Abstract
Background
Self-monitoring of blood glucose (SMBG) and continuous glucose monitoring (CGM) have been proven effective in improving hemoglobin A1c (HbA1c) and in reducing hypoglycemia in patients with type 1 diabetes mellitus (T1DM). It is not clear, however, if CGM provides further efficacy and safety benefits beyond SMBG in the management of T1DM.
Methods
MEDLINE (1966–November 2009), COCHRANE REGISTRY (all years), and EMBASE (1980–November 2009), and article bibliographies were searched for randomized controlled trials (RCTs) investigating the use of CGM in patients with T1DM, with clinical outcomes, including HbA1c and hypoglycemia and/or hyperglycemia.
Results
Fourteen RCTs met eligibility criteria [n = 1188 patients, 97.4% with T1DM, age 29.0 ± 14.3 years, diabetes duration 11.7 ± 7.0 years, and baseline HbA1c 8.3 ± 0.8% (mean ± standard deviation)]. Compared with SMBG, the use of CGM was associated with a greater reduction in HbA1c [-0.3% (confidence interval: 0.4, -0.2), p < .0001]. The number of hypoglycemic events was not significantly different between the CGM and SMBG groups (0.52 ± 0.52 versus 0.52 ± 0.63 events/day, p = .5), but duration of hypoglycemia was shorter for the CGM group (75 ± 39 versus 89 ± 19 min/day), with an incremental reduction of hypoglycemia duration of -15.2 min/day, p < .0001. Continuous glucose monitoring also resulted in a shorter duration of hyperglycemia than SMBG (172 ± 125 versus 217 ± 152 min/day, p = .04).
Conclusions
The use of CGM is associated with improvement in metabolic control in T1DM, with significant short- and long-term reductions in HbA1c and reduction in the duration of periods of hypoglycemia and hyperglycemia versus SMBG.
Keywords: glucose monitoring, meta-analysis, monitoring device, type 1 diabetes
Introduction
Diabetes is the most important chronic metabolic illness, accounting for the majority of blindness, kidney failure, and nontraumatic amputations of the lower extremities.1–3 It is well established that improved glycemic control can prevent and reduce the progression of microvascular1,2 and macrovascular4 complications. Self-monitoring of blood glucose (SMBG) is the most important tool in implementing tight glucose control in diabetes patients. Despite frequent blood glucose (BG) measurements throughout the day, SMBG is associated with gaps in glycemic control and the inability to follow glycemic trends to prevent hypoglycemia.5,6 To overcome this limitation, continuous glucose monitoring (CGM) has been introduced and provides more detailed information on glycemic control than SMBG.7
Two types of CGM devices are commercially available—retrospective and real-time. Retrospective CGM is a Holter-type device that measures interstitial glucose levels and stores the information during a period of 48 to 72 h to facilitate insulin adjustment, recognition of daily BG swings, and prevention of hypoglycemia.8 Its retrospective nature, however, represents a significant limitation with patients unable to react to BG changes before they reach abnormal ranges.9–13 In contrast, real-time CGM technology provides current BG estimates and direction and magnitude of glucose trends, allowing patients to take action that might reduce glycemic excursions outside a target range.8 Real-time CGM technology has been shown to facilitate glycemic control and reduce hypoglycemia in insulin-treated patients.5,6,8,14
Comparison studies on the efficacy and clinical benefits of CGM devices and SMBG have produced mixed results, in part, due to small sample size and variability across studies, including differences in age of subjects, type of CGM utilized (retrospective versus real time), and duration of follow-up. Two previous meta-analyses reported no significant difference in improving glycemic control between CGM and SMBG,15,16 with one focused solely on T1DM16 while the other was limited to retrospective data because it was performed prior real-time CGM availability.15 Accordingly, this meta-analysis aims to determine (1) overall efficacy and safety of retrospective and real-time CGM and SMBG and (2) differences in glycemic control between real-time and retrospective CGM patients with type 1 diabetes mellitus (T1DM) across the age continuum.
Material and Methods
Data Sources and Searches
This systematic review and meta-analysis was conducted in accordance with the QUOROM (Quality of Reporting of Meta-Analyses) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statements.17,18 We searched electronic databases for relevant articles: MEDLINE (1966–November 2009), EMBASE (1980–November 2009), and the Cochrane Registry (all years). We used a combination of medical subject headings and text terms that included “diabetes mellitus,” “continuous glucose monitoring,” and/or “continuous glucose monitoring system,” with the following limits activated: “humans,” “randomized controlled trials,” and “English language.”
Study Selection
Selection criteria included English language randomized controlled trials (RCTs), subjects with T1DM, use of subcutaneous CGM in outpatient setting, and reporting changes in hemoglobin A1c (HbA1c) and/or hyperglycemia and hypoglycemia. Randomized controlled trials focusing on medication or psychological effects, evaluating nutritional or exercise-related interventions, and including pregnant patients, oral antidiabetic agents, or inpatient settings were excluded. Abstracts were reviewed independently by two authors (Baraka Floyd and Stephanie Hall) based on the inclusion and exclusion criteria without the use of masking.19
Data Extraction and Quality Assessment
Authors rated studies for quality using the Jadad score20 and extracted study characteristics, subjects’ clinical demo-graphics, and outcome measures using a standardized data abstraction instrument. The primary outcome measure was the absolute HbA1c change from baseline to study end. Secondary outcome measures included HbA1c change during intervention, hypoglycemia frequency (BG <70 mg/dl), durations of hypoglycemia (min/day), profound hypoglycemia (min/day BG ≤55 mg/dl), normoglycemia (min/day BG 71–180 mg/dl), and hyper-glycemia (min/day BG ≥250 mg/dl).
Data Synthesis and Analysis
For each study, we calculated weighted mean difference for primary and secondary outcome measures with a 95% confidence interval (CI). We pooled individual study results using both fixed and random effects models, the latter incorporating inverse variance according to methods developed by DerSimonian and Laird.21 We used standard approaches to sensitivity analysis in subgroups defined by duration of follow-up, age group, use of real-time or retrospective CGM devices, and use of an intermittent CGM scheme (72 h increments with varying rest periods) or continuous monitoring scheme (24 h/day≥ 6 days/week) to assess for potential confounding. We explored qualitative and quantitative heterogeneity using Chi-square tests, with a significance value of p < .05. We also assessed publication bias using Begg’s funnel plot method.22,23 Final results are reported using random effects meta-analysis unless no quantitative heterogeneity was present.
Results
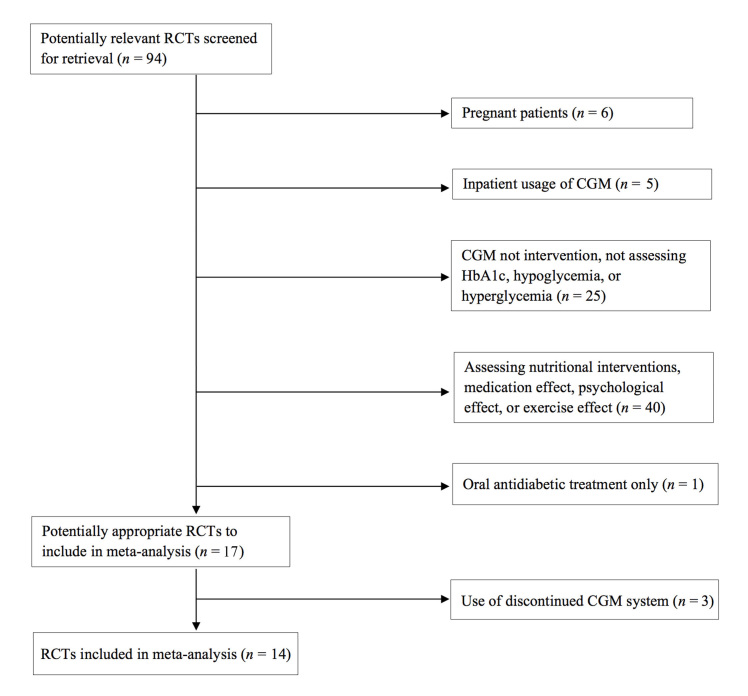
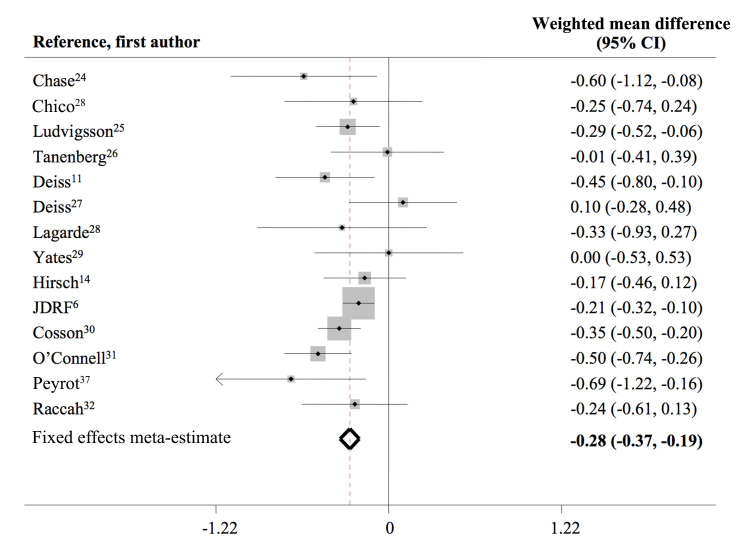
A total of 96 studies were identified electronically; of them, 25 studies were potentially eligible and retrieved for review. Sixteen studies met eligibility criteria and were included in the meta-analysis (Figure 1). Overall quality of selected studies was good, with a median Jadad score of 3 (range 1 to 3). We found no significant evidence of quantitative heterogeneity (Figure 2) or publication bias via the Begg’s funnel plot.
Figure 1.
Study selection process
Figure 2.
Forrest plot for changes in HbA1c (%) from baseline to end of study among different studies
The pooled population comprised 1188 participants, 97.4% with T1DM, mean age of 29.0 ± 14.3 years, diabetes duration of 11.7 ± 7.0 years, and baseline HbA1c of 8.3% ± 0.8% (mean ± standard deviation). Eight studies utilized real-time CGM, and eight used retrospective CGM (Table 1). Compared with SMBG, CGM was associated with a significant HbA1c reduction [∆HbA1c -0.5% ± 0.5% (p = .002) versus -0.2% ± 0.3% (p = .006); p = .006 between groups] and an incremental HbA1c reduction of 0.3% (-0.3, -0.2), p < .0001 (Table 2, Figure 2).
Table 1.
Characteristics of Included Studiesa
| Reference,first author | Number of patients | Age, years | Diabetes duration, years | % CSII | Baseline HbA1c | CGM frequency | SMBG frequency |
|---|---|---|---|---|---|---|---|
| Chase24b | 11 | 13.4 ± 13.6 | 6.2 ± 9.9 | 54.6% | 9.5 ± 0.95 | 72 h/5 days | Not reported |
| Chico33b | 75 | 38.7 ± 11 | 19 ± 11 | 0% | 8.2 ± 1.5 | 72 h/3 months | Not reported |
| Ludvigsson25b | 32 | 12.5 ± 3.3 | 7.0 ± 3.9 | 51.9% | 7.7 ± 0.95 | 72 h/2 weeks | ≥2x/day + 7-point SMBG/week |
| Tanenberg26b | 109 | 44.3 ± 11.4 | 19.9 ± 9.9 | 45.8% | 9.1 ± 1.1 | 72 h/3 months | ≥4x/day |
| Deiss11c | 162 | 26.8 ± 13.6 | Not reported | 48.1% | 9.6 ± 1.1 | Continuous or 72 h/2 weeks | Not reported |
| Deiss27b | 30 | 11.4 ± 13.6 | 2.2 ± 9.9 | Not reported | 8.1 ± 1.1 | 72 h/3 months | Not reported |
| Lagarde28b | 27 | 11.4 ± 13.6 | 4.4 ± 9.9 | 70.4% | 7.85 ± 1.3 | 72 h/2 months | ≥4x/day + 2 AM/week |
| Yates29b | 36 | 14.4 ± 13.6 | Not reported | 47% | 7.9 ± 0.85 | Eight 72 h periods | 4–6x/day |
| Hirsch14c | 138 | 33.1 ± 15.5 | 18.7 ± 11.6 | 100% | 8.45 ± 0.7 | Continuous | Not reported |
| JDRF6c | 322 | 24.4 ± 5.5 | 12.5 ± 6 | 79.7% | 7.85 ± 0.6 | Continuous | ≥4x/day |
| Cosson30b | 41 | 53.8 ± 13.6 | 15.9 ± 9.9 | 11.1% | 9.05 ± 1.05 | Two 48 h periods | Not reported |
| O’Connell31c | 62 | 23.2 ± 8.4 | 10.2 ± 7.4 | 100% | 8.4 ± 1.65 | Continuous | ≥4x/day |
| Peyrot36c | 28 | 47.2 ± 13.2 | 25 ± 12.6 | 100% | 8.6 ± 1.0 | Continuous | Not reported |
| Raccah32c | 115 | 28.5 ± 15.9 | 11.8 ± 8.9 | 100% | 9.2 ± 1.24 | Continuous | ≥3x/day |
Continuous, wearing the device ≥6 days/week or 70% of the time; % CSII, percentage of patients using continuous subcutaneous insulin infusion.
Retrospective CGM.
Real-time CGM.
Table 2.
Hemoglobin A1c Outcomes and Number and Duration of Hypoglycemic, Hyperglycemic, and Normoglycemic Eventsa
| Outcome | SMBG | CGM | Meta-estimate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c baseline | HbA1c study end | ∆ HbA1c from baseline | p value ∆ HbA1c from baseline | HbA1c baseline | HbA1c study end | ∆ HbA1c from baseline | p value ∆ HbA1c from baseline | p value CGM versus SMBG | ∆ HbA1c from baseline (%) [95% CI]b | p value absolute ∆ HbA1c from baseline | |
| All studies, n = 14 | 8.3 ± 0.7 | 8.0 ± 0.6 | -0.2 ± 0.3 | 0.006 | 8.3 ± 0.8 | 7.8 ± 0.6 | -0.5 ± 0.5 | 0.002 | 0.006 | -0.3[-0.3, -0.2] | <0.0001 |
| Duration of intervention | |||||||||||
| 4–8 weeksc | 8.9 ± 0.7 | 8.6 ± 0.8 | -0.3 ± 0.2 | 0.04 | 9.0 ± 0.8 | 8.6 ± 0.7 | -0.5 ± 0.2 | 0.03 | 0.1 | -0.2[-0.3, 0.01] | 0.06 |
| 12–16 weeksc | 8.2 ± 0.7 | 7.9 ± 0.6 | -0.3 ± 0.4 | 0.02 | 8.3 ± 0.8 | 7.7 ± 0.6 | -0.6 ± 0.5 | 0.01 | 0.01 | -0.3[-0.3, -0.2] | <0.0001 |
| 24–26 weeksc | 8.0 ± 0.6 | 7.8 ± 0.5 | -0.2 ± 0.2 | 0.04 | 8.1 ± 0.5 | 7.7 ± 0.4 | -0.4 ± 0.3 | 0.05 | 0.1 | -0.2[-0.3, -0.1] | <0.0001 |
| Age group (years) | |||||||||||
| <25c | 7.8 ± 0.6 | 7.8 ± 0.4 | -0.1 ± 0.2 | 0.003 | 7.8 ± 0.9 | 7.5 ± 0.6 | -0.3 ± 0.4 | 0.05 | 0.04 | -0.2[-0.3, -0.2] | <0.0001 |
| ≥25c | 8.9 ± 0.6 | 8.5 ± 0.8 | -0.4 ± 0.2 | 0.2 | 9.0 ± 0.4 | 8.3 ± 0.6 | -0.7 ± 0.4 | 0.02 | 0.01 | -0.3[-0.4, -0.2] | <0.0001 |
| Monitoring scheme | |||||||||||
| Intermittentc | 8.7 ± 0.6 | 8.4 ± 0.6 | -0.3 ± 0.2 | 0.02 | 8.7 ± 0.8 | 8.2 ± 0.6 | -0.6 ± 0.4 | 0.03 | 0.04 | -0.3[-0.4, -0.2] | <0.0001 |
| Continuousc | 8.0 ± 0.8 | 7.8 ± 0.4 | -0.2 ± 0.4 | 0.06 | 8.0 ± 0.8 | 7.6 ± 0.6 | -0.6 ± 0.5 | 0.02 | 0.03 | -0.4[-0.5, -0.2] | <0.0001 |
| Type of CGM | |||||||||||
| Retrospective CGMc | 8.6 ± 0.5 | 8.3 ± 0.5 | -0.3 ± 0.3 | 0.03 | 8.7 ± 0.8 | 8.1 ± 0.5 | -0.5 ± 0.4 | 0.04 | 0.06 | -0.3[-0.4, -0.2] | <0.0001 |
| Real-time CGMc | 8.0 ± 0.8 | 7.9 ± 0.7 | -0.2 ± 0.4 | 0.06 | 8.0 ± 0.8 | 7.6 ± 0.7 | -0.4 ± 0.5 | 0.02 | 0.03 | -0.3[-0.5, -0.2] | <0.0001 |
| Outcome | Mean SMBG value | Mean CGM value | p value CGM versus SMBG | p value weighted mean difference [95% CI]d | p value weighted mean difference |
|---|---|---|---|---|---|
| Hypoglycemic events (episodes/day BG ≤ 70 mg/dl)c | 0.52 ± 0.63 | 0.52 ± 0.52 | 0.5 | 0.01 [-0.21,0.23] | 0.1 |
| Duration of hypoglycemia (min/day BG ≤ 80 mg/dl)c | 89.53 ± 19.22 | 75.34 ± 39.21 | 0.1 | -15.2 [-20.3, -10.1] | <0.0001 |
| Duration of profound hypoglycemia (min/day BG ≤55 mg/dl)c | 30.63 ± 14.09 | 27.65 ± 31.10 | 0.2 | -8.8 [-11.8, -5.7] | <0.0001 |
| Duration of normo-glycemia (min/day BG = 71–180 mg/dl)e | 751.44 ± 263.67 | 810.06 ± 286.40 | 0.05 | 67.17 [24.06, 107.49] | <0.0001 |
| Duration of hyperglycemia (min/day BG ≥ 240 mg/dl)e | 217.53 ± 152.94 | 172.26 ± 125.90 | 0.04 | -45.3 [-65.5, -25.0] | <0.0001 |
Data are weighted mean ± standard error of the mean.
Meta-estimate is the absolute ∆ HbA1c generated from the difference between end of study HbA1c and baseline HbA1c for CGM and SMBG groups where the value for each study is weighted by the inverse variance.
Based on random effects meta-analysis.
Weighted mean difference is generated from the difference between the CGM and SMBG groups weighted by inverse variance.
Based on fixed effects meta-analysis.
The hypoglycemia frequency was not significantly different between the CGM and SMBG groups; however, the duration of hypoglycemia was shorter for the CGM group, with CGM producing an incremental reduction of hypoglycemia duration of -15.2 min/day, p < .0001. In addition, the duration of profound hypoglycemia and hyperglycemia were significantly shorter in the CGM group compared with the SMBG group (Table 2).
Duration of Glucose Monitoring and Glycemic Control
Improvements in HbA1c after 4–8 weeks of intervention were greater with CGM (∆HbA1c -0.5% ± 0.2%, p = .03) than SMBG (∆HbA1c -0.3% ± 0.2%, p = .04). Changes in HbA1c from baseline between CGM and SMBG were not significantly different (p = .1). Continuous glucose monitoring was associated with an incremental reduction of HbA1c compared with SMBG. After 12 to 16 weeks of follow-up, CGM was associated with a significant reduction in HbA1c from baseline of -0.6% ± 0.5%,p = .01, greater than the use of SMBG, which resulted in ∆HbA1c from baseline of -0.3% ± 0.4%, p = .02, with a statistically significant difference between groups [-0.3% (-0.3, -0.2), p < .0001]. Long-term studies of 24–26 weeks reported ∆HbA1c from baseline of -0.4% ± 0.3% (p = .05) with CGM and -0.2% ± 0.2% (p = .04) with SMBG but were not statistically different (p = .1). In long-term studies, the incremental HbA1c reduction estimated by WMD between groups was -0.2% (-0.3, -0.1), p < .0001 (Table 2).
Monitoring Scheme, Type of Continuous Glucose Monitoring, and Glycemic Control
Continuous use of CGM was associated with significant improvement in glycemic control versus SMBG [∆HbA1c -0.6% ± 0.5% (p = .02) versus -0.2% ± 0.4% (p = .06); p = .03 between groups], with an incremental HbA1c reduction between CGM and SMBG of -0.4% (-0.5, -0.2), p < .0001. Compared with SMBG, intermittent use of CGM also resulted in significantly better glycemic control [∆HbA1c -0.6% ± 0.4% (p = .03) versus -0.3% ± 0.2% (p = .02); p = .004 between groups], producing an incremental HbA1c reduction over SMBG of -0.3% (-0.4, -0.2), p < .0001. The use of retrospective CGM was also associated with a greater reduction in HbA1c levels than SMBG [∆HbA1c -0.5% ± 0.4% (p = .04) versus -0.3% ± 0.3% (p = .03)], with no significant difference between groups (p = .06). However, the incremental reduction in HbA1c over time between retrospective CGM and SMBG was significant at -0.3% (-0.4, -0.2), p < .0001. For real-time CGM, differences in HbA1c levels from baseline were significantly greater than SMBG [∆HbA1c -0.4% ± 0.5% (p = .02) versus -0.2% ± 0.4% (p = .06); p = .03 between groups], with an incremental HbA1c reduction with real-time CGM when compared with SMBG of -0.3% (-0.5, -0.2), p < .0001 (Table 2).
Age and Continuous Glucose Monitoring
We performed subgroup analyses to determine the impact of age of study patients on glycemic control. Studies were divided into two groups, mean age under 25 years and over 25 years. In patients <25 years, CGM resulted in a significantly greater HbA1c reduction than SMBG [-0.3% ± 0.4% (p = .05) versus -0.1% ± 0.2% (p = .003); p = .04 between groups], with an incremental HbA1c reduction of -0.2% (-0.3, -0.2), p < .0001. Similarly, in patients ≥ 25 years of age, CGM resulted in greater improvement in glycemic control than SMBG [∆HbA1c -0.7% ± 0.4% (p = .02) versus -0.4% ± 0.2% (p = .2); p = .01 between groups], producing an incremental HbA1c reduction over SMBG of -0.3% (-0.4, -0.2), p < .0001 (Table 2).
Discussion
The results of this meta-analysis indicate that the use of CGM and SMBG are associated with improved glycemic control, as evidenced by HbA1c reduction from baseline and that both retrospective and real-time CGM are associated with significant incremental benefit in HbA1c reduction over SMBG. The use of real-time CGM technology37 results in slightly greater reductions in HbA1c levels than retrospective CGM. Greater benefits of CGM over SMBG were observed in studies of more than 12 weeks of intervention and in subjects >25 years old, with the incremental benefit of CGM in patients <25 years old just below the level of clinical significance (0.3%). While continuous use of CGM was associated with the greatest incremental benefit, intermittent CGM use also produced a clinically significant HbA1c reduction. In addition, CGM resulted in less time spent in hypoglycemic ranges than SMBG, but there was no difference in the hypoglycemia frequency. Continuous glucose monitoring was also associated with a reduction in time spent in hyperglycemic ranges and in greater time spent within normoglycemia, suggesting that more detailed information provided by CGM on glucose trends attenuates glycemic derangements and glucose swings.
The duration of CGM monitoring is an important factor in improving glycemic control. Studies using CGM for less than 8 weeks resulted in no significant improvement in HbA1c from baseline compared with SMBG. The incremental benefit in HbA1c reduction with CGM use was observed in most of the studies of 12–16 and 24–26 weeks’ duration. Real-time CGM provided a benefit over retrospective technology; however, the use of retrospective CGM was also associated with improved HbA1c from baseline over SMBG. In addition, we observed benefits with intermittent as well as continuous CGM use, indicating that even a small increase in the amount of information about glycemic changes is helpful. The mechanism for improved glycemic control with CGM likely results from facilitating better planning of daily and supplemental insulin doses, ability of patients using CGM to take preemptive action for rising and falling BG levels, and avoidance of factors negatively affecting glycemic derangements over long-term periods.
Age of study population is an important factor in determining the efficacy of CGM. In patients younger than 25 years, CGM produces a small and nonsignificant incremental benefit in HbA1c reduction compared with SMBG,6 likely because of less compliance with using the device. Two studies have shown that the greatest benefit is in patients 25 years and over who are more likely to wear the device with increased frequency than the younger age group.6,35
A key limitation of this meta-analysis is that only study-level data were available for analysis, requiring grouping by mean values of the entire study population, for which the values of individual patients may have been higher or lower than the study mean. We attempted to capture this information by reporting the CI for the meta-estimates. We found no significant quantitative heterogeneity in the evaluation of the primary outcome, but the meta-analyses for hypoglycemia frequency and hypoglycemia and profound hypoglycemia durations did show significant statistical heterogeneity. Heterogeneity for hypoglycemia frequency was explained by the type of CGM, while heterogeneity for hypoglycemia and profound hypoglycemia durations was explained by age. Finally, adherence, a known factor affecting treatment successes and failures in diabetes management,35 could not be evaluated, as only two of the included studies provided a measure of adherence.
The Endocrine Society Clinical Practice Guideline Committee on the use of CGM has recommended against the use of this technology in the intensive care unit or operating room settings.38 This recommendation was based on the limited available data related to accuracy and the concerns regarding potential danger in their use in guiding insulin administration in an acute-care setting. Several studies have reported on the accuracy of CGM in critically ill patients.39–41 Most of these studies included a small number of patients and limited their analysis to retrospective comparisons of a reference point-of-care value with CGM data.38 The use of CGM may have an advantage over bedside point-of-care testing in that it has the potential to reduce the possibility of unknown hypoglycemic events that may occur between point-of-care measurements; however, some studies have raised concern on the accuracy of CGM at low BG levels and limited correlation between CGM and both capillary and arterial samples when the BG is less than 81 mg/dl.42Thus this technology must undergo larger and rigorous testing before it can be recommended for use in the hospital setting.
In summary, the results of this meta-analysis suggest that CGM is a useful clinical tool that is associated with clinically significant HbA1c reductions without an increase in hypoglycemic events. In addition, CGM provides information regarding the direction, magnitude, duration, frequency, and causes of BG excursions and helps identify periods of hypoglycemia and hyperglycemia. Evidence indicates that highly motivated and compliant patients are the best candidates for successful CGM implementation, with optimal results yielded if the sensor is worn at least 6 days per week.35 The benefits of CGM in clinical practice are welcome, but long-term prospective RCTs are needed to evaluate the impact of CGM on the prevention of acute and chronic complications of diabetes.
Acknowledgments
The authors thank Jackie Ali and Dr. Alexander Quarshie from the Master of Science in Clinical Research Program, Rondereo Sidney from the Atlanta Clinical and Translational Science Institute, Gwinnett Bates and Catherine Williams-Parker of the Morehouse School of Medicine Library, and the staff of the Clinical Research Center.
Glossary
- (BG)
blood glucose
- (CGM)
continuous glucose monitoring
- (CI)
confidence interval
- (HbA1c)
hemoglobin A1c
- (RCT)
randomized controlled trial
- (SMBG)
self-monitoring of blood glucose
- (T1DM)
type 1 diabetes mellitus
Funding
This study was supported by Grant Numbers U54 RR026137, 2R25RR017694-06A1, UL1 RR025008, TL1 RR025010, and 5P20RR011104 from the National Center for Research Resources, a component of the National Institutes of Health. Dr. Umpierrez is supported by research grants from the American Diabetes Association (7-03-CR-35) and National Institutes of Health (UL1 RR025008; Atlanta Clinical and Translational Science Institute).
References
- 1.American Diabetes Association, Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gore MO, McGuire DK. The 10-year post-trial follow-up of the United Kingdom Prospective Diabetes Study (UKPDS): cardiovascular observations in context. Diab Vasc Dis Res. 2009;6(1):53–55. doi: 10.3132/dvdr.2009.012. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, Zinman B, Jacobson A, Sun W, Lachin JM, Nathan DM. DCCT/EDIC Research Group. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55(12):3556–3565. doi: 10.2337/db06-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV. A randomized prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27(7):1554–1558. doi: 10.2337/diacare.27.7.1554. [DOI] [PubMed] [Google Scholar]
- 6.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 7.Weintrob N, Schechter A, Benzaquen H, Shalitin S, Lilos P, Galatzer A, Phillip M. Glycemic patterns detected by continuous subcutaneous glucose sensing in children and adolescents with type 1 diabetes mellitus treated by multiple daily injections vs continuous subcutaneous insulin infusion. Arch Pediatr Adolesc Med. 2004;158(7):677–684. doi: 10.1001/archpedi.158.7.677. [DOI] [PubMed] [Google Scholar]
- 8.Skyler JS. Continuous glucose monitoring: an overview of its development. Diabetes Technol Ther. 2009;11(Suppl 1):S5–10. doi: 10.1089/dia.2009.0045. [DOI] [PubMed] [Google Scholar]
- 9.Buckingham B, Block J, Wilson DM. Continuous glucose monitoring. Curr Opin Endocrinol Diabetes. 2005;12:273–279. doi: 10.1097/MED.0b013e32825a675e. [DOI] [PubMed] [Google Scholar]
- 10.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350(22):2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 11.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 12.Klonoff DC. Continuous glucose monitoring delivers detailed diabetes data. Point Care. 2006;5(3):105–111. [Google Scholar]
- 13.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c. Diabetes Care. 2002;25(2):275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–383. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 15.Chetty VT, Almulla A, Odueyungbo A, Thabane L. The effect of continuous subcutaneous glucose monitoring (CGMS) versus intermittent whole blood finger-stick glucose monitoring (SBGM) on hemoglobin A1c (HBA1c) levels in Type I diabetic patients: a systematic review. Diabetes Res Clin Pract. 2008;81(1):79–87. doi: 10.1016/j.diabres.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Golicki DT, Golicka D, Groele L, Pankowska E. Continuous glucose monitoring system in children with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2008;51(2):233–240. doi: 10.1007/s00125-007-0884-9. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Rev Esp Salud Publica. 2000;74(2):107–118. [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6((7)):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justice AC, Cho MK, Winker MA, Berlin JA, Rennie D. Does masking author identity improve peer review quality? A randomized controlled trialPEER Investigators. JAMA. 1998;280(3):240–242. doi: 10.1001/jama.280.3.240. [DOI] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, Garg SK. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107(2):222–226. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics. 2003;111((5 Pt 1)):933–938. doi: 10.1542/peds.111.5.933. [DOI] [PubMed] [Google Scholar]
- 26.Tanenberg R, Bode B, Lane W, Levetan C, Mestman J, Harmel AP, Tobian J, Gross T, Mastrototaro J. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526. doi: 10.4065/79.12.1521. [DOI] [PubMed] [Google Scholar]
- 27.Deiss D, Hartmann R, Schmidt J, Kordonouri O. Results of a randomised controlled cross-over trial on the effect of continuous subcutaneous glucose monitoring (CGMS) on glycaemic control in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2006;114((2)):63–67. doi: 10.1055/s-2006-923887. [DOI] [PubMed] [Google Scholar]
- 28.Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: a single-blind, randomized, controlled trial. Pediatr Diabetes. 2006;7((3)):159–164. doi: 10.1111/j.1399-543X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 29.Yates K, Hasnat Milton A, Dear K, Ambler G. Continuous glucose monitoring-guided insulin adjustment in children and adolescents on near-physiological insulin regimens: a randomized controlled trial. Diabetes Care. 2006;29((7)):1512–1517. doi: 10.2337/dc05-2315. [DOI] [PubMed] [Google Scholar]
- 30.Cosson E, Hamo-Tchatchouang E, Dufaitre-Patouraux L, Attali JR, Pariès J, Schaepelynck-Bélicar P. Multicentre, randomised, controlled study of the impact of continuous sub-cutaneous glucose monitoring (GlucoDay) on glycaemic control in type 1 and type 2 diabetes patients. Diabetes Metab. 2009;35((4)):312–318. doi: 10.1016/j.diabet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 31.O’Connell MA, Donath S, O’Neal DN, Colman PG, Ambler GR, Jones TW, Davis EA, Cameron FJ. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52((7)):1250–1257. doi: 10.1007/s00125-009-1365-0. [DOI] [PubMed] [Google Scholar]
- 32.Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire H, Jeandidier N, Nicolino M. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32((12)):2245–2250. doi: 10.2337/dc09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chico A, Vidal-Ríos P, Subirà M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care. 2003;26((4)):1153–1157. doi: 10.2337/diacare.26.4.1153. [DOI] [PubMed] [Google Scholar]
- 34.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29((1)):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 35.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, Chase P, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Huang ES, Kollman C, Kowalski AJ, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer SA, Wilson DM, Wolpert H, Wysocki T, Xing D. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32((8)):1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peyrot M, Rubin RR. Patient-reported outcomes for an integrated real-time continuous glucose monitoring/insulin pump system. Diabetes Technol Ther. 2009;11((1)):57–62. doi: 10.1089/dia.2008.0002. [DOI] [PubMed] [Google Scholar]
- 37.Hirsch IB. Continuous glucose monitoring: understanding our current culture. Diabetes Technol Ther. 2009;11(Suppl 1):S131–2. doi: 10.1089/dia.2009.0021. [DOI] [PubMed] [Google Scholar]
- 38.Klonoff DC, Buckingham B, Christiansen JS, Montori VM, Tamborlane WV, Vigersky RA, Wolpert H. Endocrine Society. Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96((10)):2968–2979. doi: 10.1210/jc.2010-2756. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg PA, Siegel MD, Russell RR, Sherwin RS, Halickman JI, Cooper DA, Dziura JD, Inzucchi SE. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol Ther. 2004;6((3)):339–347. doi: 10.1089/152091504774198034. [DOI] [PubMed] [Google Scholar]
- 40.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, Jaksic T, Agus MS. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006;118(3):1176–1184. doi: 10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]
- 41.Logtenberg SJ, Kleefstra N, Snellen FT, Groenier KH, Slingerland RJ, Nierich AP, Bilo HJ. Pre- and postoperative accuracy and safety of a real-time continuous glucose monitoring system in cardiac surgical patients: a randomized pilot study. Diabetes Technol Ther. 2009;11(1):31–37. doi: 10.1089/dia.2008.0028. [DOI] [PubMed] [Google Scholar]
- 42.Price GC, Stevenson K, Walsh TS. Evaluation of a continuous glucose monitor in an unselected general intensive care population. Crit Care Resusc. 2008;10(3):209–216. [PubMed] [Google Scholar]