Abstract
Background
We reviewed the care of a large cohort of patients with diabetes mellitus on insulin pump therapy who required an inpatient stay.
Methods
Records were reviewed of patients hospitalized between January 1, 2006, and December 31, 2011.
Results
A total of 136 patients using insulin pumps had 253 hospitalizations. Mean (standard deviation) patient age was 55 (16) years, diabetes duration was 29 (15) years, and pump duration was 6 (5) years. Insulin pump therapy was continued in 164 (65%) hospitalizations. Adherence to core process measures improved over time: by 2011, 100% of cases had an endocrinology consultation, 100% had the required insulin pump order set completed, and 94% had documentation of the signed agreement specifying patient responsibilities for continued use of the technology while hospitalized. Documentation of the insulin pump flow sheet also increased but could still be located in only 64% of cases by the end of 2011. Mean glucose was not significantly different among patients who remained on insulin pump therapy compared to those for whom it was discontinued (p > .1), but episodes of severe hyperglycemia (>300 mg/dl) and hypoglycemia (<40 mg/dl) were significantly less common among pump users. No pump site infections, mechanical pump failures, or episodes of diabetic ketoacidosis were observed among patients remaining on therapy.
Conclusions
With appropriate patient selection and usage guidelines, most patients using insulin pumps can safely have their therapy transitioned to the inpatient setting. Further study is needed to determine whether this approach can be translated to other hospital settings.
Keywords: diabetes mellitus, hospitalization, inpatients, insulin infusion systems
Introduction
Continuous subcutaneous insulin infusion (CSII) therapy (also called insulin pump therapy) has become a familiar sight to practitioners who treat patients with diabetes mellitus. More than 400,000 patients in the United States are now estimated to use CSII to achieve optimal glucose control.1Intended for use in the ambulatory setting, insulin pumps are now being encountered as patients are treated in various clinical situations where the technology was not intended to be used, and physicians are increasingly being confronted with how to manage CSII therapy in these settings. One example of such a scenario is when patients receiving outpatient CSII therapy are admitted to the hospital.
In 2005, our institution developed, implemented, and published guidelines to assist practitioners in the management of insulin pump technology within the hospital setting.2 These guidelines were originally developed to address an institutional gap in care and to respond to the then-quite-limited availability of peer-reviewed published guidelines on how best to manage the person on CSII as an inpatient.3The objectives of these inpatient guidelines were to allow for a successful transition of outpatient insulin pump therapy to the inpatient setting and to promote the patient’s independence in the use of an insulin pump during hospitalization while maximizing safety.2
Information in the medical literature on CSII use in the hospital has since expanded. Guidelines have been proposed for its use among pediatric inpatients.4 Recent data suggest that CSII may be superior to other methods of delivering insulin to hospital inpatients in that it is better able to achieve desired glucose targets.5,6 Developing separate inpatient units with specially trained staff dedicated to the management of insulin pump-treated patients has been proposed4,7 and might be reasonable for institutions that care for a large volume of such persons. With the development and application of specific procedures to guide their use, CSII technology in the hospital is safe.5,6,8–11
Since our original 2005 publication2 on inpatient application of insulin pump therapy, we have had increased experience with the use of this technology in the hospital.8–10 This review first summarizes the approach used to transition insulin pump-treated patients from the outpatient to the inpatient setting, and then it provides a synopsis of care provided to 253 cases seen at our institution from 2006 to 2011—the largest and longest experience reported to date on inpatient CSII use.
Summary of Inpatient Continuous Subcutaneous Insulin Infusion Procedures
Details of our inpatient insulin pump guidelines have been published previously.2,9 Guidelines and procedures were formalized as a written policy comprising three general components: (1) contraindications for continued use of an insulin pump within the hospital; (2) procedures to guide the medical staff in managing an insulin pump after admission; and (3) a signed patient agreement that details the conditions for CSII use in the hospital. Contraindications to the continued use of an insulin pump include an altered state of consciousness in the patient; presence of diabetic ketoacidosis/hyperosmolar nonketotic diabetes at admission; critical illness (i.e., admission to the intensive care unit); suicide risk; inability to participate in pump management and without a family member who can assist; or any another reason deemed appropriate by the physician who is primarily responsible for the individual’s care in the hospital.
Operationally, the inpatient insulin pump core procedures are an endocrinology consultation, a signed patient agreement, completion of an order set, and use of a bedside flow sheet. The patient agreement instructs patients to provide their own insulin pump supplies because these are not stocked in the hospital. The bedside flow sheet is used by the patient to record basal rates, prandial and correction insulin amounts, carbohydrate intake, and bedside glucose values. The bedside flow sheet is the primary means of information exchange between the patient and the hospital medical staff about what the patient is doing regarding self-management. The patient does not input new parameters into the device without a physician order. The order set systematically guides the practitioner through ordering basal rates, mealtime amounts, and correction factors.2,9 Since 2005, the order set has evolved from a written to an electronic format. The electronic order itself has experienced two separate transitions. The first was in 2007, during the hospital’s conversion from a paper-based to a computerized order-entering system, and the second was in 2010, during the conversion to a new electronic medical record vendor.
Implementation of Inpatient Continuous Subcutaneous Insulin Infusion Guidelines
Implementation of the new policy and procedure required thorough education for all staff involved in the care of patients with insulin pumps. Because our institution does not have a designated unit for insulin pump patients, all nurses must also be updated with any changes to the insulin pump policy, order set, and procedures. Initially, a mandatory inservice on use of insulin pumps in the hospitalized patient was provided to all nurses. New nursing staff must attend a diabetes management class as part of their orientation, which includes presentation of the insulin pump policy guidelines, order set, and nurse and patient responsibilities related to continuation of pump therapy in the hospital. Updates regarding changes in policy, order sets, and documentation are communicated via a weekly newsletter sent to nurses electronically. In addition, our institution utilizes quarterly “Team Days” for each nursing unit, in which key educational topics are presented. Review of the care of a patient with an insulin pump has been presented at the team days as needed. The inpatient endocrinology team serves as the point of contact for questions that inpatient staff physicians, nurse practitioners, physician assistants, or any other staff may have regarding the insulin pump policy and procedures.
Summary of Institutional Experience (2006–2011)
Description of Facility
Our institution is an academic medical center located in metropolitan Phoenix, AZ, that provides care to adult general medical and surgical patients. Bed capacity increased from 200 to 244 over the period of analysis. Pediatric and obstetrical care are not provided. Therefore, the inpatient CSII guidelines were developed with only the nonpregnant adult patient in mind.
Chart Review
This analysis was approved by our institutional review board. A patient registry was developed to track insulin pump patients as they were admitted to the hospital and referred to the inpatient endocrinology consultation service. Electronic medical records of any patients who were admitted between January 1, 2006, and December 31, 2011, were reviewed. The records of each case were also reviewed to determine if any hospitalizations occurred that were unknown to the inpatient endocrinology consultation service. Data were retrieved on age, sex, race or ethnicity, length of hospital stay, type of diabetes, duration of diabetes, and self-reported length of time on CSII therapy. Compliance with process measures was evaluated by reviewing the electronic medical record and determining whether the following documentation had occurred: endocrinology consultation in all patients, a signed patient agreement, completed insulin pump orders, and completion of a bedside flow sheet for those utilizing CSII in the hospital.8–10
Glycemic Control Data
Point-of-care blood glucose monitoring was conducted as previously described with an instrument (ACCU-CHEK® Inform, Roche Diagnostics, Indianapolis, IN) that scans and stores patient identification from a bar code. These values were then retrieved by linking patient identifiers to the electronic laboratory information system. Commercial software (Medical Automation Systems, Inc., Charlottesville, VA) facilitated the glucometer interface with the electronic laboratory file.8–10 Hemoglobin A1c (HbA1c) values were obtained from chart review.
Definition of Inpatient Insulin Pump Categories
Insulin pump cases were placed into one of three categories by how they were managed during the hospital stay:10 (1) “pump on,” (2) “pump off,” and (3) “intermittent pump.” The “pump on” group comprised hospitalized patients who were deemed at admission to be candidates for CSII per our policy and who remained “on” until discharge. “Pump on” patients continued to meet criteria for remaining on the pump throughout the hospital stay and were discharged with instructions regarding any changes in basal rates that may have occurred. Individuals who did not meet the conditions for inpatient CSII use at any time during hospitalization were defined as “pump off” and had CSII discontinued at admission and remained “off” even at the time of discharge. “Pump off” cases were discharged on alternative insulin regimens with instructions to follow-up with their diabetes care provider as to whether CSII should be reinitiated. “Intermittent pump” cases were either those cases in which CSII therapy was continued at admission but then stopped during hospitalization because of changing clinical circumstances that warranted discontinuation, or those patients whose CSII was stopped at admission but then restarted when their clinical status improved and they satisfied institutional criteria for use of the devices.
Data Analysis
Multiple admissions occurred for some patients. However, each hospitalization was considered to be an independent opportunity for implementation of the inpatient insulin pump policy.8–10 The unit of analysis was therefore the hospital stay rather than the unique patient.
We determined compliance with our CSII process measures for overall hospitalizations across the 6-year review period. There is currently no consensus on how to best analyze and report inpatient glucose data.12 To be consistent with our previous methods of reporting glycemic control data in this population, we averaged the bedside glucose measurements for the entire length of stay for each patient and calculated a composite bedside glucose average (BedGluc avg).8–10 The percentage of hypoglycemic and hyperglycemic values in the bedside glucose measurement data was calculated by dividing the number of events per patient by the number of bedside measurements per patient and then multiplying by 100. Hypoglycemic bedside glucose values were stratified into <40, <50, <60, or <70 mg/dl, whereas hyperglycemic bedside glucose values were stratified into >180, >200, >250, >300, >350, or >400 mg/dl.8–10
We also examined records for any evidence of adverse events possibly specific to CSII. These included any evidence of mechanical failure, infusion site problems (e.g., infection, kinking of infusion catheter), and diabetic ketoacidosis while on insulin pump therapy. The frequency of hypoglycemic and hyperglycemic measurements was compared by inpatient insulin pump status. Data were reported as mean [standard deviation (SD)], where applicable. Statistical differences were tested using non-parametric methods.
Results
Patient Characteristics
We identified 255 hospital admissions between January 1, 2006, and December 31, 2011. Two admissions were for pancreas transplant to treat type 1 diabetes and were excluded from additional analysis because the insulin pump was discontinued perioperatively. The remaining 253 hospital cases represented 136 unique patients (Table 1). The number of hospitalizations involving CSII patients increased over time (24 hospitalizations in 2006, 19 in 2007, 40 in 2008, 48 in 2009, 59 in 2010, and 63 in 2011). Only 89 patients had a single admission; of those with multiple admissions during the study period, 1 patient had a total of 14 hospitalizations during the review period.
Table 1.
Characteristics of 136 Patients on Insulin Pump Therapy with 253 Hospitalizations
| Characteristic | Valuea |
|---|---|
| Age, years | 55 (16) |
| Body mass index, kg/m2 | 28.9 (12.9) |
| Duration of diabetes mellitus, years | 29 (15) |
| Duration of insulin pump therapy, years | 6 (5) |
| Type 1 diabetes mellitus, % | 82 |
| Female sex, % | 60 |
| White race, % | 98 |
| HbA1c, % | 7.5 (1.3) |
| Length of hospital stay, days | 4.1 (4.9) |
| Bedside glucose measurements per person per day | 6.2 (3.3) |
| BedGlucavg, mg/dl | 178 (47) |
Values are mean (SD) unless indicated otherwise.
The average duration of diabetes was nearly 30 years (Table 1). Most patients were female, white, and had type 1 diabetes (Table 1). Outpatient glycemic control, based on the most recent HbA1c value, was fair (Table 1). Hemoglobin A1c measurements were obtained on average 11 (20) days prior to the hospitalization (range: 84 days prior, to 5 days into the hospitalization). The average length of stay for the 253 hospitalizations was 4.1 (4.9) days. Some lengths of stay were extremely short: ≤1 day in 64 (25%) cases. Typically, more than four point-of-care blood glucose measurements per patient per day were obtained. Bedside glucose average was 178 (47) mg/dl (Table 1). Additionally, 110 (81%) of the 136 patients used a CSII device manufactured by a single vendor (not listed).
Patients were admitted for a variety of reasons, including elective procedures (e.g., knee replacement, thyroidectomy, laminectomy) or more acute reasons (e.g., nausea and vomiting, appendectomy, suicide attempt, altered mental status). Nine hospitalizations were for cases where the patients presented with diabetic ketoacidosis.
Inpatient Pump Status
Most cases were able to remain on CSII for the duration of the hospital stay. Of the 253 hospitalizations, 164 (65%) cases were “pump on,” 50 (20%) were “intermittent pump,” and 38 (15%) were “pump off” cases. Insulin pumps were discontinued at admission for various reasons, including patient and physician preference, poor patient understanding of the pump, and 1 suicide attempt. If the pump was discontinued, patients received alternate therapy (e.g., basal and short-acting insulin). In 1 case, the status of the pump during the hospital stay could not be determined.
Adherence to Process Measures
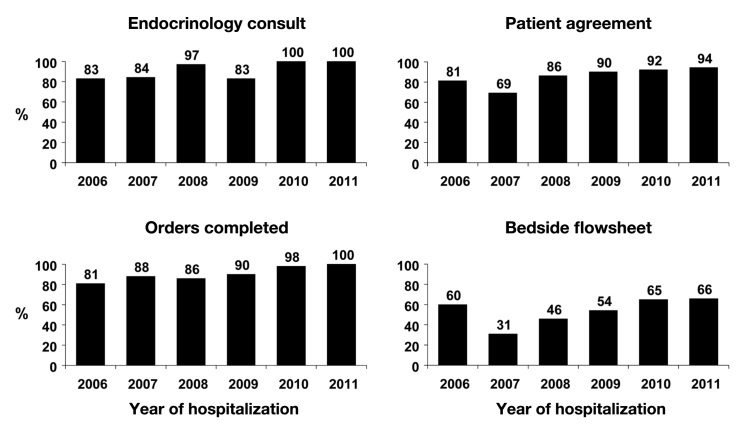
During 2010 and 2011, 100% of all cases received an inpatient endocrinology consultation (Figure 1). For evaluation of the other core measures, we combined the “pump on” and “intermittent pump” hospital cases, because all of these individuals should have had the same required procedures and documentation completed.10 In general, both adherence to completion of the patient agreement and use of the order set measures were high (Figure 1). The percentage of patient agreements that were signed improved over time, as did completion of the orders, such that in 2011, all cases that used CSII in the hospital had the required order set. Although documentation of the bedside flow sheet in the electronic medical record improved over time, it still could not be located in a substantial number of cases.
Figure 1.
Compliance with inpatient insulin pump core measures.
Glycemic Control
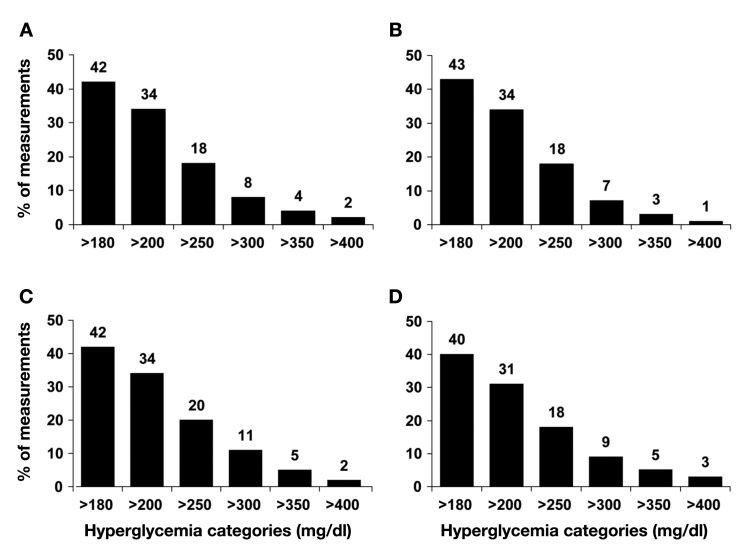
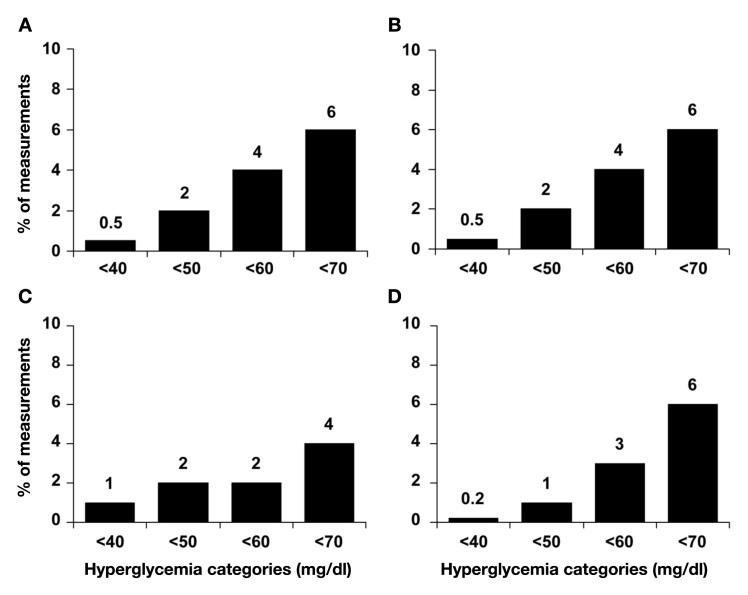
BedGlucavg values were comparable among “pump on,” “pump off,” and “intermittent pump” users (p > .1 among groups). The prevalence of hyperglycemia was common overall and among all three classes of insulin pump hospitalizations, with more than 40% of values being >180 mg/dl (Figure 2). However, the proportion of measurements that were >300 mg/dl, >350 mg/dl, and >400 mg/dl was significantly lower in “pump on” vs “pump off” cases (all p < .02; Figure 2). For values >350 mg/dl and >400 mg/dl, “pump on” cases had fewer extremely high glucose values compared to “intermittent pump” cases (both p < .01; Figure 2). The proportion of measurements that would be considered hypoglycemic, particularly severely hypoglycemic (<50 mg/dl or <40 mg/dl) was uncommon (Figure 3). “Pump on” patients had significantly fewer values that were <40 mg/dl compared to “pump off” patients (p = .03; Figure 3), with no difference compared to “intermittent pump cases.”
Figure 2.
Percentage of bedside glucose measurements per person with hyperglycemia for (A) all insulin pump hospitalizations (combined) and by category of hospitalization: (B) “pump on,” (C) “pump off,” and (D) “intermittent pump”. “Pump on” patients had significantly fewer extremely high values (see text).
Figure 3.
Percentage of bedside glucose measurements per person with hypoglycemia (A) all insulin pump hospitalizations (combined) and by category of hospitalization: (B) “pump on,” (C) “pump off,” and (D) “intermittent pump”. “Pump on” cases had significantly fewer values <40 mg/dl than “pump off” cases (see text).
Adverse Events
Adverse events have been reported previously,10 and in that study consisted of only one instance of an infusion catheter kinking, which resulted in correctable nonfatal hyperglycemia. Since the last analysis,10 we have encountered no additional complications due to insulin pump use by inpatients (e.g., insertion site infections, mechanical pump failure, or diabetic ketoacidosis), nor has there been any inpatient mortality among CSII users.
Discussion
This report presents an analysis of the largest number of cases involving insulin pump users who required hospitalization and the status of their technology after admission. There are no national data on hospitalization rates among CSII users, but the number of admissions— at least at our facility—has been increasing, and this trend is likely to continue as the use of these devices becomes more widespread. During the 6-year period of data accrual, nearly 2/3 of insulin pump users were able to transition their outpatient therapy to the inpatient setting, with only one adverse event.
In general, institutional compliance was high for three of the core measures—placement of an endocrinology consultation, use of the order set, and signing of the patient agreement. Compliance with the endocrinology consultation and use of the order set has now reached 100%. In the case of the patient agreement, this is a paper document that has to be scanned into the electronic medical record. Hence, not locating the agreement in some cases may only reflect that it was not scanned into the record rather than noncompliance with this policy requirement.
Underperformance with the bedside flow sheet was a consistent shortfall, but completion did improve over time. However, like the patient agreement, the flow sheet is also a paper document that must be scanned into the electronic medical record. We could not determine whether the absence of flow sheets meant that they had not been completed or simply that the documents had not been scanned. The flow sheet is the primary means by which the patient informs hospital staff about activities occurring with the pump (e.g., basal insulin rates, bolus amounts, and glucose data) that should be reported in the medical record. Ongoing staff education may be necessary to ensure documented completion of the form.
The efficacy of CSII vs multiple daily insulin injections to control hyperglycemia in the hospital has not been established. The BedGluc avg in the “pump on” cases was no different than that in the “pump off” patients who were managed with multiple daily injections, which suggests that allowing patients to continue insulin pump therapy in the hospital does not result in inferior glycemic control versus subcutaneous insulin regimens. This similarity in overall glucose control may simply be a consequence of the endocrinology involvement in most cases. However, the data in this analysis do indicate a possible advantage of CSII with regard to reducing the number of extreme hyperglycemic values and maybe to limiting the number of severe hypoglycemic episodes.
An ongoing challenge continues to be how best to provide continuing education to inpatient staff about insulin pump procedures. In our institution, a reeducation process of staff had to occur upon the conversion of paper ordering to computerized order entry, and then again during the transition to the new electronic medical record. Some newer initiatives that are being piloted to enhance staff comfort with the inpatient CSII process are computer-based simulations that guide a practitioner through the ordering process, and a simulation laboratory (targeting resident physicians for now) that is designed to familiarize staff with the technology.
One reason for the successful implementation of insulin pump use in our facility may be the involvement of the endocrinology team. It is not clear how the approach reviewed here of transitioning outpatient CSII to the hospital would function in the absence of a specialty endocrinology team. Assessment is needed on how well these proposed process steps might work in a setting without access to such specialists.
The generally high (and improving) compliance with inpatient insulin pump core process measures indicates that a policy on inpatient CSII use can be implemented successfully. Most patients undergoing outpatient treatment can have their therapy transitioned safely to the hospital if desired. Hospital glycemic control among patients who remained on CSII was no worse than that achieved in patients who had to discontinue treatment. Greater experience in the process of transitioning insulin pump therapy to the inpatient setting is needed in other types of hospital settings (e.g., nonacademic). A broader discussion of the topic of CSII use in the hospital should take place so that consensus guidelines can be developed.
Glossary
Abbreviations
- (BedGlucavg)
bedside glucose average
- (CSII)
continuous subcutaneous insulin infusion
- (HbA1c)
hemoglobin A1c
- (SD)
standard deviation
References
- 1.JDRF and BD Collaborate to Improve Insulin Pump Delivery Juvenile Diabetes Research Foundation. http://www.jdrf.org/index.cfm?fuseaction=home.viewPage&page_id=47980498-1321-C834-030811F5029F3DA2. Accessed December 23, 2011.
- 2.Cook CB, Boyle ME, Cisar NS, Miller-Cage V, Bourgeois P, Roust LR, Smith SA, Zimmerman RS. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital setting: proposed guidelines and outcome measures. Diabetes Educ. 2005;31(6):849–857. doi: 10.1177/0145721705281563. [DOI] [PubMed] [Google Scholar]
- 3.Lee SW, Im R, Magbual R. Current perspectives on the use of continuous subcutaneous insulin infusion in the acute care setting and overview of therapy. Crit Care Nurs Q. 2004;27(2):172–184. doi: 10.1097/00002727-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Einis SB, Mednis GN, Rogers JE, Walton DA. Cultivating quality: a program to train inpatient pediatric nurses in insulin pump use. Am J Nurs. 2011;111(7):51–55. doi: 10.1097/01.NAJ.0000399316.21279.0e. [DOI] [PubMed] [Google Scholar]
- 5.Bodur HA, Saygili E, Saygili S, Doganay LH, Yesil S. Continuous infusion of subcutaneous compared to intravenous insulin for tight glycaemic control in medical intensive care unit patients. Anaesth Intensive Care. 2008;36(4):520–527. doi: 10.1177/0310057X0803600421. [DOI] [PubMed] [Google Scholar]
- 6.Lee IT, Liau YJ, Lee WJ, Huang CN, Sheu WH. Continuous subcutaneous insulin infusion providing better glycemic control and quality of life in Type 2 diabetic subjects hospitalized for marked hyperglycemia. J Eval Clin Pract. 2010;16(1):202–205. doi: 10.1111/j.1365-2753.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 7.Morviducci L, Di Flaviani A, Lauria A, Pitocco D, Pozzilli P, Suraci C, Frontoni S, CSII Study Group Of Lazio Region, Italy Continuous subcutaneous insulin infusion (CSII) in inpatient setting: unmet needs and the proposal of a CSII unit. Diabetes Technol Ther. 2011;13(10):1071–1074. doi: 10.1089/dia.2011.0056. doi: 10.1089/dia.2011.0056 Epub 2011 Jun 29. [DOI] [PubMed] [Google Scholar]
- 8.Bailon RM, Partlow BJ, Miller-Cage V, Boyle ME, Castro JC, Bourgeois PB, Cook CB. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract. 2009;15(1):24–29. doi: 10.4158/EP.15.1.24. [DOI] [PubMed] [Google Scholar]
- 9.Leonhardi BJ, Boyle ME, Beer KA, Seifert KM, Bailey M, Miller-Cage V, Castro JC, Bourgeois PB, Cook CB. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol. 2008;2(6):948–962. doi: 10.1177/193229680800200605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nassar AA, Partlow BJ, Boyle ME, Castro JC, Bourgeois PB, Cook CB. Outpatient-to-inpatient transition of insulin pump therapy: successes and continuing challenges. J Diabetes Sci Technol. 2010;4(4):863–872. doi: 10.1177/193229681000400415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noschese ML, DiNardo MM, Donihi AC, Gibson JM, Koerbel GL, Saul M, Stefanovic-Racic M, Korytkowski MT. Patient outcomes after implementation of a protocol for inpatient insulin pump therapy. Endocr Pract. 2009;15(5):415–424. doi: 10.4158/EP09063.ORR. [DOI] [PubMed] [Google Scholar]
- 12.Cook CB, Wellik KE, Kongable GL, Shu J. Assessing inpatient glycemic control: what are the next steps? J Diabetes Sci Technol. 2012;6(2):421–427. doi: 10.1177/193229681200600230. [DOI] [PMC free article] [PubMed] [Google Scholar]