Abstract
Background
The accuracy of systems for self-monitoring of blood glucose is important, as reliable measurement results are a prerequisite for therapeutic decisions.
Methods
This system accuracy evaluation study was performed according to DIN EN ISO 15197:2003 for 43 Conformité Européenne (CE)-labeled blood glucose (BG) monitoring systems. Measurement results of each system were compared with results of the designated comparison method (manufacturer’s measurement procedure): glucose oxidase method (YSI 2300 glucose analyzer) or hexokinase method (Hitachi 917/ cobas 501).
Results
Complete assessment according to the International Organization for Standardization (ISO) standard was performed for 34 out of 43 systems, and 27 (79.4%) meet the requirements of the standard, i.e., ≥95% of their results showed at least the minimum acceptable accuracy. For 9 of the 43 systems, complete accuracy assessment was not performed due to an oxygen sensitivity (manufacturer’s labeling). The bias (according to Bland and Altman) of all 43 evaluated systems ranged from -14.1% to +12.4%.
Conclusions
From the 34 systems completely assessed, 7 systems did not fulfill the minimal accuracy requirements of the ISO standard. The CE mark apparently does not guarantee that all BG systems provide accuracy according to the standard. Because inaccurate systems bear the risk of false therapeutic decisions, regular and standardized evaluation of BG meters and test strips should be requested in order to ensure adherence to quality standards.
Keywords: blood glucose monitoring systems, Conformité Européenne mark, DIN EN ISO 15197:2003, self-monitoring of blood glucose, system accuracy
Introduction
Self-monitoring of blood glucose (SMBG) with glucose monitoring systems is widely recognized as an integral component of adequate diabetes management that enables patients to control their blood glucose (BG) levels effectively.1–4 Several studies have demonstrated that tight BG control is essential for diabetes patients to avoid late complications.1,5 The clinical benefits of SMBG in diabetes patients are widely accepted, and today, SMBG is recommended for all people with diabetes, particularly for adjustment of insulin in patients with multiple daily injections.2,6–8
A multitude of SMBG systems is available on the market. An increasing number of new systems have been introduced. Physicians and patients are looking for guidance to choose between systems with different price ranges, including well-established systems as well as completely new systems, e.g., systems providing new technologies.9–13
The accuracy of a SMBG measurement is imperative for the reliability of results and, finally, the medical outcome in diabetes therapy. DIN EN ISO 15197:200314 is an internationally accepted standard defining performance requirements for BG systems for SMBG, e.g., concerning accuracy. The standard states that ≥95% of the BG system measurement results shall fall within ±15 mg/dl of the results of the manufacturer’s measurement procedure at glucose concentrations <75 mg/dl and within ±20% at glucose concentrations ≥75 mg/dl. A revised version of the International Organization for Standardization (ISO) standard, expected to be published in 2012, includes tighter criteria for the minimum accuracy of BG systems.15 The current draft revision of ISO 15197 states that ≥95% of the system measurement results shall fall within ±15 mg/dl of the results of the manufacturer’s measurement procedure at glucose concentrations <100 mg/dl and within ±15% at glucose concentrations ≥100 mg/dl.
In Europe, manufacturers of BG systems have to provide evidence of conformity with the ISO standard in order to get the Conformité Européenne (CE) mark for their products. However, an evaluation study published in 2010 showed that more than 40% of the systems investigated did not fulfill the minimum accuracy criteria of the ISO standard.16
The aim of this study was to evaluate measurement quality standard of a broad range of current BG systems available on the market, i.e., all of them are CE marked. In total, 43 BG systems from 19 manufacturers were evaluated according to the accuracy requirements requested by DIN EN ISO 15197:2003.
Materials and Methods
The study was conducted from 2009 to 2011 in compliance with the German Medical Devices Act at the Institut für Diabetes-Technologie GmbH in Ulm, Germany. The study protocol was approved by the Ulm University ethics committee, and the competent authority was notified. Informed consent forms were signed by all participants. The procedure to evaluate system accuracy applied in this study is described in detail in DIN EN ISO 15197:2003.14 Deviations from this standard are described here.
Subjects and Test Procedure
Adult patients (≥18 years old) with diabetes type 1 and type 2 as well as subjects without diabetes were included. Exclusion criteria were as follows: pregnancy or lactation period for female subjects, severe acute disease, and severe chronic disease endangering the subject due to the study. Interruption criteria for individual subjects were retraction of written informed consent and incidences or adverse events interfering with the study continuation. At least 100 subjects were included for each system tested. For each BG system (BG meter with test strips), two individual BG meters were used. The BG meters were replaced in case of failure. For each system, measurements were performed on at least 10 days. Suitable control procedures were performed daily prior to the test procedure. The tests were performed by clinical personnel well trained to the limitations of the BG system, the manufacturer’s device labeling, the safety practices, and the test protocol. The tests were performed in a laboratory setting with controlled room temperature (23 ± 5 °C) and humidity (according to the manufacturers’ specifications).
Self-Monitoring of Blood Glucose Systems
The 43 evaluated BG systems are listed in Table 1. These BG systems have been selected in order to give a comprehensive overview of systems with the CE label. The following two criteria have been defined as prerequisites: the product must have a CE mark and BG meter and test strips must have been available in the required quantities for an ISO assessment. In addition, the investigator tried to ensure a representative overview from an international perspective. Selection also should give insights about accuracy of BG systems from established manufacturers as well as from new providers and BG systems for which new technologies are claimed. This is why products from different countries fulfilling these prerequisites have been considered. None of the 43 BG systems were already assessed in the study published in 2010.16 The Accu-Chek® Active system was an older version, and for Accu-Chek Aviva and FreeStyle Lite®, test strips with another chemistry have been used. Inclusion criteria for the evaluation of BG systems were as follows: Only systems labeled for SMBG were included. For each system, one test strip lot was evaluated. Test strips were taken from at least seven different packages or vials. The packages or vials were changed after approximately 10 subjects. For the strip-free Accu-Chek Mobile system, which incorporates 50 tests in a cassette, a new test cassette was used for each subject.
Table 1.
Forty-Three Blood Glucose Systems Evaluated (Listed Alphabetically)a
| BG system | Manufacturer | Reference method | Calibration | Test strip enzyme | Study date | Test strip lot | Expiry date (test strip) | O2 dependency in labeling |
|---|---|---|---|---|---|---|---|---|
| Accu-Chek Active | Roche Diagnostics GmbH, Germany | HK | Plasma | GDH | 04/2011–05/2011 | 23433231 | 05/2012 | No |
| Accu-Chek Aviva | Roche Diagnostics GmbH, Germany | HK | Plasma | GDH | 11/2010–02/2011 | 490018 | 11/2011 | No |
| Accu-Chek Aviva Nano | Roche Diagnostics GmbH, Germany | HK | Plasma | GDH | 04/2011–05/2011 | 490068 | 03/2012 | No |
| Accu-Chek Compact Plus | Roche Diagnostics GmbH, Germany | HK | Plasma | GDH | 01/2009 | 20684541 | 10/2009 | No |
| Accu-Chek Go | Roche Diagnostics GmbH, Germany | HK | Whole blood | GDH | 01/2009 | 22472532 | 11/2009 | No |
| Accu-Chek Mobileb | Roche Diagnostics GmbH, Germany | HK | Plasma | GDH | 11/2010–02/2011 | 27705231 | 09/2011 | No |
| Accu-Chek Mobileb | Roche Diagnostics GmbH, Germany | HK | Plasma | GDH | 05/2011–06/2011 | 27802741 | 09/2012 | No |
| Accu-Chek Performab | Roche Diagnostics, GmbH, Germany | HK | Plasma | GDH | 01/2009 | 320137 | 12/2009 | No |
| Accu-Chek Performab | Roche Diagnostics GmbH, Germany | HK | Plasma | GDH | 02/2011–03/2011 | 470049 | 12/2011 | No |
| Accu-Chek Performa Nano | Roche Diagnostics GmbH, Germany | HK | Plasma | GDH | 05/2011–06/2011 | 470137 | 05/2012 | No |
| Bayer Contour® usb | Bayer Consumer Care AG, Switzerland | GOx | Whole blood | GDH | 03/2010 | 9FC3A02 | 06/2011 | No |
| Beurer GL32 | Beurer GmbH, Germany | GOx | Plasma | GOx | 04/2011–05/2011 | V43/4 | 10/2012 | No |
| Beurer GL40 | Beurer GmbH, Germany | GOx | Plasma | GOx | 03/2010 | U13/001 | 12/2010 | No |
| BGStar™ | AgaMatrix Inc., USA | GOx | Plasma | GOx | 07/2011–08/2011 | HD14WB26C | 10/2012 | Yes |
| Biocheck TD-4225 | TaiDoc Technology Corporation, Taiwan | GOx | Plasma | GOx | 07/2009–10/2009 | TD08J123-B06 | 04/2010 | No |
| Element™ | Infopia Co. Ltd., Korea | HK | Plasma | GOx | 06/2010–07/2010 | S4ND09L14 | 12/2011 | Yes |
| FreeStyle Freedom Lite® | Abbott Diabetes Care Inc., USA | GOx | Plasma | GDH | 05/2011–06/2011 | 1067212 | 12/2011 | No |
| FreeStyle Lite | Abbott Diabetes Care Inc., USA | GOx | Plasma | GDH | 06/2010–07/2010 | 1055813 | 08/2011 | No |
| Futura Monometer® | TaiDoc Technology Corporation, Taiwan | GOx | Whole blood | GOx | 06/2010–07/2010 | TD09C117-B02 | 12/2010 | No |
| GlucoCheck Classic | TaiDoc Technology Corporation, Taiwan | GOxc | Plasma | GOx | 05/2011–06/2011 | TD10C129-B04 | 12/2011 | No |
| GlucoCheck Comfort | aktivmed GmbH, Germany | HK | Plasma | GOx | 11/2010–02/2011 | S3FC10E27 | 05/2012 | Yes |
| GlucoCheck XL | aktivmed GmbH, Germany | GOx | Plasma | GDH | 04/2011–05/2011 | TD10J114-B0E | 04/2012 | No |
| GlucoHexal® IId | Med-WatchDoc GmbH & Co. KG, Germany | GOx | Plasma | GDH | 03/2010 | E09D012577 | 11/2010 | No |
| GlucoRx (TD-4230) | TaiDoc Technology Corporation, Taiwan | GOxc | Plasmae | GOx | 02/2011–04/2011 | TD09L109-B85 | 09/2011 | No |
| GlucoSmart® Swing | MSP bodmann GmbH, Germany | GOx | Whole blood | GDH | 03/2010 | A09F05222 | 01/2011 | No |
| GlucoTel | BodyTel Europe GmbH, Germany | GOx | Plasma | GDH | 07/2009–10/2009 | 7121403 | 12/2009 | No |
| Gluco-test Plus+ TD-4230 | TaiDoc Technology Corporation, Taiwan | GOx | Plasma | GOx | 07/2009–10/2009 | TD08E114-E06 | 11/2009 | No |
| iBGStar™ | AgaMatrix Inc., USA | GOx | Plasma | GOx | 07/2011–08/2011 | HS01WZ34B | 07/2012 | Yes |
| iDia™ | IME-DC GmbH, Germany | GOxc | Plasma | GDH | 09/2010–10/2010 | GS005A | 12/2011 | No |
| IME-DC Fidelity | IME-DC GmbH, Germany | GOxc | Whole bloode | GOx | 07/2009–10/2009 | DS159A2 | 02/2010 | No |
| iXell | Genexo Sp, zo.o., Poland | GOxf | Plasmaf | GOx | 05/2011–06/2011 | TD10K112-B0C | 08/2012 | No |
| iXell OLED | Genexo Sp, zo.o., Poland | GOxf | Plasmaf | GOx | 04/2011–05/2011 | TD10K112-B0C | 08/2012 | No |
| microdot®+ | Cambridge Sensors Limited, UK | GOx | Plasma | GDH | 07/2011–08/2011 | 0060802 | 06/2012 | No |
| Omnitest® 3 | B. Braun Melsungen AG, Germany | GOx | Plasma | GOx | 07/2011–08/2011 | G5KI14 | 09/2012 | Yes |
| OneTouch® Verio™ | LifeScan Inc., USA | GOx | Plasma | GDH | 03/2010 | 2993603 | 12/2010 | No |
| OneTouch Verio Pro | LifeScan Europe, Switzerland | GOx | Plasma | GDH | 02/2011–03/2011 | 3078405 | 01/2012 | No |
| OneTouch VITA™ | LifeScan Inc., USA | GOx | Plasma | GOx | 01/2009 | 2841992 | 12/2009 | Yes |
| Pura™ | Bionime Corporation, Taiwan | HK | Plasma | GOx | 03/2010 | 1196232 | 05/2011 | No |
| SeniorLine GM210 | Bionime Corporation, Taiwan | GOx | Whole blood | GOx | 07/2009–10/2009 | 1186235 | 05/2010 | No |
| smartLAB® genie | HMM Diagnostics GmbH, Germany | GOxc | Plasmae | GOx | 07/2009–10/2009 | 022081101 | 11/2009 | Yes |
| smartLAB global | HMM Diagnostics GmbH, Germany | GOxc | Plasmae | GOx | 06/2010–07/2010 | 045100303 | 10/2011 | Yes |
| WaveSense™ Jazz™ | AgaMatrix Inc., USA | GOx | Plasma | GOx | 11/2010–02/2011 | HJ29WT32C | 11/2011 | Yes |
| Wellion® CALLA light | MED TRUST Handelsges.m.b.H., Austria | GOx | Plasma | GOx | 07/2011–08/2011 | TJS002L | 11/2011 | No |
Reference methods (GOx or HK), calibration (plasma or whole blood), and test strip enzyme (glucose dehydrogenase or GOx) according to the manufacturer’s labeling. GDH, glucose dehydrogenase.
Accu-Chek Mobile and Accu-Chek Performa were both tested with different test strip chemistries. The test strip chemistry was either maltose dependent (test strips evaluated first) or maltose independent (test strips evaluated second).
Clear information about the reference method was not available in manufacturer’s labeling; requests were made to provide information.
GlucoHexal test strip lot was recalled from the market in June 2010, after at least 11 months availability on the market.
Clear information about the calibration was not available in manufacturer’s labeling; requests were made to provide information.
Information was not available; repeated requests were unanswered at the time of manuscript submission.
Reference Measurement
Reference measurements were performed with the following two different methods for all BG systems: glucose oxidase (GOx) (YSI 2300 STAT Plus™ glucose analyzer, YSI Life Sciences, Yellow Springs, OH; measurements were performed at the study site) and hexokinase (HK) [Hitachi 917 (from January 2009 to August 2010)/cobas® 6000 c501 (since August 2010), Roche Diagnostics GmbH, Mannheim, Germany; measurements were performed at a Deutsche Akkreditierungsstelle-accredited calibration laboratory of Roche Diagnostics GmbH].
The accuracy of the GOx method was verified measuring NERL Glucose Standards (Thermo Fisher Scientific, East Providence, RI), verified against National Institute of Standards and Technology (NIST) (Gaithersburg, MD) reference material. The accuracy of the HK method was verified measuring NIST Standard Reference Material 965a (from January 2009 to February 2011) or 965b (since February 2011). In addition, for both systems, internal and external quality control measurements were performed, as required by the German national standard.17
The accuracy of the measurement results of each BG system was evaluated in comparison with the results of the reference measurement specified by the manufacturer (manufacturer’s measurement procedure).
The BG meters displayed either whole blood BG values or plasma equivalent BG values in mg/dl or mmol/liter (calibration, Table 1). Reference measurements with the GOx method were performed from capillary whole blood samples; reference measurements with the HK method were performed from hemolyzed and deproteinized whole blood samples. Both reference measurement methods provided whole blood BG values in mg/dl. For plasma-calibrated systems, whole blood BG values were converted to plasma equivalent values, and these results were used for comparison with the BG system results. Measurement results from the GOx method were converted from whole blood BG values to plasma equivalent BG values as follows: plasma equivalent BG value (in mg/dl) = whole blood BG value (in mg/dl)/[1 - (0.0024 × hematocrit value [in %])].18 Results from the HK method were converted from whole blood BG values to plasma equivalent BG values as follows: plasma equivalent BG value (in mg/dl) = 1.11 × whole blood BG value (in mg/dl). For the GlucoTel™ system, a conversion factor was described in the manufacturer’s manual that was used: plasma equivalent value (in mg/dl) = 1.12 × whole blood BG value (in mg/dl).
For 8 systems, complete or clear information about the reference measurement procedure and/or the calibration, required for system accuracy evaluation according to DIN EN ISO 15197:2003, were not documented in the manufacturers’ labeling. Even though repeated requests were made, for 2 systems (iXell® and iXell OLED), information about the reference measurement procedure and information about the calibration have not been provided (Table 1). For evaluation of iXell and iXell OLED, we used the GOx method as reference measurement procedure, and we used plasma equivalent values, because most of the available BG systems are plasma calibrated.
Test Protocol
DIN EN ISO 15197:2003 specifies the distribution of the blood samples into different concentration categories. In this evaluation, we used slightly modified limits of these concentration categories, because the limits are not clearly defined and differ between the English and the German version of the standard (Table 2). In deviation to the current standard (but in accordance with the 2011 draft of the new ISO standard), blood samples are distributed into the different concentration categories based on the mean reference results of the manufacturer’s measurement procedure instead of the determined BG values with the systems.
Table 2.
Distribution of Glucose Concentration according to DIN EN ISO 15197 with Slight Modifications
| Percentage of samples | Glucose concentration mmol/liter (mg/dl) |
|---|---|
| 5 | <2.8 (≈ <50) |
| 15 | ≥2.8–<4.35 (≈ ≥50–<80) |
| 20 | ≥4.35–<6.7(≈ ≥80–<120) |
| 30 | ≥6.7–<11.15 (≈ ≥120–<200) |
| 15 | ≥11.15–<16.65 (≈ ≥200–<300) |
| 10 | ≥16.65–<22.2 (≈ ≥300–<400) |
| 5 | ≥22.2 (≈ ≥400) |
Native capillary blood samples were used at BG concentrations of 50 to 400 mg/dl. If sufficient numbers of native samples with BG concentrations <50 mg/dl were not available, additional samples were prepared as follows: the blood samples were collected in lithium heparin tubes, incubated at room temperature to allow for glycolysis, and gently mixed before testing. If sufficient numbers of native samples with BG concentrations ≥400 mg/dl were not available, additional samples were prepared as follows: the blood samples were collected in lithium heparin tubes, supplemented with concentrated glucose solution (40% glucose in 0.9% NaCl), and gently mixed before testing.
At least 100 fresh capillary blood samples from 100 subjects were collected (distribution of BG concentrations as described earlier). For each subject, the hematocrit value was checked to be within 30% and 55%. For determination of the hematocrit, capillary whole blood was collected in heparinized capillaries (double test). After centrifugation, the hematocrit was read on an alignment chart.
Samples were collected from fingertips by skin puncture. The steps of the sample sequence for BG systems were as follows:
Sample collection for the two reference measure-ment procedures: (a) a sample (100 µl) for the GOx method was collected using a lithium heparin tube, and the BG concentration was measured in duplicate; (b) a sample (20 µl) for the HK method was hemolyzed and deproteinized in tubes containing 400 µl of 0.33 mmol/liter perchloric acid—these tubes were centrifuged, and the supernatants were transferred to fresh tubes and stored at -20 °C for later triplicate testing.
BG measurements with up to three BG systems (meter 1 and meter 2, respectively).
Taking of samples for the two reference measurement procedures (sample collection and measurement as described earlier).
Residual blood was wiped off the finger before the sample collection for each reference measurement procedure and before measurement with each BG system. Measurements with meter 1 and meter 2 of the respective system were normally carried out from the same drop of blood, except for systems with test fields (Accu-Chek Active system and Accu-Chek Mobile system), where blood was wiped off before the measurement with each BG meter.
Statistical Analyses
The entire data evaluation was performed at the study site. Data were excluded from statistical analysis if a handling error occurred, no reference value was available, a technical error was documented, the data set was not complete, the hematocrit value was outside the defined range (30% to 55%), the maximum number of samples in a given BG concentration category was already reached, or the drift between the first and second reference measurement was >4 mg/dl at BG concentrations ≤100 mg/dl or >4% at BG concentrations >100 mg/dl. Data of 100 subjects were included in the system accuracy evaluation for each system according to the ISO 15197 standard. Calculations were performed in mmol/liter, with a conversion factor of 18.02.
The accuracy of each of the 43 SMBG system results was evaluated by comparison with respective mean result of the reference measurement obtained immediately before and after the measurements with the system.
According to the ISO standard, at BG concentrations <75 mg/dl, the relative number of system results within ±15, ±10, and ±5 mg/dl and, at BG concentrations ≥75 mg/dl, the relative number of system results within ±20%, ±15%, ±10%, and ±5% of the reference measurement were calculated. For assessment of the overall accuracy of a system, the number of system results within ±15 mg/dl at BG concentrations <75 mg/dl was added to the number of system results within ±20% at BG concentrations ≥75 mg/dl.
In this study, the preparation procedure of modified blood samples with BG concentrations <50 and ≥400 mg/dl (as described earlier) did not ensure constant oxygen concentrations of the blood samples. This might cause systematic measurement bias on BG systems with an oxygen dependency (as mentioned in the manufacturer’s labeling; Table 1). Therefore, data of modified blood samples (BG concentration <50 and ≥400 mg/dl) were excluded from overall system accuracy calculation of these 9 systems (Table 1). In these cases, a complete system accuracy assessment and determination of acceptability of the system according to the ISO standard was not performed.
To illustrate the accuracy of the 43 systems according to the ISO standard, the agreement between each BG system and the mean reference result was plotted in a difference plot. The difference plot shows the deviation of single measurement results of a BG system from the reference measurement. It shows both random and systematic deviations, which reflect the total measuring error of a system. The average bias (%) of the results of each BG system was calculated according to Bland and Altman19 using the formula
where BG is a single measurement result, reference is the mean value of the reference measurements before and after the BG system measurement, and n is the number of BG system results. For the calculation of the average bias of each system, only 180 data sets of native blood samples with BG concentrations ≥50 and <400 mg/dl were taken into account. The average bias is shown with 95% limits of agreement (≈1.96 × standard deviation).
Additionally, system accuracy of each BG system was evaluated in accordance to the current draft revision of ISO 15197 with the BG concentration threshold of 100 mg/dl (previously 75 mg/dl). The blood samples were distributed into the different concentration categories as mentioned earlier according to DIN EN ISO 15197:2003 with slight modifications. At BG concentrations <100 mg/dl, the relative number of system results within ±15, ±10, and ±5 mg/dl and, at BG concentrations ≥100 mg/dl, the relative number of system results within ±15%, ±10%, and ±5% of the reference measurement was calculated. For assessment of the overall accuracy of a system, the number of system results within ±15 mg/dl at BG concentrations <100 mg/dl was added to the number of system results within ±15% at BG concentrations ≥100 mg/dl.
Results
The percentage of BG system results within different deviation ranges is shown in Tables 3 and 4. According to the current ISO standard, system results within ±15, ±10, and ±5 mg/dl of the reference results at BG concentrations <75 mg/dl and system results within ±20%, ±15%, ±10%, and ±5% of the reference results at BG concentrations ≥75 mg/dl are calculated (Tables 3 and 4). For the completely assessable 34 of 43 BG systems, the overall accuracy assessment and the conformity of the system according to the ISO standard are shown in Table 3. For these 34 systems, all 200 obtained results per system from 100 subjects could be compared with the reference results (Table 3). For 9 systems with an oxygen dependency (manufacturer’s labeling), only 180 results from 90 subjects were calculated, and complete system accuracy assessment was not performed (Table 4).
Table 3.
Accuracy Results of the Completely Assessable 34 of 43 Blood Glucose Systemsa
| DIN EN ISO 15197:2003 | Current draft revision of ISO 15197 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BG concentration <75 mg/dl | BG concentration ≥75 mg/dl | BG concentration <100 mg/dl | BG concentration ≥100 mg/dl | |||||||||||||||
| BG system | Reference method | Within accuracy limits (±15 mg/dl and ±20%) | ±15 mg/dl | ±10 mg/dl | ±5 mg/dl | ±20% | ±15% | ±10% | ±5% | Within accuracy limits (±15 mg/dl and ±15%) | ±15 mg/dl | ±10 mg/dl | ±5 mg/dl | ±15% | ±10% | ±5% | ||
| n | % | % | % | % | % | % | % | % | n | % | % | % | % | % | % | % | ||
| Accu-Chek Active | HK | (200/200) | 100.0 | 100 | 100 | 61 | 100 | 100 | 100 | 81 | (200/200) | 100.0 | 100 | 100 | 72 | 100 | 100 | 79 |
| Accu-Chek Aviva | HK | (200/200) | 100.0 | 100 | 100 | 87 | 100 | 99 | 91 | 64 | (198/200) | 99.0 | 100 | 97 | 80 | 99 | 91 | 64 |
| Accu-Chek Aviva Nano | HK | (200/200) | 100.0 | 100 | 100 | 84 | 100 | 99 | 94 | 65 | (199/200) | 99.5 | 100 | 96 | 80 | 99 | 95 | 65 |
| Accu-Chek Compact Plus | HK | (200/200) | 100.0 | 100 | 88 | 23 | 100 | 100 | 91 | 63 | (200/200) | 100.0 | 100 | 86 | 27 | 100 | 94 | 69 |
| Accu-Chek Go | HK | (200/200) | 100.0 | 100 | 100 | 97 | 100 | 100 | 97 | 78 | (200/200) | 100.0 | 100 | 100 | 94 | 100 | 96 | 79 |
| Accu-Chek Mobileb | HK | (199/200) | 99.5 | 98 | 98 | 75 | 100 | 100 | 97 | 67 | (199/200) | 99.5 | 98 | 98 | 73 | 100 | 96 | 66 |
| Accu-Chek Mobileb | HK | (200/200) | 100.0 | 100 | 100 | 78 | 100 | 100 | 93 | 64 | (200/200) | 100.0 | 100 | 98 | 71 | 100 | 94 | 66 |
| Accu-Chek Performab | HK | (199/200) | 99.5 | 100 | 100 | 79 | 99 | 99 | 93 | 67 | (199/200) | 99.5 | 100 | 95 | 72 | 99 | 94 | 68 |
| Accu-Chek Performab | HK | (198/200) | 99.0 | 100 | 98 | 75 | 99 | 98 | 93 | 68 | (196/200) | 98.0 | 98 | 97 | 78 | 98 | 92 | 66 |
| Accu-Chek Performa Nano | HK | (200/200) | 100.0 | 100 | 100 | 93 | 100 | 100 | 96 | 66 | (200/200) | 100.0 | 100 | 98 | 87 | 100 | 96 | 64 |
| Bayer Contour usb | GOx | (194/200) | 97.0 | 100 | 84 | 61 | 96 | 88 | 63 | 31 | (182/200) | 91.0 | 90 | 69 | 45 | 91 | 68 | 34 |
| Beurer GL32 | GOx | (192/200) | 96.0 | 80 | 48 | 30 | 100 | 98 | 90 | 56 | (189/200) | 94.5 | 85 | 62 | 40 | 99 | 91 | 56 |
| Beurer GL40 | GOx | (198/200) | 99.0 | 97 | 95 | 53 | 99 | 94 | 78 | 43 | (192/200) | 96.0 | 98 | 90 | 52 | 95 | 78 | 42 |
| Biocheck TD-4225 | GOx | (187/200) | 93.5 | 73 | 40 | 13 | 99 | 96 | 81 | 48 | (183/200) | 91.5 | 76 | 52 | 22 | 97 | 80 | 49 |
| FreeStyle Freedom Lite | GOx | (200/200) | 100.0 | 100 | 100 | 98 | 100 | 100 | 98 | 91 | (200/200) | 100.0 | 100 | 100 | 98 | 100 | 98 | 90 |
| FreeStyle Lite | GOx | (200/200) | 100.0 | 100 | 100 | 95 | 100 | 100 | 100 | 86 | (200/200) | 100.0 | 100 | 100 | 93 | 100 | 100 | 86 |
| Futura Monometer | GOx | (182/200) | 91.0 | 93 | 75 | 23 | 91 | 78 | 59 | 28 | (165/200) | 82.5 | 90 | 68 | 27 | 79 | 59 | 26 |
| GlucoCheck Classic | GOx | (191/200) | 95.5 | 100 | 93 | 60 | 94 | 86 | 66 | 38 | (177/200) | 88.5 | 97 | 84 | 55 | 85 | 66 | 38 |
| GlucoCheck XL | GOx | (191/200) | 95.5 | 98 | 95 | 63 | 95 | 89 | 67 | 40 | (182/200) | 91.0 | 97 | 92 | 58 | 88 | 65 | 40 |
| Glucohexal IIc | GOx | (162/200) | 81.0 | 61 | 24 | 8 | 86 | 74 | 57 | 28 | (143/200) | 71.5 | 50 | 21 | 7 | 80 | 64 | 32 |
| GlucoRx (TD-4230) | GOx | (170/200) | 85.0 | 98 | 45 | 13 | 82 | 63 | 38 | 14 | (141/200) | 70.5 | 83 | 41 | 14 | 65 | 40 | 14 |
| GlucoSmart Swing | GOx | (193/200) | 96.5 | 95 | 63 | 24 | 97 | 88 | 60 | 28 | (182/200) | 91.0 | 84 | 53 | 15 | 94 | 67 | 33 |
| GlucoTel | GOx | (190/200) | 95.0 | 87 | 71 | 37 | 97 | 93 | 70 | 40 | (183/200) | 91.5 | 89 | 70 | 33 | 92 | 72 | 41 |
| Gluco-test Plus+ TD-4230 | GOx | (198/200) | 99.0 | 100 | 98 | 75 | 99 | 94 | 80 | 45 | (190/200) | 95.0 | 96 | 94 | 76 | 95 | 79 | 42 |
| iDia | GOx | (191/200) | 95.5 | 100 | 85 | 50 | 94 | 90 | 70 | 41 | (184/200) | 92.0 | 96 | 80 | 47 | 90 | 71 | 40 |
| IME-DC Fidelity | GOx | (183/200) | 91.5 | 80 | 48 | 18 | 94 | 88 | 78 | 44 | (175/200) | 87.5 | 72 | 43 | 18 | 94 | 85 | 48 |
| iXell | GOxd | (199/200) | 99.5 | 100 | 100 | 70 | 99 | 91 | 77 | 42 | (185/200) | 92.5 | 100 | 98 | 68 | 89 | 75 | 38 |
| iXell OLED | GOxd | (198/200) | 99.0 | 95 | 78 | 45 | 100 | 98 | 81 | 42 | (194/200) | 97.0 | 97 | 85 | 52 | 97 | 79 | 39 |
| microdot+ | GOx | (198/200) | 99.0 | 97 | 97 | 71 | 99 | 94 | 81 | 43 | (190/200) | 95.0 | 98 | 95 | 62 | 94 | 83 | 43 |
| OneTouch Verio | GOx | (199/200) | 99.5 | 100 | 87 | 34 | 99 | 96 | 90 | 62 | (198/200) | 99.0 | 97 | 78 | 40 | 100 | 95 | 64 |
| OneTouch Verio Pro | GOx | (193/200) | 96.5 | 93 | 63 | 20 | 98 | 90 | 70 | 38 | (183/200) | 91.5 | 88 | 53 | 21 | 93 | 75 | 40 |
| Pura | HK | (200/200) | 100.0 | 100 | 92 | 55 | 100 | 100 | 75 | 30 | (200/200) | 100.0 | 100 | 95 | 48 | 100 | 74 | 30 |
| SeniorLine GM210 | GOx | (144/200) | 72.0 | 10 | 0 | 0 | 88 | 72 | 48 | 26 | (120/200) | 60.0 | 15 | 0 | 0 | 79 | 55 | 29 |
| Wellion CALLA Light | GOx | (182/200) | 91.0 | 68 | 33 | 10 | 97 | 85 | 68 | 48 | (165/200) | 82.5 | 68 | 33 | 13 | 89 | 73 | 52 |
200 results from 100 subjects were evaluated.
Accu-Chek Mobile and Accu-Chek Performa were both tested with different test strip chemistries. The test strip chemistry was either maltose dependent (test strips evaluated first) or maltose independent (test strips evaluated second).
GlucoHexal test strip lot was recalled from the market in June 2010, after at least 11 months availability on the market.
Information was not available.
Table 4.
Accuracy Results of Nine Blood Glucose Systems with an Oxygen Dependency on Measurement Results (as Mentioned in the Manufacturer’s Labeling)a
| DIN EN ISO 15197:2003 | Current draft revision of ISO 15197 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BG concentration <75 mg/dl | BG concentration ≥75 mg/dl | BG concentration <100 mg/dl | BG concentration ≥100 mg/dl | |||||||||||||
| BG system | Reference method | Within accuracy limits (±15 mg/dl and ±20%) | ±15 mg/dl | ±10 mg/dl | ±5 mg/dl | ±20% | ±15% | ±10% | ±5% | Within accuracy limits (±15 mg/dl and ±15%) | ±15 mg/dl | ±10 mg/dl | ±5 mg/dl | ±15% | ±10% | ±5% |
| n | % | % | % | % | % | % | % | n | % | % | % | % | % | % | ||
| BGStar | GOx | (179/180) | 100 | 93 | 83 | 99 | 96 | 87 | 62 | (175/180) | 98 | 92 | 80 | 97 | 87 | 62 |
| Element | HK | (172/180) | 97 | 90 | 53 | 95 | 83 | 63 | 29 | (153/180) | 90 | 79 | 48 | 83 | 64 | 30 |
| GlucoCheck Comfort | HK | (178/180) | 97 | 87 | 60 | 99 | 95 | 75 | 42 | (171/180) | 96 | 86 | 56 | 95 | 74 | 41 |
| iBGStar | GOx | (173/180) | 100 | 93 | 57 | 95 | 90 | 74 | 36 | (165/180) | 96 | 90 | 52 | 90 | 72 | 35 |
| Omnitest 3 | GOx | (172/180) | 97 | 90 | 70 | 95 | 91 | 79 | 48 | (165/180) | 94 | 86 | 64 | 91 | 79 | 48 |
| OneTouch VITA | GOx | (180/180) | 100 | 100 | 83 | 100 | 99 | 85 | 51 | (178/180) | 98 | 93 | 72 | 99 | 87 | 50 |
| smartLAB genie | GOx | (172/180) | 75 | 36 | 11 | 99 | 98 | 86 | 56 | (170/180) | 84 | 52 | 23 | 98 | 87 | 57 |
| smartLAB global | GOx | (173/180) | 86 | 75 | 43 | 98 | 94 | 80 | 51 | (167/180) | 91 | 85 | 57 | 93 | 79 | 48 |
| WaveSense Jazz | GOx | (178/180) | 100 | 75 | 39 | 99 | 94 | 78 | 45 | (173/180) | 100 | 75 | 38 | 95 | 80 | 47 |
180 results from 90 subjects were evaluated. Data of prepared blood samples (BG concentration <50 and ≥400 mg/dl) were excluded. GOx, glucose oxidase; HK, hexokinase.
Twenty-seven (79.4%) of the 34 completely assessable systems fulfilled the minimum accuracy requirements of the ISO standard (Table 3). According to the current draft revision of ISO 15197, only 18 (52.9%) of 34 systems fulfilled the minimum accuracy requirements: ≥95% of the BG system results fall within ±15 mg/dl of the reference measurement results at glucose concentrations <100 mg/dl and within ±15% at glucose concentrations ≥100 mg/dl.
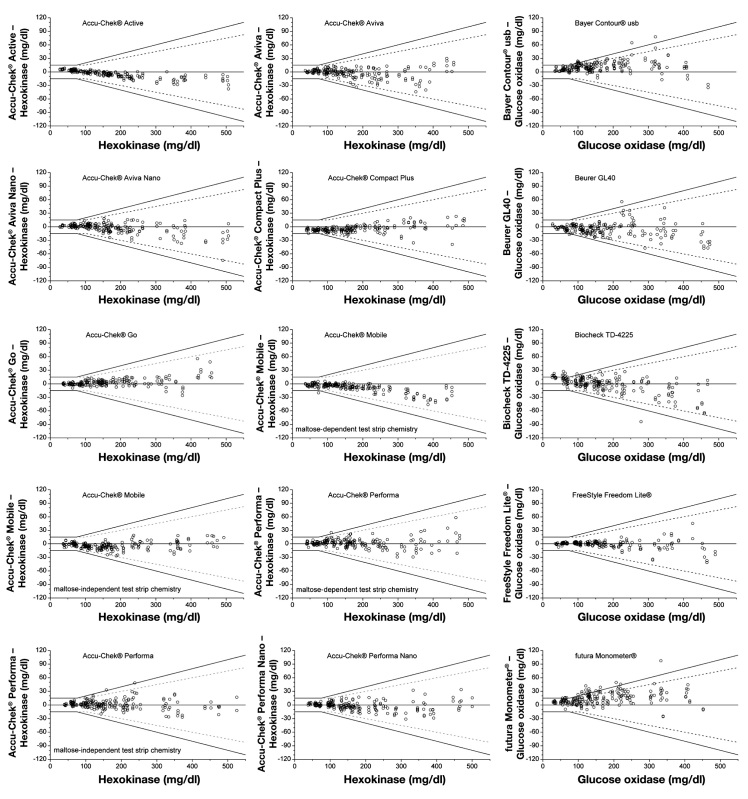
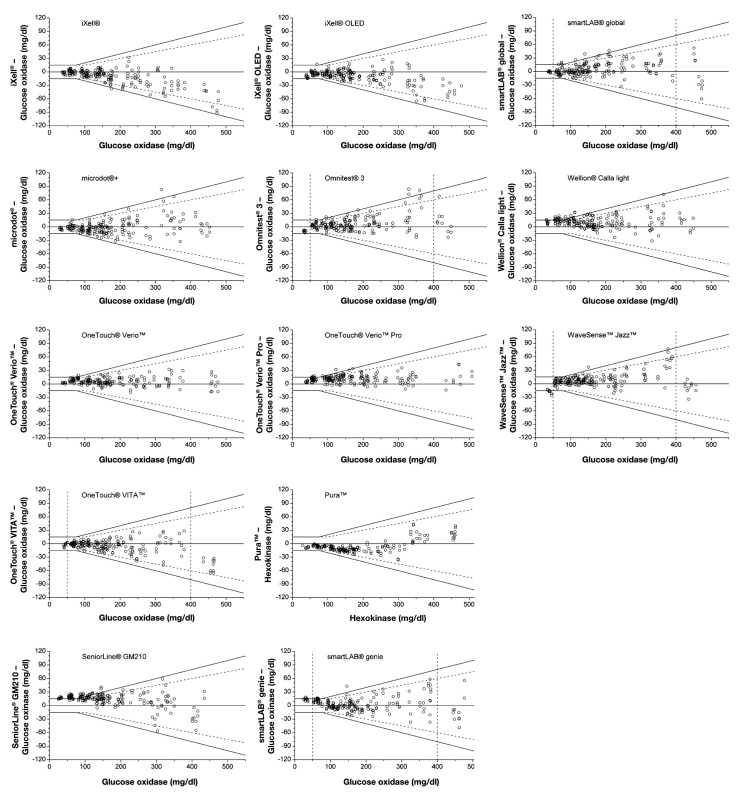
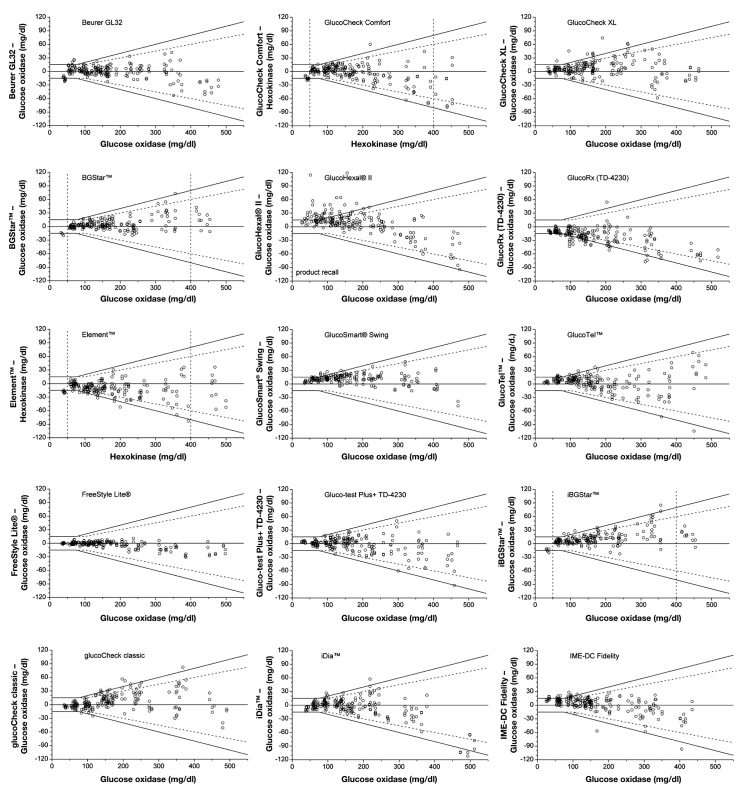
For all evaluated 43 BG systems, the agreement between the BG system results and the mean reference results according to DIN EN ISO 15197:2003 is illustrated in Figures 1A–1C. For each system, all 200 obtained results (BG concentration <50 to ≥400 mg/dl) are shown (Figures 1A–1C).
Figure 1A.
Difference plots of 43 BG systems. Black lines, system accuracy in accordance with DIN EN ISO 15197:2003; dashed lines, system overall accuracy determination according to the current draft revision of ISO 15197. For 9 BG systems with oxygen dependency (as mentioned in the manufacturer’s labeling), data of modified blood samples were excluded from overall system accuracy evaluation. For these 9 systems, the boundaries of concentration categories, including only unprepared blood samples (BG concentration ≥50 and <400 mg/dl) and categories that may include prepared blood samples (BG concentration <50 and ≥400 mg/dl), are marked by dashed perpendicular lines.
Figure 1C.
Difference plots of 43 BG systems. Black lines, system accuracy in accordance with DIN EN ISO 15197:2003; dashed lines, system overall accuracy determination according to the current draft revision of ISO 15197. For 9 BG systems with oxygen dependency (as mentioned in the manufacturer’s labeling), data of modified blood samples were excluded from overall system accuracy evaluation. For these 9 systems, the boundaries of concentration categories, including only unprepared blood samples (BG concentration ≥50 and <400 mg/dl) and categories that may include prepared blood samples (BG concentration <50 and ≥400 mg/dl), are marked by dashed perpendicular lines.
Figure 1B.
Difference plots of 43 BG systems. Black lines, system accuracy in accordance with DIN EN ISO 15197:2003; dashed lines, system overall accuracy determination according to the current draft revision of ISO 15197. For 9 BG systems with oxygen dependency (as mentioned in the manufacturer’s labeling), data of modified blood samples were excluded from overall system accuracy evaluation. For these 9 systems, the boundaries of concentration categories, including only unprepared blood samples (BG concentration ≥50 and <400 mg/dl) and categories that may include prepared blood samples (BG concentration <50 and ≥400 mg/dl), are marked by dashed perpendicular lines.
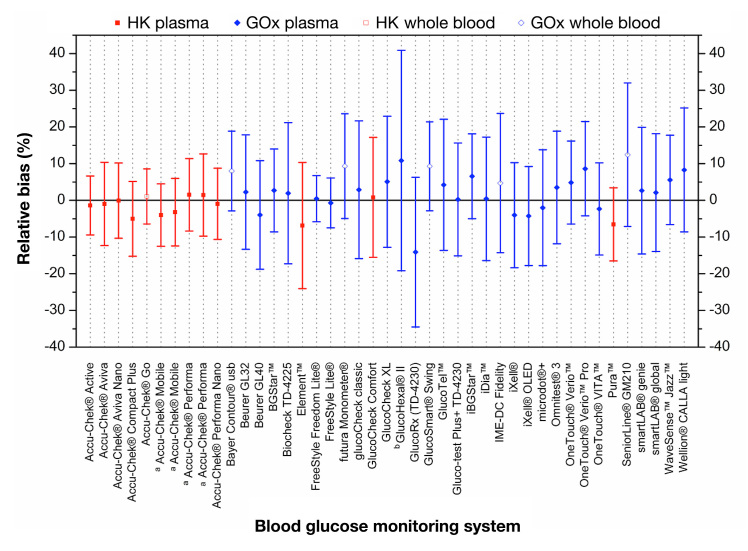
The bias according to Bland and Altman with limits of agreement of all investigated systems is shown in Figure 2 for the 43 evaluated BG systems. For the calculation of the bias, only data of native samples (BG concentration ≥50 and <400 mg/dl) were taken into account. The bias ranged from -14.1% (GlucoRx [TD-4230]) to +12.4% (SeniorLine® GM210; Figure 2). The bias was smallest for the Accu-Chek Aviva Nano system (bias, -0.1% with ±10.3% limits of agreement) and highest for GlucoRx (TD-4230; bias, -14.1% with ±20.4% limits of agreement).
Figure 2.
Bias according to Bland and Altman. Error bars represent 95% limits of agreement (≈ 1.96 × standard deviation). For the calculation of the bias of each system, only data of 180 unprepared blood samples (BG concentrations ≥50 and <400 mg/dl) were included. a: Accu-Chek® Mobileand Accu-Chek® Performa were both tested with different test strip chemistries. The test strip chemistry was either maltose dependent (left) or maltose independent (right). b: GlucoHexal® test strip lot was recalled from the market in June 2010, after at least 11 months’ availability on the market.
Discussion
Assessment of the system’s overall accuracy and deter-mination of conformity to DIN EN ISO 15197:2003 were performed with 43 systems. Of the 34 systems, for which a complete system accuracy assessment could be performed, 27 (79.4%) fulfilled the minimum accuracy requirements of the standard. Considering the tighter criteria of the current draft revision of ISO 15197, only 18 (52.9%) of these 34 systems fulfilled these minimum accuracy requirements. For 9 of the 43 evaluated systems, complete system accuracy assessment was not performed because of an oxygen dependency specified in the package insert.
The present study is focused on analytical accuracy of the BG systems under laboratory conditions and does not represent their total system accuracy20,21 when used by patients. However, the study provides an overview about the measurement quality of a broad range of CE-marked products available on the market. In a study published in 2010, 59% of 27 investigated BG systems fulfilled the minimum accuracy requirements of the ISO standard.16 Both studies demonstrate that the CE mark of a BG system does not ensure that the minimum required accuracy criteria are fulfilled in all cases. An important issue in this context is the tendency of health insurance companies and pharmacy associations (e.g., in Germany) to require the automatic supply of low-priced systems for SMBG to patients with diabetes in order to reduce health care spending.22 Our study shows that systems with a CE mark do not necessarily exhibit equal quality and therefore should not be used interchangeably without further considerations such as evaluation of measurement accuracy.23
The minimum accuracy requirements defined by the ISO standard apply to BG measurements over the full clinical relevant range. However, the accuracy of a BG system is probably not constant over the complete range of BG values and may exhibit different measurement qualities at different BG ranges. Previous discussions have already mentioned the evaluation of a system for the different clinically relevant BG ranges (hypoglycemic range, euglycemic range, and hyperglycemic range) separately.21,24 This would ensure more detailed information about the analytical quality of a system, which is needed for a better categorization of systems to ensure correct therapeutic decisions. The categorization of BG systems into different quality classes and for different patient groups with specific needs for accuracy is frequently discussed.21,25,26
Detailed comparison of different BG systems is difficult and has certain limitations. The evaluated systems are calibrated with either the GOx method or the HK method. Measurement errors of both reference methods as well as measurement differences of approximately up to 8% between these methods contribute to inaccuracy of the overall result that is not due to the systems per se.27 Additionally, the results may vary depending on whether whole blood or plasma samples are used for reference measurements. Furthermore, the conversion factor from whole blood BG values to plasma equivalent BG values is specific for the manufacturer. Not all manufacturers stick to the recommendations of the International Federation of Clinical Chemistry28 on reporting of BG results. In order to improve the comparability of system assessments by manufacturers, it would be useful to standardize the manufacturer’s reference measurement method and to further complete the standardization of the calibration mode to plasma calibration.
For nine systems, the test strip chemistry is labeled to be sensitive to blood oxygen content variations. For some of these nine systems, measurement results obtained in blood samples with glucose concentrations <50 or ≥400 mg/dl were remarkably different from the reference method. According to the ISO standard, blood samples with glucose concentrations <50 mg/dl can be obtained by incubation of capillary blood samples to allow glucose to hydrolyze, whereas glucose concentrations >400 mg/dl can be obtained by supplementation with glucose. However, different effects like oxygen consumption by blood cells as well as rapid equilibration with the oxygen in the ambient air, e.g., by air bubbles as well as by diffusion through gas-permeable blood collection tubes, make it quite difficult to maintain constant oxygen content in these modified samples. The preparation procedure employed in this study could also not ensure constant oxygen partial pressure in the modified samples. Several previous studies reported that some test strips, especially those with glucose oxidase enzyme reaction, are sensitive to oxygen and that high oxygen concentrations may lead to system results lower than the true value.29–33 Most of the published system accuracy evaluation studies either do not evaluate samples with BG concentration <80 and ≥300 mg/dl (or not sufficient numbers) or use venous blood.34–39 Main reasons for doing so are most likely the difficulty of designing a controlled human study or an adequate procedure to obtain capillary blood samples in hypoglycemic and hyperglycemic ranges.
Conclusions
In summary, 34 out of 43 BG systems were completely assessed, and 27 (79.4%) of these 34 systems fulfill the minimal accuracy requirements of the standard DIN EN ISO 15197:2003. Only 18 (52.9%) of 34 systems fulfilled the minimal accuracy requirements if tighter criteria of the current draft revision of ISO 15197 are considered. Because inaccurate systems bear the risk of false therapeutic decisions, regular and standardized evaluation of BG meters and test strips should be requested in order to ensure adherence to quality and accuracy standards.
Acknowledgments
We thank Prof. Dr. Theodor Koschinsky for his valuable input in data evaluation and discussion. We also thank Prof. Dr. Lutz Heinemann and Dr. Oliver Schnell for contributing their scientific expertise to the discussion.
Glossary
- (BG)
blood glucose
- (CE)
Conformité Européenne
- (GOx)
glucose oxidase
- (HK)
hexokinase
- (ISO)
International Organization for Standardization
- (NIST)
National Institute of Standards and Technology
- (SMBG)
self-monitoring of blood glucose
Funding
This study was funded by a grant from Roche Diagnostics GmbH, Mannheim, Germany.
Disclosures
Guido Freckmann received speaker’s honoraria and refunding of traveling expenses for congress participation from Roche Diagnostics GmbH, Mannheim, Germany, the sponsor of this study, and from Berlin-Chemie AG, Berlin, Germany.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Blonde L, Karter AJ. Current evidence regarding the value of self-monitored blood glucose testing. Am J Med. 2005;118((Suppl 9A)):20S–26S. doi: 10.1016/j.amjmed.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 3.IDF Clinical Guidelines Task Force Global Guideline for Type 2 Diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23(6):579–593. doi: 10.1111/j.1464-5491.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 5.Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: a single-blind, randomized, controlled trial. Pediatr Diabetes. 2006;7(3):159–164. doi: 10.1111/j.1399-543X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 6.Bergenstal RM, Gavin JR., 3rd Global Consensus Conference on Glucose Monitoring Panel. The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med. 2005;118((Suppl 9A)):1S–6S. doi: 10.1016/j.amjmed.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 7.Martin S, Schneider B, Heinemann L, Lodwig V, Kurth HJ, Kolb H, Scherbaum WA. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49(2):271–278. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- 8.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Petersen B, Schweitzer M, Wagner RS. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng WY, Tiong CC, Jacob E. Maltose interference-free test strips for blood glucose testing at point-of-care: a laboratory performance evaluation. Diabetes Technol Ther. 2010;12(11):889–893. doi: 10.1089/dia.2010.0095. [DOI] [PubMed] [Google Scholar]
- 10.Rao A, Wiley M, Iyengar S, Nadeau D, Carnevale J. Individuals achieve more accurate results with meters that are codeless and employ dynamic electrochemistry. J Diabetes Sci Technol. 2010;4(1):145–150. doi: 10.1177/193229681000400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice MJ. Dynamic electrochemistry: a step in the right direction. J Diabetes Sci Technol. 2011;5(5):1176–1178. doi: 10.1177/193229681100500521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musholt PB, Schipper C, Thomé N, Ramljak S, Schmidt M, Forst T, Pfützner A. Dynamic electrochemistry corrects for hematocrit interference on blood glucose determinations with patient self-measurement devices. J Diabetes Sci Technol. 2011;5(5):1167–1175. doi: 10.1177/193229681100500520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempf K, Heinemann L. Dynamic electrochemistry: an innovative method for high-quality blood glucose monitoring. Diabetologie und Stoffwechsel. 2012;7(2):121–126. [Google Scholar]
- 14.International Organization for Standardization In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. 2003. DIN EN ISO 15197.
- 15.International Organization for Standardization In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. 2011. (Draft.) ISO/DIS 15197.
- 16.Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, Heister F, Haug C. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010;12(3):221–231. doi: 10.1089/dia.2009.0128. [DOI] [PubMed] [Google Scholar]
- 17.Bundesärztekammer Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen. Dtsch Ärztebl. 2008;105(7):341–355. [Google Scholar]
- 18.Astles JR, Sedor FA, Toffaletti JG. Evaluation of the YSI 2300 glucose analyzer: algorithm-corrected results are accurate and specific. Clin Biochem. 1996;29(1):27–31. doi: 10.1016/0009-9120(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 20.Krouwer JS, Cembrowski GS. A review of standards and statistics used to describe blood glucose monitor performance. J Diabetes Sci Technol. 2010;4(1):75–83. doi: 10.1177/193229681000400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinemann L, Lodwig V, Freckmann G. Accuracy in blood glucose measurement: what will a tightening of requirements yield? J Diabetes Sci Technol. 2012;6(2):435–443. doi: 10.1177/193229681200600232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arzneiversorgungsvertrag (gültig ab 01. Oktober 2010) zwischen den Ersatzkassen und dem Deutschen Apothekerverband e. V. http://www.vdek.com/vertragspartner/apotheken/Arzneiversorgungsvertrag_1_10_2010.pdf (accessed August 28, 2012)
- 23.Freckmann G, Haug C, Heinemann L. Blutzuckerselbstmessung heute: Sind alle Geräte gleich? Diabetes Stoffwechsel Herz. 2011;20(4):235–241. [Google Scholar]
- 24.Klonoff DC. The need for separate performance goals for glucose sensors in the hypoglycemic normoglycemic, and hyperglycemic ranges. Diabetes Care. 2004;27(3):834–836. doi: 10.2337/diacare.27.3.834. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg BH. We need tighter regulatory standards for blood glucose monitoring, but they should be for accuracy disclosure. J Diabetes Sci Technol. 2010;4(5):1265–1268. doi: 10.1177/193229681000400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh J, Roberts R, Vigersky RA, Schwartz F. New Criteria for Assessing the Accuracy of Blood Glucose Monitors Meeting, October 28, 2011. J Diabetes Sci Technol. 2012;6(2):466–474. doi: 10.1177/193229681200600236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twomey PJ. Plasma glucose measurement with the Yellow Springs Glucose 2300 STAT and the Olympus AU640. J Clin Pathol. 2004;57(7):752–754. doi: 10.1136/jcp.2003.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Orazio P, Burnett RW, Fogh-Andersen N, Jacobs E, Kuwa K, Külpmann WR, Larsson L, Lewenstam A, Maas AH, Mager G, Naskalski JW, Okorodudu AO. International Federation of Clinical Chemistry Scientific Division Working Group on Selective Electrodes and Point of Care Testing. Approved IFCC recommendation on reporting results for blood glucose (abbreviated) Clin Chem. 2005;51(9):1573–1576. doi: 10.1373/clinchem.2005.051979. [DOI] [PubMed] [Google Scholar]
- 29.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3(4):903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hönes J, Müller P, Surridge N. The technology behind glucose meters: test strips. Diabetes Technol Ther. 2008;10((S1)):S10–26. [Google Scholar]
- 31.Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062–1070. doi: 10.1097/00003246-200105000-00038. [DOI] [PubMed] [Google Scholar]
- 32.Tang Z, Louie RF, Payes M, Chang KC, Kost GJ. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2(3):349–362. doi: 10.1089/15209150050194215. [DOI] [PubMed] [Google Scholar]
- 33.Kurahashi K, Maruta H, Usuda Y, Ohtsuka M. Influence of blood sample oxygen tension on blood glucose concentration measured using an enzyme-electrode method. Crit Care Med. 1997;25(2):231–235. doi: 10.1097/00003246-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Kristensen GB, Monsen G, Skeie S, Sandberg S. Standardized evaluation of nine instruments for self-monitoring of blood glucose. Diabetes Technol Ther. 2008;10(6):467–477. doi: 10.1089/dia.2008.0034. [DOI] [PubMed] [Google Scholar]
- 35.Kuo CY, Hsu CT, Ho CS, Su TE, Wu MH, Wang CJ. Accuracy and precision evaluation of seven self-monitoring blood glucose systems. Diabetes Technol Ther. 2011;13(5):596–600. doi: 10.1089/dia.2010.0223. [DOI] [PubMed] [Google Scholar]
- 36.Tack C, Pohlmeier H, Behnke T, Schmid V, Grenningloh M, Forst T, Pfützner A. Accuracy evaluation of five blood glucose monitoring systems obtained from the pharmacy: a european multicenter study with 453 subjects. Diabetes Technol Ther. 2012;14(4):330–337. doi: 10.1089/dia.2011.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essack Y, Hoffman M, Rensburg M, Van Wyk J, Meyer CS, Erasmus R. A comparison of five glucometers in South Africa. JEMDSA. 2009;14(2):102–105. [Google Scholar]
- 38.Harrison B, Leazenby C, Halldorsdottir S. Accuracy of the CONTOUR® blood glucose monitoring system. J Diabetes Sci Technol. 2011;5(4):1009–1013. doi: 10.1177/193229681100500425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfützner A, Mitri M, Musholt PB, Sachsenheimer D, Borchert M, Yap A, Forst T. Clinical assessment of the accuracy of blood glucose measurement devices. Curr Med Res Opin. 2012;28(4):525–531. doi: 10.1185/03007995.2012.673479. [DOI] [PubMed] [Google Scholar]