Abstract
Dulaglutide (dula, LY2189265) is a once-weekly glucagon-like peptide-1 analog in development for the treatment of type 2 diabetes mellitus. An adaptive, dose-finding, inferentially seamless phase 2/3 study was designed to support the development of this novel diabetes therapeutic. The study is divided into two stages based on two randomization schemes: a Bayesian adaptive scheme (stage 1) and a fixed scheme (stage 2). Stage 1 of the trial employs an adaptive, dose-finding design to lead to a dula dose-selection decision or early study termination due to futility. If dose selection occurs, the study proceeds to stage 2 to allow continued evaluation of the selected dula doses. At completion, the entire study will serve as a confirmatory phase 3 trial. The final study design is discussed, along with specifics pertaining to the actual execution of this study and selected baseline characteristics of the participants.
Keywords: adaptive dose finding, dulaglutide, GLP-1, inferentially seamless
Introduction
Analogs of the incretin hormone glucagon-like peptide-1 (GLP-1) are a growing class of therapeutic agents for diabetes that provide enhanced glucose-dependent insulin secretion1 and glycemic control.2–4 Dulaglutide (dula, LY2189265) is a long-acting GLP-1 analog currently in development.5 Early studies showed that dula exhibited the expected GLP-1-mediated pharmacological effects on insulin secretion and glucose lowering6,7with a half-life that supported once-weekly dosing. Gastrointestinal (GI) adverse events (AEs) were consistent with those reported for other GLP-1 analogs. Small dose-dependent increases were observed in mean heart rate (HR) at doses ≥1 mg6,7and in mean diastolic blood pressure (DBP) at doses ≥3 mg.6The clinical significance of these hemodynamic findings was uncertain, but similar observations have since been reported with other GLP-1 mimetics.8–10
Given this, continued development of dula meant identification of doses that would strike an optimal balance between safety and efficacy parameters. Two options for dose finding were considered: a conventional fixed dose trial design and an adaptive design. Traditional dose-finding studies explore a limited number of doses because of the large sample sizes required to estimate pairwise differences. Since all doses are treated equally, a number of patients may be allocated to suboptimal doses, resulting in less informative learning about the dose–response relationships, particularly if multiple dose-determining safety and efficacy parameters need to be evaluated simultaneously. With adaptive dose-finding, a greater number of doses can be investigated and, through iterative learning, dose assignment can be optimized. Implementation of early stopping rules and adaptive allocation schemes can limit patient exposure to ineffective or unsafe doses and increase exposure to more effective doses. These efficiencies in characterizing the dose response can enable better data-driven decisions, notably dose selection for phase 3. Based on these considerations, and that the previously observed hemodynamic changes might be dose-limiting, the adaptive design was deemed the more efficient dose finding approach.
To illustrate the utility of an adaptive approach, a dose-escalation, proof-of-concept study was conducted to investigate the dose-toxicity and dose-response relationship of dula.7 Dose-toxicity and dose-response models were created from early-phase clinical trial data with predictive biomarkers of response [e.g., fasting blood glucose (FBG)], leveraging the extensive amount of published data on other diabetes drugs. These models were used to predict long-term safety and efficacy [hemoglobin A1c (HbA1c)] responses through iterative clinical trial simulation scenarios. Cohorts were evaluated sequentially in a dose-escalating fashion. Adaptive allocation based on a modified continual reassessment method11 was implemented to guide dose escalation and to assess the maximum tolerated dose (MTD). The adaptive algorithm included an assessment of the tolerability of all prior doses. Dose escalation continued until the MTD was estimated between 5 mg and 8 mg based on the incidence rates of nausea and vomiting. After the MTD range was estimated, additional patients were allocated to lower doses (0.3 mg and 1 mg) based on analysis of interim data to refine the dose response for fasting and postprandial plasma glucose. The results supported the theory that adaptive allocation and use of exposure-response models could improve the estimate of the MTD and biomarker (FBG and HbA1c) response.
While it was recognized that adaptive dose finding might improve dula dose selection, it was thought that further efficiencies in development could be gained by incorporating a seamless design feature that addresses, within a single trial, objectives that are normally achieved through separate trials.12,13 Specifically, dose selection (usually associated with phase 2) and confirmation of treatment and efficacy (usually associated with phase 3) could be combined into a single trial, both operationally and inferentially, where all data would be used in the final analysis. This approach could reduce the development time line by approximately 9 to 12 months, the typical lag time observed in a traditional development paradigm in which a dose-finding trial and pivotal phase 3 trial are conducted sequentially.
Thus, the first adaptive, dose-finding, inferentially seamless phase 2/3 study was designed for the development of a diabetes drug [Assessment of Weekly AdministRation of LY2189265 in Diabetes-5 (AWARD-5)]. Additional details of this study, entitled “A Study of LY2189265 Compared to Sitagliptin in Patients With Type 2 Diabetes Mellitus on Metformin,” can be found at http://clinicaltrials.gov as NCT00734474.
The study is divided into two stages based on two randomization schemes: a Bayesian adaptive scheme (stage 1) and a fixed scheme (stage 2). During stage 1, adaptive treatment allocation will be used to assign patients to the dula doses. Predictive algorithms will inform decisions to select doses or to terminate the study. The adaptations will be informed by a clinical utility index (CUI), a single metric that reflects four prespecified safety and efficacy response measures. If dula dose selection occurs, stage 2 will begin, and the doses selected will continue to be evaluated for safety and efficacy. The entire study will be a phase 3 confirmatory study.
Trial simulation was used to demonstrate the operating characteristics of this design and to compare the efficiencies of our adaptive approach to that of a conventional fixed-dose design.14 In this article, we report the final design, actual implementation metrics, and selected baseline characteristics. Statistical methodologies are discussed by Skrivanek and coauthors,14 and operational challenges to study implementation are discussed by Spencer and coauthors.15
Methods
Study Design and Objectives
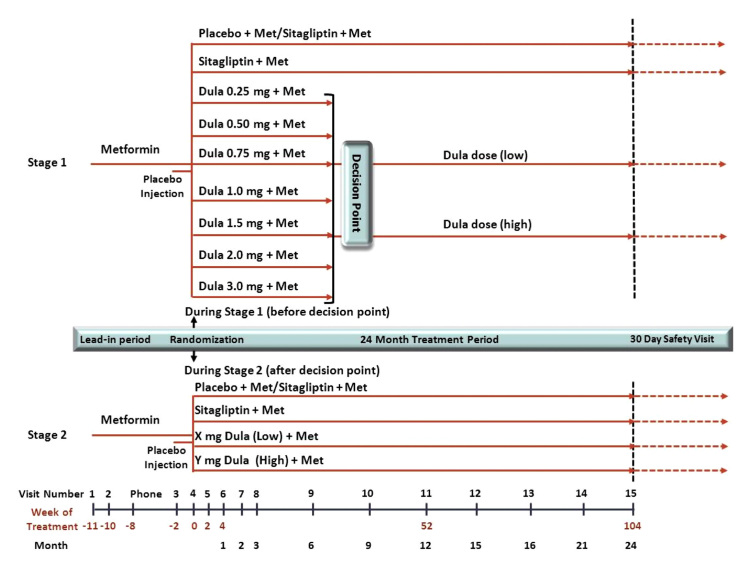
AWARD-5 is a multicenter, randomized, double-blind, placebo-controlled, 24-month, parallel clinical trial comparing dula with sitagliptin (Merck, Whitehouse Station, NJ) in patients with type 2 diabetes mellitus (T2DM) on metformin (Met) (Figure 1). The study is divided into two stages based on two randomization schemes: a Bayesian adaptive scheme (stage 1) and a fixed scheme (stage 2).
Figure 1.
Adaptive seamless phase 2/3 study design. AWARD-5 is a double-blind randomized, placebo-controlled, adaptive, dose-finding, inferentially seamless phase 2/3 study. Two randomization schemes are used which divide the study into two stages: adaptive randomization (stage 1) and fixed randomization (stage 2). Seven doses of dula will be evaluated in stage 1 along with comparators. Patients will be adaptively allocated across these doses based on accumulating data. The decision point is defined as the time when sufficient information has accrued to either select dula doses or to stop the trial. If dose selection occurs, stage 2 will begin. Only patients assigned the selected doses and comparators will continue to be followed; all others will stop further participation in the trial. Additional patients will enroll and be assigned to the comparators or dula. Patients will be followed for 24 months and complete a safety visit 30 days after drug discontinuation.
The objective of stage 1, using an adaptive dose-finding approach, is to identify up to 2 safe and efficacious doses of dula, referred to as high and low doses, for further study in this trial and all phase 3 clinical trials, or to terminate the study for futility.
If dose selection occurs, the study will proceed to stage 2. At completion, the entire study will serve as a phase 3 study. The primary objective is to demonstrate that glycemic control of the high dula dose is noninferior to that of sitagliptin at 12 months (52 weeks), as measured by HbA1c change from baseline using a noninferiority margin of 0.25%. A prespecified testing strategy will be used to assess glycemic efficacy of the low dula dose to sitagliptin after 12 months of treatment, and of both dula doses to placebo after 6 months of treatment.14 Secondary objectives include evaluating the effects of the 2 dula doses compared to sitagliptin at 12 and 24 months on FBG, weight, safety, and quality of life measures. Data from patients assigned to the 2 selected doses and the comparator arms, before and after adaptation, will be included in the primary analysis (i.e., inferentially seamless), using appropriate statistical methodology to avoid inflation of the type I error rate. The sample size for this study is not fixed and will be determined adaptively. Study powerwas assessed by simulations across a range of alternative hypotheses. A drug-disease model for diabetes that incorporated data from literature and other dula studies to predict patient response was considered the most likely scenario. Under this model, assuming a 20% dropoutrate and an accrual rate of five patients per week, approximately 89% of the studies simulated were statistically significant for the primary analysis.14
AWARD-5 is being conducted in accordance with regulatory standards and good clinical practice guidelines rooted in the Declaration of Helsinki. All patients will provide signed informed consent. The protocol was approved by the ethical review board at investigative sites.
Adaptive Treatment Allocation and Dulaglutide Dose Selection
Based on scientific understanding of diabetes, experience with dula, and expert opinion, the study team decided that HbA1c, weight, HR, and DBP were the four key measures that had to be balanced to yield optimal dula dose selection; thus, these measures were included in the adaptive algorithm. HbA1c is an acceptable primary efficacy end point for regulatory approval of therapies for T2DM; GLP-1 analogs have been associated with weight reduction. Both HR and DBP were believed to be potentially dose-limiting based on epidemiological data showing that resting HR and BP are predictors of cardiovascular (CV) morbidity16,17 and mortality.18 Nausea and vomiting are commonly associated with GLP-1 analogs; however, while both are important outcomes, they are not easily quantified and thus were not included in the algorithm.
Dose- and exposure-response models for glucose (i.e., HbA1c), weight, HR, and DBP were created using data from early dula studies6,7, leveraging population variability estimates and longitudinal time course of response from larger and longer studies on other diabetes drugs. Simulations based on these models, along with prior pharmacodynamic, AE, and vital sign data,7 were used to define the dula dose range (0.25 mg to 3.0 mg) for this study. Within this range, 7 doses (0.25, 0.5, 0.75, 1.0, 1.5, 2.0, and 3.0 mg) with overlapping concentrations, within a 12-fold dose range, were selected to ensure adequate exploration of the dose-response curve and risk-benefit profiles, as well as to enable identification of the most optimal doses to carry forward into phase 3.
Adaptive allocation across the 7 dula doses will be based on a single metric referred to as the CUI. The CUI is the result of a mathematical formula in which the four measures are differentially quantified. The CUI is defined in a multiplicative fashion.14 There will be uncertainty associated with the CUI of a dose because the CUI will be calculated based on observed data. Therefore, the decision rules will be implemented with probability statements around the CUI. The dose selection rules also are based on these same four measures. If 2 doses of dula are selected, the high dose should be the dose estimated to have the maximum utility, and the low dose should be the lowest dose estimated to have meaningful clinical benefit based on the CUI.14 The low dose cannot be adjacent to the high dose; it must be at least one-half the dose of the high dose as long as efficacy is maintained. If none of the doses fulfill the prespecified decision criteria, the algorithm will terminate the trial for futility.
Screening/Lead-In Period
Eligible patients are those aged 18 to 75 years with T2DM (≥ 6 months) who meet the following criteria: an HbA1c value of > 8% and ≤ 9.5% on diet and exercise alone or of ≥ 7% and ≤ 9.5% on monotherapy [Met or other oral antihyperglycemic medication (OAM)] or combina-tion therapy (Met plus another OAM); a body mass index (BMI) between 25 kg/m2to 40 kg/m2; and a stable weight (prior 3 months). Patients will be excluded if they have type 1 diabetes, a CV event within 6 months of screening, uncontrolled hypertension, a significant gastric-emptying abnormality, hepatic insufficiency, a history of chronic or idiopathic pancreatitis, uncontrolled diabetes, renal impairment, or use of excluded medications (e.g., insulin, other GLP-1 analogs, or weight-loss drugs).
Eligible patients will enter the lead-in period, which may last between 4 to 11 weeks, depending on the patient’s diabetes regimen at entry. During this time, Met dose titration and stabilization occur, and other OAMs are washed out to ensure a stable HbA1c value at randomization. Patients must tolerate Met at a dose of at least 1500 mg/day for a minimum of 6 weeks before randomization. Patients will be educated about adherence to diet and exercise, hypoglycemia, blood glucose testing, and injection of study drug, and will participate in a 2-week placebo run-in prior to randomization.
Stage 1
Patients who meet all inclusion criteria and are taking Met ≥ 1500 mg/day will be randomized to 1 of 9 treatment arms: placebo, sitagliptin 100 mg, or 1 of 7 dula doses of 0.25 mg to 3.0 mg (Figure 1). Patients will administer one injection of study medication subcutaneously once weekly and take one tablet of study medication orally once daily. Those assigned to the placebo arm will receive placebo tablets and injections for 6 months and then transition in a blinded manner to sitagliptin 100 mg once daily and placebo injections weekly.
The first 45 patients will be allocated equally across the nine treatment arms using a fixed randomization scheme.The time it takes to enroll these patients is called the burn-in period. The purpose of the burn-in is to accumulate data on the four response measures, which can then be used in the adaptive algorithm. It was determined through simulation that approximately five patients per treatment arm would be needed to have adequate power to detect a glycemic response (i.e., would separate from placebo).
After the burn-in period, new patients will be randomized to placebo, sitagliptin, or dula in a 1:1:3 ratio. A Bayesian adaptive randomization scheme will assign patients to the 7 dula doses. To accomplish this, a limited subset of accu-mulating data, using prespecified safety (HR, DBP, and weight) and efficacy (HbA1c) measures, will be analyzed every 2 weeks. These measures will be transformed into a single metric, the CUI, to assess relative benefit/risk for each dula dose. The CUI will be used to adapt thetreatment allocation probabilities to the 7 dula doses every 2 weeks. When adaptive randomization begins, patients may be equally allocated to each of the 7 dula doses because data may be too limited to allow for differentiation between doses. As information accumulates, if there is evidence of differentiation between doses, allocation will increase to doses that are therapeutically optimal; thus, a newly enrolling patient will have a higher probability of being assigned to a dose predicted to provide therapeutic benefit (greater CUI) than to a less effective dose (lesser CUI). An enrollment rate of approximately 5 to 8 patients per week is to be maintained to support optimal performance of the algorithm.14,15
After 200 patients enroll, the adaptive algorithm will assess each dula dose to determine whether the prespecified safety and efficacy decision rules have been met. One of three decisions can be made: dula dose selection occurs, the trial is stopped for futility, or new patients will continue to enroll. If new patients continue to randomize, the treat-ment allocation probabilities will continue to be updated every 2 weeks and, concomitantly, the algorithm will assess whether the decision rules have been met. Stage 1 will continue until sufficient data have accumulated to make a decision or until 400 patients have enrolled. If the algorithm cannot make a decision after 400 patients, the trial will terminate. Based on this design, the sample size for stage 1 may vary from 200 to 400 patients.
An independent Data Monitoring Committee (DMC) will review interim data approximately bimonthly to monitor the performance of the adaptive algorithm and will conduct routine safety reviews. An independent Statistical Analysis Center (SAC) will prepare the interim reports. The DMC may pause or stop randomization to a treatment arm and/or discontinue patients already randomized to an arm due to safety concerns. Under select circumstances predefined in the DMC Charter, an Eli Lilly and Company Internal Review Committee (IRC) may be asked to review the same interim data that the DMC reviews (e.g., if the DMC recommends discontinuing a treatment arm). The IRC is a small group of executive physicians and a biostatistician, independent of the dula team, and selected prior to study initiation.
The roles, responsibilities, and decisions of the DMC and IRC were prespecified in the DMC charter and agreed on by all parties before study initiation. Appropriate firewalls, including secure data access and transfer procedures, were implemented to ensure that access to the interim results are limited and that these data are known only to this small group of decision-makers.15These provisions were discussed with regulatory authorities to ensure that limited sponsor involvement in the decision process was appropriate and acceptable.
Decision Point
The decision point (DP) is defined as the time when sufficient information has been gathered to enable the adaptive algorithm to either select up to 2 dula doses or to stop the trial for futility (Figure 1). After the algorithm renders a decision, the DMC will review the available data. If the algorithm selects 2 doses, the DMC can endorse 1, both, or neither of the doses. If 1 dose is selected, the DMC determines whether or not to endorse that dose. The DMC cannot recommend doses that the algorithm does not select, they cannot stop the trial due to overwhelming efficacy, nor can they override an algorithm decision to stop the trial for futility.
Following issuance of the DMC recommendation, the Lilly IRC will review the same data package provided to the DMC. If dose selection occurs and the DMC endorses the doses chosen, the IRC can sanction all, some, or none of these doses. The IRC can terminate the trial if none of the doses chosen would provide potential treatment options to patients that offer efficacy and/or safety advantages over those antidiabetic agents currently or soon to be commercially available. The IRC cannot recommend any dose the algorithm does not select nor can they overrule a recommendation to stop the trial for futility.
To maintain trial integrity, if dose selection occurs, only the dula doses to be carried forward into the remainder of the trial will be communicated to the dula team, study investigators, ethics review boards, and regulatory authorities. Patients assigned to the selected dula doses and comparator arms will continue on their assigned treatments for a maximum duration of 24 months. Patients assigned to nonselected doses will be discontinued from the trial.
Stage 2
If dose selection occurs, stage 2 will begin. The SAC will perform a predefined sample size re-estimation. The sample size will be either 263 or 333 patients for each dula and sitagliptin arm (and half that number for the placebo arm), based on the predictive probability of showing superiority to sitagliptin after 52 weeks of treatment.14 To show noninferiority to sitagliptin in a typical fixed design, 263 patients in each arm would be sufficient. However, 263 patients per arm may not be sufficient to show superiority; therefore, 333 patients will be assigned to each arm to increase the probability of showing superiority. At least 70% of the patients from each treatment arm will be randomized during stage 2. If a regulatory agency does not accept the results from the entire trial (data from both stages combined), then data from stage 2 alone will be used to test the primary hypothesis and will serve as a phase 3 confirmatory trial.
Sites participating in stage 1 may enroll additional patients into stage 2. New sites will be added to ensure that sample size requirements are met. Enrollment during stage 2 may proceed as quickly as feasible. The eligibility criteria will remain unchanged to mitigate against heterogeneity. Patients will be randomized to the selected dula doses, sitagliptin, or placebo, using a fixed randomization scheme (2:2:2:1 for low dose:high dose:sitagliptin:placebo if 2 dula doses are selected, or 4:3:2 for dula:sitagliptin:placebo if only 1 dula dose is selected). Randomization will be stratified by HbA1c (≤8.5% and > 8.5%) and by country. Stage 2 patients will follow the same study schedule as stage 1 patients. Patients randomized to the primary treatment arms in either stage will be exposed to treatment for up to 24 months.
After all patients have completed 12 months of treatment, an interim database lock will be executed. Analyses of the primary measure will be performed and sponsor unblinding will be restricted. The DMC will review interim safety data until this data lock. Patients and investigators will remain blinded for the treatment period. Analyses of the 24-month data will be performed on data collected through the last patient visit.
Data Collection
All patients will follow the same visit schedule regardless of whether they enroll during stage 1 or 2 (Figure 1). All randomized patients will complete a safety visit 30 days after discontinuation of study drug. At baseline, participants will undergo a standard clinical evaluation, electrocardiogram (ECG), and laboratory testing. Hemo-globin A1c will be measured at baseline, at months 1, 2, and 3, and every 3 months thereafter. Heart rate and BP, measured in triplicate using standardized procedures, and weight will be measured at baseline and each visit. Blood and urine samples will be collected routinely. ECGs were recorded at 2 and 4 weeks and at 3, 6, 12, and 24 months after randomization.
Patients will be discontinued if the DMC stops randomi-zation to a dula arm; if the patient is assigned to a dula arm not selected at the DP; or if the patient develops severe and persistent hyperglycemia, acute pancreatitis, or hepatic or renal impairment, becomes pregnant, or requires chronic insulin therapy.
Results
Recruitment for stage 1 began in August 2008 and involved six countries (> 50 sites). The first patient was randomized in October 2008, thus commencing the burn-in period, which lasted approximately 9 weeks, during which time 47 patients enrolled. Thereafter new patients were assigned to placebo, sitagliptin, or dula in a 1:1:3 ratio, and randomization to the 7 dula doses was done adaptively. The algorithm was updated every 2 weeks using accumulated HbA1c, weight, HR, and DBP data. Sites were required to enter data within 48 hafter each patient visit so that the adaptive algorithm functioned using the most current and maximal amount of data available.14,15 To optimize performance of the algorithm, enrollment was controlled at a rate of five to eight patients per week.14,15 The DMC reviewed the performance of the algorithm bimonthly and routinely reviewed interim safety data. Approximately 24 weeks after the first patient was randomized, 200 patients were enrolled, and the algorithm began assessment of each dula dose to determine if the dose decision criteria had been met. Shortly afterwards, the DMC recommended that further randomization to the dula 3.0-mg arm cease and patients on that dose be discontinued due to a potential long-term safety risk (although there was no imminent safety concern); the IRC endorsed this recommendation. This decision was executed promptly according to a prespecified action plan.15 The DP was reached a few weeks later; the algorithm selected 2 dula doses. The DMC and the IRC endorsed both doses. The dula doses selected were 0.75 mg and 1.5 mg. Patients on the nonselected dula doses were discontinued from the study according to a predetermined action plan. All other patients remained on their assigned treatment to be followed for a maximum of 24 months.
Recruitment for stage 2 began immediately following the DP. The SAC conducted a sample-size re-estimation, and the study team was informed of the targeted enrollment number. Existing sites continued to enroll patients, and six new countries were initiated. Recruitment completed in May 2010. Overall, 2195 patients signed an informed consent to participate; 993 did not meet eligibility criteria or discontinued before randomization for other reasons, and 1202 patients were randomized (stages 1 and 2 combined). Baseline characteristics for patients in all treatment arms during stage 1 and stage 2 are summarized in Table 1. The study is ongoing.
Table 1.
Baseline Characteristics for Patients in All Treatment Arms Randomized during Stage 1 and Stage 2
| Variable | Patients Randomized Total (N = 1202) | |
|---|---|---|
| Stage 1 (n = 230) | Stage 2 (n = 972) | |
| Age, years (mean ± SD) | 53.4 ± 10.3 | 54.3 ± 9.7 |
| Gender, % Female | 60.4 | 51.9 |
| Race, % White | 44.8 | 52.4 |
| Body mass index, kg/m2 (mean ± SD) | 31.9 ± 4.5 | 31.1 ± 4.3 |
| Body weight, kg (mean ± SD) | 87.3 ± 18.0 | 86.2 ± 17.1 |
| Duration of diabetes, years (mean ± SD) | 7.5 ± 5.5 | 7.0 ± 5.1 |
| Seated systolic BP, mm Hg (mean ± SD) | 128.0 ± 14.4 | 127.7 ± 13.1 |
| Seated diastolic BP, mm Hg (mean ± SD) | 77.9 ± 7.9 | 77.6 ± 8.6 |
| Seated heart rate, bpm (mean ± SD) | 74.5 ± 9.6 | 75.2 ± 10.0 |
SD, standard deviation; BP, blood pressure; bpm, beats per minute.
Discussion
To our knowledge, this is the first adaptive, dose-finding, inferentially seamless phase 2/3 trial. The Study was designed and executed for the development of dula, a once-weekly GLP-1 analog. The initial objective of the trial was fulfilled when the adaptive design approach resulted in selection of two doses of dula that were carried forward to the remainder of the trial. The prespecified decision-making processes and committees functioned according to plan, and the trial continued seamlessly into stage 2. Additional phase 3 trials were subsequently initiated, enabling continued evaluation of the 2 selected dula doses.
Because the trial is ongoing, it remains to be determined if the assumptions and advantages of the adaptive approach will be fulfilled when compared with a traditional develop-ment paradigm. For example, how were patients allocated across the 7 doses (i.e., were sample sizes increased for those doses in the therapeutic range while minimizing exposure across less optimal doses)? Did the CUI function as desired? Were patient resources optimized? Does the lower dose exhibit meaningful benefit? Was the target dose-range of benefit identified? Despite these uncertainties, the seamless design feature has enabled collection of 24-month safety and efficacy data for dula, which will be available at the time of marketing application. Such long-term data is unlikely to have been available had a typical development paradigm been pursued. With the current regulatory CV risk assessment require-ments for drugs to treat T2DM,19 which necessitate that trials be of longer duration to accrue sufficient numbers of CV events, having longer-term data will be useful.19
Compared to a conventional fixed-dose design, there are many more assumptions inherent in this adaptive approach. The design is based on limited priors from early clinical trials about the timing of both metabolic and adverse effects. Knowledge about treatment options available in the marketplace was incorporated to the extent possible. The development team clinicians and scientists chose the safety and efficacy measures used in the algorithm and estimated the effects of dula on each of these variables to construct a priori the dose-decision algorithm. In the CUI, differential weighting was applied to each of the four components. Thresholds were defined for the safety measures to minimize or prevent randomization of patients to doses that met or exceeded these limits, which were determined to be clinically relevant. Assumptions regarding treatment effects on glycemic response at 1 year were highly model-based, but the estimated treatment effect was also considered clinically relevant to the treatment of diabetes. Trial simulation was used to test and optimize the design assumptions14under a variety of plausible and implausible scenarios.
In addition, human oversight was built into the trial execution and decision-making processes. The SAC and DMC closely monitored the performance of the algorithm. Rules were prespecified to allow the DMC to overrule the algorithm’s decisions to ensure the safety of study participants and to minimize patient exposure to potentially less optimal doses.15 These responsibilities extended beyond the traditional role of a DMC. Similarly, although the algorithmic dose selection was based on four key outcomes, the DMC and IRC were involved in the final decision-making processes and evaluated all data available at the time of dose selection. This allowed for a review of AEs, in particular GI AEs, discontinuation rates, and other data to decide whether or not to continue investigation and further development of the selected doses. It was equally imperative that there be some limited sponsor involvement to factor in changes in the regulatory environment and/or in the competitive diabetes marketplace that could have occurred after trial inception but before the DP (assuming dose selection). The IRC needed to ensure that the doses selected would offer safety and/or efficacy advantages over those anti-diabetic agents currently (or soon to be) commercially available. This was not a decision the sponsor could place solely in the hands of an external committee because the development team would not be privy to the study results for a long time, and the company intended to initiate additional phase 3 trials.
Designing this trial required more advanced planning than that for a traditional study design given that all adaptations and many operational processes15 had to be prespecified. As this study was intended to be a confirmatory trial, early and frequent interactions with the regulatory authorities, especially the U.S. Food and Drug Administration (FDA), were needed to ensure successful implementation and acceptance of this trial design. Recommendations for modifications to the trial design had to be incorporated; this translated into additional trial simulations to re-evaluate the operating design characteristics and control of the type I error. The acceptability of an inferentially seamless approach to the primary analysis was a topic of much discussion. To mitigate this, the study was designed to enroll the majority of patients during stage 2 so that the stage 2 data could stand alone for hypothesis testing, should this be needed for registration. Also, the final analyses will use traditional frequentist statistical methods. The FDA was also concerned that dula was being evaluated as an add-on to Met therapy and that this evaluation could yield results not representative of dula monotherapy or dula in combination with other diabetes drugs, given that Met has positive effects on secretion of endogenous GLP-1, which may lead to an overestimation of the treatment effect of dula when used without Met. To address this, a phase 2, dose-ranging, monotherapy trial was conducted in parallel with AWARD-5 to confirm optimal selection of the doses to be taken into phase 3.20
Conclusion
This adaptive, seamless trial for dulaglutide remains ongoing. When the final data are available for analysis, we will then be able to realize the strengths of this design, the utility and influence of the assumptions, and the potential advantages and/or disadvantages to more traditional development approaches.
Acknowledgments
The authors thank Angela B. Thompson (Eli Lilly and Company, Indianapolis, IN), Gordon Berry (Eli Lilly and Company), Chris Mundy (Eli Lilly and Company), Nicole Johnston (INC Research, Raleigh, NC), Anne Holway (INC Research), Rosemary Procko (INC Research), and Elizabeth Wagner (INC Research) for assisting with manuscript preparation, which was supported by Eli Lilly and Company.
Glossary
- (AE)
adverse event
- (AWARD-5)
Assessment of Weekly AdministRation of LY2189265 in Diabetes-5
- (BMI)
body mass index
- (CUI)
clinical utility index
- (CV)
cardiovascular
- (DBP)
diastolic blood pressure
- (DMC)
Data Monitoring Committee
- (DP)
Decision Point
- (dula)
dulaglutide
- (ECG)
electrocardiogram
- (FBG)
fasting blood glucose
- (FDA)
Food and Drug Administration
- (GI)
gastrointestinal
- (GLP-1)
glucagon-like peptide-1
- (HbA1c)
hemoglobin A1c
- (HR)
heart rate
- (IRC)
Internal Review Committee
- (Met)
metformin
- (MTD)
maximum tolerated dose
- (OAM)
oral antihyperglycemic medication
- (SAC)
Statistical Analysis Center
- (T2DM)
type 2 diabetes mellitus
Funding
This work is sponsored by Eli Lilly and Company.
Disclosures
Mary Jane Geiger, Zachary Skrivanek, Brenda Gaydos, and Jenny Chien are full-time employees of and own stock or stock options for Eli Lilly and Company. James Anderson owns stock or stock options for Eli Lilly and Company. Donald Berry and Scott Berry are consultants to Eli Lilly and Company through contracts between Eli Lilly and Company and Berry Consultants, LLC.
References
- 1.Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28(9):704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- 2.Gentilella R, Bianchi C, Rossi A, Rotella CM. Exenatide: a review from pharmacology to clinical practice. Diabetes Obes Metab. 2009;11(6):544–556. doi: 10.1111/j.1463-1326.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- 3.Hansen KB, Knop FK, Holst JJ, Vilsboll T. Treatment of type 2 diabetes with glucagon-like peptide-1 receptor agonists. Int J Clin Pract. 2009;63:1154–1160. doi: 10.1111/j.1742-1241.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- 4.Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31(7):665–670. doi: 10.3109/00365529609009147. [DOI] [PubMed] [Google Scholar]
- 5.Glaesner W, Vick AM, Millican R, Ellis B, Tschang SH, Tian Y, Bokvist K, Brenner M, Koester A, Porksen N, Etgen G, Bumol T. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26(4):287–296. doi: 10.1002/dmrr.1080. [DOI] [PubMed] [Google Scholar]
- 6.Barrington P, Chien JY, Tibaldi F, Showalter HD, Schneck K, Ellis B. LY2189265, a long-acting glucagon-like peptide 1 analogue, showed a dose dependent effect on insulin secretion in healthy subjects. Diabetes Obes Metab. 2011;13(5):434–443. doi: 10.1111/j.1463-1326.2011.01365.x. [DOI] [PubMed] [Google Scholar]
- 7.Barrington P, Chien JY, Showalter HD, Schneck K, Cui S, Tibaldi F, Ellis B, Hardy TA. A 5 week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide 1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(5):426–443. doi: 10.1111/j.1463-1326.2011.01364.x. [DOI] [PubMed] [Google Scholar]
- 8.Gustavson SM, Chen D, Somayaji V, Hudson K, Baltrukonis DJ, Singh J, Boyden TL, Calle RA. Effects of a long-acting GLP-1 mimetic (PF 04603629) on pulse rate and diastolic blood pressure in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2011;13(11):1056–1058. doi: 10.1111/j.1463-1326.2011.01479.x. [DOI] [PubMed] [Google Scholar]
- 9.Kothare PA, Linnebjerg H, Isaka Y, Uenaka K, Yamamura A, Yeo KP, de la Peña A, Teng CH, Mace K, Fineman M, Shigeta H, Sakata Y, Irie S. Pharmacokinetics, pharmacodynamics, tolerability, and safety of exenatide in Japanese patients with type 2 diabetes mellitus. J Clin Pharmacol. 2008;48(12):1389–1399. doi: 10.1177/0091270008323750. [DOI] [PubMed] [Google Scholar]
- 10.Halbirk M, Nørrelund H, Møller N, Holst JJ, Schmitz O, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjaer H, Bøtker HE, Wiggers H. Cardiovascular and metabolic effects of 48-hour glucagon-like peptide-1 infusion in compensated chronic heart failure patients. Am J Physiol Heart Circ Physiol. 2010;298(3):H1096–1102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- 11.Faries D. Practical modifications of the continual reassessment method for phase I cancer clinical trials. J Biopharm Stat. 1994;4(2):147–164. doi: 10.1080/10543409408835079. [DOI] [PubMed] [Google Scholar]
- 12.Gallo P, Chuang-Stein C, Dragalin V, Gaydos B, Krams M, Pinheiro J. Adaptive designs in clinical drug development – an executive summary of the PhRMA working group. J. Biopharm Stat. 2006;16:275–283. doi: 10.1080/10543400600614742. [DOI] [PubMed] [Google Scholar]
- 13.Gallo P, Krams M. PhRMA working group on adaptive designs: introduction to the full white paper. Drug Inf J. 2006;40(4):421–423. [Google Scholar]
- 14.Skrivanek Z, Berry S, Berry D, Chien J, Geiger MJ, Anderson JH, Jr., Gaydos B. Application of adaptive design methodology in development of a long-acting glucagon-like peptide-1 analog (dulaglutide): statistical design and simulations. J Diabetes Sci Technol. 2012;6(6):1305–1318. doi: 10.1177/193229681200600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer K, Colvin K, Braunecker B, Brackman M, Ripley J, Hines P, Skrivanek Z, Gaydos B, Geiger MJ. Operational challenges and solutions with implementation of an adaptive, seamless phase 2/3 study. J Diab Sci Tech. 2012;6(6):1296–1304. doi: 10.1177/193229681200600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 17.Cook S, Togni M, Schaub MC, Wenaweser P, Hess OM. High heart rate: a cardiovascular risk factor? Eur Heart J. 2006;27(20):2387–2393. doi: 10.1093/eurheartj/ehl259. [DOI] [PubMed] [Google Scholar]
- 18.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 19.[FDA] Food and Drug Administration. US Department of Health and Human Services, Center for Drug Evaluation and Research (CDER). Guidance for Industry. Diabetes Mellitus – Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008a. Available from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf. Accessed on July 29, 2012.
- 20.Grunberger G, Chang A, Garcia-Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once weekly GLP-1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet Med. 2012:1464–5491. doi: 10.1111/j.1464-5491.2012.03745.x. [DOI] [PubMed] [Google Scholar]