Abstract
Background
Growing technological improvements in insulin pump design have increased the use of these devices in young children. To better understand the types of infusion pump-related problems and associated adverse events in this age group, we performed a comprehensive evaluation of pump-related adverse event reports received by the U.S. Food and Drug Administration (FDA) for children ages 1–12 years.
Methods
A query was conducted of FDA‘s Manufacturer and User Facility Device Experience database from January 1, 1996, through December 31, 2009, in children ages 1–12 years involving insulin pumps. Report narratives were individually reviewed for age, gender, and seriousness of outcomes. Device or patient problems and potential contributory factors were assessed.
Results
Over the past 14 years, 1774 (7%) of all insulin pump adverse event reports were identified in children ages 1–12. Of these reports, 777 (43%) resulted in hospitalization. In hospitalized cases (n = 614), diabetic ketoacidosis and/or hyperglycemia were the predominant patient problems, and in other cases (n = 98), hypoglycemia was evident. There were 106 emergency room visits, 19 cases requiring paramedic attention, and five deaths. The majority of reports indicated that the devices were not returned to the manufacturer, and root causes were not always confirmed.
Conclusions
Younger children with diabetes deserve careful consideration of the risk and benefit of insulin pump technology. Studies are needed to better understand pediatric safety issues and to identify the root cause of adverse events. Problems related to patient education, device misuse, and malfunctions were found, highlighting the need to strengthen user training for children and their caregivers.
Keywords: adverse event reports, children, device-related problems, diabetes, diabetic ketoacidosis, hospitalization, hyperglycemia, hypoglycemia, insulin pump, malfunction, manufacturer evaluation, pediatric, postmarket, use error
Introduction
The growing experience with insulin pumps in the adult population has increased the use of these devices in the pediatric population. As the technology advances and insulin pumps are promoted as achieving better glucose control, it is imperative to ensure safe use in children.
The Center of Devices and Radiological Health defines the pediatric population as birth through 21 years. Therefore, it is necessary to evaluate the various subpopulations to determine whether there are any differences in serious adverse events such as hospitalizations or emergency department visits, and to better understand device problems that might result in diabetic ketoacidosis (DKA), hypoglycemia, and seizures.
Insulin pumps are being used to treat younger children ages 1–12 years with increasing regularity. In a previous publication,1 adverse events and the use of pump technology in older adolescents were evaluated. This article will focus on adverse events and safety concerns in younger children 1–12 years of age and the use of insulin infusion pumps.
It is important to understand the regulatory background and context for which pump devices are used. The majority of insulin pumps that are used for treatment of diabetes are cleared by the U.S. Food and Drug Administration (FDA) through the 510(k), or premarket notification process. The 510(k) process is one type of regulatory pathway for medical devices to enter the market. It allows a manufacturer to show substantial equivalence to a previously approved and marketed device.
Insulin pumps may be set for continuous use, programmed to deliver a basal rate, or deliver a bolus. The delivery mechanism may include components such as a drug reservoir, pump tubing, user interface, programming unit, power supply, battery, software, display screens, alarms, driver arms, and tube connectors. These systems may have accuracy and variability issues, particularly at lower volumes, leading to inaccurate insulin dosing. Priming and occlusion problems may also lead to serious adverse events.
Insulin pumps that are designed for use with continuous glucose monitoring systems are approved through the premarket approval process and require a more rigorous assessment of safety and effectiveness. These devices will not be reviewed in this article, and further studies are needed to evaluate their use.2
Methods
A review was conducted of all medical device reports for insulin pumps for patients ages 1–12 years, using the FDA Manufacturer and User Facility Device Experience (MAUDE), a postmarket database, from January 1, 1996, through December 31, 2009. The FDA requires reporting of adverse events when the manufacturer or user facility has information that reasonably suggests that a device may have caused or contributed to death or serious injury of the patient while using the device.
All reports were identified by using codes specific for insulin pumps, and duplicate reports were merged. Reports were reviewed individually. Device-related problems were categorized, and educational issues and use-related events were assessed. Device problems were classified as: component failure; damaged, broken, separated, or missing component; damaged or defective pump; pump corrosion; device leakage; noninfusion of insulin; software failure; alarm and/or alerts; water ingress; and environmental interferences, such as moisture and climatic conditions. Patient-related problems were assessed by looking at outcomes by age subgroups, injury, extent of medical intervention, and severity of device-related events. Reports of hyperglycemia and reports of hypoglycemia based on the presence or absence of seizures, in addition to dead-in-bed reports that might relate to nocturnal hypoglycemia, were reviewed and further assessed to determine whether there were hospitalizations, emergency department visits, paramedic attention, or other severe events. Additionally, report narratives were analyzed to further identify the device problem and were categorized as device misuse, parental oversight issues, device maintenance education, knowledge, and familiarity with insulin pumps.
All manufacturer reports (supplemental and final reports) for recalls and returned devices were further evaluated for root causes of the device failure and/or patient-related problems. The narrative texts of reports with no age information were reviewed, and no additional pediatric reports were found for ages 1–12 years.
Results
A search of the MAUDE database for insulin pump adverse event reports with known age information from January 1, 1996, through December 31, 2009, revealed a total of 21,769 reports for all ages. Of these, 1774 (8%) reports were for children ages 1–12 years. The majority were manufacturer reports. Approximately 94% of reports were from the United States. Other countries included Canada and Germany, and a few reports were from Italy, Finland, the United Kingdom, and Switzerland. During the 14-year period, the annual number of insulin pump reports for young children increased (Figure 1). These included device problems such as component failures, damaged or missing component(s), alarm failures, failure to prime, battery failures, environmental interferences, damaged cannulas, leakage, and use errors.
Figure 1.
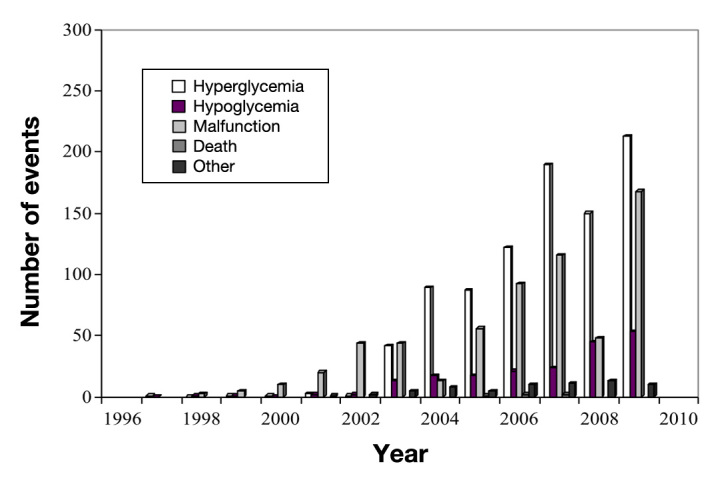
Food and Drug Administration adverse event reports for insulin pumps in children ages 1–12, January 1, 1996, through December 31, 2009.
Of the 1774 reported pediatric events, more than half resulted in serious patient outcomes (Table 1). These included hospitalization (n = 614) for hyperglycemia with or without DKA, or hypoglycemia with or without seizures. There were five patient deaths (5-year-old, 9-year-old, and 10-year-old patients with hyperglycemia and/or DKA, a 9-year-old with hypoglycemia, and a 7-year-old with no information). The causality of deaths could not be confirmed.
Table 1.
Summary of FDA Insulin Pump Adverse Event Reports for Ages 1–12 Years, January 1, 1996, through December 31, 2009
| Total: 1774 (846 males, 907 females, 21 unknown gender) | |
|---|---|
| Types of events | |
| Overall hospitalizations | 777 |
| Hospitalizations for hyperglycemia and/or DKA | 614 |
| Hospitalizations for hypoglycemia | 98 |
| Hospitalized with erratic blood glucose | 2 |
| Hospitalized for abscess infection at pump site | 1 |
| Hospitalized for unrelated bicycle accident | 1 |
| Other | 61 |
| Emergency Department visits, total | 106 |
| With hyperglycemia and/or DKA | 77 |
| With hypoglycemia | 25 |
| With erratic blood glucose | 3 |
| With insulin squirting out pump | 1 |
| Paramedic assistance required | 19 |
| For hypoglycemia | 18 |
| For hyperglycemia | 1 |
| Deaths | 5 |
| Other/nonserious | 867 |
An assessment of hospitalization reports for hypoglycemia, hyperglycemia, and/or DKA was also conducted. Narratives were reviewed to identify any comorbid conditions. There were 614 reported hospitalizations for hyperglycemia and/or DKA. Of these, 15 were hospitalizations for DKA in association with other illnesses—8 cases of “stomach virus,” 2 with other viral infections, 2 with otitis, 1 with gastroenteritis, 1 with Shigella, and 1 with possible appendicitis. There were 98 reported cases of patients hospitalized for hypo-glycemia, 10 of which were accompanied by seizures; 2 hospitalized with erratic blood glucose; 1 hospitalized for abscess infection at pump site; and 1 unrelated bicycle accident. In addition, there were 106 emergency department visits: 77 with hyperglycemia and/or DKA; 25 with hypoglycemia; 3 with erratic blood glucose; and 1 with insulin squirting out of the pump. There were 18 additional reports of hypoglycemia requiring paramedic attention.
When we evaluated all 1774 reports for possible device-related problems, approximately one-third of these reports indicated that the device problem was ‘unknown.’ Device-related issues ranged from component failures, alarm failures, battery failures, damaged cannulas, leakage, and use errors.
Additional analysis of hospitalization reports was conducted for possible device-related problems (Table 2). Common device problems that were identified included use error, battery issues, alarm problems, and bent cannulas. Most reports of hospitalization stated that the device problem was unknown. In most instances, when testing was conducted, the insulin pump operated according to specifications, i.e., the pump functioned normally.
Table 2.
Reports of Device-Associated Problems for Hospitalized Children and Manufacturer Evaluation (n = 284)
| Operated according to specification | 121 |
| User error | 36 |
| Bent cannula | 28 |
| Battery issues | 25 |
| Alarm–related problems | 19 |
| Priming issue | 9 |
| Delivery problems | 9 |
| Breakage/cracked/corroded/damaged | 6 |
| Infusion site issues | 6 |
| Software failure | 5 |
| Environmental issues | 4 |
| Reservoir issues | 3 |
| Component separation | 3 |
| Shut down | 2 |
| Kinked tubing | 2 |
| Leakage | 2 |
| Disconnections | 2 |
| Button issues | 2 |
Table 3 provides examples of the types of use error and device-related narrative reports for the young children who were hospitalized.
Table 3.
Examples of Device-Related Adverse Events in Hospitalized Children 1–12 Years of Age
| Age | Gender | Patient Problem | Reported Problem | Pump Evaluation |
|---|---|---|---|---|
| 35 months | f | Hyperglycemia | The ACT button did not respond properly | Pump operated OK but moisture on keypad |
| 7 | m | Hypoglycemia, seizures | Parent stated that she gave too much correction before going to bed | Pump not returned Use error |
| 8 | m | Hypoglycemia | Family uncomfortable with pump and wants replacement | Pump tested OK |
| 8 | m | Hyperglycemia, DKA | Pump not correct size for body | Battery was reported to be out of limit; pump tested OK |
| 8 | m | Hyperglycemia, DKA | Parent did not understand pump performance | Pump not returned |
| 8 | m | Hyperglycemia, DKA | Parent not familiar with pump operation | Pump not returned Education needed |
| 9 | f | Hyperglycemia, DKA | Nondelivery during the middle of the night | Pump not returned |
| 9 | f | Hyperglycemia, DKA | Numerous nondelivery alarms | Pump not returned |
| 10 | m | Hyperglycemia | Hospital and family did not want to troubleshoot | Pump not returned |
| 11 | f | Hyperglycemia | Bent cannula, which came out during sleep; two hospitalizations | Pump not returned |
| 11 | m | Hypoglycemia | Parent activated the ACT button with pump in prime mode | Pump not returned Use error |
Based on the manufacturers’ evaluation, most returned devices showed that the device operated and functioned according to specification. Overall, only one-third of the 1774 reports (34%) indicated that the device was returned to the manufacturer. The majority of these were evaluated by the manufacturer.
Discussion
Our analysis of FDA adverse event reports for young children highlights the need for a better understanding of the safe use of these devices in the pediatric population. Within the 14-year study period, we found over 1700 unique reports of insulin pump-related problems within the younger pediatric age group. We lookedat the pediatric age subgroups and found that the majority of patients were between 5 and 11 years, with preponderance in the older age group, 9–11 years. There were many cases in which the underlying device problem was unknown or inconclusive with respect to whether the device may have caused or contributed to the event. Often, devices were not returned to the manufacturer, and root causes could not be confirmed. This highlights the need for insulin pumps to be returned to the manufacturer for full evaluation. In approximately 43% of hospitalized cases, where devices were evaluated and tested by the manufacturer, the device was found to operate according to specifications. However, some pumps were found to be defective due to missing or damaged components, and a replacement device was sent to the patient. In many of the reports, the narratives revealed that pump instructions were not followed. A common problem was failure to prime the pump, and in several reports, the priming was done incorrectly with the tubing still attached to the child. In summary, this article underscores that many adverse events were due to use error and point to the importance of education and training.
Hypoglycemia is a major concern in the treatment of diabetes mellitus, especially in children.4 In a recent systematic review of continuous subcutaneous insulin infusion versus multiple daily injections, Fatourechi and colleagues5 found that children had a higher risk of mild hypoglycemia when treated with pump therapy. Severe or serious hypoglycemia was not evaluated because the number of severe hypoglycemic episodes was quite small, precluding further analysis of this important variable. We focused on serious adverse events and hospitalizations.
However, another systematic review of clinical trials in very young children (≤6 years) found a decreasing trend in the frequency of hypoglycemia among pump users. They also observed that the definition used for hypoglycemia in the different trials was inconsistent.6
While not a major finding in our evaluation, there were many reports of serious hypoglycemia that resulted in hospitalization, emergency department visits, or paramedic intervention. We did not find any narrative reports of nocturnal hypoglycemia. One death was attributed to hypoglycemia. No dead-in-bed reports were found. We noted only a few reports of hypoglycemia with seizures. However, several articles have reported hypo-glycemic seizures in children occurring during sleep and an increased risk of dead-in-bed syndrome.7–10
In our review, the reports of hyperglycemia exceeded those with hypoglycemia and resulted in more hospitalizations. Shalitin and colleagues9 report DKA secondary to dislodgment or occlusion of the infusion set or pump failure. They report that interruption of insulin delivery may be unintentional (e.g., caused by catheter occlusion, battery failure, or depleted insulin supply) or intentional, to allow children to participate in certain activities. They also noted that device alarms do not always warn against leakage or dislodgment, and therefore “blood glucose should be monitored frequently and consistently with parental supervision.” We found six reported problems at the infusion site—three with infections, one with scar tissue at the site, and two others with a problem or issue at the intravenous site. In clinical practice, infusion site problems are quite common. When adverse events are expected and occur frequently, these events are not usually reported to FDA.
Parental oversight and monitoring may vary, and Zhang and colleagues11–12 suggest that use errors contribute to a significant portion of pump adverse events. Users may operate their pumps without sufficient guidance or supervision by experts. They also found three major issues: users do not follow pump operation instructions, do not understand the features of their pumps, and fail to program the pump with appropriate delivery profiles. This highlights the importance of caregiver oversight, and in the case of younger children, adequate training of parents and guardians is vital. In addition, a review article by Fuld and colleagues13 highlighted the many unique challenges of insulin pump therapy in very young children, suggesting that selection of pump therapy in this age group should be individualized. They also outline that the benefits and risk need to be further studied.
We found similar issues in the FDA reports describing parental stress and feeling “uncomfortable with the pump.” There were reports of parental difficulty in understanding pump operation and performance. Maahs and colleagues14 suggest that while insulin pump use is becoming more and more popular in children with type 1 diabetes, candidate selection should be carefully considered. They highlight that good communication and education are the keys to successful insulin pump use in the school setting. Additionally, they point out that many parents utilize baby monitors for youths who sleep through alarms during the night.
According to Scrimgeour and colleagues,15 pediatric rates of discontinuation of insulin pump therapy in use are usually between 7 and 18% in the United States. These rates may be related to the strict criteria for starting or initiating pump therapy. Wood and colleagues16 evaluated a cohort of pediatric pump users and causes for pump discontinuation, which they grouped into five categories, including DKA, diabetes burnout, minor problems, body image, and weight gain concerns. However, there were no details on pump-specific adverse events leading to pump discontinuation. We could not find any information on pump discontinuation following device-related adverse events.
Limitations
There were a number of limitations to this study. Most of the adverse event reports were from manufacturers. Completeness and accuracy of the reports are not verified by FDA. By limiting our search to the specific age group (1–12 years), we were unable to capture reports with missing age information. Medical device reports did not clearly establish an underlying cause-and-effect relationship of an injury or illness with the device, use error, or both. In addition, it is often not clear whether changes in blood glucose are device-related, drug-related or due to patient comorbidities. It is challenging to estimate the rates of adverse events and information on pump use, and sales figures are not available to provide the denominator factor. National estimates for the prevalence of pump use in children are unavailable to more accurately interpret the incidence of insulin pump adverse events in this age group. There is often a paucity of information about the patient’s medical history, disease duration, severity, duration of pump therapy, or type of insulin used. Often, details of hospitalization are not accessible. In most reports, causation could not be determined because of the low rate of pump returns to the manufacturer. In some reports, it was difficult to assess whether an emergency room visit resulted in hospitalization.
Conclusions
Pumps have revolutionized the treatment of diabetes mellitus, and many physicians have found these devices to be of great benefit in controlling glucose. While the benefits far outweigh the risks, efforts should be made to minimize the occurrence of pump-related adverse events by strengthening user training and establishing better education for children and their caregivers. As demonstrated in this article, use error accounted for many pump-related issues. In order to better understand the root cause of a pump malfunction, it is imperative that pumps are returned to the manufacturer for a full evaluation. In addition, clinicians should include device-related issues in their differential diagnosis when assessing poor glucose control. Better patient education may help to make these devices of even more benefit to all patients, particularly children.
The use of insulin pumps for the treatment of diabetes in young children must take into consideration the benefits of using these devices while balancing the risks to minimize the adverse events. This article provides a summary of pediatric-related adverse events reported to the FDA. These events are likely to be under-reported in the MAUDE database and in the published literature. The precise rates of device-related complications, use error, and misuse should be further studied in this young age group. Health-care practitioners, medical care facilities, and consumers are encouraged to report device-related problems through the FDA MedWatch program (www.fda.gov/medwatch).17
The FDA has made a concerted effort to ensure safety of all infusion pumps, including insulin infusion pumps. Through a number of initiatives and interactive public workshops, the FDA has developed a draft guidance document to assist the industry in preparing premarket notification submissions for infusion pumps and to identify device features that manufacturers should address throughout the total product life cycle.18 The FDA believes that these steps will help to mitigate some of the adverse events reported and will ensure safer infusion pumps. In addition, the FDA emphasizes the importance of taking into consideration human factors, specifically the interface between the user and the device. It is believed that specific attention to this interaction of the user with the device will help reduce use errors. Many families may be uncomfortable or apprehensive with the daily use of newer insulin pump technologies in younger children. More studies are needed to further understand the type of requisite training that should be in place to support pediatric patients on insulin pump therapy. Therefore, future research should place more emphasis on device safety in the pediatric population.
Glossary
- (DKA)
diabetic ketoacidosis
- (FDA)
U.S. Food and Drug Administration
- (MAUDE)
Manufacturer and User Facility Device Experience
Funding
All authors are FDA employees.
References
- 1.Cope JU, Morrison AE, Samuels-Reid J. Adolescent use of insulin and patient-controlled analgesia pump technology: a 10-year Food and Drug Administration retrospective study of adverse events. Pediatrics. 2008;121(5):e1133–8. doi: 10.1542/peds.2007-1707. [DOI] [PubMed] [Google Scholar]
- 2.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Beck RW, Buckingham B, Miller K, Wolpert H, Xing D, Block JM, Chase HP, Hirsch I, Kollman C, Laffel L, Lawrence JM, Milaszewski K, Ruedy KJ, Tamborlane WV. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32(11):1947–1953. doi: 10.2337/dc09-0889. Epub 2009 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.How to Report a Problem (Medical Devices). U.S. Food and Drug Administration. http://www.fda.gov/MedicalDevices/Safety/ReportaProblem/default.htm Accessed February 29, 2012.
- 4.Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125(2):177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 5.Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: Hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab. 2009;94(3):729–740. doi: 10.1210/jc.2008-1415. Epub 2008 Dec 16. [DOI] [PubMed] [Google Scholar]
- 6.Churchill JN, Ruppe RL, Smaldone A. Use of continuous insulin infusion pumps in young children with type 1 diabetes: a systematic review. J Pediatr Health Care. 2009;23(3):173–179. doi: 10.1016/j.pedhc.2008.07.002. Epub 2008 Aug 23. [DOI] [PubMed] [Google Scholar]
- 7.Buckingham B, Caswell K, Wilson DM. Real-time continuous glucose monitoring. Curr Opin Endocrinol Diabetes Obes. 2007;14(4):288–295. doi: 10.1097/MED.0b013e32825a675e. [DOI] [PubMed] [Google Scholar]
- 8.De Vries L, Grushka Y, Lebenthal Y, Shalitin S, Phillip M. Factors associated with increased risk of insulin pump discontinuation in pediatric patients with type 1 diabetes. Pediatr Diabetes. 2011;12(5):506–512. doi: 10.1111/j.1399-5448.2010.00701.x. doi: 10.1111/j.1399-5448.2010.00701.x. Epub 2010 Aug 15. [DOI] [PubMed] [Google Scholar]
- 9.Shalitin S, Phillip M. The use of insulin pump therapy in the pediatric age group. Horm Res. 2008;70(1):14–21. doi: 10.1159/000129673. Epub 2008 May 21. [DOI] [PubMed] [Google Scholar]
- 10.Sovik O, Thordarson H. Dead-in-bed syndrome in young diabetic patients. Diabetes Care. 1999;22(Suppl 2):B40–2. [PubMed] [Google Scholar]
- 10.Zhang Y, Jones PL, Klonoff DC. Second insulin pump safety meeting: summary report. J Diabetes Sci Technol. 2010;4(2):488–493. doi: 10.1177/193229681000400232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Jones PL, Jetley R. A hazard analysis for a generic insulin infusion pump. J Diabetes Sci Technol. 2010;4(2):263–283. doi: 10.1177/193229681000400207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuld K, Conrad B, Buckingham B, Wilson DM. Insulin pumps in young children. Diabetes Technol Ther. 2010;12(Suppl 1):S67–71. doi: 10.1089/dia.2009.0182. [DOI] [PubMed] [Google Scholar]
- 14.Maahs DM, Horton LA, Chase HP. The use of insulin pumps in youth with type 1 diabetes. Diabetes Technol Ther. 2010;12(Suppl 1):S59–65. doi: 10.1089/dia.2009.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scrimgeour L, Cobry E, McFann K, Burdick P, Weimer C, Slover R, Chase HP. Improved glycemic control after long-term insulin pump use in pediatric patients with type 1 diabetes. Diabetes Technol Ther. 2007;9(5):421–428. doi: 10.1089/dia.2007.0214. [DOI] [PubMed] [Google Scholar]
- 16.Wood JR, Moreland EC, Volkening LK, Svoren BM, Butler DA, Laffel LM. Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care. 2006;29(11):2355–2360. doi: 10.2337/dc06-1141. [DOI] [PubMed] [Google Scholar]
- 17.MedWatch: The FDA Safety Information and Adverse Event Reporting Program. The U.S. Food and Drug Administration. http://www.fda.gov/Safety/MedWatch/default.htm Accessed February 29, 2012.
- 18.Draft Guidance for Industry and FDA Staff - Total Product Life Cycle: Infusion Pump - Premarket Notification [510(k)] Submissions. http://www.fda.gov/medicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm206153.htm Accessed August 1, 2012. [Google Scholar]


