Abstract
Urea breaks hydrogen bonds among biopolymers leading to structural destabilization. In the case of hydrocolloids urea addition is thought to impact gelation. Detailed information about its pertinent role on influencing the structure-function relationships of hydrocolloids is still elusive, however. The present investigation is aimed at delineating hydrocolloids structural behavior in the presence of urea employing iota-carrageenan as a model system. X-ray fiber diffraction, rheological and thermal properties of two iota-carrageenan solutions with weight concentrations 4.5 and 6.0% w/w at two urea molar concentrations (0.5 and 2.0 M) with and without heat treatments have been analyzed. X-ray results suggest that the canonical double helical structural arrangement of iota-carrageenan is maintained even after urea addition. However, improved crystallinity, ordering and altered unit cell dimensions especially with heat treatments of the binary mixtures indicate the promotion of favorable interactions among carrageenan helices in the presence of urea. Increased elastic modulus and onset temperature of melting endotherm with the heat treatment compared to cold addition further attests the X-ray observations of enhanced structural ordering. Overall, results suggest that urea molecules synergistically aid iota-carrageenan interactions and stabilize structure of junction zones. Our findings are deemed to be helpful in the design and development of novel non-food applications of hydrocolloids.
Keywords: X-ray Diffraction, Gelation, DSC, Iota-carrageenan, Urea, Sulfated polysaccharide
Introduction
Among the several widespread marine algal polysaccharides, carrageenans are important to food scientists due to their ability to form thickeners, viscosifiers and gels. These sulfated galactans are heterogeneous in terms of sources, life stages and recovery procedures (Campo, Kawano, da Silva & Carvalho, 2009). Carrageenans have a niche in pharmaceutical and medical areas owing to their anticoagulant (Farias, Valente, Pereira & Mourao, 2000), antiherapetic (Carlucci, Ciancia, Matulewicz, Cerezo & Damonte, 1999) and antitumor (Zhou, Sun, Xin, Zhang, Li & Xu, 2004) activities. Furthermore, they serve as inhibitors of herpes simplex virus (Carlucci, Scolaro & Damonte, 1999), human papillomar virus (Roberts et al., 2007) and human immunodeficiency virus (Coggins et al., 2000). As carrageenans are not assimilated by the human body, they have the potential to be dietary fibers with zero nutritional value. Reduction of blood cholesterol and lipid levels subsequent to carrageenans consumption (Panlasigui, Baello, Dimatangal & Dumelod, 2003) further accentuates their utility in the design and development of novel functional foods and medicinal foods. Their synergistic interactions with hydrocolloids and proteins also lend favorable opportunities to fine-tune gel texture; e.g. carrageenan binary mixtures with locust bean gum (Chronakis, Borgstrom & Piculell, 1999; Dunstan, Chen, Liao, Salvatore, Boger & Prica, 2001), konjac mannan (Williams, Clegg, Langdon, Nishinari & Piculell, 1993) and casein (Nono, Durand & Nicolai, 2012) are helpful in developing novel dairy products.
The disaccharide repeat →3)-β-D-Galp-(1→4)-α-D-Galp-(1→ and sulfation at O-2H, O-4H and O-6H hydroxyl positions, and with or without 3, 6-anhydro-bridge in the α-D-galactose residue results in fifteen varieties of carrageenans. However, only the three types – kappa (κ), iota (ι) and lambda (λ) – are exploited most due to their versatility and subjected to extensive structural and functional studies. Their viscoelastic properties are especially intriguing: upon adding mono- and di-valent ions, kappa- and iota-carrageenans form thermally reversible gels (Piculell, 1995; Therkelsen, 1993) but lambda-carrageenan forms only viscous solutions; however, tri-valent iron ions impart gelation to lambda-carrageenan (Running, Falshaw & Janaswamy, 2012). Iota-carrageenan gels are relatively clear and display larger increase in storage modulus (G') with calcium than sodium ions (Michel, Mestdagh & Axelos, 1997). It’s gelation mechanism involves initial helix formation followed by salt and/or temperature-induced aggregation of helices, and are better understood by examining structural arrangements and interactions at atomic level. X-ray diffraction studies on oriented fibers demonstrate that iota-carrageenan adopts a stable double helical structure in which the peripheral sulfate groups promote inter-helical interactions via cations (Janaswamy & Chandrasekaran, 2001). These interactions are stronger with calcium ions than sodium causing noticeable differences in gel strengths.
Urea is known to break hydrogen bonds leading to structural destabilization and network collapse of biopolymers. Several studies have examined the effect of cations on the solid-state structure and rheological behaviors of iota-carrageenan, but none with urea. Herein, we report the effect of urea on the structural, viscoelastic and thermal properties of iota-carrageenan. Our choice of iota-carrageenan as a model hydrocolloid stems from the fact that its robust molecular structure, stabilized by strong inter-chain hydrogen bonds, is unperturbed by type of cation used. Thus, it would be interesting to examine to what extent urea could destabilize the molecular and packing structure and alter the physicochemical properties of iota-carrageenan.
2. Experimental Section
2.1 Materials
Measured amounts of the sodium salt of iota-carrageenan, gift from CP Kelco, were dispersed in distilled deionized water to obtain 4.5 and 6.0% (w/w) solutions and heated in a boiling water bath for 45 mins with periodic vortexing and later cooled to room temperature. In the first set of experiments, calculated weights of 0.5 M and 2.0 M urea were added to the above solutions, at room temperature, and stirred homogeneously. In another set of experiments, the four urea added carrageenan solutions were subjected to heat treatment by placing the vials in a heat bath (95 °C) with intermediate mixing for 30 mins, and cooled to room temperature. Thus, the two polysaccharide concentrations and two urea amounts coupled with no-heat (room temperature mixing) and heat treatments resulted in eight sample sets. For brevity, in the subsequent discussion these eight solutions are labeled as IC45U05C, IC45U20C, IC45U05H, IC45U20H, IC60U05C, IC60U20C, IC60U05H and IC60U20H: IC45 and IC60 signify 4.5 and 6.0% of IC solutions, U05 and U20 refer to 0.5 and 2.0 M of urea amounts, and C and H designate urea mixing without heat (room temperature) and with heat, respectively.
2.2 Fiber preparation and intensity data
Around 20 µL of solution was suspended between two glass rods in a fiber puller maintained at 66% relative humidity and allowed to dry for about four hours before stretching to twice the original length of 2–3 mm.
Synchrotron X-ray intensities from the oriented fibers were collected using BioCARS 14-BMC beamline at Argonne National Laboratory (ANL), Chicago, IL. Wavelength of the X-ray beam was set to 0.979 Å and the exposure lasted 5 seconds. Calcite powder (3.035 Å characteristic spacing) was used for internal calibration. The pattern center, detector to fiber distance, fiber tilt and rotation were estimated using FibreFix version 1.3.1 from CCP13 (Rajkumar, Al-Khayat, Eakins, Knupp & Squire, 2007). Reflection positions in each quadrant were measured and corresponding ρ (distance between the origin and reflection position in the reciprocal space) was estimated. For a trigonal system (a = b ≠ c, γ = 120°) the relationship between lateral component ξ, vertical component ζ and ρ of each reflection is given by: ρ2 = ξ2 + ζ2, where ξ = a*(h2+hk+k2)1/2 and ζ = lc*. The dimensions of the reciprocal unit cell a* and c* as well as the Miller indices (h, k, l) for each reflection and the direct unit cell parameters a and c are estimated using in-house programs.
2.3 Rheology
Viscoelastic measurements were conducted on an ARG2 mechanical spectrometer from TA Instruments, New Castle, DE. Linear viscoelastic region was identified from strain sweep measurements in the range 0.01 to 20% strain at 1Hz and 5 °C. Frequency sweeps in the range 0.1 to 100 Hz at 5% strain (linear region) were obtained at three selected temperatures: 5, 25 and 45 °C; two measurements below the gel-sol temperature and one above. Average values from duplicate experiments are reported.
2.4 Thermal analysis
Melting behaviors were recorded using Model Q2000 differential scanning calorimeter (DSC) from TA Instruments, New Castle, DE. Around 4 mg of sample was sealed hermetically in aluminum pans and thermal transitions were registered in the temperature range −20 to 95°C at 10°C/min heating rate. Universal Analysis 2000 software (TA Instruments, New Castle, DE) was used to estimate onset temperature, peak temperature and enthalpy of melting endotherms. Samples were tested in duplicate and average values are reported.
3. Results
3.1 X-ray analysis
3.1.1 Effect of urea on the diffraction patterns
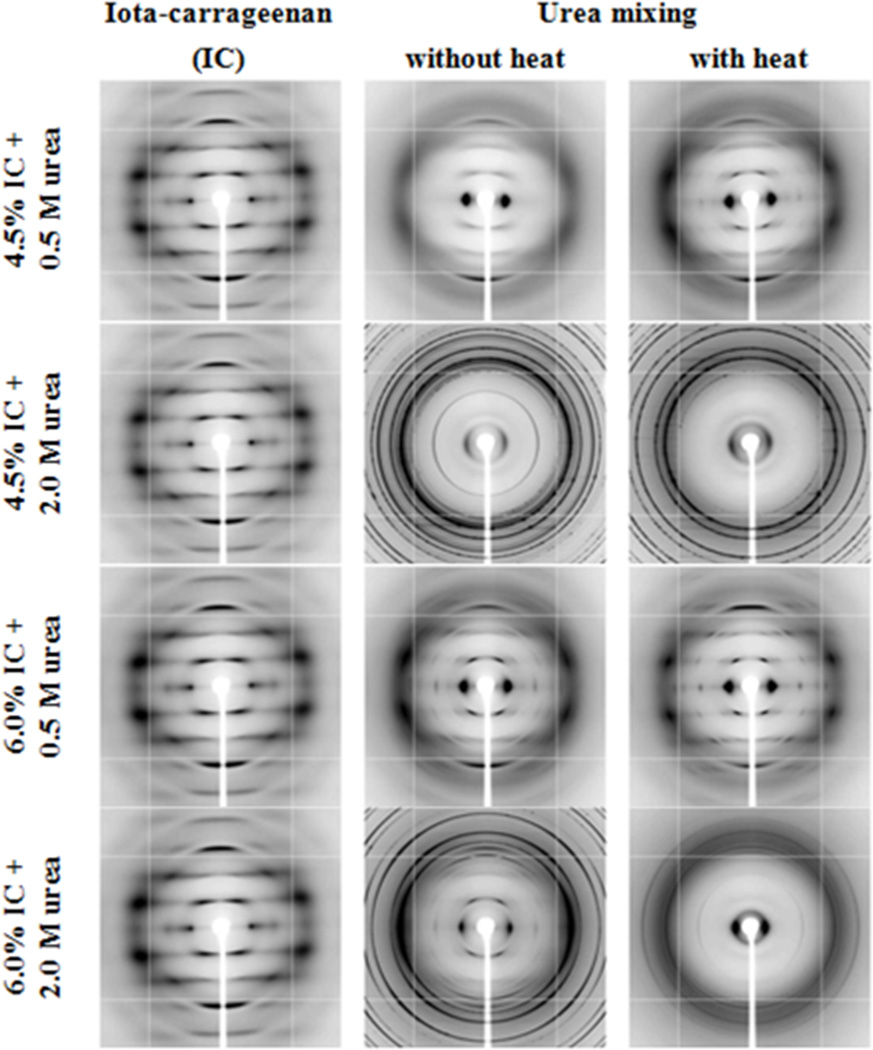
X-ray fiber diffraction patterns of IC45 and IC60 (Fig. 1) contain slightly diffuse reflections and resemble well with those obtained from 1.5% iota-carrageenan solutions (Janaswamy & Chandrasekaran, 2001, 2006). All the 12 reflections – zero through four layers – are indexable on a trigonal unit cell of dimensions a = 24.1 and c = 13.1 Å, similar to the values reported for sodium iota-carrageenan (Janaswamy & Chandrasekaran, 2001). Urea addition induced substantial alterations in the diffraction patterns. For example, long arcs with feeble intensities are characteristic of IC45U05C along with one strong reflection on the equator at inter-planar spacing of 13.7 Å. This intense reflection at roughly 2 Å more than that for sodium iota-carrageenan indicates a larger basal net caused by the presence of urea in the samples. After heating the solutions, much stronger and well-resolved reflections emerge in IC45U05H, suggesting the active role of urea molecules in promoting iota-carrageenan’s structural organization. However, the occurrence of urea rings and absence of carrageenan diffraction at higher urea concentrations (2.0 M) in IC45U20C and IC45U20H hint the loss of network. On the other hand, measurable ordering for IC60 with 0.5 M urea is evident in IC60U05C, which increases further after heating the solutions (IC60U05H). In association with 2.0 M urea, IC60U20C and IC60U20H contain weak Bragg reflections from iota-carrageenan network blanketed with intense urea rings. Retention of iota-carrageenan network in IC60 even after subjecting to elevated urea amounts demonstrates the resilient nature of iota-carrageenan in maintaining organized gel network at higher hydrocolloid concentrations; however, the dual role of urea – participation in the network destabilization as well as network promotion – cannot be discounted.
Fig. 1.
X-ray fiber diffraction patterns of 4.5 and 6.0% iota-carrageenan solutions in the presence of 0.5 and 2.0 M urea subjected to no-heat and heat-treatments. The fibers are almost perpendicular to the incident X-ray beam. The well-resolved Bragg reflections in 6.0% iota-carrageenan in the presence of 0.5 M urea coupled with solution heating indicate ordering among the iota-carrageenan chains.
3.1.2 Unit cell dimensions
IC60 gels with 0.5 M urea and heat-treatment (IC60U05H) yield well-resolved diffraction patterns suggesting that fibers drawn from them are well-oriented and polycrystalline. The first meridional reflection is on the 3rd layer line and there are 38 Bragg reflections extending out to 2.0 Å resolution up to 6 layer lines. These reflections are sharp and arc length increases toward the edge of the pattern indicating the formation of larger crystallites with shorter alignment along the fiber axis.
The lowest ξ value reflections are on the first and second layers and their average, 0.043 Å−1, is used as the smallest reciprocal vector (ξs) in the rest of the calculations. Reflections with similar ξ values across the six layer lines are grouped together and subsequent analysis reveal that these higher order ξ values are in multiples of √3, √4, √7, √9, √12, √13, √16, √19, √21, √27 and √31 of ξs. Assigning Miller indices (1, 0) as (h, k) for ξs, the higher order reflections are (1, 1), (2, 0), (2, 1), (3, 0), (2, 2), (3, 1), (4, 0), (3, 2), (4, 1), (3, 3) and (5, 1) in a trigonal lattice arrangement. Least-squares refinement of observed ρs with these Miller indices yield the unit cell dimensions as a = b = 27.16(5) Å and c = 12.82(2) Å for IC60U05H.
Table 1 summarizes the unit cell constants for iota-carrageenan (from the present and previous studies) bound to various co-solutes. In all these cases, the layer line spacing (c) maintained around 13 Å demonstrates intact molecular structure of iota-carrageenan but different basal net dimensions reveal a host of packing possibilities. In the case of sodium iota-carrageenan, there are three double helices spaced at 13.9 Å in a 24.0 Å trigonal net; however, helices are drawn closer to 13.6 Å upon switching to calcium ions with a concomitant shrinkage in the basal net to 23.6 Å. Interestingly, calcium ions also invoke bigger basal planes of dimension 27.4 Å (Janaswamy & Chandrasekaran, 2008) that allows four double helices set apart 13.7 Å on the cell edge and 23.8 Å along the short diagonal (1̅10) in the basal plane. As the cell edge of IC60U05H (27.16 Å) is close to one of the calcium iota-carrageenans, it can be reasoned that IC60U05H might favor four helices packing arrangement; if so, the resulting tight sheet packing – parallel to cell edges a and b – will retain fewer urea molecules located mainly in the empty spaces available between helices along the longer diagonal, parallel to the (110) plane, of the basal net. Alternatively, if the packing contains three double helices, similar to sodium iota-carrageenan, more urea molecules are likely to reside between the helices that are separated by about 15.7 Å in the (110) plane. Tertiary structure analyses will reveal actual numbers of double helices and urea molecules and their mode of interactions in bestowing stability, however.
Table 1.
Comparison of unit cell dimensions of iota-carrageenan in different allomorphs
| Solute/ Cation |
Unit cell dimensions (Å) | Lattice | Reference | ||
|---|---|---|---|---|---|
| a | b | c | |||
| Urea | 27.16 | 27.16 | 12.82 | Trigonal | Present work |
| Na+ (I) | 24.02 | 24.02 | 12.96 | Trigonal | (Janaswamy & Chandrasekaran, 2001) |
| Na+ (II) | 13.70 | 20.08 | 13.16 | Orthorhombic | (Janaswamy & Chandrasekaran, 2006) |
| Na+ (III) | 21.80 | 21.80 | 13.10 | Trigonal | (Janaswamy & Chandrasekaran, 2006) |
| Ca2+ (I) | 13.70 | 13.70 | 13.30 | Trigonal | (Arnott, Scott, Rees & McNab, 1974) |
| Ca2+ (II) | 23.61 | 23.61 | 13.21 | Trigonal | (Janaswamy & Chandrasekaran, 2002) |
| Ca2+ (III) | 27.44 | 27.44 | 13.01 | Trigonal | (Janaswamy & Chandrasekaran, 2008) |
| Sr2+ | 13.70 | 13.70 | 13.30 | Trigonal | (Arnott, Scott, Rees & McNab, 1974) |
| Mg2+ | 14.70 | 14.70 | 13.00 | Trigonal | (Arnott, Scott, Rees & McNab, 1974) |
| Rb+ | 54.60 | 54.60 | 12.30 | Trigonal | (Janaswamy & Chandrasekaran, 2005) |
| K+ | 68.20 | 68.20 | 13.10 | Trigonal | (Janaswamy & Chandrasekaran, 2005) |
3.2 Viscoelastic properties
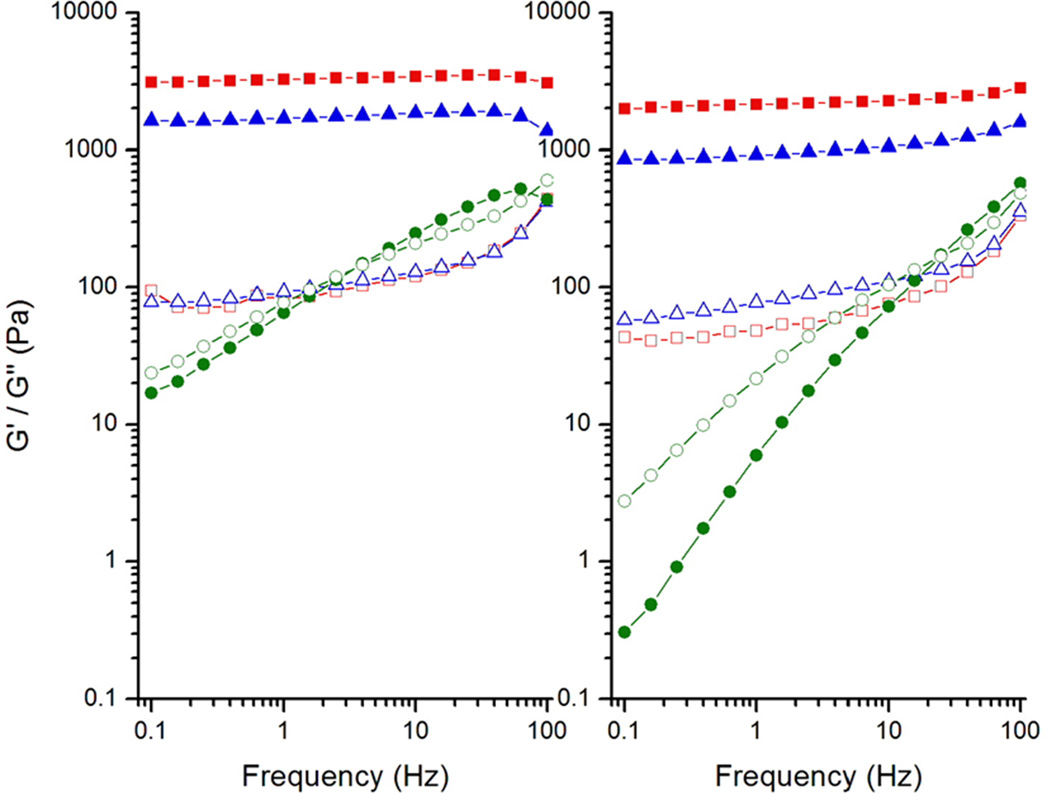
The elastic (G') and loss (G") moduli variations of IC45 and IC60 solutions in the frequency range 0.1 – 100 Hz at 5, 25 and 45 °C are shown in Figure 2. As expected, increase in carrageenan concentration resulted in higher G' values. In IC45, G' and G" at 5 °C are 2130 and 50 Pa, respectively, whereas in IC60 they rise to 3200 and 80 Pa. Although these values are little less at 25 °C, the nature of G' greater than G" continues suggesting that the gel network is intact. At 45 °C this trend reverses i.e. G" is greater than G' at lower frequencies signifying the viscous behavior; however, at higher frequencies (above 30 Hz for IC45 and 2 Hz for IC60) G' is greater than G" indicating the temporary association of iota-carrageenan helices during short oscillation time-periods.
Fig. 2.
Variation in the viscoelastic properties of G' (filled symbols) and G" (open symbols) 4.5 and 6.0% iota-carrageenan gels as a function of frequency at 5 (■), 25 (▲) and 45 (●) °C.
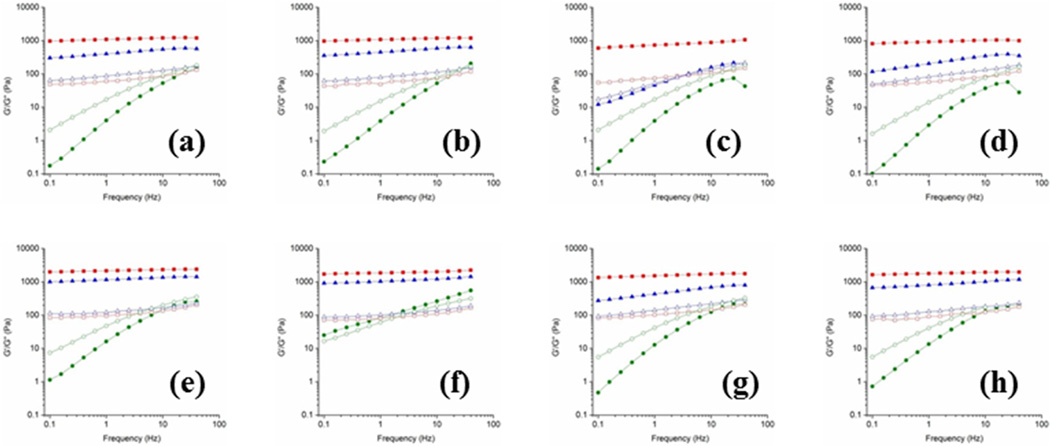
Addition of urea to IC60 altered its rheological behavior. At 5 °C, for 0.5M urea, G' decreased by about 30%, without heating the solutions, indicating the weakening of gel network (Fig. 3e), which further recedes by about 50% with 2.0 M addition (Fig. 3g). Similar trend is noticed in IC45, but the reductions are substantial – 50% and 65% decrease for 0.5 M (Fig. 3a) and 2.0 M (Fig. 3c) urea, respectively – suggesting that the carrageenan concentration is relatively important in maintaining the network. Heating the iota-carrageenan:urea solutions resulted in interesting observations, especially at higher urea concentrations. For example, G', at 5 °C, increases by about 16% in IC60U20H (Fig. 3h) compared to IC60U20C (Fig. 3g). Similarly, around 25% increase in noticed in IC45U20H (Fig. 3d) compared to IC45U20C (Fig. 3c). The enhancement of G' clearly implies the involvement of urea molecules in maintaining the iota-carrageenan gel network and importantly the role of heat energy in promoting the interactions of urea molecules with the carrageenan chains. Though, at lower urea amounts (0.5 M) this phenomenon is not that pronounced (Figs. 3b and 3f), similar G' values in both heat-treated and non-heat-treated solutions indeed point out that urea stabilizes the junction zone architecture of iota-carrageenan.
Fig. 3.
Variation in the viscoelastic properties of G' (filled symbols) and G" (open symbols) of iota-carrageenan (IC) gels in the presence of urea as a function of frequency at 5 (■), 25 (▲) and 45 (●) °C for the solutions of (a) 4.5% IC + 0.5 M urea (b) 4.5% IC +0.5 M urea heat treated, (c) 4.5% IC + 2.0 M urea, (d) 4.5% IC + 2.0 M urea heat treated, (e) 6.0% IC + 0.5 M urea, (f) 6.0% IC + 0.5 M urea heat treated, (g) 6.0% IC + 2.0 M urea, and (h) 6.0% IC + 2.0 M urea heat treated.
3.3 Thermal properties
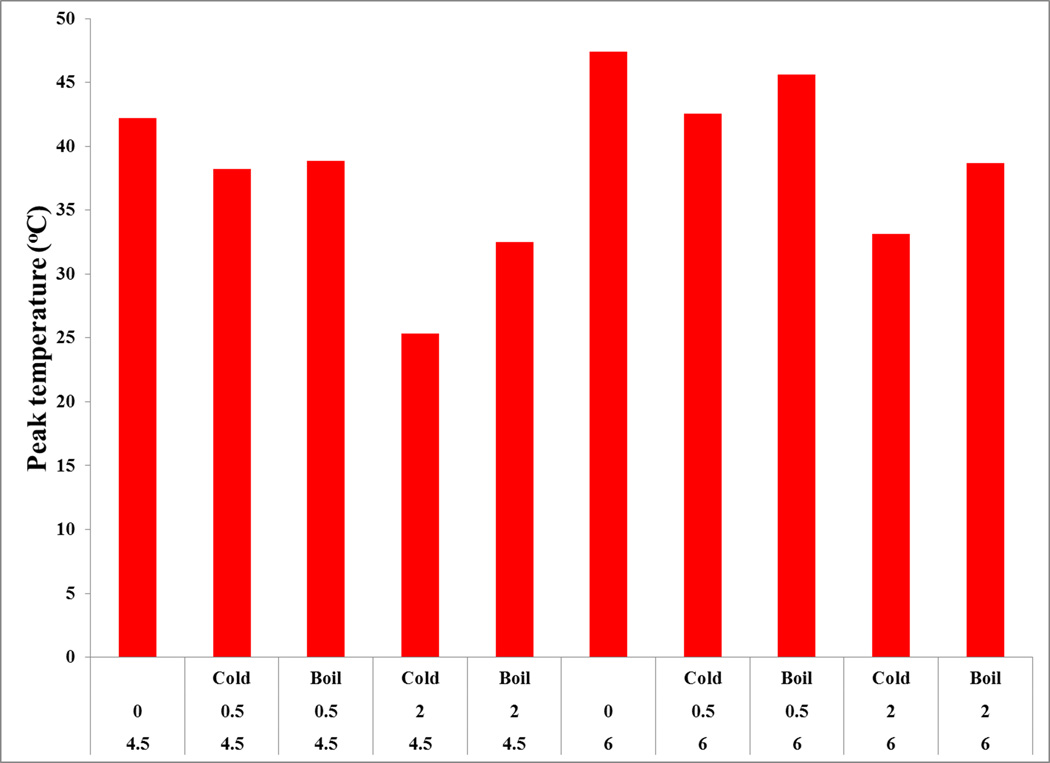
The peak temperature (Tm) of IC45 and IC60 melting endotherm is around 42 and 47 °C, respectively, and is in agreement with the reported values (Watase & Nishinari, 1987). In the presence of urea, Tm decreases, but springs back by heating the solutions (Fig. 4). The decrease in Tm shows dependence on the added urea amounts. For example, Tm lowers by about 4 °C in IC45U05C that further decreases by 17 °C in the presence of 2.0 M urea in IC45U20C. Similar behavior is noticed for IC60 but the subtle reduction (9 °C) in IC60U20H undoubtedly suggests the retention of carrageenan network. This trend indicates that the decrease in Tm is dependent on carrageenan concentration as well. The melting endotherm shift to lower temperatures is reduced when urea addition is coupled with heat-treatment. This behavior can be better explained by understanding the order-disorder transitions involving coil to helix to dimer formation of iota-carrageenan chains. We hypothesize that, urea mixing without any heating results only in the disruption of dimers, reflecting in the loss of G' as well as orientation and organization of helices in fiber. However, upon heating the solutions though the iota-carrageenan chains undergo helix to coil transition, network formation during cooling is altered due to urea participation leading to slightly elevated gel strengths and Tm values. Further research is necessary to validate this hypothesis.
Fig. 4.
Effect of urea coupled with no-heat and heat-treatments on the peak onset temperature endothermic peak of 4.5 and 6.0% iota-carrageenan solutions.
Generally, melting enthalpy reflects the amount of heat energy required during gel to sol transition. The melting enthalpies of IC45 and IC60 0.094 and 0.217 J/g, respectively, are very low. The rise in enthalpy due to increased carrageenan concentration in IC60 is expected since more energy is required to melt the junction zones. On dry weight basis, the calculated value 3.6 mJ/mg is close to 3.1 mJ/mg for IC60 (Watase & Nishinari, 1987). Addition of urea results in decreased enthalpy values for IC45 and IC60 as well. In the case of IC45, with 0.5M addition the enthalpy drops to 0.027 J/g and further reduces to 0.018 J/g with 2.0 M urea. However, upon heating the solutions these values increase to 0.284 and 0.338 J/g, respectively. Similarly, for IC60 the values are 0.900, 0.800, 0.578 and 0.777 J/g, in the same order. The increase in the enthalpies after heating the solutions is in agreement with those reported for kappa-carrageenan (Nishinari, Watase, Williams & Phillips, 1990). Decrease in the melting temperature and enthalpy is also noticed for recombinant human deoxyribonuclease (Chan, AuYeung & Gonda, 1996).
Increase in the enthalpy as a result of urea addition to IC60 does not indicate structural destabilization; however, viscoelastic properties do not reflect such improvement since G' is lowered. It appears that at higher iota-carrageenan amounts (IC60) either with or without heating the solutions, urea plays interactive role in promoting the carrageenan network, which is not the case for IC45 without heating the solutions. However, similar mechanism appears to increase the enthalpy for samples with urea upon heating the solutions. The X-ray analysis support this argument that heating the iota-carrageenan and urea together improves structural ordering.
4. Discussion
Chaotropic agents such as guanidinium chloride, magnesium chloride, lithium perchlorate, sodium dodecyl sulfate, thiourea and urea possess the intrinsic ability to interfere with intra and inter-molecular interactions of biopolymers resulting in structural destabilization. For example, urea is used to understand the unfolding of proteins (Bennion & Daggett, 2003; Vanzi, Madan & Sharp, 1998; Zou, Habermann-Rottinghaus & Murphy, 1998). Generally, urea pairs with peptide groups and disrupts internal hydrogen bonds and van der Waals forces leading to protein unfolding. Urea also disrupts nucleic acids stability – DNA and RNA – (Hackl & Blagoi, 2004; Shcherbakova & Brenowitz, 2005) by influencing the hydrogen bonding arrangement in the base pairs. In the case of polysaccharides higher amounts of urea thwart gelation in amylose (Welsh, Bailey, Chandarana & Norris, 1982), xanthan (Dintzis, Babcock & Tobin, 1970; Frangou, Morris, Rees, Richardson & Rossmurphy, 1982), iota-carrageenan (Morris & Belton, 1982), kappa-carrageenan (Nishinari, Watase, Williams & Phillips, 1990) and agarose (Watase & Nishinari, 1986). However, urea in association with sodium hydroxide is reported to be beneficial for studying the functional behaviors of water insoluble cellulose (Cai & Zhang, 2006) and chitosan (Tsaih & Chen, 1997) as well as those of water soluble glucan (Zhang, Zhang & Cheng, 2000) and aeromoans gum (Xu, Zhang & Zhang, 2005) aggregates.
Our current study of urea’s effect on iota-carrageenan has revealed interesting results. Though, iota-carrageenan maintains viscoelastic nature, at and below room temperature, urea seems to alter the properties by interfering with the gel network. Observation of increased G' values in heat-treated iota-carrageenan:urea solutions coupled with well-resolved fiber diffraction patterns and elevated onset temperatures during DSC measurements unequivocally suggest the promotion of favorable interactions among the iota-carrageenan helices and urea molecules. Thus, it appears that urea can participate in the junction-zones of iota-carrageenan gels, despite its innate nature of disrupting network arrangements.
A close examination of the chemical structure, molecular and packing details of iota-carrageenan suggests that there are two free hydroxyl groups O-2H and O-6H per disaccharide repeat, and urea can weaken the network in two possible ways: (1) breaking the hydrogen bonds involving the hydroxyls, and (2) disrupting the water coordination around carrageenan chains. Several physicochemical studies reveal the existence of iota-carrageenan doublets, i.e. two chains together even at dilute concentrations (Hjerde, Smidsrod & Christensen, 1999; McIntire & Brant, 1999; Viebke, Borgstrom & Piculell, 1995). X-ray studies confirm that these two chains are intertwined and held strongly by O-6H⋯O-2 hydrogen bonds and ordered helical arrangements are present in the junction zones. Each hydrogen bond is further bridged to a water molecule linked to sulfate group oxygen atoms from adjoining anhydrogalactan residue signifying that iota-carrageenan molecular structure is quite robust (Janaswamy & Chandrasekaran, 2001, 2002, 2008). In the double helix, O-2H resides deep inside the core while O-6H is on the periphery and adjacent helices communicate via string-like interactions 2-S⋯W⋯W⋯W⋯4-S, 2-S⋯Na⋯W⋯2-S, 4-S⋯Na⋯W⋯Na⋯4-S, and 4-S⋯W⋯W⋯O-6H, where 4-S, 2-S and W denote 4-sulfate group, 2-sulfate group and water molecule, respectively. Hence, to destabilize iota-carrageenan assembly, first urea molecules need to break the inter-helical water bridges, isolate the helices singly and then gain access to exterior O-6H; O-2H might be still inaccessible as it is in the helix interior. These events can readily take place with higher amounts of urea but at lower concentrations it seems that only inter-helical interactions are broken and the ensuing disengaged carrageenan helices with weak interactions can only bestow reduced structural ordering and viscoelastic properties, as noted in IC45U05C and IC60U05C. However, upon heat-treatment, carrageenan helices re-organize themselves and regain the network by utilizing urea molecules as space fillers as in IC45U05H and IC60U05H. Overall, our results suggest that urea synergistically promotes structural ordering in iota-carrageenan and this phenomenon is likely to hold good with other hydrocolloids. Reports on increased folding of proteins (Weissman & Kim, 1991) and RNA (Pan & Sosnick, 1997) by urea support our findings.
We believe that our results will aid in the design and development of novel exoteric creams, ointments and solutions based on inexpensive and FDA approved hydrocolloids to address skin and eye related issues. Topical creams containing urea are generally employed as tissue softeners as well as to combat skin diseases such as psoriasis (Shemer, Nathansohn, Kaplan, Weiss, Newman & Trau, 2000) and xerosis (Ademola, Frazier, Kim, Theaux & Saudez, 2002).
Similarly, topical ophthalmic preparations containing urea and/or urea derivatives are effective for dryness, noninfectious keratitis and ocular scarring (Charlton, Schwab & Stuchell, 1996), to name a few. Hydrocolloids are also exploited as delivery vehicles in transdermal compositions. Thus, we strongly envisage that binary mixtures of urea and iota-carrageenan, as well as other carrageenans and water soluble polysaccharides, will be advantageous in developing effective topical agents. Furthermore, products containing fine suspensions or translucently dispersed immiscible components should be able to control gel behavior such that the resulting elastic characteristics are endurable to suspend particles, improve their stability and increase bioavailability. In this regard, the soft viscoelastic behavior of iota-carrageenan:urea binary mixtures and corresponding gel constants at room temperature (25 °C) – the preferred temperature to store pharmaceutical products – especially for the heat-treated IC45U05H and IC60U05H appear to be suitable in developing stable formulations. Overall, our results suggest the applicability of highly utilized food hydrocolloids towards developing novel topical creams and gels as well as non-food products for addressing skin and eye related ailments. Further research is necessary to take full advantage of our findings, however.
Highlights.
Urea substantially modifies solution and thermal properties of iota-carrageenan.
Lower urea concentrations induces stability in iota-carrageenan gels.
Highly ordered iota-carrageenan networks are seen in the presence of urea.
Acknowledgements
We thank Whistler Center for Carbohydrate Research for support; Dr. Irina Koshelev and Dr. Robert Henning at BioCARS, Argonne National Laboratory, Chicago, IL for help during synchrotron X-ray intensity data collection. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract No. DE-AC02-06CH11357. Use of the BioCARS Sector 14 was supported by the National Institutes of Health (NIH), National Center for Research Resources, under grant number RR007707.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ademola J, Frazier C, Kim SJ, Theaux C, Saudez X. Clinical evaluation Of 40% urea And 12% ammonium lactate in the treatment of xerosis. American Journal Of Clinical Dermatology. 2002;3(3):217–222. doi: 10.2165/00128071-200203030-00007. [DOI] [PubMed] [Google Scholar]
- Arnott S, Scott WE, Rees DA, Mcnab CGA. Iota-carrageenan - molecular-structure and packing of polysaccharide double helices in oriented fibers of divalent-cation salts. Journal Of Molecular Biology. 1974;90(2):253–267. doi: 10.1016/0022-2836(74)90371-4. [DOI] [PubMed] [Google Scholar]
- Bennion BJ, Daggett V. The molecular basis for the chemical denaturation of proteins by urea. Proceedings Of The National Academy of Sciences of the United States of America. 2003;100(9):5142–5147. doi: 10.1073/pnas.0930122100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang L. Unique gelation behavior of cellulose in NaOH/Urea aqueous solution. Biomacromolecules. 2006;7(1):183–189. doi: 10.1021/bm0505585. [DOI] [PubMed] [Google Scholar]
- Campo VL, Kawano DF, Da Silva DB, Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis - A Review. Carbohydrate Polymers. 2009;77(2):167–180. [Google Scholar]
- Carlucci MJ, Ciancia M, Matulewicz MC, Cerezo AS, Damonte EB. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antiviral Research. 1999;43(2):93–102. doi: 10.1016/s0166-3542(99)00038-8. [DOI] [PubMed] [Google Scholar]
- Carlucci MJ, Scolaro LA, Damonte EB. Inhibitory action of natural carrageenans on herpes simplex virus infection of mouse astrocytes. Chemotherapy. 1999;45(6):429–436. doi: 10.1159/000007236. [DOI] [PubMed] [Google Scholar]
- Chan HK, Auyeung KL, Gonda I. Effects of additives on heat denaturation of rhdnase in solutions. Pharmaceutical Research. 1996;13(5):756–761. doi: 10.1023/a:1016007818575. [DOI] [PubMed] [Google Scholar]
- Charlton JF, Schwab IR, Stuchell R. Topical urea as a treatment for non-infectious keratopathy. Acta Ophthalmologica Scandinavica. 1996;74(4):391–394. doi: 10.1111/j.1600-0420.1996.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Chronakis IS, Borgstrom J, Piculell L. Conformation and association of kappa-carrageenan in the presence of locust bean gum in mixed NaI/CsI solutions from rheology and cryo-tem. International Journal Of Biological Macromolecules. 1999;25(4):317–328. doi: 10.1016/s0141-8130(99)00050-1. [DOI] [PubMed] [Google Scholar]
- Coggins C, Blanchard K, Alvarez F, Brache V, Weisberg E, Kilmarx PH, Lacarra M, Massai R, Mishell D, Salvatierra A, Witwatwongwana P, Elias C, Ellertson C. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sexually Transmitted Infections. 2000;76(6):480–483. doi: 10.1136/sti.76.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintzis FR, Babcock GE, Tobin R. Studies on dilute solutions and dispersions of polysaccharide from xanthomonas-campestris NRRL B-1459. Carbohydrate Research. 1970;13(2) 257-&. [Google Scholar]
- Dunstan DE, Chen Y, Liao ML, Salvatore R, Boger DV, Prica M. Structure and rheology of the Κ-carrageenan/locust bean gum gels. Food Hydrocolloids. 2001;15(4–6):475–484. [Google Scholar]
- Farias WRL, Valente AP, Pereira MS, Mourao PAS. Structure and anticoagulant activity of sulfated galactans - isolation of a unique sulfated galactan from the red algae botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. Journal Of Biological Chemistry. 2000;275(38):29299–29307. doi: 10.1074/jbc.M002422200. [DOI] [PubMed] [Google Scholar]
- Frangou SA, Morris ER, Rees DA, Richardson RK, Rossmurphy SB. Molecular-origin of xanthan solution rheology - Effect of urea on chain conformation And interactions. Journal Of Polymer Science Part C-Polymer Letters. 1982;20(10):531–538. [Google Scholar]
- Hackl EV, Blagoi YP. Urea effect on Cu2+-induced DNA structural transitions in solution. Journal Of Inorganic Biochemistry. 2004;98(11):1911–1920. doi: 10.1016/j.jinorgbio.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Hjerde T, Smidsrod O, Christensen BE. Analysis of the conformational properties of kappa- and iota-carrageenan by size-exclusion chromatography combined with low-angle laser light scattering. Biopolymers. 1999;49(1):71–80. [Google Scholar]
- Janaswamy S, Chandrasekaran R. Three-dimensional structure of the sodium salt of iota-carrageenan. Carbohydrate Research. 2001;335(3):181–194. doi: 10.1016/s0008-6215(01)00219-1. [DOI] [PubMed] [Google Scholar]
- Janaswamy S, Chandrasekaran R. Effect of calcium ions on the organization of iota-carrageenan helices: An X-ray investigation. Carbohydrate Research. 2002;337(6):523–535. doi: 10.1016/s0008-6215(02)00017-4. [DOI] [PubMed] [Google Scholar]
- Janaswamy S, Chandrasekaran R. Cation-induced polymorphism in iota-carrageenan. Carbohydrate Polymers. 2005;60(4):499–505. [Google Scholar]
- Janaswamy S, Chandrasekaran R. Sodium iota-carrageenan: A paradigm of polymorphism and pseudopolymorphism. Macromolecules. 2006;39(9):3345–3349. [Google Scholar]
- Janaswamy S, Chandrasekaran R. Heterogeneity in iota-carrageenan molecular structure: insights for polymorph II -> III transition in the presence of calcium ions. Carbohydrate Research. 2008;343(2):364–373. doi: 10.1016/j.carres.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Mcintire TM, Brant DA. Imaging of carrageenan macrocycles and amylose using noncontact atomic force microscopy. International Journal Of Biological Macromolecules. 1999;26(4):303–310. doi: 10.1016/s0141-8130(99)00097-5. [DOI] [PubMed] [Google Scholar]
- Michel AS, Mestdagh MM, Axelos MAV. Physico-chemical properties of carrageenan gels in presence of various cations. International Journal Of Biological Macromolecules. 1997;21(1–2):195–200. doi: 10.1016/s0141-8130(97)00061-5. [DOI] [PubMed] [Google Scholar]
- Morris VJ, Belton PS. The influence of the cations sodium, potassium and calcium on the gelation of iota-carrageenan. Progress In Food And Nutrition Science. 1982;6(1–6):55–66. [Google Scholar]
- Nishinari K, Watase M, Williams PA, Phillips GO. Kappa-carrageenan gels - Effect of sucrose, glucose, urea, and guanidine-hydrochloride on the rheological and thermal-properties. Journal Of Agricultural And Food Chemistry. 1990;38(5):1188–1193. [Google Scholar]
- Nono M, Durand D, Nicolai T. Rheology and structure of mixtures of iota-carrageenan and sodium caseinate. Food Hydrocolloids. 2012;27(1):235–241. [Google Scholar]
- Pan T, Sosnick TR. Intermediates and kinetic traps in the folding of a large ribozyme revealed by circular dichroism and UV absorbance spectroscopies and catalytic activity. Nature Structural Biology. 1997;4(11):931–938. doi: 10.1038/nsb1197-931. [DOI] [PubMed] [Google Scholar]
- Panlasigui LN, Baello OQ, Dimatangal JM, Dumelod BD. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pacific Journal Of Clinical Nutrition. 2003;12(2):209–214. [PubMed] [Google Scholar]
- Piculell L. Gelling carrageenans. In: Stephen AM, editor. Food Science And Technology. New York, New York 10016: Marcel Dekker, Inc., 270 Madison Avenue; 1995. pp. 205–244. Usa Basel, Switzerland. [Google Scholar]
- Rajkumar G, Al-Khayat HA, Eakins F, Knupp C, Squire JM. The CCP13 Fibrefix program suite: Semi-automated analysis of diffraction patterns from non-crystalline materials. Journal Of Applied Crystallography. 2007;40:178–184. doi: 10.1107/S0021889806048643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by Nonoxynol-9 and inhibited by carrageenan. Nature Medicine. 2007;13(7):857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Running CA, Falshaw R, Janaswamy S. Trivalent iron induced gelation in lambda-carrageenan. Carbohydrate Polymers. 2012;87:2735–2739. doi: 10.1016/j.carbpol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbakova I, Brenowitz M. Perturbation of the hierarchical folding of a large RNA by the destabilization of its scaffold's tertiary structure. Journal Of Molecular Biology. 2005;354(2):483–496. doi: 10.1016/j.jmb.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Shemer A, Nathansohn N, Kaplan B, Weiss G, Newman N, Trau H. Treatment of scalp seborrheic dermatitis and psoriasis with an ointment of 40% urea and 1% bifonazole. International Journal Of Dermatology. 2000;39(7):532–534. doi: 10.1046/j.1365-4362.2000.00986-3.x. [DOI] [PubMed] [Google Scholar]
- Therkelsen GH. Carrageenan. In: WRL, BJN, editors. Industrial Gums. Polysaccharides And Their Derivatives. New York: Academic Press; 1993. pp. 145–180. [Google Scholar]
- Tsaih ML, Chen RH. Effect of molecular weight and urea on the conformation of chitosan molecules in dilute solutions. International Journal of Biological Macromolecules. 1997;20(3):233–240. doi: 10.1016/s0141-8130(97)01165-3. [DOI] [PubMed] [Google Scholar]
- Vanzi F, Madan B, Sharp K. Effect of the protein denaturants urea and guanidinium on water structure: A structural and thermodynamic study. Journal of The American Chemical Society. 1998;120(41):10748–10753. [Google Scholar]
- Viebke C, Borgstrom J, Piculell L. Characterization of kappa-carrageenan and iota-carrageenan coils and helices by MALLS/GPC. Carbohydrate Polymers. 1995;27(2):145–154. [Google Scholar]
- Watase M, Nishinari K. Rheological and thermal properties of agarose and kappa-carrageenan gels containing urea, guanidine hydrochloride or formamide. Food Hydrocolloids. 1986;1(1):25–36. [Google Scholar]
- Watase M, Nishinari K. Rheological and thermal-properties of carrageenan gels - Effect of sulfate content. Makromolekulare Chemie-Macromolecular Chemistry And Physics. 1987;188(9):2213–2221. [Google Scholar]
- Weissman JS, Kim PS. Reexamination of the folding of BPTI predominance of native intermediates. Science (Washington D C) 1991;253(5026):1386–1393. doi: 10.1126/science.1716783. [DOI] [PubMed] [Google Scholar]
- Welsh EJ, Bailey J, Chandarana R, Norris WE. Physical characterization of interchain association in starch systems. Progress in Food And Nutrition Science. 1982;6(1–6):45–53. [Google Scholar]
- Williams PA, Clegg SM, Langdon MJ, Nishinari K, Piculell L. Investigation of the gelation mechanism in kappa-carrageenan konjac mannan mixtures using differential scanning calorimetry and electron-spin-resonance spectroscopy. Macromolecules. 1993;26(20):5441–5446. [Google Scholar]
- Xu XJ, Zhang LN, Zhang YY. Urea/NAOH aqueous solution as new solvent of aeromonas gum. Journal Of Applied Polymer Science. 2005;97(4):1710–1713. [Google Scholar]
- Zhang PY, Zhang LN, Cheng SY. Effects of urea and sodium hydroxide on the molecular weight and conformation of alpha-(1 -> 3)-D-glucan from lentinus edodes in aqueous solution. Carbohydrate Research. 2000;327(4):431–438. doi: 10.1016/s0008-6215(00)00077-x. [DOI] [PubMed] [Google Scholar]
- Zhou GF, Sun YP, Xin H, Zhang YN, Li Z, Xu ZH. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from chondrus ocellatus. Pharmacological Research. 2004;50(1):47–53. doi: 10.1016/j.phrs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Zou Q, Habermann-Rottinghaus SM, Murphy KP. Urea effects on protein stability: Hydrogen bonding and the hydrophobic effect. Proteins-Structure Function And Genetics. 1998;31(2):107–115. [PubMed] [Google Scholar]