Abstract
Background
A new development in the field of telehealth is the use of mobile health technologies (mhealth) to help patients record and track medical information. Mhealth appears particularly advantageous for conditions that require intense and ongoing monitoring, such as diabetes, and where people are of working age and not disabled. This review aims to evaluate the evidence for the effectiveness of mhealth interventions in diabetes management on glycosylated hemoglobin.
Method
A comprehensive search strategy was developed and applied to eight electronic databases to identify studies that investigated the clinical effectiveness of mobile-based applications that allowed patients to record and send their blood glucose readings to a central server. The eligibility of 8543 papers was assessed against the selection criteria, and 24 papers were reviewed. All studies reviewed were assessed for quality using a standardized quality assessment tool.
Results
Results for patients with type 1 and type 2 diabetes were examined separately. Study variability and poor reporting made comparison difficult, and most studies had important methodological weaknesses. Evidence on the effectiveness of mhealth interventions for diabetes was inconsistent for both types of diabetes and remains weak.
Keywords: diabetes, glycemic control, mobile health, monitoring, self-management
Introduction
Telemonitoring refers to the recording and tracking of medical data by patients and health care professionals (HCPs) at a distance. This method of care may be particularly relevant for the management of chronic conditions, such as diabetes, which require intensive daily monitoring and behavioral adjustment. Diabetes self-management includes self-monitoring of blood glucose (SMBG) readings, taking medication, exercise, dietary management, and foot care. Evidence suggests that SMBG alone may be of limited clinical effectiveness. This may be because patients are unable to interpret results and hence make adjustments to self-care.1,2 By providing patients with the tools needed to review, interpret data, and receive feedback, telemonitoring could facilitate self-management.
In the past, telemonitoring applications relied on home-based technologies, but with mobile devices, patients can transmit data in real time, at any time and in any place. This also means feedback can be received when it is most relevant. The ubiquitous nature of these wireless technologies is an important development, with potential to impact diabetes management.
A number of systematic reviews have examined the use of telehealth in diabetes, looking at a range of technologies, including fixed and mobile equipment.3–5 Where reviews focused only on mobile platforms, a variety of interventions were included; for example, interventions aiming to increase peer support, educate, or remind patients of appointments or self-care activities.6–9 Some reviewed both pediatric and adult samples, despite their differences in the management of diabetes and use of technology. Inclusive reviews are useful to gain a better understanding of ongoing research in the field. When looking at clinical effectiveness, however, reviews that focus on specific interventions or intervention components are needed for conclusions to be precise and reliable.
This review aims to examine the evidence for the clinical effectiveness [glycosylated hemoglobin (HbA1c)] of mobile telemonitoring to support diabetes management in adult patients. It focuses on interventions including the transfer of data to a Web server to receive feedback.
Methods
Search Strategy
Six electronic databases were searched in August 2009, with a subsequent update in January 2012. The search combined diabetes and mobile platform terms: “HbA1c,” “metabolic control,” “glycemic control,” “glycosylated hemoglobin,” “glycated hemoglobin,” “diabetes complications,” “blood glucose,” “hypoglycemia,” “plasma glucose,” “insulin,” “mobile phone,” “cell phone,” “PDA,” “personal digital assistant,” “personal smart assistant,” “pocket computer,” “pocket PC,” “short message service,” “SMS,” “text messaging,” “wireless,” “iPhone,” “smartphone,” “electronic diary,” “real-time,” and “pager.”
Inclusion and Exclusion Criteria
Studies included for review investigated the clinical effectiveness of interventions requiring patients to transmit blood glucose (BG) readings to an online server via a mobile device. Studies involving an adult population (>18 years) with type 1 or type 2 diabetes were eligible. Glycosylated hemoglobin had to be a clinical outcome. Case studies, papers with simulated HbA1c data, devices designed for use by HCPs, and studies with a sample consisting of more than 20% insulin pump users were excluded. Only English language papers were reviewed.
Data Extraction
A data extraction form was developed, piloted, and used by Justine Baron to extract data. Authors were contacted for clarification when needed.
Quality Assessmen
An adapted version of the McMaster University quality assessment tool10 was used to assess papers. Using the tool and its dictionary, studies were rated as poor, moderate, or strong. Ten areas were covered: selection bias, research objectives, study design, power, blinding, data collection methods, withdrawals and dropouts, intervention integrity, suitability of analyses, and suitability of interpretation of findings. Studies that were not randomized controlled trials (RCTs) or controlled trials were assessed against nine of these areas, as blinding was not relevant. To achieve a “strong” rating, RCTs and controlled trials had to be rated “strong” in at least 6 of the 10 areas and have no areas rated “weak.” Other study designs required five or more strong ratings out of nine and no weak ratings.
Results
Study Selection
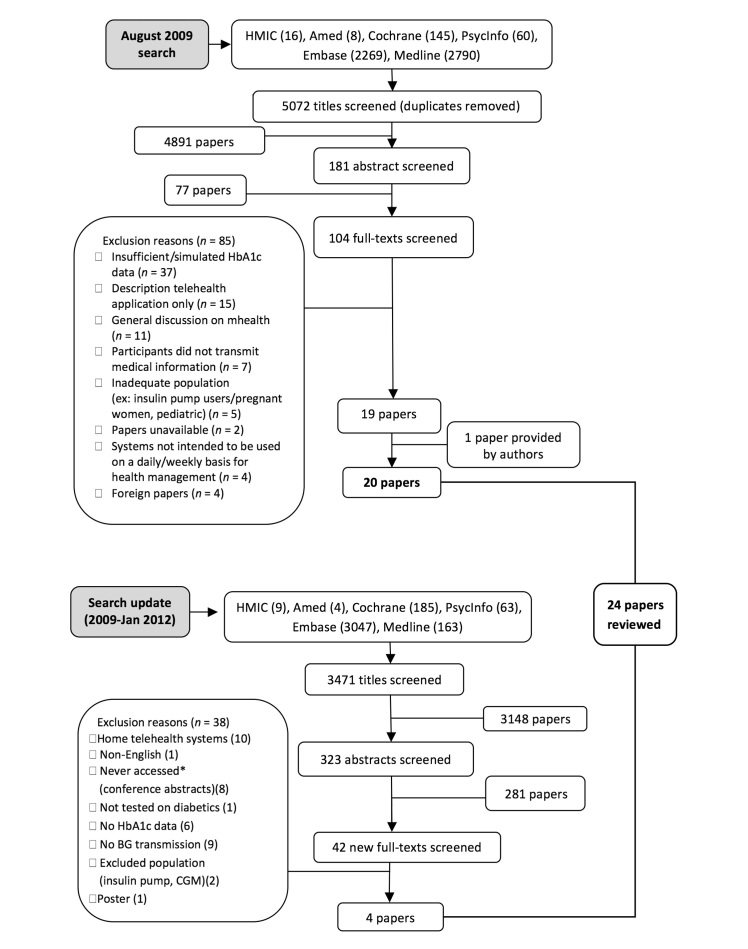
Paper selection was conducted independently by two of the authors. Disagreements were resolved through discussion until consensus was reached. Figure 1 illustrates stages of the paper selection process for the 2009 search and 2012 update. Titles and abstracts were screened, leading to the review of a total of 146 full texts. A total of 24 publications matched the selection criteria. Three additional papers11–13were used for data extraction purposes; they provided no additional clinical data but further information on the methodology or intervention tested in two of the reviewed studies.
Figure 1.
Study selection process for the 2009 search and 2012 update.
The 24 identified publications described 20 studies. Seven papers14–20published by the same group of authors evaluated the same intervention with some appearing to describe the same sample. The seven papers were independently examined by two authors and divided into three different studies. One study (2 papers18,20) focused on an intervention delivered to obese patients. A second study (4 papers15–17,19) evaluated the same intervention in a population not restricted to obese patients. Each of these 4 articles presented different follow-up periods, and 1 paper presented a subgroup analysis based on baseline HbA1c. Finally, a third study (1 paper14) used a single group before and after design. In this review, all papers referring to what was defined earlier as one study are grouped.
Description of Included Studies
Papers were published between 2002 and 2011. Studies were conducted in Asia (n = 8), Europe (n = 8), and the United States (n = 3); one was a multinational trial. Seven studies involved a population with type 1 diabetes, 11 with type 2. Two studies included a mixed population,21,22 but as the percentage with type 1 diabetes was minimal (8% and 16%), they were grouped with studies on type 2 diabetes.
Tables 1 and 2 summarize intervention components. Tables 3 and 4 summarize study and participant characteristics; they include the quality assessment results. These results suggest that overall quality was poor. Sixteen studies were rated weak, three moderate, and one strong.
Table 1.
Intervention Components of Studies on Type 1 Diabetes
| Intervention group | ||||||
|---|---|---|---|---|---|---|
| Reference, first author | Control group | Mobile platform | Data inputted | Recommended frequency of data input | Type and nature of feedback | Frequency of HCP feedback |
| Dietary interventions | ||||||
| Tsang23 | Standard care | Personal digital assistant | Meal content BG readings | 2x per week | Automated text: CHO daily intake, proteins, calories, fat | Not applicable |
| Rossi24 | Standard care (education on CHO counting) | Mobile phone | Meal content BG readings Insulin dose Exercise | 2–3x per day | Automated text feedback: CHO daily intake, proteins, calories, fat, suggested insulin dose HCP feedback (behavioral advice) | Not reported |
| Rossi25 | Not applicable | |||||
| Nondietary interventions | ||||||
|---|---|---|---|---|---|---|
| Gómez26 | Standard care | Personal digital assistant | BG readings Free text | At least 1x fortnightly | HCP feedback to patients with out-of-range BG readings or queries Graphical feedback for different time periods | 24 h |
| Kollman27 | Not applicable | Mobile phone | BG readings Medication Exercise Wellbeing | 2–4x per day | Color-coded graphical feedback | Not applicable |
| Farmer28 | Mobile-phone intervention with minimal feedback (no HCP feedback + non-color-coded graphical feedback for one time period only) | Mobile phone | BG readings Exercise Medication CHO | 2–3x per day | HCP feedback and color-coded graphical feedback for different time periods | Fortnightly |
| Vähätalo29 | Standard care | Mobile phone | BG readings | Not reported | HCP feedback to all patients whether changes needed to be made to regimen or not | Weekly during first month then biweekly |
Table 2.
Intervention Components of Studies on Type 2 Diabetesa
| Intervention group | ||||||
|---|---|---|---|---|---|---|
| Reference, first author | Control group | Data inputted | Recommended frequency of data input | Reminders and exclusions | Type and nature of feedback | Frequency of HCP feedback |
| Cho30 | Web/personal computer transmission of medical data (BG readings, lifestyle, hypoglycemic events, medication, blood pressure, weight, free text) with HCP and graphical feedback + diabetes education | BG readings | Not reported | Exclusions after 3 weeks of nontransmission | HCP feedback: treatment recommendations, corrections to lifestyle factors, encouragements, remindersb | Fortnightly |
| Faridi31 | Standard care + pedometer | BG readings | Daily | Not reported | Automated feedback: text message tailored to the BG readings and selected from a bank of predetermined messages | Not applicable |
| Kim14 | Not applicable | BG readings, diet, medication, exercise | Daily | Reminders after 1 week of nontransmission Exclusion after nontransmission for 4 weeks | HCP feedback Graphical feedback | Weekly |
| Kim16 Kim17 Hee-Sung15 Yoon19 |
Standard care | BG readings, CHO, medication, exercise | Daily | Reminders after 1 week of nontransmission, Exclusion after nontransmission for 4 weeks | HCP feedback Graphical feedback | Weekly |
| Istepanian21 | Standard care + 2 h diabetes education course | BG readings | Personalized (4–9x/week) | Reminders when personalized monitoring schedule not respected | Automated feedback: letters sent through the post to HCPs and patients with amalgamated readings and treatment recommendationsb | Not applicable |
| Kim18 Kim20 |
Standard care | BG readings Medication, diet, exercise | Daily | Reminders after 1 week of nontransmission Exclusion after nontransmission for 4 weeks | HCP feedback | Weekly |
| Kwon22 | Not applicable | BG and blood pressure readings, Weight | Not reported | Not reported | HCP feedback Graphical feedback | Not reported |
| Quinn32 | Faxing/phoning in BGs until stable | BG readings, Medication, CHO | Not reported | Not reported | Automated feedback for patients within range BG values HCP feedback for those with troubling BG values | Not reported |
| Rodríguez-Idígoras33 | Standard care | BG readings | Not reported | Not reported | HCP feedback to those patients signaled by the system | When signaled |
| Larsen34 | Not applicable | BG readings, blood pressure, weight | Not reported | Reminders after 3 days of non-transmission | HCP feedback + graphical feedback | Data reviewed every 2–3 days |
| Kim35 | Standard care + 1 h 20 diabetes education | BG readings | 3x/week | Exclusion if less than 3 fasting readings in 20 days | Daily automated feedback messages on insulin adjustmentb | Not applicable |
| Yoo36 | Standard care | BG and blood pressure readings, weight, and exercise | Daily | Not reported | Automated feedback: text messages to encourage/remind/motivate | Not reported |
| Quinn37 | Standard care | 3 active treatment groups transmitted BG and blood pressure readings, weight, medication | Not reported | Not reported | The three treatment groups received automated feedback: action plan to support diabetes self-management sent electronically every 2.5 months HCP feedback | Min. 1x/2–3 months, max. 4x/month, depending on patient risk status |
In all the studies on type 2 diabetes, the mobile platform in the intervention group was a mobile phone.
The intervention group received the same diabetes education as the control group.
Table 3.
Study and Participant Characteristics and Outcomes (Type 1 Studies)
| Clinical outcomes (HbA1c) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference, first author | Design and quality | Research groups | Duration (months) | Recruited/completed (n) | Age mean (standard deviation) | Gender (males, %) | Baseline | % change at last follow-up (except for crossover trials) | |
| Baseline to 3 months | 3 to 6 months | ||||||||
| Tsang23 | Crossover (pilot)a | (1) Standard care then transmission via personal digital assistant versus (2) Transmission via Personal digital assistant then standard care |
6 (2x3) | 20/19 | 32.5 ± 8.2 | 63.2% | (1): 8.76% (2): 8.56% |
(1): -0.05% (2): -1.01%b |
(1): +0.36% (2): +0.29% |
| Rossi24 | Multinational RCTc | (1) Standard CHO education versus (2) shortened version + Mobile phone transmission | 6 | 130/119 | 35.7 ± 9.4 | 43% | (1): 8.4% (2): 8.2% | 1: -0.5%b 2: -0.4%b |
|
| Rossi25 | Single-group pre and post (pilot)a | Mobile phone transmission | 9 | 41/Not reportedd | 31.6 ± 11.9 | 61% | 7.6% | -0.33% | |
| Gómez26 | Crossover (pilot)a | (1) Standard care followed by transmission via personal digital assistant (2) Transmission via personal digital assistant followed by standard care |
12 (2x6) | 10/Not reported | Not reported | Not reported | (1) 8.10%d for control study (2) 8.4% for mhealth study |
(1) 8.15% for control study (2) 7.9% for mhealth study | |
| Kollman27 | Single-group pre and posta | Mobile phone transmission | 3 | 10/10 | 36.6 ± 11.0 | 60% | 7.9% | -0.4%b | |
| Farmer28 | RCTa | Mobile phone transmission with (1) nurse + graphical feedback (2) Graphical feedback trial only |
9 | 93/81 | 23.8 ± 4.2 | 59.1% | (1): 9.2% (2):9.3% | (1): -0.6%b (2): -0.4%b |
|
| Vähätalo29 | Controlled trial (pre and post)a | (1) Standard care versus (2) mobile phone transmission | 12 | 203/Not reported | 42.9 ± 12.5 | 55.7% | (1): 7.7% (2): 7.9% | (1): +0.45% (2): +0.35% | |
Poor quality rating.
Significant within group difference.
Moderate quality rating.
Pre and post HbA1c values reported are medians. No percentage change presented.
Table 4.
Study and Participant Characteristics and Outcomes (Type 2 Studies)
| Clinical outcomes (HbA1c) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference, first author | Design and quality | Research groups | Duration (months) | Recruited/ completed (n) | Age (mean, standard deviation) | Gender (males, %) | Baseline | % change at last follow-up |
| Cho30 | RCTa | (1) Mobile phone transmission versus (2) Computer/Web-based transmission |
3 | 69/63 | 48.1 ± 12.5 | 78.3% | (1): 8.3% (2): 7.6% |
(1): -0.7%b (2): -1.2%b |
| Faridi31 | RCTa | (1) Standard care + pedometer versus (2) Mobile phone transmission + pedometer |
3 | 30/Not reported | 56.45 ± 9.6 | 36.6% | (1): 6.5% (2): 6.4% |
(1): +0.3% (2): -0.1% |
| Kim14 | Single-group pre and posta | Mobile phone transmission | 3 | 45/33 | 43.5 ± 12.6 | 42.4% | 8.1% | -1.1%b |
| Kim16 Kim17 Hee-Sung15 Yoon19 |
RCTc | (1) Standard care versus (2) Mobile-phone transmission |
12 | 60/51 | 47.1 ± 8.9 | 43.1% | (1): 7.59% (2): 8.09% |
(1): +0.81% (2): -1.32%b,d |
| Istepanian21 | RCTa | (1) Standard care + 2h diabetes education course versus (2) Mobile phone transmission + 2h diabetes education |
9 | 137/87 | 58.6 ± 12.5 | Not reported | (1): 8.1% (2): 7.9% |
(1): +0.1% (2): 0% |
| Kim18 Kim20 |
RCTa | (1) Standard care versus (2) Mobile phone transmission |
12 | 40/34 | 46.9 ± 8.6 | 52.9% | (1): 7.66% (2): 8.16% |
(1): +0.53% (2): -1.49%b,d |
| Kwon22 | Single-group pre and posta | Mobile phone transmission | 3 | 185/Not reported | 42.4 (4–79) | 28.1 | 7.5% | -0.5%b |
| Quinn32 | RCTa | (1) Faxing/phoning in BGs until stable versus (2) Mobile phone transmission |
3 | 30/26 | 51.04 ± 11.03 | 65% | (1): 9.05% (2): 9.51% |
(1): -0.68% (2): -2.03%d |
| Rodríguez-Idígoras33 | RCTe | (1) Standard care versus (2) Mobile phone transmission |
12 | 328/297 | 63.9 ± 0.60 | 51.5% | (1): 7.41% (2): 7.62% |
(1): -0.09% (2): -0.22%b At 6 months,d not at 12 months |
| Larsen34 | Single-group pre and posta | Mobile phone transmission | 6 | 23/Not reported | 57.6 ± 12 | 80% | 9.5% | -0.66%b |
| Kim35 | RCTa | (1) Standard care + 1 h 20 diabetes education versus (2) Mobile phone transmission + 1 h 20 diabetes education |
3 | 100/92 | 48.4 ± 7.46 | 50% | (1): 9.8% (2): 9.8% Overall: 9.8% |
(1): -2.0% (2): -2.4%d Overall: -2.2%b |
| Yoo36 | RCTa | (1) Standard care versus (2) Mobile phone transmission |
3 | 123/111 | 58.2 ± 8.73 | 58.5% | (1): 7.4% (2): 7.6% |
(1): +0.29%b
(2): -0.4%b |
| Quinn37 | 4-group cluster RCTc | (1) Standard care versus (2, 3, 4) mobile phone transmission with increasing levels of HCP access to data | 12 | 163/163 | 52.8 ± 8.66 | 49.7% | (1): 9.2%f (2): 9.3% (3): 9.0% (4): 9.9% |
(1): -0.7% (2): -1.6%d (3): -1.1% (4): -2.0%d |
Poor quality rating.
Significant difference within group.
Moderate quality rating.
Significant difference between groups.
Strong quality rating.
Group 1 receiving standard care is the reference group. Between group differences calculated by Quinn in relation to the reference group.
Of the 20 studies, 12 were RCTs of which 1 was a four-group cluster RCT, 1 was a controlled trial, 2 were crossover studies, and 5 were single before and after designs. Of the 15 two-group studies, 9 evaluated mhealth compared with standard care and 6 with another intervention. This was either another mhealth intervention, a Web- or fax/phone-based intervention, pedometer monitoring, or diabetes education. A four-group RCT compared both mhealth with standard care and different mhealth groups with varying HCP access to patient data.
The mhealth interventions evaluated were similar across type 1 and type 2 diabetes, with the exception of dietary interventions, which occurred in type 1 diabetes. Three23–25of the type 1 diabetes studies had a specific focus on dietary management. The purpose of these mhealth systems was to provide patients with support in calculating the appropriate insulin dose to match food consumed. Participants were required to transmit information on meal content and received automated feedback on protein, carbohydrate (CHO), calorie, and fat intake. An algorithm-based insulin dose was suggested in two of these studies. Of these, one study investigated whether the use of such a system could reduce the amount of hours usually spent on CHO-counting education; the mhealth group received a shortened version of the standard CHO education and used the device while the control group received the full version.
In the remaining studies on both type 1 and type 2 diabetes, participants transferred a combination of one or more of the following to a Web server: BG readings, blood pressure readings, weight, exercise, diet, medication, free text, and/or their level of wellbeing. Reminders to transmit were part of the intervention protocol in seven studies. Of these, two27,29 were on type 1 diabetes; in one,29 patients were reminded to transmit when there were too few readings for clinical judgment, and in another,27 patients were reminded to transmit if less than three readings were sent daily. In some studies on type 2 diabetes, patients who did not transmit sufficient data were withdrawn.14–20,30,35
Health care professional feedback was provided in the majority of studies and included treatment recommenda-tions, encouragements, reminders, advice, and corrections to lifestyle. In some cases, only patients with an out-of-range BG reading or high-risk profiles were contacted, while in others, all participants received feedback regardless of their BG values. Automated feedback was an intervention component in nine studies and was delivered via text message, on an accompanying patient Web portal, or via letter. Graphical feedback was provided in seven studies and was a representation, sometimes color-coded, of BG values over time. This was offered in addition to HCP feedback in five studies. Only one study included both automated text and HCP feedback. It suggests that providing automated text feedback is considered as a good alternative to HCP feedback when resources are limited.
Clinical Effectiveness of Studies on Type 1 Diabetes
For studies evaluating a diet-focused intervention, results were mixed. The single-group trial,25which was rated poor quality, failed to find any significant change in HbA1c post-intervention. The sample size remained small (n = 41), making the generalizability of the findings limited, and authors failed to report the number of participants completing the study. In addition, the frequency at which HCPs reviewed patient data and provided feedback was not specified. When the mhealth technology plus a short version of standard CHO education was compared with standard CHO education in the multinational RCT,24 no difference was found between groups, but significant reductions in HbA1c were observed at 6 months in both groups. Although results are useful in suggesting mhealth could effectively replace part of the standard CHO-counting education, this study was rated of moderate quality. Little detail was provided on the content of the education sessions, which makes it difficult to identify which intervention components are necessary for intervention effectiveness. Authors also failed to report outcome differences between countries, although variations in dietary habits and intervention delivery (despite efforts to standardize) might have influenced results. Finally, the remaining dietary inter-vention compared twice-weekly transmission of BG and diet information with feedback from a HCP to standard care in a crossover trial.23 A significant reduction in HbA1c was reported only in the group with a significantly shorter diabetes duration (5.3 versus 11.8 years), suggesting this tool might be particularly useful for patients recently diagnosed. This study, however, was also rated as poor quality. It included only 20 participants, and there was no washout period between study periods to avoid carry-over effects.
Results of studies on non-dietary interventions were inconclusive with two27,28 of the four studies supporting the effectiveness of mhealth. Monitoring patients via mhealth led to significant improvements in HbA1c after 3 months in a before-and-after study27 involving submission of data via mobile phone and access to graphics via a Web portal. Participants were sent a reminder to transfer every day on which less than three BG readings had been transmitted. This particularly intensive protocol for reminders may have ensured high use of the equipment and contributed to success of the intervention. This study, however, was rated poor in quality, a sample size of 10 being the major issue. A RCT28 comparing mhealth with either intensive graphical feedback and nurse support or minimal graphical feedback only found significant improvements in HbA1c in both groups at months 4 and 9. This suggests that significant changes can occur regardless of the intensity of the graphical feedback provided and that HCP input may not be an essential ingredient to intervention success. Although the study had a larger sample size than many of the studies reviewed, it was slightly below the number of participants required for adequate power. Authors also failed to report if outcome assessors were blinded. Interestingly, the response rate in this study was relatively low (52%) despite recruiting an age group (18–30 years) that may be keen to use technology. Finally, no significant clinical changes were observed in the two remaining studies examining transfer of BG readings via personal digital assistant and mobile phone plus HCP and graphical feedback.26,29 Unlike the majority of studies reviewed, patients were limited to transferring BG readings only in these two studies. Asking patients to transfer more information may increase awareness and understanding of the relationship between BG readings and lifestyle factors, making it possible for patients to act upon them in an effective way. In addition, Gómez and colleagues26 asked patients to transmit BG readings fortnightly, which is considerably less frequent than other studies. Research regarding optimal transmission frequency is, however, lacking. In terms of methodological quality, intervention participants in the study by Vähätalo and associates29 received twice as many testing strips as control participants, thus enabling increased monitoring and thereby introducing bias. In addition, the trend toward HbA1c deterioration in both groups was linked to the calibration differences between the machines used to test HbA1c. This suggests lack of methodological rigor in the conduct of the study, potentially biasing results.
Clinical Effectiveness of Studies on Type 2 Diabetes
In the studies published by the same group of authors, the intervention included transmission of BG readings by mobile phone and weekly text message recommendations. In the 12-week program using a single-group design,14 a significant pre-to-post reduction in HbA1c was found. Although the reduction from baseline to follow-up was clinically significant (1.1%), the sample size was small. With 26% (n = 12) of the sample excluded from the analysis, it would have been particularly relevant to investigate differences between patients who did not engage with the equipment or dropped out and those who completed the research. However, such analyses were not reported by the authors, nor were reasons for nontransmission of patient data. When applied to patients with a body mass index >23,18,20 this intervention led to a significant improvement in HbA1c in the intervention group compared with the control group. Following a group of participants longitudinally,15–17,19 significant differences between the intervention and control groups were found at 3, 9, and 12 months. In a subgroup analysis,15 significant improvements in HbA1c were observed at 3 months for intervention group participants with a baseline HbA1c of ≥7% but not for the control group participants with the same baseline HbA1c. As might have been expected, however, no significant improvement was noted in intervention group participants already well controlled at baseline (HbA1c <7%). In fact, these participants maintained good glycemic control, whereas participants in the control group starting the study with a HbA1c of <7% deteriorated significantly. These results suggest that mhealth is effective for people with poorly controlled diabetes while also being more effective than standard care in helping people with well-controlled diabetes maintain glycemic control.
Of the 10 remaining studies, 7 found mhealth to be significantly more effective than other telehealth interventions and standard care. Two single-group studies22,34 led to similar and significant improvements in HbA1c at 3 and 6 months, particularly so for those with a baseline HbA1c of ≥7.0%.31 In a trial35 evaluating a system that provided patients with an insulin dose adjustment based on fasting BG readings, overall, a clinically significant reduction in HbA1c was observed, but the reduction was significantly greater in the intervention group. Three RCTs32,33,36 found significant reductions in HbA1c for the mhealth group. One compared the mhealth intervention to a fax- or telephone-based intervention.32 In this study, however, the control group phoned or faxed in their BG readings fortnightly until these were stable. It was unclear whether HCP feedback was provided to this group, and no criteria defined a stable BG readings pattern. The two other RCTs33,36 compared mhealth with standard care. In one of them,33 improvements were significant at 6 months but were not at 12 months, therefore suggesting only short-term effectiveness. Finally, the four-group RCT37 found significantly greater reductions in HbA1c at 12 months in two of the three active treatment groups compared with the control group after controlling for baseline HbA1c. Unlike Rodríguez-Idígoras and coworkers,33 these between-group differences were still significant at 12 months. Interestingly, there were no significant differences between the three active treatment groups, although these differed in the level of access HCPs had to patient data. Similar to Farmer and colleagues,28 in type 1 diabetes, it appears the key and active driver to success may be the transmission of patient data, regardless of whether those data are reviewed by HCPs or used to provide feedback.
The remaining three21,30,31 RCT studies failed to find mhealth to be more effective than standard care or other telehealth interventions. These included mhealth and pedometer monitoring compared with standard care with pedometer monitoring,31 HCP feedback via letter including amalgamated readings and treatment recommendations to standard care,21 and a computer versus mhealth intervention.30 For Faridi and associates,31 this is unsurprising considering the low levels of adherence to protocol among 15 intervention group patients. Only 2 patients were completely adherent and transmitted readings daily, while 9 patients were found to either transmit only for a week (n = 4) or not at all (n = 5) and the remaining four only for 1–2 months out of 3. Important methodological issues led to this study being rated as poor. For example, the control group wore pedometers as part of the objective assessment of physical activity. Although this was not intended as an intervention, reviewing daily step counts could have influenced participants’ levels of exercise and biased results. Istepanian and coworkers21 found mhealth to be ineffective in reducing HbA1c with patients receiving feedback in a letter format. Unfortunately, authors did not report the frequency at which letters were sent to patients or the type of treatment recommendations made. The immediacy of feedback displayed via mobile platforms as a result of data transmission may be more likely to facilitate data interpretation and promote active and prompt reactions to physiological states. The third RCT30 did find significant improvements in HbA1c for both mhealth and a computer-based Web monitoring intervention; however, differences between groups were not significant. Both groups improved significantly and similarly despite the computer group being able to transfer considerably more diabetes-related information than the mobile phone group. The portability of the device, which may act as a reminder and prompt to self-care, may therefore be as effective as being able to provide more information. The behavioral mechanisms involved in fixed and mobile technology may differ and require further examination.
Discussion
This systematic review summarizes the evidence base for the clinical effectiveness of mhealth interventions in which patients transmit diabetes-related information to receive automated text, graphical, and/or HCP feedback. Systematic searching found 13 studies on type 2 diabetes and 7 on type 1 diabetes. None of the studies reviewed found mhealth to be harmful. Overall, the findings from the studies reviewed are somewhat mixed but do appear to be more consistently positive for studies in type 2 diabetes as was reported by Azar and Gabbay.38 Ten ofthe 13 studies in type 2 diabetes and 4 of 7 studies on type 1 diabetes found mhealth to lead to benefits. Studies without HCP feedback led to improved HbA1c, suggesting HCP feedback might not be necessary for intervention success. The recording and tracking of data could be the key factor for increasing patients’ awareness, understanding, and motivation to self-manage. Knowledge that the data are accessible to HCPs may also be an incentive to adhere to a regimen. The graphical and automated text feedback might also be an effective incentive to engage patients. It may help patients identify relationships between their lifestyle and BG patterns. Future research needs to determine which patients benefit most from HCP feedback and which patient characteristics predict intervention effectiveness. This will guide future mhealth deployment tactics and increase cost-effectiveness.
The methodological quality of the reviewed studies was poor, with many involving small sample sizes, no power calculations, and poor study designs. Many studies excluded patients who failed to engage with the devices from the analysis; this implies they assessed intervention efficacy and not effectiveness. If a “per-protocol analysis” rather than an intention-to-treat analysis is presented, as might be the case especially with studies with smaller sample sizes, this should be supplemented by an analysis of differences between completers and those who dropped out or were withdrawn along with a discussion on the possible implications and effects of the missing participants. Some of these criticisms reflect the observations made by Whitten and colleagues39 in their review of the methodology adopted in telehealth research. In addition, poor reporting in these studies made interpretations difficult; additional papers, Web pages, or diagrams should be made available to ensure transparency. The CONSORT-eHealth guidelines40 for the reporting of telehealth interventions are available for researchers to use.
Finally, the costs incurred in the delivery and running of these telemonitoring interventions was not discussed in this review. Without this information, it remains impossible to know whether implementing such services is cost-effective.
This systematic review has limitations. It does not consider research exclusively on specific subgroups such as pregnant women or insulin pump users. Non-English language papers were not reviewed, and a publication bias could have occurred since grey literature was not searched.
In view of the considerations raised earlier and their implications on the interpretation of study results, this review cannot reliably conclude on the clinical effectiveness of mhealth interventions for diabetes management. Results do show potential for beneficial change, but higher-quality studies with better standard of reporting are urgently needed and will provide a strong evidence base for policy makers.
Acknowledgments
We thank members of Health Services Research Group, City University, London, UK, for participating in the development of the quality assessment tool used in this systematic review. In particular, we also acknowledge the contribution of Janet Stygall, Dr. Anna Davies, and Sophie Cleanthous who helped with paper selection and data extraction, as well as providing useful guidance and advice in the conduct of this review. Justine Baron was supported by a Ph.D. studentship from the Department of Health, UK.
Glossary
- (BG)
blood glucose
- (CHO)
carbohydrate
- (HbA1c)
glycosylated hemoglobin
- (HCP)
health care professional
- (RCT)
randomized controlled trial
- (SMBG)
self-monitoring of blood glucose
References
- 1.Davidson MB. Counterpoint: self-monitoring of blood glucose in type 2 diabetic patients not receiving insulin: a waste of money. Diabetes Care. 2005;28(6):1531–1533. doi: 10.2337/diacare.28.6.1531. [DOI] [PubMed] [Google Scholar]
- 2.Clar C, Barnard K, Cummins E, Royle P, Waugh N. Aberdeen Health Technology Assessment Group. Self-monitoring of blood glucose in type 2 diabetes: systematic review. Health Technol Assess. 2010;14(12):1–140. doi: 10.3310/hta14120. [DOI] [PubMed] [Google Scholar]
- 3.Montori VM, Helgemoe PK, Guyatt GH, Dean DS, Leung TW, Smith SA, Kudva YC. Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care. 2004;27(5):1088–1094. doi: 10.2337/diacare.27.5.1088. [DOI] [PubMed] [Google Scholar]
- 4.Farmer A, Gibson OJ, Tarassenko L, Neil A. A systematic review of telemedicine interventions to support blood glucose self-monitoring in diabetes. Diabet Med. 2005;22(10):1372–1378. doi: 10.1111/j.1464-5491.2005.01627.x. [DOI] [PubMed] [Google Scholar]
- 5.Krishna S, Boren SA. Diabetes self-management care via cell phone: a systematic review. J Diabetes Sci Technol. 2008;2(3):509–517. doi: 10.1177/193229680800200324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell-Minda E, Jutai J, Speechley M, Bradley K, Chudyk A, Petrella R. Health technologies for monitoring and managing diabetes: a systematic review. J Diabetes Sci Technol. 2009;3(6):1460–1471. doi: 10.1177/193229680900300628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatara N, Arsand E, Nilsen H, Hartvigsen G. A review of mobile terminal-based applications for self- management of patients with diabetes. Proc International Conference on eHealth, Telemedicine, and Social Medicine; February 1-7, 2009; Cancun, Mexico. [Google Scholar]
- 8.Liang X, Wang Q, Yang X, Cao J, Chen J, Mo X, Huang J, Wang L, Gu D. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28(4):455–463. doi: 10.1111/j.1464-5491.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- 9.Holtz B, Lauckner C. Diabetes management via mobile phones: a systematic review. Telemed J E Health. 2012;18(3):175–184. doi: 10.1089/tmj.2011.0119. [DOI] [PubMed] [Google Scholar]
- 10.Effective Public Health Practice Project. Quality assessment tool for quantitative studies. http://www.ephpp.ca/tools.html Accessed January 18, 2012.
- 11.Istepanian RS, Sungoor A, Earle KA. Technical and compliance considerations for mobile health self-monitoring of glucose and blood pressure for patients with diabetes. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:5130–5133. doi: 10.1109/IEMBS.2009.5334580. [DOI] [PubMed] [Google Scholar]
- 12.Earle KA, Istepanian RS, Zitouni K, Sungoor A, Tang B. Mobile telemonitoring for achieving tighter targets of blood pressure control in patients with complicated diabetes: a pilot study. Diabetes Technol Ther. 2010;12(7):575–579. doi: 10.1089/dia.2009.0090. [DOI] [PubMed] [Google Scholar]
- 13.Quinn CC, Gruber-Baldini AL, Shardell M, Weed K, Clough SS, Peeples M, Terrin M, Bronich-Hall L, Barr E, Lender D. Mobile diabetes intervention study: testing a personalized treatment/behavioral communication intervention for blood glucose control. Contemp Clin Trials. 2009;30(4):334–346. doi: 10.1016/j.cct.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Kim NC, Ahn SH. Impact of a nurse short message service intervention for patients with diabetes. J Nurs Care Qual. 2006;21(3):266–271. doi: 10.1097/00001786-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Hee-Sung K. Impact of Web-based nurse’s education on glycosylated haemoglobin in type 2 diabetic patients. J Clin Nurs. 2007;16(7):1361–1366. doi: 10.1111/j.1365-2702.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS. A randomized controlled trial of a nurse short-message service by cellular phone for people with diabetes. Int J Nurs Stud. 2007;44(5):687–692. doi: 10.1016/j.ijnurstu.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Jeong HS. A nurse short message service by cellular phone in type-2 diabetic patients for six months. J Clin Nurs. 2007;16(6):1082–1087. doi: 10.1111/j.1365-2702.2007.01698.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Song MS. Technological intervention for obese patients with type 2 diabetes. Appl Nurs Res. 2008;21(2):84–89. doi: 10.1016/j.apnr.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Yoon KH, Kim HS. A short message service by cellular phone in type 2 diabetic patients for 12 months. Diabetes Res Clin Pract. 2008;79(2):256–261. doi: 10.1016/j.diabres.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform. 2008;77(6):399–404. doi: 10.1016/j.ijmedinf.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Istepanian RS, Zitouni K, Harry D, Moutosammy N, Sungoor A, Tang B, Earle KA. Evaluation of a mobile phone telemonitoring system for glycaemic control in patients with diabetes. J Telemed Telecare. 2009;15(3):125–128. doi: 10.1258/jtt.2009.003006. [DOI] [PubMed] [Google Scholar]
- 22.Kwon HS, Cho JH, Kim HS, Lee JH, Song BR, Oh JA, Han JH, Kim HS, Cha BY, Lee KW, Son HY, Kang SK, Lee WC, Yoon KH. Development of web-based diabetic patient management system using short message service (SMS) Diabetes Res Clin Pract. 2004;66(Suppl 1):S133–7. doi: 10.1016/j.diabres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Tsang MW, Mok M, Kam G, Jung M, Tang A, Chan U, Chu CM, Li I, Chan J. Improvement in diabetes control with a monitoring system based on a hand-held, touch-screen electronic diary. J Telemed Telecare. 2001;7(1):47–50. doi: 10.1258/1357633011936138. [DOI] [PubMed] [Google Scholar]
- 24.Rossi MC, Nicolucci A, Di Bartolo P, Bruttomesso D, Girelli A, Ampudia FJ, Kerr D, Ceriello A, Mayor Cde L, Pellegrini F, Horwitz D, Vespasiani G. Diabetes Interactive Diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open-label, international, multicenter, randomized study. Diabetes Care. 2010;33(1):109–115. doi: 10.2337/dc09-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi MC, Nicolucci A, Pellegrini F, Bruttomesso D, Bartolo PD, Marelli G, Dal Pos M, Galetta M, Horwitz D, Vespasiani G. Interactive diary for diabetes: a useful and easy-to-use new telemedicine system to support the decision-making process in type 1 diabetes. Diabetes Technol Ther. 2009;11(1):19–24. doi: 10.1089/dia.2008.0020. [DOI] [PubMed] [Google Scholar]
- 26.Gómez EJ, Hernando ME, García A, Del Pozo F, Cermeño J, Corcoy R, Brugués E, De Leiva A. Telemedicine as a tool for intensive management of diabetes: the DIABTel experience. Comput Methods Programs Biomed. 2002;69(2):163–177. doi: 10.1016/s0169-2607(02)00039-1. [DOI] [PubMed] [Google Scholar]
- 27.Kollmann A, Riedl M, Kastner P, Schreier G, Ludvik B. Feasibility of a mobile phone-based data service for functional insulin treatment of type 1 diabetes mellitus patients. J Med Internet Res. 2007;9(5):e36. doi: 10.2196/jmir.9.5.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farmer AJ, Gibson OJ, Dudley C, Bryden K, Hayton PM, Tarassenko L, Neil A. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes (ISRCTN 46889446) Diabetes Care. 2005;28(11):2697–2702. doi: 10.2337/diacare.28.11.2697. [DOI] [PubMed] [Google Scholar]
- 29.Vähätalo MA, Virtamo HE, Viikari JS, Rönnemaa T. Cellular phone transferred self blood glucose monitoring: Prerequisites for positive outcome. Pract Diabetes Int. 2003;21(5):192–194. [Google Scholar]
- 30.Cho JH, Lee HC, Lim DJ, Kwon HS, Yoon KH. Mobile communication using a mobile phone with a glucometer for glucose control in type 2 patients with diabetes: as effective as an Internet-based glucose monitoring system. J Telemed Telecare. 2009;15(2):77–82. doi: 10.1258/jtt.2008.080412. [DOI] [PubMed] [Google Scholar]
- 31.Faridi Z, Liberti L, Shuval K, Northrup V, Ali A, Katz DL. Evaluating the impact of mobile telephone technology on type 2 diabetic patients’ self-management: the NICHE pilot study. J Eval Clin Pract. 2008;14(3):465–469. doi: 10.1111/j.1365-2753.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 32.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;10(3):160–168. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Idígoras MI, Sepúlveda-Muñoz J, Sánchez-Garrido-Escudero R, Martínez-González JL, Escolar-Castelló JL, Paniagua-Gómez IM, Bernal-López R, Fuentes-Simón MV, Garófano-Serrano D. Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technol Ther. 2009;11(7):431–437. doi: 10.1089/dia.2008.0114. [DOI] [PubMed] [Google Scholar]
- 34.Larsen ME, Turner J, Farmer A, Neil A, Tarassenko L. Telemedicine-supported insulin optimisation in primary care. J Telemed Telecare. 2010;16(8):433–440. doi: 10.1258/jtt.2010.100103. [DOI] [PubMed] [Google Scholar]
- 35.Kim CS, Park SY, Kang JG, Lee SJ, Ihm SH, Choi MG, Yoo HJ. Insulin dose titration system in diabetes patients using a short messaging service automatically produced by a knowledge matrix. Diabetes Technol Ther. 2010;12(8):663–669. doi: 10.1089/dia.2010.0031. [DOI] [PubMed] [Google Scholar]
- 36.Yoo HJ, Park MS, Kim TN, Yang SJ, Cho GJ, Hwang TG, Baik SH, Choi DS, Park GH, Choi KM. A ubiquitous chronic disease care system using cellular phones and the internet. Diabet Med. 2009;26(6):628–635. doi: 10.1111/j.1464-5491.2009.02732.x. [DOI] [PubMed] [Google Scholar]
- 37.Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34(9):1934–1942. doi: 10.2337/dc11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azar M, Gabbay R. Web-based management of diabetes through glucose uploads: Has the time come for telemedicine? Diabetes Res Clin Pract. 2009;83(1):9–17. doi: 10.1016/j.diabres.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 39.Whitten P, Johannessen LK, Soerensen T, Gammon D, Mackert M. A systematic review of research methodology in telemedicine studies. J Telemed Telecare. 2007;13(5):230–235. doi: 10.1258/135763307781458976. [DOI] [PubMed] [Google Scholar]
- 40.Eysenbach G. CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J Med Internet Res. 2011;13(4):e126. doi: 10.2196/jmir.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]