Abstract
Fluorescence represents a promising alternative technology to electrochemistry and spectroscopy for accurate analysis of glucose in diabetes; however, no implanted fluorescence glucose assay is currently commercially available. The method depends on the principle of fluorescence, which is the emission of light by a substance after absorbing light. A fluorophore is a molecule that will absorb energy of a specific wavelength and reemit energy at a different wavelength. A fluorescence glucose-sensing molecule can be constructed to increase or decrease in fluorescence from baseline according to the ambient concentration of glucose. A quantum dot is a semiconductor crystal that can serve as a sensor by fluorescing at a desired wavelength or color, depending on the crystal size and materials used. If receptor molecules for glucose can be adsorbed to single-wall carbon nanotubules, then the resulting binding of glucose to these receptors will alter the nanotubes’ fluorescence. Fluorescence glucose sensors can provide a continuous glucose reading by being embedded into removable wire-shaped subcutaneous or intravenous catheters as well as other types of implanted structures, such as capsules, microcapsules, microbeads, nano-optodes, or capillary tubes.
Fluorescence glucose-sensing methods, which are under development, offer four potential advantages over commercially used continuous glucose monitoring technologies: (1) greater sensitivity to low concentrations of glucose, (2) the possibility of constructing sensors that operate most accurately in the hypoglycemic range by using binding proteins with disassociation constants in this range, (3) less need to recalibrate in response to local tissue reactions around the sensor, and (4) no need to implant either a transmitter or a power source for wireless communication of glucose data.
Fluorescence glucose sensors also have four significant disadvantages compared with commercially used continuous glucose monitoring technologies: (1) a damaging foreign body response; (2) a sensitivity to local pH and/or oxygen, which can affect the dye response; (3) potential toxicity of implanted dyes, especially if the implanted fluorophore cannot be fully removed; and (4) the necessity of always carrying a dedicated light source to interrogate the implanted sensor. Fluorescence sensing is a promising method for measuring glucose continuously, especially in the hypoglycemic range. If currently vexing technical and engineering and biocompatibility problems can be overcome, then this approach could lead to a new family of continuous glucose monitors.
Keywords: fluorescence; glucose; nanotube; quantum dot; sensor, tattoo
Introduction
The three most investigated technologies for measuring glucose are electrochemistry, spectroscopy, and fluorescence. Almost every currently available glucose monitoring device uses electrochemistry to quantify glucose concentration. Only a single product that uses spectroscopy to measure glucose has been cleared for sale (in Europe only), and no product that uses fluorescence to measure glucose has been approved.
Greater accuracy is being demanded of blood glucose monitors in the outpatient and inpatient settings by government regulators.1 Continuous glucose monitors will eventually need to demonstrate better accuracy if they are ever going to be cleared for a primary indication to modify therapy without the need for a confirmatory blood glucose test. The demand for more accurate glucose monitors is spurring interest in new approaches to measuring glucose concentration in blood and interstitial fluid. Fluorescence represents a promising alternative technology to electrochemistry and spectroscopy for accurate analysis of glucose and other biological molecules.
Fluorescence
Fluorescence refers to the emission of light by a substance after it absorbs light. This phenomenon occurs when incoming light strikes a molecule or structure with fluorescent properties and promotes an electron to a higher energy level. The excited molecule or structure then loses some of its energy by emitting light. The emitted light has a lower energy than the interrogating light and is therefore shifted from the blue end of the spectrum (characterized by shorter wavelength, higher frequency, and higher energy) toward the red end of the spectrum (characterized by longer wavelength, lower frequency, and lower energy).2
Fluorescence is the mechanism for how a black light [which is a high-energy ultraviolet (UV) light] causes bright colors to appear in apparently dull-colored (under white light) fluorescent minerals or paints containing fluorescent dyes. Paper currency is commonly modified with fluorescent ink for anticounterfeiting. Bright colors can be seen because the UV light in the invisible range causes electrons in these objects to fluoresce at an energy level lower than UV. This fluorescence results in light emission at selected specific wavelengths, which therefore appear bright, in the lower-energy visible range.
Fluorophores
A fluorophore is a molecule that will absorb energy of a specific wavelength and reemit energy at a different wave-length. Fluorescent organic molecules usually are aromatic or contain conjugated bonds, which are alternating single and double bonds. These molecules contain delocalized electrons that form a cloud around the molecule. The largest conjugated systems of delocalized electrons are found in graphite, carbon nanotubes, and conducting polymers. These electrons are susceptible to becoming excited and fluorescing in response to light energy.
Förster Resonance Energy Transfer
An excited fluorophore can relax not only by emitting light, but also by dissipating energy through heat and vibration or transferring the energy to another fluorophore, which is nearby. Following absorption of a photon, if a donor fluorophore transfers its excitation energy to a ground-state acceptor fluorophore (which will then emit a lower energy photon), then the process is known as Förster (or fluorescence) resonance energy transfer (FRET).3 The amount of energy transfer depends mainly on: (1) the degree of spectral overlap between donor transfer and acceptor absorption and (2) the separation distance between the donor and acceptor pair.4 In the presence of FRET, donor fluorescence intensity and excited-state lifetime decrease as incoming light energy or electrons are transferred from the donor fluorophore to the acceptor fluorophore rather than radiated as light. If FRET between a pair of fluorophores can be made to decrease (by increased structural or charge separation), then the donor fluorophore will emit more light (exhibit greater fluorescence) in response to incoming photons. Förster resonance energy transfer can therefore be thought of as a process whereby an acceptor molecule suppresses fluorescence by draining energy from a nearby excited fluorophore and limits the amount of light emitted by that fluorophore.
Fluorescent Lifetime
With spectroscopic assays, the concentration of an analyte is proportionate to the intensity of the measured light signal, which is reflected in response to light interrogation. Instead of intensity, fluorescence sensors can also measure the characteristic time over which an excited substance emits photons, which follows an exponential decay profile. This metric is known as the fluorescent lifetime (FL; also known as fluorescence decay lifetime).5 With FL, the lifetime of the fluorophore signal, rather than its intensity, is used to measure the concentration of an analyte. This method has the advantage of minimizing the effect of photon scattering in thick layers of sampled tissues. Fluorescence-lifetime sensing generates emissions that are determined by the fluorescence lifetime of the emitting analytes. Therefore the contrast between substances with different fluorescence decay rates can be differentiated, even if they fluoresce at exactly the same wavelength. Although both FL and spectroscopy represent measurements of an interaction of a light source with glucose in the body, various factors of the incoming light and emitted light can affect these two measurements differently, as presented in Table 1.
Table 1.
How Features of Light Can Affect Measurements of Fluorescent Lifetime and Spectroscopya
| Features of incident light | FL | Spectroscopy |
|---|---|---|
| Fluctuations in incident light signal amplitude | No | Yes |
| Duration of incident light exposure | No | Yes |
| Fluorophore concentration | No | Yes |
| Fluorescence intensity | No | Yes |
| Emission spectrum | No | Yes |
| Light scattering by tissue | No | Yes |
No, effect is absent; yes, effect is present.
Fluorescence Sensor Mechanisms
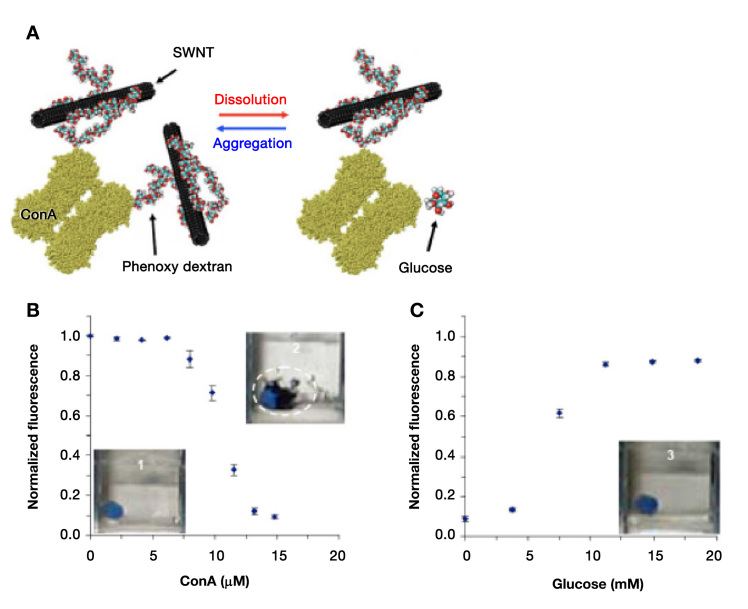
A fluorescence glucose sensor molecule can be constructed to contain a receptor for glucose, a donor fluorophore, and an acceptor of fluorescent energy or electrons, all in close proximity. When glucose binding to the receptor occurs, then the sensor molecule undergoes a structural change that brings the fluorescence donor and fluorescence acceptor farther apart or else decreases electron transfer to the donor. Glucose binding to this type of receptor–fluorophore molecule can decrease FRET by disrupting chelated bonds between the donor fluorophore and the acceptor, leading to decreased electron sharing and instead to increased fluorescence. This process is depicted in Figure 1. Conversely, in the absence of glucose, the fluorophore donor and acceptor develop increased steric and electronic interactions that result in increased electron transfer to the donor fluorophore and less fluorescence due to the increased amount of FRET between these groups. The concentration of glucose can be calculated by measuring an increase or decrease from baseline in fluorescence of the donor fluorophore associated with the glucose receptor molecule. An increase in FRET leading to decreased fluorescence of a donor fluorophore in the presence of light is known as quenching, and a decrease in FRET leading to increased fluorescence of a donor fluorophore in the presence of light is known as excitation.3 Most fluorophores used in fluorescence glucose sensing are organic dyes.
Figure 1.
A receptor–fluorophore molecule can exhibit low fluorescence because of FRET-induced quenching (left half), which is an interaction between an energy acceptor, also known as a quencher, and a fluorophore. When a glucose molecule reversibly binds to the receptor part of the molecule (right half), then the FRET becomes disrupted because of decreased electron sharing between the two interacting groups. In the absence of FRET, quenching of the fluorophore ceases and the fluorophore becomes highly fluorescent.
An additional mechanism of using fluorescence to measure the local concentration of glucose can be used with a boronic acid receptor moiety on a recognition molecule that can competitively bind to particular dyes or to glucose. When specific fluorescent dyes, such as alizarin red, are bound to the receptor in the absence of glucose, then electrons are pulled from the dye to the boronic acid, and the dye fluoresces. In the presence of glucose, the dye is displaced from the shared receptor, and in its free form, with a full complement of electrons, the dye does not fluoresce.6 In this type of indicator displacement assay (unlike most FRET-based fluorescent assays), the greater the glucose concentration, the less fluorescence is emitted.7
Quantum Dots
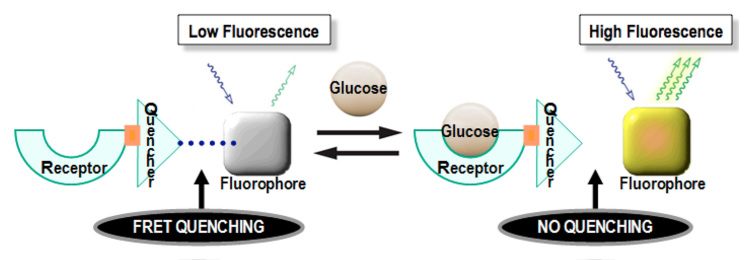
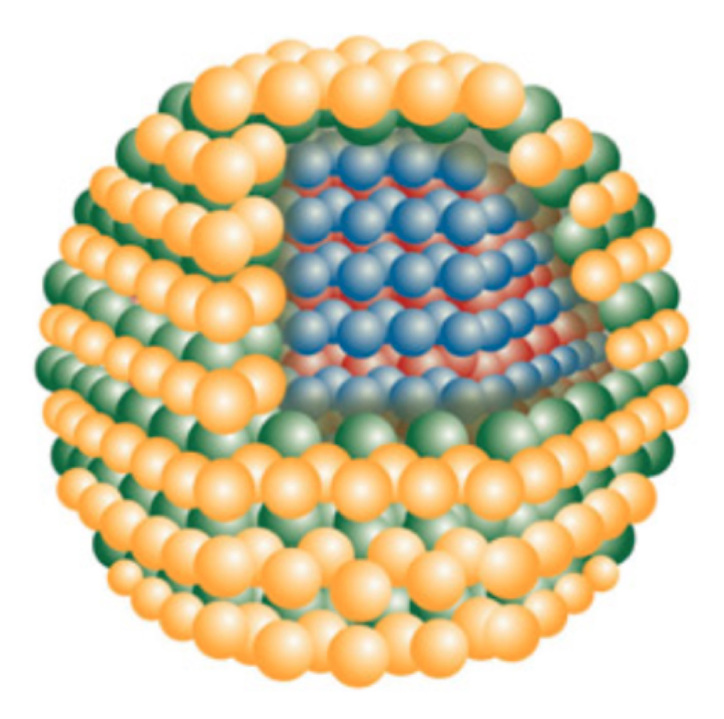
The intensity and duration of the light emission in a fluorescence assay depend on the shape and charge of the donor fluorophore. A quantum dot (QD) can be used as part of a fluorescence sensor. A QD is an inorganic (non-natural) nanometer-sized semiconductor crystal that can be designed to fluoresce at a desired wavelength or color over a given range, depending on the crystal size and materials used.8 Quantum dots are typically composed of a cadmium selenide core and a zinc sulfide shell (Figure 2) and are extremely photostable and bright. They absorb light very efficiently over a broad spectrum, and they have narrow and spectrally tunable emission profiles. Because they have a broad absorption spectrum, QDs can be excited far from their emission wavelengths. Therefore their emission light is separated from the excitation light, which maximizes the efficiency of measuring an output signal. In contrast, organic fluoro-phores typically have narrower absorption bands and broader fluorescence spectra.9 Photobleaching refers to the destruction of a fluorescence donor as a light emitter caused by strong illumination at its excitation wavelength.10 Quantum dots exhibit negligible photobleaching and longer fluorescence lifetimes than organic fluorophores.11 A QD can serve both as an acceptor and donor of light energy and can be made to fluoresce at a single wavelengthor multiple narrow wave-lengths simultaneously. A QD can be used to generate fluorescence in response to glucose binding with a receptor molecule that is also bound to the QD (Figure 3). Alternatively, a QD can fluoresce at multiple distinct wavelengths and trigger fluorescence of multiple other fluorophores (which must serve both as acceptors and then as light emitters).12 This process leads to an analyte-specific pattern of emission in response to light interrogation by the QD for measurement of the analyte. This property of QDs is known as multiplexing.13,14
Figure 2.
Cadmium selenide/zinc sulfide core-shell QDs have an inner spherical cadmium selenide semiconductor crystal surrounded by a shell of zinc sulfide semiconductor. Core diameters typically range from 2 to 10 nm, and shell thickness may vary from 0.5 to 4 nm. Reprinted with permission from Evident Technologies Inc.
Figure 3.
Schematic description of properties of a solution-phase QD–GBP sensing complex that targets glucose. On the left is a solution containing QDs comprised of cadmium selenide/zinc sulfide conjugated with multiple moieties of an engineered GBP, which also binds to a dextran–quencher complex. The quencher is in close proximity to the QD center, where it quenches outgoing QD emissions by way of FRET. On the right, when glucose is added to a solution containing QDs bound to dextran–quencher complexes, then glucose displaces dextran and the quencher. Without quenching, the result is a concentration-dependent increase in emissions. D, dextran; Q, quencher; ; GLUC, glucose.
The large size of QD crystals and their need for surface functionalization, as well as their limited pH solubility, reduce their capability to undergo bioconjugation with receptors for fluorescent assays.4 Potential toxicity from heavy metals that are usually contained in QDs might be mitigated by specialized coatings or via encapsulation within polyethylene glycol microcapsules.15 The emergence of QD technology, which is at its infancy in glucose sensing, represents a convergence of biotechnology and nanotechnology for development of hybrid synthetic and natural particles with unique recognition and photonic properties to decrease the limit of detection and improve sensitivity and specificity.16
Recognition Molecules for Glucose
Three types of recognition molecules for glucose can be attached to fluorophores for creating fluorescence sensors that can bind glucose reversibly: (1) enzymes, (2) boronic acid derivatives, and (3) glucose-binding proteins (GBPs), including concanavalin A (ConA) and glucose/galactose binding protein.17–19 Following such reversible glucose binding, fluorophores that are attached in various ways to these recognition molecules can undergo changes in their local properties, potentially leading to altered fluorescence. At this time, enzymes and engineered proteins are being studied most intensively for potential use as fluorescence-based glucose sensors because of boronic acid’s sensitivity to ambient pH and ConA’s risk of toxicity.20–22 Non-natural proteins can be genetically engineered to exhibit variable binding in predetermined ranges of ambient glucose and can be made to vary their binding and therefore their fluorescence across desired physiologic ranges.
Single-Walled Carbon Nanotubes
An additional approach to generating variable amounts of fluorescence instead of using a natural organic dye as a fluorescing agent is to use single-walled carbon nanotube (SWCNT) molecules complexed with glucose recognition molecules.23 Single-walled carbon nanotube molecules are cylinder-shaped tubes of graphite whose diameters are in the range of 1 nm and whose lengths can be up to several millimeters. They are essentially one-dimensional structures, and all their atoms are surface atoms. Single-walled carbon nanotubes are highly fluorescent, and their light emissions are tunable in response to local electronic changes. Functionalization of SWCNTs can disrupt their one-dimensional electronic structure. If receptor molecules for glucose can be adsorbed to SWCNTs, then the resulting binding of glucose to these receptors will alter the nanotubes’ fluorescence. The change in fluorescence can then be linked to the ambient glucose concentration. Unlike most organic fluorophore dyes, which undergo photobleaching with exposure to a continuous light source,SWCNTs remain photostable. Therefore, these molecules are well-suited to being developed into continuous glucose sensors that can operate with constant light interrogation.24 The potential risks to using SWCNT glucose sensors are foreign body encapsulation, membrane biofouling, and possible toxicity of the nanotubes.25
Three examples of SWCNT-based fluorescence glucose sensors are presented here. First is a protein-based sensor. In this type of system, molecules of glucose oxidase and ferricyanide, an electron acceptor, are both adsorbed to SWCNTs. In the absence of glucose, the ferricyanide decreases SWCNT fluorescence, but in the presence of glucose, the enzyme breaks down glucose, generates current, and reduces the ferricyanide, which decreases the inhibitory effect of ferricyanide on fluorescence. Therefore, a greater concentration of glucose leads to greater fluorescence.26 Second is a polymer-based sensor, wherein SWCNTs are wrapped in dextran, which is a polymer of glucose molecules. Adding ConA, which binds glucose, to the system, induces aggregation of the SWCNTs and decreases fluorescence. Introducing glucose in a solution of unknown concentration competitively decreases the binding of ConA to the SWCNTs, dissolves the aggregates, and causes fluorescence to increase in proportion to the Novo Nordisk Pharmaceuticals Inc.). The order form utilized is shown in Figure 4).27 A second polymer-based sensor can also serve as the basis of a fluorescence glucose sensor. Hydrogels are biocompatible polymers that swell in the presence of water. A hydrogel embedded with SWCNTs inside will swell in response to local fluctuations in analyte concentrations, and the swelling will alter fluorescent emissions by SWCNTs. Cross-linking with glucose oxidase can render the hydrogel to be reversibly glucose responsive. In a mouse model, a glucose-responsive SWCNT-studded hydrogel has been demonstrated to respond reversibly to increasing ambient glucose concentrations with increasing fluorescence. The shifts in fluorescence appear to be related not only to lattice deformations, but also to dielectric changes around the nanotubes.28 Third is a protein/polymer-based SWCNT sensor in which changes in fluorescence are directly determined by changes in protein conformation. This method uses periplasmic proteins that contain nonenzymatic receptors. These proteins can adopt an open form in the absence of ligand and a closed form in the presence of ligand.29 This conformational change from open to closed in the presence of glucose can be used to induce a change in FRET when such GBPs are bound to nanotubes. The reversible conformational change induces a reversible decrease in fluorescence.30
Figure 4.
Schematic representation of a carbon nanotube glucose sensor. (A) Dextran-coated nanotubes will aggregate in the presence of ConA, decreasing measured flourescence. The addition of glucose results in aggregate dissoltion and fluorescence recovery. (B) The addition of ConA to dextran-suspended nanotubes results in a 90% decrease in SWCNT fluorescence and visible nanotube aggregation. (C) The subsequent addition of glucose causes nanotube aggregate dissolution and fluorescence recovery. This figure is reproduced from an article by Barone and Strano.25.
Structures of Fluorescence Glucose Sensors
Configurations of fluorescent sensors for measurement of glucose in interstitial fluid include (1) removable wire-shaped subcutaneous probe sensors containing fluorescing dye;31,32 (2) removable intravenous catheters;33 (3) contain-ment within implanted structures, such as capsules,34,35 microbeads,36 microcapsules,37–39 or nano-optodes;40 or (4) implantable nonremovable carbon nanotube sensors within a capillary tube coated with a hydrogel.28 In all these cases, the sensor would respond to interrogation by an extracorporeal light source with the amount of fluorescence related to the ambient glucose concentration.
For a subcutaneously implanted glucose oxidase sensor to measure glucose accurately, the concentration of ambient oxygen must exceed that of glucose or else the reaction will be limited by a lack of oxygen.41 The typical interstitial fluid or blood concentrations of glucose are approximately 3–33 mM (50–600 mg/dl), but the typical concentrations of oxygen are only approximately 0.01– 0.15 mM, which correspond to partial pressure of oxygen levels of 7–100 mmol.42 This disparity can be addressed by decreasing the transport of glucose to the sensor, but increasing oxygen transport is not practical. Glucose transport into microcapsules of fluorescing glucose sensors can be tuned by varying the thickness and composition of transport-controlling nanofilm coatings43 as well as varying the porosity of the microspheres.42
Development of a robust dedicated optoelectronic system is required for adequate interrogation of implanted fluorescence glucose sensors. The optoelectronic system must excite the sensor, collect emitted light, analyze the glucose concentration, and communicate the results. Five barriers that must be overcome to build a robust fluorescence glucose-sensing system include (1) photon loss at fiber connections; (2) tradeoff between signal resolution and inexpensive input light power; (3) light scatter from tissue; (4) autofluorescence, defined as the natural emission of light by biological structures or molecules after they have absorbed light and which must be distinguished from light that originates from artificially added fluorescent markers (fluorophores); and (5) photobleaching, which is the photochemical destruction of a fluorophore.
These five barriers will need to be overcome with a combination of solutions. The first two barriers might be amenable to measurement of two wavelengths simul-taneously and elimination of fiber coupling between the spectrofluorometer and the hardware for spectrum analysis.44 The light scatter problem can be overcome with the use of longer wavelengths to reduce tissue scattering. A fluorescent glucose-sensing marker can be distinguished from autofluorescence signals by specifically measuring the FL of the marker fluorophore. Unlike organic dyes that are susceptible to photobleaching, QDs and SWCNT fluorophores are not susceptible to this gradual loss of function. Eight remedies to reduce photobleaching in a fluorescence glucose-sensing system are listed in Table 2.
Table 2.
Remedies to Decrease Photobleaching in a Fluorescence Glucose-Sensing System
| 1 | Decrease intensity of light |
| 2 | Decrease duration of light exposure |
| 3 | Lower energy (higher wavelength) of light for interrogation |
| 4 | Increase concentration of fluorophore |
| 5 | Substitution with a fluorophore that is less susceptible to photobleaching |
| 6 | Add an oxygen scavenger to restore function of a photobleached glucose binder |
| 7 | Use QD as a fluorescing agent (does not photobleach) |
| 8 | Use SWCNT as a fluorescing agent (does not photobleach) |
Novel methods for attenuating fibrosis and inflammation are also being developed, including deposition of dexamethasone in the vicinity of the sensor. The main drawback to a subcutaneously implanted wire or capillary tube could be a local reaction causing skin irritation and a need to change sensors frequently.38 The main drawback to an intravenous sensor is the risk of formation of thrombus or emboli. The main drawback to an implanted tattoo is the risk of toxicity if the dye, QD, SWCNT, or other fluorophore were to leach out of the sensor matrix particles over time. A drawback to any of these sensors might be skin photodamage from prolonged UV or infrared light exposure.
Advantages and Disadvantages of Fluorescence Sensors
An implanted fluorescence sensor, compared with an implanted electrochemical sensor, offers four advantages: (1) potentially greater sensitivity to low concentrations of analytes, depending on the binding properties of the electrochemical sensor’s enzyme; (2) the potential to create sensors that operate most accurately in the hypoglycemic range with synthetic binding proteins that have disassociation constants in this range and that fluoresce nonlinearly in response to increasing glucose concentrations; (3) less need to recalibrate in response to local tissue reactions around the sensor; (4) no need to implant either a transmitter or a power source for wireless communication of glucose data. An implanted fluorescence sensor, compared with an implanted ampero-metric sensor also offers four disadvantages, which, in the aggregate, are so significant that no implanted fluorescence glucose assay is currently available outside of a testing laboratory. These disadvantages include (1) a foreign body response that can damage the sensor; (2) a sensitivity to local pH and/or oxygen, which can affect the dye response; (3) potential toxicity of implanted dyes, especially if the implanted fluorophore cannot be fully removed upon development of photobleaching or a local reaction; and (4) the necessity of always carrying a dedicated light source for interrogation of the implanted sensor, although it should be noted that a dedicated optical system for interrogation could be miniaturized and worn much like current continuous glucose monitoring units. Finally, the cost of sensor material and handheld spectrofluorometers is currently not competitive with portable electrochemistry sensors.
Conclusions
Fluorescence sensors for measuring glucose offer great promise for patients who need accurate measurements of their glucose levels, especially in the hypoglycemic range. No product based on this type of technology is currently available; however, several companies are currently developing such products for subcutaneous or intravenous use. These systems will need to be tested carefully to ensure that they can maintain (1) accuracy in clinical settings, (2) stability following prolonged light exposure without photobleaching, and (3) safe implantation without chronic local inflammation or toxicity. The costs of these systems relative to existing technologies for continuous glucose monitoring will need to come down through engineering advances and economies of scale from mass production. Fluorescence glucose sensors are most suitable for subcutaneous or intravenous implantation, and combined with nonbleaching light sources, they can be configured as continuous glucose monitors. Current electrochemical sensing technology appear to be so effective and well established that near-term introduction of fluorescence-based self-monitoring of blood glucose devices for spot testing is not likely to be introduced in the foreseeable future. However, given the sensitivity, specificity, and stability of fluorescence glucose sensors, it is likely that several products using this technology will become available for subcutaneous or intravenous implantation in the 2020s. Fluorescence glucose sensing has a bright future.
Acknowledgments
The author thanks Michael McShane for his helpful suggestions for this article.
Glossary
- (ConA)
concanavalin A
- (FL)
fluorescent lifetime
- (FRET)
Föer resonance energy transfer
- (GBP)
glucose-binding protein
- (QD)
quantum dot
- (SWCNT)
single-walled carbon nanotube
- (UV)
ultraviolet
References
- 1.Klonoff DC. The Food and Drug Administration is now preparing to establish tighter performance requirements for blood glucose monitors. J Diabetes Sci Technol. 2010;4(3):499–504. doi: 10.1177/193229681000400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams RT, Bridges JW. Fluorescence of solutions: a review. J Clin Pathol. 1964;17:371–394. doi: 10.1136/jcp.17.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaláb P, Soderholm J. The design of Förster (fluorescence) resonance energy transfer (FRET)-based molecular sensors for Ran GTPase. Methods. 2010;51(2):220–232. doi: 10.1016/j.ymeth.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapp AR, Medintz IL, Mattoussi H. Förster resonance energy transfer investigations using quantum-dot fluorophores. Chem Phys Chem. 2006;7(1):47–57. doi: 10.1002/cphc.200500217. [DOI] [PubMed] [Google Scholar]
- 5.Berezin MY, Achilefu S. Fluorescence lifetime measurements and biological imaging. Chem Rev. 2010;110(5):2641–2684. doi: 10.1021/cr900343z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springsteen G, Wang B. Alizarin Red S. as a general optical reporter for studying the binding of boronic acids with carbohydrates. Chem Commun. 2001:1608–1609. doi: 10.1039/b104895n. [DOI] [PubMed] [Google Scholar]
- 7.Cao H, Diaz DI, DiCesare N, Lakowicz JR, Heagy MD. Monoboronic acid sensor that displays anomalous fluorescence sensitivity to glucose. Org. Lett. 2002;4:1503–1505. doi: 10.1021/ol025723x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, Liu B, Zhang H, Wang Y. The fluorescence bioassay platforms on quantum dots nanoparticles. J Fluoresc. 2005;15(5):729–733. doi: 10.1007/s10895-005-2980-5. [DOI] [PubMed] [Google Scholar]
- 9.Grabolle M, Ziegler J, Merkulov A, Nann T, Resch-Genger U. Stability and fluorescence quantum yield of CdSe-ZnS quantum dots--influence of the thickness of the ZnS shell. Ann N Y Acad Sci. 2008;1130:235–241. doi: 10.1196/annals.1430.021. [DOI] [PubMed] [Google Scholar]
- 10.Eggeling C, Volkmer A, Seidel CA. Molecular photobleaching kinetics of Rhodamine 6G by one- and two-photon induced confocal fluorescence microscopy. Chemphyschem. 2005;6(5):791–804. doi: 10.1002/cphc.200400509. [DOI] [PubMed] [Google Scholar]
- 11.Ghasemi Y, Peymani P, Afifi S. Quantum dot: magic nanoparticle for imaging, detection and targeting. Acta Biomed. 2009;80(2):156–165. [PubMed] [Google Scholar]
- 12.Cordes DB, Miller A, Gamsey S, Singaram B. Simultaneous use of multiple fluorescent reporter dyes for glucose sensing in aqueous solution. Anal Bioanal Chem. 2007;387(8):2767–2773. doi: 10.1007/s00216-007-1128-z. [DOI] [PubMed] [Google Scholar]
- 13.Han M, Gao X, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat Biotechnol. 2001;19(7):631–635. doi: 10.1038/90228. [DOI] [PubMed] [Google Scholar]
- 14.Dave SR, White CC, Gao X, Kavanagh TJ. Luminescent quantum dots for molecular toxicology. Adv Exp Med Biol. 2012;745:117–137. doi: 10.1007/978-1-4614-3055-1_8. [DOI] [PubMed] [Google Scholar]
- 15.Romoser A, Ritter D, Majitha R, Meissner KE, McShane M, Sayes CM. Mitigation of quantum dot cytotoxicity by microencapsulation. PLoS One. 2011;6(7):e22079. doi: 10.1371/journal.pone.0022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Li R, Li CM, Wu N. Electrochemical and optical biosensors based on nanomaterials and nanostructures: a review. Front Biosci (Schol Ed). 2011;3:1308–1331. doi: 10.2741/228. [DOI] [PubMed] [Google Scholar]
- 17.Ballerstadt R, Schultz JS. A fluorescence affinity hollow fiber sensor for continuous transdermal glucose monitoring. Anal Chem. 2000;72(17):4185–4192. doi: 10.1021/ac000215r. [DOI] [PubMed] [Google Scholar]
- 18.Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJ. Fluorescence-based glucose sensors. Biosens Bioelectron. 2005;20(12):2555–2565. doi: 10.1016/j.bios.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Weidemaier K, Lastovich A, Keith S, Pitner JB, Sistare M, Jacobson R, Kurisko D. Multi-day pre-clinical demonstration of glucose/galactose binding protein-based fiber optic sensor. Biosens Bioelectron. 2011;26(10):4117–4123. doi: 10.1016/j.bios.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Ye K, Schultz JS. Genetic engineering of an allosterically based glucose indicator protein for continuous glucose monitoring by fluorescence resonance energy transfer. Anal Chem. 2003;75(14):3451–3459. doi: 10.1021/ac034022q. [DOI] [PubMed] [Google Scholar]
- 21.Veetil JV, Jin S, Ye K. A glucose sensor protein for continuous glucose monitoring. Biosens Bioelectron. 2010;26(4):1650–1655. doi: 10.1016/j.bios.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin S, Veetil JV, Garrett JR, Ye K. Construction of a panel of glucose indicator proteins for continuous glucose monitoring. Biosens Bioelectron. 2011;26(8):3427–3431. doi: 10.1016/j.bios.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boghossian AA, Zhang J, Barone PW, Reuel NF, Kim JH, Heller DA, Ahn JH, Hilmer AJ, Rwei A, Arkalgud JR, Zhang CT, Strano MS. Near-infrared fluorescent sensors based on single-walled carbon nanotubes for life sciences applications. Chem Sus Chem. 2011;4(7):848–863. doi: 10.1002/cssc.201100070. [DOI] [PubMed] [Google Scholar]
- 24.Barone PW, Parker RS, Strano MS. In vivo fluorescence detection of glucose using a single-walled carbon nanotube optical sensor: design, fluorophore properties, advantages, and disadvantages. Anal Chem. 2005;77(23):7556–7562. doi: 10.1021/ac0511997. [DOI] [PubMed] [Google Scholar]
- 25.Barone PW, Strano MS. Single walled carbon nanotubes as reporters for the optical detection of glucose. J Diabetes Sci Technol. 2009;3(2):242–252. doi: 10.1177/193229680900300204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barone PW, Baik S, Heller DA, Strano MS. Near-infrared optical sensors based on single-walled carbon nanotubes. Nat Mater. 2005;4(1):86–92. doi: 10.1038/nmat1276. [DOI] [PubMed] [Google Scholar]
- 27.Barone PW, Strano MS. Reversible control of carbon nanotube aggregation for a glucose affinity sensor. Angew Chem Int Ed Engl. 2006;45(48):8138–8141. doi: 10.1002/anie.200603138. [DOI] [PubMed] [Google Scholar]
- 28.Barone PW, Yoon H, Ortiz-García R, Zhang J, Ahn JH, Kim JH, Strano MS. Modulation of single-walled carbon nanotube photo-luminescence by hydrogel swelling. ACS Nano. 2009;3(12):3869–3877. doi: 10.1021/nn901025x. [DOI] [PubMed] [Google Scholar]
- 29.Borrok MJ, Kiessling LL, Forest KT. Conformational changes of glucose/galactose-binding protein illuminated by open, unliganded, and ultra-high-resolution ligand-bound structures. Protein Sci. 2007;16(6):1032–1041. doi: 10.1110/ps.062707807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon H, Ahn JH, Barone PW, Yum K, Sharma R, Boghossian AA, Han JH, Strano MS. Periplasmic binding proteins as optical modulators of single-walled carbon nanotube fluorescence: amplifying a nanoscale actuator. Angew Chem Int Ed Engl. 2011;50(8):1828–1831. doi: 10.1002/anie.201006167. [DOI] [PubMed] [Google Scholar]
- 31.Heo YJ, Shibata H, Okitsu T, Kawanishi T, Takeuchi S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc Natl Acad Sci U S A. 2011;108(33):13399–13403. doi: 10.1073/pnas.1104954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxl T, Khan F, Ferla M, Birch D, Pickup J. A fluorescence lifetime-based fibre-optic glucose sensor using glucose/galactose-binding protein. Analyst. 2011;136(5):968–972. doi: 10.1039/c0an00430h. [DOI] [PubMed] [Google Scholar]
- 33.Peyser T, Zisser H, Khan U, Jovanovič L, Bevier W, Romey M, Suri J, Strasma P, Tiaden S, Gamsey S. Use of a novel fluorescent glucose sensor in volunteer subjects with type 1 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):687–693. doi: 10.1177/193229681100500323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen JK, Christiansen JS, Kristensen JS, Toft HO, Hansen LL, Aasmul S, Gregorius K. Clinical evaluation of a transcutaneous interrogated fluorescence lifetime-based microsensor for continuous glucose reading. J Diabetes Sci Technol. 2009;3(1):98–109. doi: 10.1177/193229680900300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. BioPortfolio. First implantable continuous glucose sensor to achieve high accuracy performance in people with diabetes. http://www.bioportfolio.com/news/article/796206/First-Implantable-Continuous-Glucose-Sensor-To-Achieve-High-Accuracy-Performance-In-People.html. Accessed October 15, 2012.
- 36.Shibata H, Heo YJ, Okitsu T, Matsunaga Y, Kawanishi T, Takeuchi S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proc Natl Acad Sci U S A. 2010;107(42):17894–17898. doi: 10.1073/pnas.1006911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinnayelka S, McShane MJ. Microcapsule biosensors using competitive binding resonance energy transfer assays based on apoenzymes. Anal Chem. 2005;77(17):5501–5511. doi: 10.1021/ac050755u. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava R, Jayant RD, Chaudhary A, McShane MJ. “Smart tattoo” glucose biosensors and effect of coencapsulated anti-inflammatory agents. J Diabetes Sci Technol. 2011;5(1):76–85. doi: 10.1177/193229681100500111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Jayant RD, McShane MJ, Srivastava R. In vitro and in vivo evaluation of anti-inflammatory agents using nanoengineered alginate carriers: towards localized implant inflammation suppression. Int J Pharm. 2011;403(1-2):268–275. doi: 10.1016/j.ijpharm.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 40.Balaconis MK, Billingsley K, Dubach MJ, Cash KJ, Clark HA. The design and development of fluorescent nano-optodes for in vivo glucose monitoring. J Diabetes Sci Technol. 2011;5(1):68–75. doi: 10.1177/193229681100500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh S, McShane M. Role of porosity in tuning the response range of microsphere-based glucose sensors. 2011;26(5):2478–2483. doi: 10.1016/j.bios.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gough DA, Lucisano JY, Tse PH. Two-dimensional enzyme electrode sensor for glucose. Anal Chem. 1985;57(12):2351–2357. doi: 10.1021/ac00289a042. [DOI] [PubMed] [Google Scholar]
- 43.Stein EW, Singh S, McShane MJ. Microscale enzymatic optical biosensors using mass transport limiting nanofilms. 2. Response modulation by varying analyte transport properties. 2008;80(5):1408–1417. doi: 10.1021/ac701738e. [DOI] [PubMed] [Google Scholar]
- 44.Long R, McShane M. High-efficiency optical systems for interrogation of dermally-implanted sensors. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:1033–1036. doi: 10.1109/IEMBS.2010.5628059. [DOI] [PubMed] [Google Scholar]