periods [8].
Figure 1. Heterogeneity of hematopoietic stem cells.
Subsets of hematopoietic stem cells (HSCs) differ in terms of surface marker expression, dye efflux and function. In murine bone marrow, most are defined as Lin− c-Kit+ Sca-1+ Flt3− CD34− CD150+ CD48−. Recent studies suggest there are at least three major HSC classes, distinguished according to the spectrum of blood cells they produced [1]. That is, some are relatively balanced, others are biased to produce myeloid cells and still others preferentially generate lymphocytes. Ones with high densities of CD150 tend to be robust and myeloid biased. Additional variability relates to cell cycle status and potential for marrow engraftment on transplantation, as well as the kinetics and duration of blood cell formation. Stem cells can change with respect to composition and properties as a result of normal aging [10•] and during chronic infectious disease [12•]. Lymphoid progenitors arising from these HSCs are themselves heterogeneous and acquire B cell properties in an asynchronous manner.
CD150 is an extremely valuable marker for long-term HSCs, and its density reflects lineage bias [6••,9,10•]. That is, CD150Hi HSCs are myeloid biased whereas CD150low HSCs are more competent to generate lymphocytes. While the CD150Hi subset in marrow of young mice is robust with respect to serial transplantation, a phenotypically similar population accumulates in aged mice and has reduced lymphopoietic potential [7••,10•,11]. We found a partially similar phenomenon occurred in mice repeatedly given low doses of the TLR4 ligand lipopolysaccharide (LPS) [12•]. A distinct category of CD150HiCD86− HSCs accumulated in LPS treated mice and was poor at replenishing the adaptive immune system.
The basis for HSC heterogeneity is not completely understood and might be explained in multiple ways. For example, most HSCs rarely divide and their engraftment efficiency on transplantation is reduced when they are driven into cycle [13]. That is, slowly-dividing HSCs have the best long-term reconstitution capacity [14]. The majority of HSCs are generally quiescent and approximately 5% of them appear to be dormant. Experiments done with dye dilution methods suggest this subset may divide only five times in the entire lifespan of a mouse [15]. Another study applied serial transplantation of labeled cells to conclude HSCs can shift between quiescent and dividing states [16]. Nakauchi and colleagues used a panel of monoclonal antibodies and CD150 density to reveal HSC heterogeneity [6••]. Of particular interest, some CD150Hi HSCs were dormant and only generated blood cells when transplanted a second time. As mentioned above, a lymphoid biased subset of HSCs, i.e. the “upper side population” was more likely to be in cycle than other HSCs, and their proliferation was inhibited by TGF-β[7••]. While this cytokine was previously found to maintain HSC quiescence, responses may be both dose and subset dependent [17]. For example, the “lower side population” proliferated and differentiated in response to low concentrations of TGFβ [7••]. Thus, differences in metabolic and proliferation status might account for some HSC heterogeneity.
Some attempts have been made to establish precursor-product relationships between HSC subsets. For example, CD150Hi HSCs tend to generate CD150Hi HSCs as well as CD150Lo/− HSCs, but CD150Lo/− HSCs cannot become CD150Hi [6••,10•]. Hematopoiesis is usually viewed as a one-way process, where HSCs give rise to progenitors with progressively fewer options. However, experiments done with a multipotential cell line suggest that HSCs may oscillate between states [18]. Similarly, we found that lymphoid progenitors became lineage unstable and could be re-directed into erythropoiesis with canonical Wnt signaling [19]. There is precedent for such reversible differentiation in studies of Drosophila germ cells where position within niches is critical [20]. However, the extent to which it occurs in normal hematopoiesis is unknown. HSCs are thought to reside in multiple niches, but how that influences their properties is not clear [21]. There is one report that HSCs in spleen are more likely to be in cycle than those in marrow [22].
Complete agreement has not been reached about what marker combinations define all, or subsets of HSCs [3,17,23]. Also unclear is whether heavily lymphoid biased HSCs lacking serial transplantation potential should more properly be considered lymphoid progenitors [5]. Lymphocytes can self-renew and have extremely long lifespans, regardless of whether they arise from early lymphoid progenitors or true stem cells.
Partial lineage commitment and early lymphoid gene expression
A crisp definition for the “earliest” stage in B lymphopoiesis is probably unattainable, because initial events are not synchronous, and many changes appear to be gradual. Our lab originally noted that lin−c-KitHiFlt3+ cells from bone marrow were uniquely sensitive to estrogen and capable of generating T and B lineage cells [24]. Importantly, they were heterogeneous in that some expressed TdT, some were positive for a human immunoglobulin transgene and some were positive for both markers. The availability of RAG-1/GFP knock-in and RAG-1/tdRed reporter mice made it possible to sort viable early lymphoid progenitors (ELPs) on that basis and again appreciate that they were not synchronized [25]. RAG-1+ ELPs are potent at replenishing T, natural killer (NK) and B cells when transplanted, and they are characteristically slow to generate lymphocytes in culture [24].
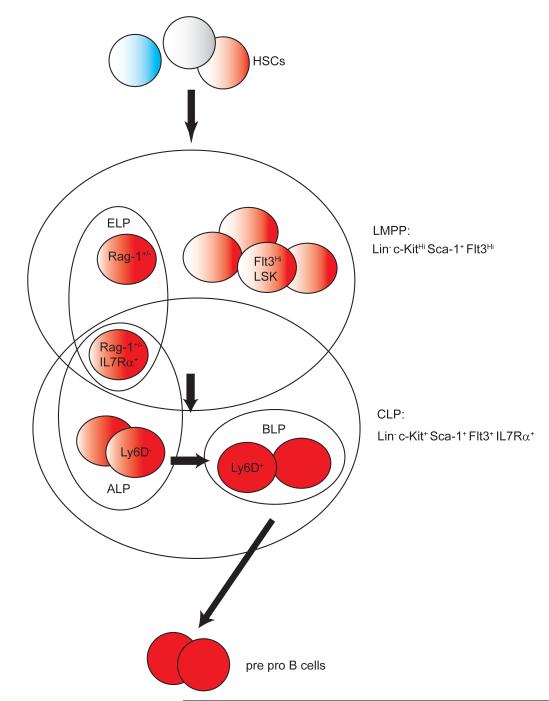
It has been known for some time that HSCs and progenitors express transcripts for genes required by mature blood cells [2,26,27]. The phenomenon is known as “priming” and could reflect an initial opening of chromatin, as well as anticipatory loading of promoters [28••]. Single-cell PCR results indicate variable expression of these genes, and individual HSCs only contain transcripts of one or two lymphoid genes. Although most of these genes are essential for B and/ or T cell development, it is not clear if they directly lead to early lineage decisions. Given the lack of standard definitions of HSCs, it is not surprising that there is poor agreement about what HSC expressed genes are most closely affiliated with “lymphoid” lineages. Thus, there is a degree of ambiguity associated with the term lymphoid primed multipotent progenitors (LMPPs) [29]. Nevertheless, LMPPs are phenotypically defined as lin−c-KitHi cells with a very high density of Flt3, a category that includes ELPs (Figure 2). Kondo and Lai used progressive loss of VCAM-1 to track formation of overlapping populations of primitive lymphoid progenitors [30]. Importantly, LMPPs have greatly reduced potential for generating megakaryocytes and erythrocytes [29]. As with ELPs, they have very low, residual ability to respond to myeloid supporting cytokines and proliferate in standard Methocel cultures [24]. We regard these cells as heterogeneous, partially lineage committed progenitors. Elegant single cell PCR approaches have shown that the order of expression of particular lymphoid genes is not rigid [2,27]. Hence, we refer to the process as asynchronous.
Figure 2. Terminology used for B Lymphoid Progenitors.
Significant progress has been made with respect to the cartography of B lymphopoiesis. However, progression from one arbitrarily defined “stage” to another is not abrupt and the precise sequence of events can differ. As another complication, terms used to describe these categories are not standardized. Loss of erythroid and megakaryocytic potential is paralleled by marked up-regulation of Flt3, generating cells referred to as early lymphoid progenitors (ELPs) or lymphoid primed multipotent progenitors (LMPPs) [24,29]. These are largely overlapping groups of cells that, like HSCs, still have high densities of c-Kit. Originally defined as weakly IL-7Rα+ and c-KitLo, many labs now omit c-Kit as a sort parameter for common lymphoid progenitors (CLPs), selecting instead for cells still bearing Flt3. As the sensitivity of IL-7Rα detection has increased, the distinction between ELPs/LMPPs and CLPs has been reduced. With some strains of mice, progression in the B lineage can be distinguished by increased expression of RAG genes [24] and acquisition of Ly6D [33].
Chromatin immunoprecipitation (ChIP) sequencing is providing additional insight into epigenetic changes, relationships between key transcriptional regulators and modulation of gene expression during B cell differentiation. For example, Lin and colleagues found associations between E2A DNA binding, certain histone methylation patterns and a wide spectrum of cis-regulatory elements [31•]. They also found coordinated DNA occupancy between E2A, Ebf-1 and Foxo-1. Treiber and colleagues focused on Ebf-1 binding sites and discovered that genes regulated by Ebf-1 encoded molecules involved in B cell receptor signaling, cell migration and adhesion [32•]. They observed interactions between Ebf-1 and other transcription factors, such as Pax5, Runx1 and Ets1. It will be important to integrate this new information with knowledge of environmental cues for HSC maintenance and early events in lymphopoiesis.
Common Lymphoid Progenitors (CLPs)
CLPs were originally defined in terms of a binary fate decision, where progenitors destined to make T, NK and B cells diverge from those related to all other blood cell lineages [24]. While the separation may be more gradual and dynamic than originally proposed, and their phenotypic definition has evolved, several recent papers suggest that it occurs. Expression of IL-7Rα has been consistently used to discriminate CLPs, but thresholds are affected by generally low density, antibodies, fluorochromes and sensitivity of flow cytometers. Our own experience of many years was that IL-7Rα transcripts were present in ELPs, but surface display was negative. More recently, and with more sensitive laser/fluorochrome/filter combinations, small numbers of c-KitHi Flt3Hi cells are seen to be IL-7Rα+. This is noteworthy, because that subset could confusingly be designated LMPPs, ELPs or CLPs (Figure 2). Adding to the complexity, the name all lymphoid progenitors, or “ALPs” has also been proposed for those cells [33]. These are c-KitHiIL7Rα+Flt3Hi and able to generate NK and dendritic cells (DCs) as well as B cells. Given the approach used, a small number of ELP that express IL7Rα would be included in this ALP subset (Figure 2). The same group described Ly6D as a marker for a potent B cell restricted subset of CLPs (referred to as BLPs). Expression of Ly6D is partially dependent on IL7Rα signaling [34]. Ly6D+ BLPs highly overlap with subsets marked by RAG-1/GFP and λ5 reporters [35]. However, expression of Ly6D is not dependent on the Ebf-1 transcription factor [34]. Thus, Ly6D may be used as a phenotypic marker but whether it is functionally involved in B lineage development is unknown. In RAG-1/tdRed fate mapping mice, half of conventionally defined CLPs are marked [25]. Indeed, RAG-1 expressing CLPs are more potent and restricted B cell progenitors than non-labeled CLPs.
Lymphoid progenitors with high levels of c-Kit (LMPPs/ELPs) take much longer than c-KitLo CLPs (BLPs) to generate CD19+ lymphocytes in culture, providing evidence for a precursor-product relationship. However, rate-limiting factors are not sufficiently well understood and ELPs differentiate rapidly under the influence of retinoids [24].
Functional definitions are also confusing, particularly with respect to the ability of progenitors to replenish the adult thymus. Transfer to unirradiated or immunodeficient mice represents the most stringent definition while intrathymic injections and stromal cell co-culture assays are more permissive [36]. Most reports indicate that ELPs/LMPPs are the most potent T progenitors when thymus homing is required while IL-7Rα+ CLPs efficiently expand and differentiate when placed on DLL1-transduced stromal cells [37]. Other subsets have T lineage potential but seem unlikely to colonize the thymus under normal circumstances. As one interesting example, a recently described subset of CD150−Flt3+CMPs can generate myeloid and T lymphocyte lineage cells but lack chemokine receptors essential for thymic homing [38•].
A more important issue is whether cells with restricted T/B/NK potential normally colonize the thymus, but that again depends on definitions and experimental approaches used. Fate mapping with RAG-1/tdRed mice showed that while all T and B lymphocytes have a history of RAG-1 expression, labeling of peripheral neutrophils and macrophages is extremely low [24,25]. The same was true of a labeling system based on IL7Rα+ [39•]. These results would be consistent with a clean separation between lymphoid and myeloid lineages, supporting the existence of “common” lymphoid progenitors.
On the other hand, cells that seed the thymus retain potential for generating neutrophils, macrophages and dendritic cells under at least some experimental circumstances [40,41]. In addition, we found that an extraordinarily high percentage of dendritic and NK cells in the thymus have a history of RAG-1 expression [24,25]. Conventional dendritic cells are often included in the “myeloid” category, although they can be made with some efficiency from lymphoid progenitors. Additionally, many plasmacytoid dendritic cells express transcripts for traditional B lineage lymphoid genes and have DH-JH gene rearrangements [24].
B lymphopoiesis and aging
HSC numbers are normal to slightly elevated in aged mice, while their phenotypes and functions show corresponding changes [7••,42-44]. Importantly, there is selective loss of ability to replenish the adaptive immune system. The basis of this is incompletely understood, but is characterized by reduced numbers of lymphoid progenitors, as well as declines in requisite Ebf-1 and E2A transcription factors [45].
Telomere shortening occurs in stem cells during aging, and HSCs from mice with defective telomerase can undergo fewer rounds of transplantation than those from wild type mice [46]. However, recent studies suggest that telomerase deficiency also alters BM environment. [47].
Until recently, there was little evidence to suggest that lymphopoiesis in bone marrow is controlled by peripheral B cell numbers. That is, the rate of new B cell production was not known to be linked to demand. However, an interesting report by Melamed and colleagues demonstrated that chronic depletion of mature lymphocytes “re-activated” the aged bone marrow [48••]. Several methods of depletion elevated numbers of lymphocyte progenitors in old mice and diversified the repertoires of mature B cells. Furthermore, old mice with chronic B cell deficiency from birth displayed no age-related changes in progenitors.
Alterations in B lymphopoiesis during infectious disease
Some pathogens, such as human immunodeficiency virus, herpes viruses, parvovirus, cytomegalovirus, varicella zoster virus, Leishmania and Measles virus can infect hematopoietic or stromal cells in bone marrow [49-51]. Other agents alter hematopoiesis by eliciting production of inflammatory cytokines, such as TNFα that mobilizes Pre-B and newly formed B cells [24,52]. Also, HSCs and progenitors are responsive to interferons (IFNs) [53]. Some LPS negative intracellular pathogens affect hematopoiesis through IFNγdependent pathways. For example, Malaria infection induces a novel IL7Rα+ c-KitHi progenitor population, while conventional erythroid and lymphoid progenitors decline [54]. The occurrence of these progenitors is dependent on IFNγ but not TLR signaling. Infection with E. muris, an intracellular bacterium, induces myelopoiesis, and this response was also IFNγ dependent [55].
Pathogen products and endogenous danger signal ligands released by infected cells can also influence lymphopoiesis. This follows from the discovery that HSCs/progenitors express functional Toll-like receptors, and their ligation acts to bias hematopoiesis towards production of innate effecter cells [24]. Culture and in vivo studies showed that LPS also drove HSCs out of quiescence [16,24]. Chronic treatment of young mice with LPS partially reproduced some changes associated with aging [12•]. That is, there is an accumulation of CD150Hi HSCs, loss of transplantation potency and reduction of B lymphopoiesis. Unlike the situation with normal aging, an abnormally high number of HSCs are in cycle, and they continue to divide many months after LPS exposure and serial transplantation. This suggests that infectious episodes might contribute to age-related immunosenescence. CLPs in Herpes infected mice lose their potential for B cell production, becoming instead better progenitors for dendritic and NK-like cells [24]. Ligation of TLR-9 by a Herpes product rather than indirect effects of cytokines caused those dramatic changes. In C. albicans infected mice, Lin− c-Kit+ Sca-1+ (LSK) cells expanded rapidly while B cell lymphopoiesis was reduced, and a new monocyte-derived DC population was detected [56]. In most cases, inflammation favors myelopoiesis rather than lymphopoiesis. However, myeloid differentiation was blocked at progenitor stages and lymphopoiesis was less affected by P. aeruginosa or high dose LPS in a sepsis model [57]. This selective suppression of myelopoiesis was partially dependent on TLR4.
Bone marrow stromal cells also express TLRs [50]. When cultured with TLR ligands, human BM mesenchymal stem cells secrete inflammatory cytokines and chemokines, such as TNFα and CXCL10 [58]. However, TLR signaling in stromal cells can also result in immune suppression [59]. LPS treated mouse mesenchymal stem cells secreted prostaglandin E2, which converted activated macrophages to a regulatory-like profile [60]. These macrophages became sources of IL-10 instead of TNFαIFNγ and IL-6, a shift that increased survival in sepsis mice [61]. However, most of these results are based on culture experiments, and more information is needed about influences of infectious agents on other components of marrow.
It is clear the marrow is poised to respond quickly to a variety of pathogens, and more information is needed about how discrete mechanisms are used for each agent. In many cases, there is a rapid boosting of innate immune defenses at the expense of B lymphopoiesis. While this should have survival value, chronic infections can also be harmful to stem cells and may contribute to immunosenescence.
Summary
Progression in the B lineage lymphoid pathway is a continuum, where progenitors gradually lose options for other fates. Bias towards or away from lymphopoiesis begins in HSCs and becomes more prominent with lineage progression. Considerable progress has been made in developing methods that reveal potential heterogeneity and asymmetry of the process. This information should help achieve consensus about major and minor differentiation routes used in experimental animals. Furthermore, comparable tools will be extremely valuable for studying human cells. As one example, it is important to have antibodies that discriminate human HSCs with the full spectrum of differentiation options from those that may be injured or heavily biased. It may also be possible to identify progenitors from early stages of malignant transformation. A greater understanding of environmental cues and consequences of chronic infection should help preserve humoral immunity and encourage its replenishment following transplantation or chemotherapy.
*Highlights.
Hematopoietic stems cells are more heterogeneous than previously thought.
B lineage commitment is gradual and unsynchronized.
B lymphopoiesis is altered by infectious disease and aging.
Acknowledgements
The authors thank Karla Garrett for technical assistance and Shelli Wasson for editorial assistance. This work was supported by National Institutes of Health Grants AI020069 and HL107138 (P.W.K.). P.W.K. holds the William H. and Rita Bell Endowed Chair in Biomedical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- [1].Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2006;107:2311–2316. doi: 10.1182/blood-2005-07-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ng SY, Yoshida T, Zhang J, Georgopoulos K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity. 2009;30:493–507. doi: 10.1016/j.immuni.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- [4].Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- [5].Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- [6] ••.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318.This study used antibody screening and single cell transplantation to explore heterogeneity of HSC. Of special interest, some functionally specialized HSC were deeply quiescent and only produced cells after secondary transplantation.
- [7] ••.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002.Previous descriptions of HSC heterogeneity were confirmed and extended with dye efflux, CD150 staining and single cell transplantation approaches.
- [8].Benveniste P, Frelin C, Janmohamed S, Barbara M, Herrington R, Hyam D, Iscove NN. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell. 2010;6:48–58. doi: 10.1016/j.stem.2009.11.014. [DOI] [PubMed] [Google Scholar]
- [9].Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10] •.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107.This noteworthy paper also exploited CD150 density to appreciate functional HSC subsets.
- [11].Kent DG, Copley MR, Benz C, Wohrer S, Dykstra BJ, Ma E, Cheyne J, Zhao Y, Bowie MB, Gasparetto M, et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113:6342–6350. doi: 10.1182/blood-2008-12-192054. [DOI] [PubMed] [Google Scholar]
- [12] •.Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186:5367–5375. doi: 10.4049/jimmunol.1003438.Chronic exposure to LPS injured HSC, and this was marked by changes in HSC phenotypes.
- [13].Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nygren JM, Bryder D. A novel assay to trace proliferation history in vivo reveals that enhanced divisional kinetics accompany loss of hematopoietic stem cell self-renewal. PLoS One. 2008;3:e3710. doi: 10.1371/journal.pone.0003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- [16].Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011;208:273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morita Y, Ema H, Yamazaki S, Nakauchi H. Non-side-population hematopoietic stem cells in mouse bone marrow. Blood. 2006;108:2850–2856. doi: 10.1182/blood-2006-03-010207. [DOI] [PubMed] [Google Scholar]
- [18].Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Malhotra S, Baba Y, Garrett KP, Staal FJ, Gerstein R, Kincade PW. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J Immunol. 2008;181:3955–3964. doi: 10.4049/jimmunol.181.6.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- [21].Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morita Y, Iseki A, Okamura S, Suzuki S, Nakauchi H, Ema H. Functional characterization of hematopoietic stem cells in the spleen. Exp Hematol. 2011;39:351–359. e353. doi: 10.1016/j.exphem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- [23].Weksberg DC, Chambers SM, Boles NC, Goodell MA. CD150- side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood. 2008;111:2444–2451. doi: 10.1182/blood-2007-09-115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ichii M, Shimazu T, Welner RS, Garrett KP, Zhang Q, Esplin BL, Kincade PW. Functional diversity of stem and progenitor cells with B-lymphopoietic potential. Immunol Rev. 2010;237:10–21. doi: 10.1111/j.1600-065X.2010.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Welner RS, Esplin BL, Garrett KP, Pelayo R, Luche H, Fehling HJ, Kincade PW. Asynchronous RAG-1 expression during B lymphopoiesis. J Immunol. 2009;183:7768–7777. doi: 10.4049/jimmunol.0902333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3:137–147. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- [27].Mansson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S, Al-Hashmi S, Liuba K, Thoren L, Adolfsson J, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- [28] ••.Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME, Habib N, Yosef N, Chang CY, Shay T, et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011;144:296–309. doi: 10.1016/j.cell.2011.01.004.Human stem and progenitors cells were subjected to extensive microarray and bioinformatics analyses. Anticipatory loading of promoters in HSC corresponded to previous knowledge of lineage priming and networks of cross-competing transcription factors were found to become simpler with differentiation.
- [29].Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- [30].Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- [31] •.Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, Hagman J, Espinoza CA, Dutkowski J, et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 2010;11:635–643. doi: 10.1038/ni.1891.The authors performed genome wide ChiP-sequencing analyses for DNA occupancy by E2A, Ebf-1 and Foxo-1. B lymphopoiesis requires a network of transcription regulators and histone modifications.
- [32] •.Treiber T, Mandel EM, Pott S, Gyory I, Firner S, Liu ET, Grosschedl R. Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription- independent poising of chromatin. Immunity. 2010;32:714–725. doi: 10.1016/j.immuni.2010.04.013.The study identifies a wide spectrum of genes targeted by Ebf-1 via genome wide ChiP-sequencing.
- [33].Inlay MA, Bhattacharya D, Sahoo D, Serwold T, Seita J, Karsunky H, Plevritis SK, Dill DL, Weissman IL. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tsapogas P, Zandi S, Ahsberg J, Zetterblad J, Welinder E, Jonsson JI, Mansson R, Qian H, Sigvardsson M. IL-7 mediates Ebf-1-dependent lineage restriction in early lymphoid progenitors. Blood. 2011;118:1283–1290. doi: 10.1182/blood-2011-01-332189. [DOI] [PubMed] [Google Scholar]
- [35].Mansson R, Zandi S, Welinder E, Tsapogas P, Sakaguchi N, Bryder D, Sigvardsson M. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115:2601–2609. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- [36].Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- [37].Zlotoff DA, Bhandoola A. Hematopoietic progenitor migration to the adult thymus. Ann N Y Acad Sci. 2011;1217:122–138. doi: 10.1111/j.1749-6632.2010.05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38] •.Chi AW, Chavez A, Xu L, Weber BN, Shestova O, Schaffer A, Wertheim G, Pear WS, Izon D, Bhandoola A. Identification of Flt3CD150 myeloid progenitors in adult mouse bone marrow that harbor T lymphoid developmental potential. Blood. 2011;118:2723–2732. doi: 10.1182/blood-2010-09-309989.Bhandoola and colleagues show that myeloid restricted progenitors have T, but not B lineage potential and are unlikely to colonize the thymus for lack of appropriate chemokine receptors.
- [39] •.Schlenner SM, Madan V, Busch K, Tietz A, Laufle C, Costa C, Blum C, Fehling HJ, Rodewald HR. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005.A well designed IL-7Rα based fate mapping system was used to conclude that B/T restricted progenitors differ from myelo-erythroid ones and are likely to replenish the adult thymus.
- [40].Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- [41].Wada H, Masuda K, Satoh R, Kakugawa K, Ikawa T, Katsura Y, Kawamoto H. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–772. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- [42].Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011 doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lescale C, Dias S, Maes J, Cumano A, Szabo P, Charron D, Weksler ME, Dosquet C, Vieira P, Goodhardt M. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging Cell. 2010;9:410–419. doi: 10.1111/j.1474-9726.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- [46].Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–520. doi: 10.1182/blood-2002-07-2334. [DOI] [PubMed] [Google Scholar]
- [47].Song Z, Wang J, Guachalla LM, Terszowski G, Rodewald HR, Ju Z, Rudolph KL. Alterations of the systemic environment are the primary cause of impaired B and T lymphopoiesis in telomere-dysfunctional mice. Blood. 2010;115:1481–1489. doi: 10.1182/blood-2009-08-237230. [DOI] [PubMed] [Google Scholar]
- [48] ••.Keren Z, Naor S, Nussbaum S, Golan K, Itkin T, Sasaki Y, Schmidt-Supprian M, Lapidot T, Melamed D. B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood. 2011;117:3104–3112. doi: 10.1182/blood-2010-09-307983.This is one of two related studies indicating that age-related declines in B lymphopoiesis are reversible. Feedback regulation by mature B cells to the bone marrow is implied.
- [49].Kolb-Maurer A, Goebel W. Susceptibility of hematopoietic stem cells to pathogens: role in virus/bacteria tropism and pathogenesis. FEMS Microbiol Lett. 2003;226:203–207. doi: 10.1016/S0378-1097(03)00643-8. [DOI] [PubMed] [Google Scholar]
- [50].Nemeth K, Mayer B, Mezey E. Modulation of bone marrow stromal cell functions in infectious diseases by toll-like receptor ligands. J Mol Med (Berl) 2010;88:5–10. doi: 10.1007/s00109-009-0523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nguyen Hoang AT, Liu H, Juarez J, Aziz N, Kaye PM, Svensson M. Stromal cell-derived CXCL12 and CCL8 cooperate to support increased development of regulatory dendritic cells following Leishmania infection. J Immunol. 2010;185:2360–2371. doi: 10.4049/jimmunol.0903673. [DOI] [PubMed] [Google Scholar]
- [52].Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Belyaev NN, Brown DE, Diaz AI, Rae A, Jarra W, Thompson J, Langhorne J, Potocnik AJ. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat Immunol. 2010;11:477–485. doi: 10.1038/ni.1869. [DOI] [PubMed] [Google Scholar]
- [55].MacNamara KC, Oduro K, Martin O, Jones DD, McLaughlin M, Choi K, Borjesson DL, Winslow GM. Infection-induced myelopoiesis during intracellular bacterial infection is critically dependent upon IFN-gamma signaling. J Immunol. 2011;186:1032–1043. doi: 10.4049/jimmunol.1001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yanez A, Megias J, O’Connor JE, Gozalbo D, Gil ML. Candida albicans induces selective development of macrophages and monocyte derived dendritic cells by a TLR2 dependent signalling. PLoS One. 2011;6:e24761. doi: 10.1371/journal.pone.0024761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rodriguez S, Chora A, Goumnerov B, Mumaw C, Goebel WS, Fernandez L, Baydoun H, HogenEsch H, Dombkowski DM, Karlewicz CA, et al. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood. 2009;114:4064–4076. doi: 10.1182/blood-2009-04-214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Koppel A, Tolosa E, Hoberg M, Anderl J, Aicher WK, et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells. 2009;27:909–919. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- [60].Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S, Vermeulen M, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]