Abstract
Diabetic autonomic neural imbalance is a severe complication of long-term diabetes patients and may progress to diabetic autonomic neuropathy (DAN). The prevalence of DAN is reported to be between 20 and 70%, depending on the studies. The pathogenesis of DAN remains unresolved. However, emerging evidence suggests that glycemic variability (GV) may be associated with autonomic imbalance in patients with both type 1 and type 2 diabetes. As symptoms are initially weak and uncharacteristic, the condition often remains undiagnosed until late manifestations present themselves. Predominant symptoms may include nausea, vomiting, gastroparesis, involuntary diarrhea, postural hypotension, voiding difficulties, and sexual dysfunction.
Analyzing the patterns of heart rate variability carries the potential for detection of autonomic imbalance in the subclinical and asymptomatic stages. In this context, GV may affect the sympathovagal balance by increasing oxidative stress and proinflammatory cytokines. Establishing a GV risk profile could therefore be important in determining risk factors in diabetes patients. This review addresses the issues above and in particular the possible association between diabetic autonomic imbalance and GV.
Keywords: autonomic imbalance, complications, correlation between estimates of blood glucose variability, diabetes, glycemic variability, heart rate variability, hypothalamic-pituitary-adrenal axis, neuropathy
Introduction
In the diabetes population, (both type 1 (T1DM) and type 2 (T2DM), autonomic imbalance is prevalent and may progress to diabetic autonomic neuropathy (DAN). The pathogenesis of DAN is not fully elucidated, but available evidence shows that the process is multi factorial, comprising impaired axonal transport, comprised blood supply, and metabolic disturbances, including perturbated glucose homeostasis.1 There is well-known and strong evidence suggesting that chronic hyperglycemia is involved in the development of autonomic neural imbalance.2–4 The presence of chronic hyperglycemia causes several biochemical changes, each of which may be involved in the processes of destruction of both myelin sheath and nerve fibers, which in turn is associated with increased dysfunction. Hyperglycemia-induced nerve damage may be due to one or more of the following biochemical changes: enhanced flux through the polyol pathway, oxidative stress, nonenzymatic glycosylation, and deprivation of nerve growth factor.5 However, it has been suggested that acute hyperglycemia increases circulating cytokines more than continuous hyperglycemia,6 and it has been shown that there is a chronic elevation in hypothalamic-pituitary-adrenal (HPA) axis activity in diabetes patients with DAN, thereby implying a possible association between glycemic variability (GV) and DAN.7
Symptoms, Clinical Signs, and Prevalence of Autonomic Neural Imbalance
Symptoms may be weak and uncharacteristic for years and are therefore easily overlooked. As a consequence hereof, people suffer undiagnosed and in silence, and even if symptoms are recorded, they may not be associated with diabetes. Clinical signs and symptoms may not appear until long after diabetes onset4 and depend on which organs are affected (Table 1). Predominant symptoms include nausea, vomiting, early satiety (gastroparesis), involuntary diarrhea (diabetic diarrhea), dizziness on standing (postural hypotension), voiding difficulties (neurogenic bladder), and sexual dysfunction (men and women).8 In the diabetes population (T1DM and T2DM), autonomic imbalance is prevalent and may progress to autonomic neuropathy, affecting various organs. The prevalence is estimated to be approximately 20–70%, depending on the test’s cohort,1,9–11 and is higher among individuals with T2DM compared with individuals with T1DM.12
Table 1.
| Symptoms and clinical signs | |
|---|---|
| Pupillary function | Pupil reflex dysfunction, reduced darkness adaptation |
| Cardiovascular function | Perioperative instability, profound fatigue, silent myocardial ischemia, orthostatic hypotension, orthostatic tachycardia, orthostatic bradycardia, and an inability to use heart rate as a guide to exercise intensity because of persistent tachycardia |
| Gastrointestinal function | Nausea and vomiting, diarrhea, gastroparesis, loss of bowel control, tachygastria, bradygastria |
| Sudomotor function | Gustatory sweating, hyperhidrosis, anhidrosis, dry hands and feet, heat intolerance |
| Respiratory function | Sleep apnea, reduced bronchial reactivity, shortness of breath |
| Genitourinary function | Erectile dysfunction, female sexual dysfunction, cystopathy, loss of bladder control, urinary tract infection |
| Endocrine function | Hypoglycemic unawareness |
Glycemic Variability
In individuals with normal glucose tolerance, the body’s metabolism of glucose is tightly controlled within a very narrow range (3.8–7.7 mmol⁄liter).19 This narrow range of blood glucose is maintained despite a lifestyle with irregular eating habits and activity patterns. In contrast, diabetes is characterized by glycemic disorders consisting of sustained chronic hyperglycemia—both fasting and postprandial—and acute glucose fluctuations. It has been argued whether acute glucose fluctuations should be a part of the term ‘glycemic disorder.’19–25In this light, one of the major challenges regarding GV estimation is the fact that there is no consensus on data acquisition and analysis (sampling frequency, recording time, or measure of variability).26 Table 1 shows the correlation between measures of GV in a cohort of 86 newly diagnosed T2DM patients (device: Medtronic MiniMed ®; sampling frequency: 1 per 5 min; recording time: 24 h).
As shown in Table 2, there is a weak correlation between variables of GV and glycated hemoglobin A1c (HbA1c). Hence, measurement of HbA1c alone does not reflect all-important aspects of the glycemic disorders. Despite the absence of a golden standard measure of GV in nondiabetic populations, accumulating data suggest that GV, which consists of both acute upward and downward glucose changes, is deleterious for critically ill patients.19,26,27 Furthermore, GV may play a role in the development of diabetic micro- and macrovascular complications,28hypoglycemic unawareness, and it may be associated with an increased risk of both hypo- and hyperglycemic excursions.7 Also, Monnier and coauthors25 have theorized that GV may have a more deleterious effect than chronic hyperglycemia in the development of diabetes complications. Su and colleagues29 showed, in a cohort of 344 patients with T2DM and chest pain, that GV [mean amplitude of glycemic excursions (MAGE) ≥3.4 mmol/liter; sampling frequency: 1 per 5 min; recording time: 72 h] was an independent predictor for coronary artery disease and is associated with its presence and severity. In relation to autonomic imbalance, Ohlsson and colleagues30 found, in a cohort of 20 diabetes patients complaining of symptoms suggestive of disturbances in the gastrointestinal tract, that parasympathetic dysfunction was associated with increased glucose fluctuations (coefficient of variation; sampling frequency: 1 per 5 min; recording time: 72 h). The authors hypothesized that the disturbed glucose homeostasis is possibly a result of disturbed nervous humoral reflexes.30 Flaviani and colleagues28 have shown, in a group of 26 T2DM patients receiving diet and/or metformin treatment and no hypertensive treatment or overt autonomic neuropathy, that GV (MAGE ≥3.4 mmol/liter; sampling frequency: 1 per 5 min; recording time: 48 h) affects sympathovagal balance in well-controlled diabetes patients (HbA1c 6.70 ± 1.25%). In an animal study, Jamali and colleagues31 concluded that hypoglycemia has a more severe impact on somatic motor nerves than on somatic sensory nerves, whereas hyperglycemia affects only somatic sensory nerves. There exists no coherent explanation for these findings, but it has been suggested that GV increases the risk of both hyper- and hypoglycemic excursions, which may be an important factor in increasing oxidative stress32 and proinflammatory cytokines,21 thereby implying a possible association to the HPA axis. It has been shown that diabetes patients with asymptomatic autonomic imbalance33 or established symptomatic polyneuropathy34 have an increased activity of HPA axis, and the degree of cortisol secretion is related to the degree of neuronal dysfunction.33,35 Other studies have described dysfunction in the HPA axis in the development or perpetuation of autoimmune disease.35,36 The HPA axis is a neuroendocrine system that plays an important role in the regulation of stress responses, and the end-hormones of the HPA axis are glucocorticoids.37
Table 2.
Correlation between Measures of Blood Glycemic Variabilitya
| Mean | SD | HbA1c | MAGE | CV | Hyper180 | Hyper150 | Hypo60 | Hypo72 | IQR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | — | |||||||||
| SD | 0.56 | — | ||||||||
| HbA1c | 0.39 | 0.46 | — | |||||||
| MAGE | –0.39 | –0.87 | –0.40 | — | ||||||
| CV | NS | 0.90 | 0.34 | 0.45 | — | |||||
| Hyper180 | 0.82 | 0.82 | 0.42 | 0.47 | 0.40 | — | ||||
| Hyper150 | 0.89 | 0.59 | 0.34 | 0.43 | 0.31 | 0.89 | — | |||
| Hypo60 | NS | NS | NS | NS | 0.43 | NS | NS | — | ||
| Hypo72 | –0.46 | NS | NS | NS | 0.44 | NS | NS | 0.66 | — | |
| IQR | 0.57 | 0.91 | 0.43 | 0.52 | 0.78 | 0.68 | 0.61 | NS | NS | — |
SD, standard deviation; CV, coefficient of variation; IQR, interquartile range.
Pearson’s linear correlation coefficient among common measures of GV26,38 and HbA1c. Glycemic variability measures were calculated from 86 patients (24 h of CGM, sampling each 5 min). Mean amplitude of glycemic excursions was calculated as average of positive adjacent peak greater than 1 SD. Coefficient of variation was calculated as SD/mean. HyperX and HypoX were calculated as fractions of the total time above/below a given threshold, e.g., Hyper150 was time (in percent) spent above 150 mg/dl. Interquartile range (IQR) is the difference between 75th and 25th percentiles from distribution of blood glucose values. Unpublished data.
Heart Rate Variability: a Measure of Asymptomatic Autonomic Imbalance
Detailed analysis of heart rate variability (HRV), including both cardiovascular reflex tests and time/frequency domain analysis, is an important tool to estimate autonomic balance.11,18 Imbalance in the autonomic nervous system may affect both the sympathetic and the parasympathetic nervous system and may affect any organ that is innervated by the autonomic nervous system, from the gastrointestinal tract to the skin.8 The autonomic nervous system is a multifunctional system that controls motility and is regulated by the sympathetic nervous system and parasympathetic system, which provide a rapidly responding mechanism to control a wide range of functions, e.g., cardiovascular, respiratory, gastro-intestinal, renal, exocrine and endocrine secretions, and microcirculation, and are involved in regulating immune and inflammatory processes.6The parasympathetic system may assist sympathetic functions by withdrawing and can antagonize them by increasing its activity.39,40
Heart rate variability, the study of beat-to-beat fluctuations in heart rate, has received much attention over the years as a means of measuring the balance in the autonomic nervous system.41–44 Both animal and human studies have shown an association between HRV and the degree of autonomic modulations.45 Heart rate variability is a result of both internal and external changes in breathing, blood pressure, hormone status, mental condition, and physical condition, and a number of pathophysiological conditions may decrease or increase the stimulation of the hearts sinoatrial.
Heart rate variability is most often recorded by means of electrocardiograph (ECG) but can also be extracted from pulse wave measurements.46 The HRV analysis is performed on the basis of heart rate recordings of various durations, from a few seconds to minutes, or even days or weeks.29,46–48 Usually three distinctive methods are used during continuous ECG measurement and recording:45,49–54 (1) Active tests: the patients perform a known physical response. (2) Passive tests: continuous ECG measurement during supine resting performed during spontaneous or controlled breathing. (3) Holter monitoring: the patient is continuously monitored by ECG with a portable device for at least 24 h in a hospital or in a home setting.
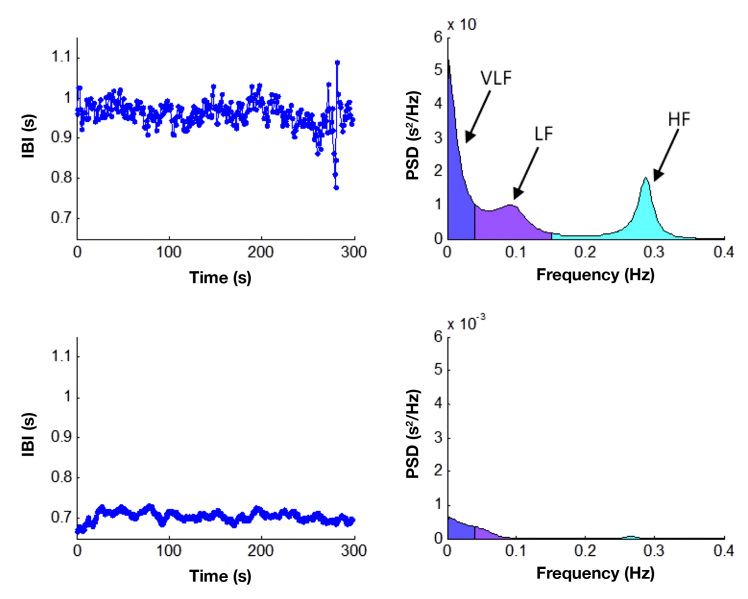
Data analysis of beat-to-beat intervals, or RR intervals, of the ECG recorded during all three methods can be analyzed by means of normal statistical descriptions (time domain), nonlinear, and/or by estimating the frequency-specific fluctuations in the RR intervals (frequency domain).41 To measure how quickly a given phenomenon repeats itself (e.g., frequency-specific fluctuations in heart rate), fast Fourier transformation, or autoregressive algorithms, known as spectral analysis, can be used to estimate the power of the frequency-specific fluctuations. By simply eyeballing RR intervals of an ECG from a healthy person [Figure 1 shows HRV from a healthy control (the two top plots) and a type 1 diabetes patient with cardiac autonomic neuropathy (CAN) (the two lower plots)],55 the signal seems almost chaotic, but by decomposing the signal into its frequency components, three distinguished peaks will be recognizable.44
Figure 1.
Time and frequency plots from an HRV passive 5 min supine test. The two top plots are healthy controls, and the two lower plots are a T1DM patient with autonomic dysfunction. In the frequency domain from the healthy control, three distinguished peaks are recognizable. Very low frequency (VLF), low frequency (LF) and high frequency (HF). Inter-beat-interval (IBI), frequency (Freq), Power Spectral Density (PSD).
The three distinguished peaks are: (1) Very low frequency, defined as the power in the frequency band less than 0.04 Hz and represents slower fluctuations caused by regulatory systems, temperature fluctuations, and day-night periodicity. (2) Low frequency, defined as the power in the frequency band 0.04–0.15 Hz.56 The low-frequency component is influenced by sympathetic, parasympathetic, and baroreflex sensitivity and is characterized by a peak at approximately 0.1 Hz, which is related to the 10-s blood pressure waves reflecting oscillations in baroreceptor and chemoreceptor reflex control systems, also called Mayer waves.57 (3) The high frequency band from 0.15–0.4 Hz is influenced by parasympathetic and spontaneous breathing, mainly contributing to power with a center frequency around 0.3 Hz.8
Use of the correct methodology, including data acquisitions and analysis, is crucial for extracting the information embedded in the variability of the heart rate.16,47 For clinical use, the gold standard HRV tests are: heart rate response to deep breathing, standing, and Valsalva maneuver.11 Active tests are crucial in order to make the distinction between autonomic imbalance and autonomic neuropathy. Time and frequency plots from an HRV passive supine test are used extensively for research purposes and may provide early additional information of both sympathetic and parasympathetic modulation.8 It is important to beaware that autonomic imbalance is not restricted to diabetes alone but may also comprise individuals without diabetes.
Autonomic Imbalance in Individuals without Diabetes
Associations between autonomic imbalance (measurements of HRV) and several pathological conditions or events are well-established,40,42,58–60 and reduced HRV has been shown to be associated with compromised health in the general population and to be predictive of mortality.42 Molgaard and coauthors61 reported an association between low 24-h HRV and sudden cardiac death in apparently healthy subjects. Tsuji and colleagues62 found that diminished HRV was significantly associated with the risk of cardiac events, including myocardial infarction and congestive heart failure. In the ARIC study, Dekker and colleagues40 found that low HRV was associated with increased risk of coronary heart disease and death from several causes, and the authors hypothesize that low HRV is indicative of general poor health. In addition, obesity, work stress, and depression have also been found to be associated with autonomic imbalance measured with HRV.63–66 Exercise has, on the other hand, been shown to improve HRV. Melanson and colleagues67 reported that time- and frequency-domain measures of HRV were increased in previously sedentary men within 12 weeks after following an exercise program. Also, weight loss has been reported to improve HRV in obese subjects, which seems to indicate that weight loss improves the health of the individuals and reduces the elevated cardiovascular risk in this group.63,64
Autonomic Imbalance in Individuals with Diabetes
The association between the finding of low HRV and the presence of symptomatic as well as asymptomatic autonomic imbalance in individuals with diabetes is well-described in the literature for both T1DM and T2DM,4 and the finding of autonomic imbalance in an individual with diabetes may indeed be an early risk marker and a marker of progression of diabetes complications. In both T1DM and T2DM populations, imbalance of the autonomic nervous system is a subclinical marker of the development of CAN.11,50,51,68 Cardiac autonomic neuropathy may provoke ischemic cardiac episodes by upsetting the balance between myocardial supply and demand.69 Testing for the presence of CAN has been suggested as a prognostic marker of microangiopathic complications,9,70 and the presence of asymptomatic CAN may occur before other complications.70 Furthermore, low HRV is associated with hypertension,71 ischemic stroke,72 and GV,73 and may be important in the detection and prevention of hypoglycemia.74,75 The described imbalance in the autonomic nervous system and the potential onset of autonomic neuropathy develop early in the disease development of diabetes.47 Furthermore, different progression patterns and pathophysiological mechanisms may account for the onset and development of CAN in T1DM and T2DM.11,14 In The DAN study, a cross-sectional observational study examining 382 T1DM and 271 T2DM subjects with diabetes, autonomic imbalance was associated with proliferative retinopathy, micro-albuminuria, and macroalbuminuria in T1DM patients, whereas in T2DM patients, it was associated with increased pulse pressure and obesity.12 These findings are in line with the Toronto consensus report.11 There is a clear association between the degree of autonomic imbalance and severity of late complications of diabetes, including retinopathy, nephropathy, and peripheral neuropathy.12 Autonomic imbalance in individuals with or without diabetes has been associated with a risk of perioperative accidental hypothermia, cardiovascular liability, and compromised cerebral blood flow.13,14 It has been suggested that preoperative screening of autonomic imbalance may be used as a risk stratification, identifying those patients who need further cardiac testing and optimization of the preoperative status.13 Overall, autonomic imbalance (reduced HRV) has been associated with compromised health in diabetes patients and is not limited to diagnosing CAN.
Discussion
Although autonomic neuropathy is clinically well-described, the pathophysiology remains elusive. As demonstrated earlier, there is no clear description of the pathogenesis of diabetic autonomic neural imbalance. Very little is known about the highly probable contribution of glycemic variability and the possible link to dysfunction in HPA axis.
With the current knowledge, optimal glycemic control is the most important clinical parameter in preventing development of DAN. Therefore, a multifactorial approachcould be beneficial focusing on continued patient education, motivation, and individualized risk assessment with ambitious goal achievements.76 However, intervention with drugs such as angiotensin-converting-enzyme (ACE) inhibitors and beta blockers may also have a potential role in future preventive strategies.14,18,77 Kontopoulos and colleagues78 found that administration of the ACE inhibitor Quinapril significantly increases parasympathetic activity measured by HRV in the time and frequency domain.
The Hoorn Study showed that cardiac autonomic imbalance in patients already at risk (diabetes, hyper-tension, or history of cardiovascular disease) may be especially hazardous.79 Therefore, early detection of autonomic imbalance and focus on diabetes control and elimination of risk factors for neuropathy (obesity, smoking, alcohol abuse, and hypertension) may delay or slow down the progression of diabetic neuropathy. Hence, early identification of asymptomatic autonomic imbalance is important to enhance risk stratification and enable initiation of individually tailored preventive measures in high-risk patients. A baseline determination of autonomic modulation is recommended upon diagnosis in T2DM and after 5 years of diagnosis in T1DM, followed by yearly repeated tests.69,80 Early detection of autonomic imbalance is also interesting due to the vasodilator effect of insulin, which increases skeletal muscle blood flow.81 This increase in blood flow may provoke postural hypotension in patients that have autonomic imbalance, causing symptoms that may be confused with hypoglycemia because of absence of the normal autonomic regulatory mechanisms to maintain blood pressure.82,83 It has been shown that arterial pressure falls, especially in the standing position, after insulin administration in diabetes patients with either abnormal baroreceptor reflexes or autonomic neuropathy.81,82 A glucose variability risk profile could therefore be important in determining risk factors in diabetes patients. One could speculate that the degree of glucose variability is associated with external factors such as the quality of medical and nursing care84 and the severity of the impact of diabetes complications. It is tempting to speculate whether autonomic imbalance is predictive of the state of glycemic variability.
Future Research
Measuring GV using continuous glucose monitoring (CGM) up to 144 h or more is a great step forward in order to study GV.85–87 Most invasive methods use an electrochemical sensor for measuring interstitial glucose concentration every 10 s. After elimination of outliers and/or noise, the reported value remains a weighted average reflecting interstitial glucose values during 1–10 min intervals.85,87 To understand the nature of GV, it is important to measure real-time GV using a high sampling frequency (1/s) for prolonged intervals. This could be achieved with the current invasive CGM but also with newer noninvasive devices. New information should be added about GV, especially when combined with other variables, such as autonomic imbalance.
The association between autonomic imbalance and GV in the development of autonomic neuropathy remains unsettled, and future prospective studies are needed.
Acknowledgments
The author would like to thank Niels Ejskjaer, M.D., Ph.D.; Hans Nygaard D.M.Sci.; Karin Ørbæk Kristensen, research secretary; Esben Laugesen M.D.; Simon Cichosz M.Sc.; Pernille Hoeyem, M.D.; Troels Krarup Hansen, D.M.Sc.; and Edith Clausen, research librarian; for contributions and advice.
Glossary
- (ACE)
angiotensin-converting-enzyme
- (CAN)
cardiac autonomic neuropathy
- (CGM)
continuous glucose monitoring
- (DAN)
diabetic autonomic neuropathy
- (ECG)
electrocardiograph
- (GV)
glycemic variability
- (HbA1c)
glycated hemoglobin
- (HPA)
hypothalamic-pituitary-adrenal
- (HRV)
heart rate variability
- (MAGE)
mean amplitude of glycemic excursions
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
References
- 1.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 2.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33(2):434–441. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev. 1994;10(4):339–383. doi: 10.1002/dmr.5610100403. [DOI] [PubMed] [Google Scholar]
- 6.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 7.Bragd J, Adamson U, Bäcklund LB, Lins PE, Moberg E, Oskarsson P. Can glycaemic variability, as calculated from blood glucose self-monitoring, predict the development of complications in type 1 diabetes over a decade? Diabetes Metab. 2008;34((6 Pt 1)):612–616. doi: 10.1016/j.diabet.2008.04.005. Epub 2008 Sep 27. [DOI] [PubMed] [Google Scholar]
- 8.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valensi P, Pariès J, Attali JR. French Group for Research and Study of Diabetic Neuropathy. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration obesity, and microangiopathic complications--the French multicenter study. Metabolism. 2003;52(7):815–820. doi: 10.1016/s0026-0495(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 10.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8(5):491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 11.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P on behalf of the Toronto Consensus Panel on Diabetic Neuropathy*. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011 doi: 10.1002/dmrr.1239. doi: 10.1002/dmrr.1239. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Fleischer J, Yderstraede K, Gulichsen E, Jakobsen PE, Lervang H, Eldrup E, Nygaard H, Tarnow L, Ejskjaer N. Assessment of subclinical diabetic autonomic neuropathy in outpatient clinics using a point-of-care device: The DAN-study (Abstract) J Diabetes Sci Technol. 2012;6(2):A46. [Google Scholar]
- 13.Mazzeo AT, La Monaca E, Di Leo R, Vita G, Santamaria LB. Heart rate variability: a diagnostic and prognostic tool in anesthesia and intensive care. Acta Anaesthesiol Scand. 2011;55(7):797–811. doi: 10.1111/j.1399-6576.2011.02466.x. doi: 10.1111/j.1399-6576.2011.02466.x. Epub 2011 Jun 9. [DOI] [PubMed] [Google Scholar]
- 14.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 15.Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68(11):928–930. doi: 10.3949/ccjm.68.11.928. 932, 934–44. [DOI] [PubMed] [Google Scholar]
- 16.Spallone V, Bellavere F, Scionti L, Maule S, Quadri R, Bax G, Melga P, Viviani GL, Esposito K, Morganti R, Cortelli P. Diabetic Neuropathy Study Group of the Italian Society of Diabetology. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis. 2011;21(1):69–78. doi: 10.1016/j.numecd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Vinik AI, Erbas T. Cardiovascular autonomic neuropathy: diagnosis and management. Curr Diab Rep. 2006;6(6):424–430. doi: 10.1007/s11892-006-0074-z. [DOI] [PubMed] [Google Scholar]
- 18.Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet Med. 2011;28(6):643–651. doi: 10.1111/j.1464-5491.2010.03184.x. doi: 10.1111/j.1464-5491.2010.03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceriello A, Ihnat MA. ‘Glycaemic variability’: a new therapeutic challenge in diabetes and the critical care setting. Diabet Med. 2010;27(8):862–867. doi: 10.1111/j.1464-5491.2010.02967.x. [DOI] [PubMed] [Google Scholar]
- 20.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch IB. Glycemic variability: it’s not just about A1C anymore! Diabetes Technol Ther. 2005;7(5):780–783. doi: 10.1089/dia.2005.7.780. [DOI] [PubMed] [Google Scholar]
- 22.Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008;31(Suppl 2):S150–4. doi: 10.2337/dc08-s241. [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick ES, Rigby AS, Atkin SL. For debate. Glucose variability and diabetes complication risk: we need to know the answer. Diabet Med. 2010;27(8):868–871. doi: 10.1111/j.1464-5491.2010.02929.x. [DOI] [PubMed] [Google Scholar]
- 24.Kilpatrick ES. Arguments for and against the role of glucose variability in the development of diabetes complications. J Diabetes Sci Technol. 2009;3(4):649–655. doi: 10.1177/193229680900300405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008;2(6):1094–1100. doi: 10.1177/193229680800200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eslami S, Taherzadeh Z, Schultz MJ, Abu-Hanna A. Glucose variability measures and their effect on mortality: a systematic review. Intensive Care Med. 2011;37(4):583–593. doi: 10.1007/s00134-010-2129-5. Epub 2011 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egi M, Bellomo R. Reducing glycemic variability in intensive care unit patients: a new therapeutic target? J Diabetes Sci Technol. 2009;3(6):1302–1308. doi: 10.1177/193229680900300610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Flaviani A, Picconi F, Di Stefano P, Giordani I, Malandrucco I, Maggio P, Palazzo P, Sgreccia F, Peraldo C, Farina F, Frajese G, Frontoni S. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care. 2011;34(7):1605–1609. doi: 10.2337/dc11-0034. Epub 2011 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, Zhou Y, Ma C. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohlsson B, Melander O, Thorsson O, Olsson R, Ekberg O, Sundkvist G. Oesophageal dysmotility, delayed gastric emptying and autonomic neuropathy correlate to disturbed glucose homeostasis. Diabetologia. 2006;49(9):2010–2014. doi: 10.1007/s00125-006-0354-9. Epub 2006 Jul 11. [DOI] [PubMed] [Google Scholar]
- 31.Jamali R, Mohseni S. Differential neuropathies in hyperglycemic and hypoglycemic diabetic rats. J Neuropathol Exp Neurol. 2006;65(12):1118–1125. doi: 10.1097/01.jnen.0000248546.13176.d4. [DOI] [PubMed] [Google Scholar]
- 32.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 33.Chiodini I, Di Lembo S, Morelli V, Epaminonda P, Coletti F, Masserini B, Scillitani A, Arosio M, Adda G. Hypothalamic-pituitary-adrenal activity in type 2 diabetes mellitus: role of autonomic imbalance. Metabolism. 2006;55(8):1135–1140. doi: 10.1016/j.metabol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Tsigos C, Young RJ, White A. Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1993;76(3):554–558. doi: 10.1210/jcem.76.3.8383141. [DOI] [PubMed] [Google Scholar]
- 35.Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, Epaminonda P, Masserini B, Beck-Peccoz P, Orsi E, Ambrosi B, Arosio M. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007;30(1):83–88. doi: 10.2337/dc06-1267. [DOI] [PubMed] [Google Scholar]
- 36.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuro-endocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 37.Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Hypothalamic-pituitary-adrenal (HPA) axis functioning in relation to body fat distribution. Clin Endocrinol (Oxf) 2010;72(6):738–743. doi: 10.1111/j.1365-2265.2009.03712.x. Epub 2009 Sep 21. [DOI] [PubMed] [Google Scholar]
- 38.Rausch JR. Measures of glycemic variability and links with psychological functioning. Curr Diab Rep. 2010;10(6):415–421. doi: 10.1007/s11892-010-0152-0. [DOI] [PubMed] [Google Scholar]
- 39.Whitsel EA, Raghunathan TE, Pearce RM, Lin D, Rautaharju PM, Lemaitre R, Siscovick DS. RR interval variation, the QT interval index and risk of primary cardiac arrest among patients without clinically recognized heart disease. Eur Heart J. 2001;22(2):165–173. doi: 10.1053/euhj.2000.2262. [DOI] [PubMed] [Google Scholar]
- 40.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102(11):1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 41.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 42.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol. 1997;145(10):899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- 43.Engel G, Beckerman JG, Froelicher VF, Yamazaki T, Chen HA, Richardson K, McAuley RJ, Ashley EA, Chun S, Wang PJ. Electrocardiographic arrhythmia risk testing. Curr Probl Cardiol. 2004;29(7):365–432. doi: 10.1016/j.cpcardiol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 44.DeBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events particularly for heart rate variability data. IEEE Trans Biomed Eng. 1984;31(4):384–387. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- 45.Freeman R. Assessment of cardiovascular autonomic function. Clin Neurophysiol. 2006;117(4):716–730. doi: 10.1016/j.clinph.2005.09.027. Epub 2006 Feb 7. [DOI] [PubMed] [Google Scholar]
- 46.Kristiansen NK, Fleischer J, Jensen MS, Andersen KS, Nygaard H. Design and evaluation of a handheld impedance plethysmograph for measuring heart rate variability. Med Biol Eng Comput. 2005;43(4):516–521. doi: 10.1007/BF02344734. [DOI] [PubMed] [Google Scholar]
- 47.Fleischer J, Charles M, Tarnow L, Jensen KS, Nygaard H, Sandbaek A, Ejskjaer N. Paper electrocardiograph strips may contain overlooked clinical information in screen-detected type 2 diabetes patients. J Diabetes Sci Technol. 2012;6(1):74–80. doi: 10.1177/193229681200600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mølgaard H, Sørensen KE, Bjerregaard P. Attenuated 24-h heart rate variability in apparently healthy subjects, subsequently suffering sudden cardiac death. Clin Auton Res. 1991;1(3):233–237. doi: 10.1007/BF01824992. [DOI] [PubMed] [Google Scholar]
- 49.Sharpey-Schafer EP, Taylor PJ. Absent circulatory reflexes in diabetic neuritis. Lancet. 1960;1(7124):559–562. doi: 10.1016/s0140-6736(60)92773-2. [DOI] [PubMed] [Google Scholar]
- 50.Wheeler T, Watkins PJ. Cardiac denervation in diabetes. Br Med J. 1973;4(5892):584–586. doi: 10.1136/bmj.4.5892.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ewing DJ, Campbell IW, Murray A, Neilson JM, Clarke BF. Immediate heart-rate response to standing: simple test for autonomic neuropathy in diabetes. Br Med J. 1978;1(6106):145–147. doi: 10.1136/bmj.1.6106.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hilsted J. Autonomic neuropathy: the diagnosis. Acta Neurol Scand. 1983;67(4):193–201. doi: 10.1111/j.1600-0404.1983.tb04563.x. [DOI] [PubMed] [Google Scholar]
- 53.Assessment: Clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46(3):873–880. [PubMed] [Google Scholar]
- 54.Fleischer J, Nielsen R, Laugesen E, Nygaard H, Poulsen PL, Ejskjaer N. Self-monitoring of cardiac autonomic function at home is feasible. J Diabetes Sci Technol. 2011;5(1):107–112. doi: 10.1177/193229681100500115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleischer J, Nygaard H, Ejskjaer N. Testing for autonomic neuropathy may support the diagnosis of diabetic gastroparesis (Abstract) J Diabetes Sci Technol. 2011;5(4):A9. [Google Scholar]
- 56.May O, Arildsen H. Long-term predictive power of heart rate variability on all-cause mortality in the diabetic population. Acta Diabetol. 2011;48(1):55–59. doi: 10.1007/s00592-010-0222-4. Epub 2010 Sep 16. [DOI] [PubMed] [Google Scholar]
- 57.Takalo R, Korhonen I, Majahalme S, Tuomisto M, Turjanmaa V. Circadian profile of low-frequency oscillations in blood pressure and heart rate in hypertension. Am J Hypertens. 1999;12((9 Pt 1)):874–881. doi: 10.1016/s0895-7061(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 58.Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32(2):293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 59.De Bruyne MC, Kors JA, Hoes AW, Klootwijk P, Dekker JM, Hofman A, van Bemmel JH, Grobbee DE. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. Am J Epidemiol. 1999;150(12):1282–1288. doi: 10.1093/oxfordjournals.aje.a009959. [DOI] [PubMed] [Google Scholar]
- 60.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 61.Mølgaard H, Sørensen KE, Bjerregaard P. Attenuated 24-h heart rate variability in apparently healthy subjects, subsequently suffering sudden cardiac death. Clin Auton Res. 1991;1(3):233–237. doi: 10.1007/BF01824992. [DOI] [PubMed] [Google Scholar]
- 62.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 63.Facchini M, Malfatto G, Sala L, Silvestri G, Fontana P, Lafortuna C, Sartorio A. Changes of autonomic cardiac profile after a 3-week integrated body weight reduction program in severely obese patients. J Endocrinol Invest. 2003;26(2):138–142. doi: 10.1007/BF03345142. [DOI] [PubMed] [Google Scholar]
- 64.Karason K, Mølgaard H, Wikstrand J, Sjöström L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83(8):1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 65.Lishner M, Akselrod S, Avi VM, Oz O, Divon M, Ravid M. Spectral analysis of heart rate fluctuations. A non-invasive, sensitive method for the early diagnosis of autonomic neuropathy in diabetes mellitus. J Auton Nerv Syst. 1987;19(2):119–125. doi: 10.1016/0165-1838(87)90005-1. [DOI] [PubMed] [Google Scholar]
- 66.Massin MM, Derkenne B, Tallsund M, Rocour-Brumioul D, Ernould C, Lebrethon MC, Bourguignon JP. Cardiac autonomic dysfunction in diabetic children. Diabetes Care. 1999;22(11):1845–1850. doi: 10.2337/diacare.22.11.1845. [DOI] [PubMed] [Google Scholar]
- 67.Melanson EL, Freedson PS. The effect of endurance training on resting heart rate variability in sedentary adult males. Eur J Appl Physiol. 2001;85(5):442–449. doi: 10.1007/s004210100479. [DOI] [PubMed] [Google Scholar]
- 68.Sharpey-Schafer EP, Taylor PJ. Absent circulatory reflexes in diabetic neuritis. Lancet. 1960;1(7124):559–562. doi: 10.1016/s0140-6736(60)92773-2. [DOI] [PubMed] [Google Scholar]
- 69.Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyörälä K, Raz I, Schernthaner G, Volpe M, Wood D. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 70.Pavy-Le Traon A, Fontaine S, Tap G, Guidolin B, Senard JM, Hanaire H. Cardiovascular autonomic neuropathy and other complications in type 1 diabetes. Clin Auton Res. 2010;20(3):153–160. doi: 10.1007/s10286-010-0062-x. Epub 2010 Mar 31. [DOI] [PubMed] [Google Scholar]
- 71.Istenes I, Keresztes K, Hermányi Z, Putz Z, Vargha P, Gandhi R, Tesfaye S, Kempler P. Relationship between autonomic neuropathy and hypertension--are we underestimating the problem? Diabet Med. 2008;25(7):863–866. doi: 10.1111/j.1464-5491.2008.02458.x. [DOI] [PubMed] [Google Scholar]
- 72.Ko SH, Song KH, Park SA, Kim SR, Cha BY, Son HY, Moon KW, Yoo KD, Park YM, Cho JH, Yoon KH, Ahn YB. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with Type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med. 2008;25(10):1171–1177. doi: 10.1111/j.1464-5491.2008.02567.x. [DOI] [PubMed] [Google Scholar]
- 73.oussay S, Alvariñas JH, Burlando G, Gonzalez C, Garcia A, Vasta S. The relation of heart rate variability with glycemic variability in type 1 diabetic patients (Abstract 658-P) Acute and Chronic Complications Diabetes. 2011 Jul;60:A133–A195. doi:10.2337/db11-478-715. [Google Scholar]
- 74.Skladnev VN, Tarnavskii S, McGregor T, Ghevondian N, Gourlay S, Jones TW. Hypoglycemia alarm enhancement using data fusion. J Diabetes Sci Technol. 2010;4(1):34–40. doi: 10.1177/193229681000400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skladnev VN, Ghevondian N, Tarnavskii S, Paramalingam N, Jones TW. Clinical evaluation of a noninvasive alarm system for nocturnal hypoglycemia. J Diabetes Sci Technol. 2010;4(1):67–74. doi: 10.1177/193229681000400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pedersen O, Gaede P. Intensified multifactorial intervention and cardiovascular outcome in type 2 diabetes: the Steno-2 study. Metabolism. 2003;52((8 Suppl 1)):19–23. doi: 10.1016/s0026-0495(03)00213-0. [DOI] [PubMed] [Google Scholar]
- 77.Hoogwerf BJ, Young JB. The HOPE study. Ramipril lowered cardiovascular risk, but vitamin E did not. Cleve Clin J Med. 2000;67(4):287–293. doi: 10.3949/ccjm.67.4.287. [DOI] [PubMed] [Google Scholar]
- 78.Kontopoulos AG, Athyros VG, Didangelos TP, Papageorgiou AA, Avramidis MJ, Mayroudi MC, Karamitsos DT. Effect of chronic quinapril administration on heart rate variability in patients with diabetic autonomic neuropathy. Diabetes Care. 1997;20(3):355–361. doi: 10.2337/diacare.20.3.355. [DOI] [PubMed] [Google Scholar]
- 79.Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24(10):1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 80.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 81.Liang C, Doherty JU, Faillace R, Maekawa K, Arnold S, Gavras H, Hood WB., Jr Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J Clin Invest. 1982;69(6):1321–1336. doi: 10.1172/JCI110572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Page MM, Watkins PJ. Provocation of postural hypotension by insulin in diabetic autonomic neuropathy. Diabetes. 1976;25(2):90–95. doi: 10.2337/diab.25.2.90. [DOI] [PubMed] [Google Scholar]
- 83.Page MM, Smith RB, Watkins PJ. Cardiovascular effects of insulin. Br Med J. 1976;1(6007):430–432. doi: 10.1136/bmj.1.6007.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kildegaard J, Christensen TF, Hejlesen OK. Sources of glycemic variability--what type of technology is needed? J Diabetes Sci Technol. 2009;3(4):986–991. doi: 10.1177/193229680900300448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waldron-Lynch F, Herold KC. Continuous glucose monitoring: long live the revolution! at Clin Pract Endocrinol Metab. 2009;5(2):82–83. doi: 10.1038/ncpendmet1044. Epub 2008 Dec 17. [DOI] [PubMed] [Google Scholar]
- 86.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 87.Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28(5):1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]