Abstract
Introduction
Neuroglycopenia in type 1 diabetes mellitus (T1DM) results in reduced cognition, unconsciousness, seizures, and possible death. Characteristic changes in the electroencephalogram (EEG) can be detected even in the initial stages. This may constitute a basis for a hypoglycemia alarm device. The aim of the present study was to explore the characteristics of the EEG differentiating normoglycemia and hypoglycemia and to elucidate potential group differences.
Methods
We pooled data from experiments in T1DM where EEG was available during both normoglycemia and hypo-glycemia for each subject. Temporal EEG was analyzed by quantitative electroencephalogram (qEEG) analysis with respect to absolute amplitude and centroid frequency of the delta, theta, alpha, and beta bands, and the peak frequency of the unified theta–alpha band. To elucidate possible group differences, data were subsequently stratified by age group (± 50 years), gender, duration of diabetes (± 20 years), and hypoglycemia awareness status (normal/impaired awareness of hypoglycemia).
Results
An increase in the log amplitude of the delta, theta, and alpha band and a decrease in the alpha band centroid frequency and the peak frequency of the unified theta–alpha band constituted the most significant hypoglycemia indicators (all p < .0001). The size of these qEEG changes remained stable across all strata.
Conclusions
Hypoglycemia-associated EEG changes remain stable across age group, gender, duration of diabetes, and hypoglycemia awareness status. This indicates that it may be possible to establish a general algorithm for hypoglycemia detection based on EEG measures.
Keywords: diabetes, electroencephalogram, human, hypoglycemia, quantitative electroencephalogram
Introduction
Hypoglycemia is the most common adverse event in type 1 diabetes mellitus (T1DM). While strict glucose control significantly reduces the overall risk of diabetes-related complications, it also increases the risk for severe hypo-glycemia.1 Normal brain metabolism depends entirely continuous glucose supply, and accordingly, the brain is particularly vulnerable to hypoglycemia. One indicator of brain function is the electroencephalogram (EEG). The effect of acute hypoglycemia on the EEG was demonstrated already in 1956 by Regan and Browne-Mayers.2 This was confirmed by other investigators who studied EEG changes during insulin-induced hypoglycemia in T1DM patients3,4 Pramming and coauthors4 found that the EEG was unaffected when the blood glucose concentration was above 3 mmol/liter. Following a gradual decline in blood glucose, the EEG changes became apparent in all patients. At a median blood glucose concentration of 2.0 mmol/liter, the alpha activity (8–12 Hz) decreased while the theta activity (4–8 Hz) increased, reflecting a cortical dysfunction. Importantly, a normal EEG was reestablished when the blood glucose concentration exceeded 2.0 mmol/liter. Thus the authors hypothesized a “glucose threshold” for normal brain function to exist.
We have shown that EEG changes occur before deterio-ration of cognitive functions in the majority of cases of T1DM patients exposed to insulin-induced hypoglycemia.5 A number of studies have further characterized the EEG changes associated with hypoglycemia.6–10 Some discrep-ancy exists with respect to the spatial location of the EEG changes and the persistence of these changes after restoration of euglycemia. However, it is well established that hypoglycemia is associated with an increased power in the low-frequency bands.
Long duration of diabetes, sleep, and recurrent and recent antecedent hypoglycemia all tend to reduce the hormonal counterregulation in acute hypoglycemia.11 However, which factors may influence the hypoglycemia-associated EEG changes is not well described.
The aim of our series of insulin-induced hypoglycemia experiments on T1DM patients is to develop an EEG-based mathematical algorithm for detecting hypoglycemia. Ultimately, this shall be incorporated into a portable alarm device and warn patients about impending severe hypoglycemia. This prediction should be timely in order for the patient to react to the alarm, thereby avoiding severe events. If such an alarm device should be applicable for T1DM patients without extensive individual calibration of the algorithm, the described EEG changes must be generally occurring, irrespective of patient-specific factors. Accordingly, the aim of the present study was to analyze EEG changes during insulin-induced hypoglycemia in T1DM patients and, in particular, to assess if these EEG characteristics depend on patient factors such as age, gender, duration of diabetes, and hypoglycemia awareness status.
Methods
Database Description
The data applied in this study is pooled from a total of five different protocols conducted between 2003 and 2011 at the Department of Endocrinology, Odense University Hospital; the Department of Medicine, Sydvestjysk Sygehus, Esbjerg; and the Department of Cardiology, Nephrology, and Endocrinology, Hillerød Hospital, Hillerød. It includes a total of 34 patients exposed to insulin-induced hypoglycemia. Table 1 lists the patient characteristics. All protocols have been approved by the local ethical committees and comply with the Declaration of Helsinki. In all cases, the study subjects were given oral and written information about the study procedures and signed informed consent. Although the protocols had different aims and different setups, the study procedures were comparable with respect to induced hypoglycemia procedure and EEG recording. The induced hypoglycemia experiments were performed at daytime. During all experiments, the subjects were awake, resting in a chair with eyes open, sometimes reading or talking. The patients met at the research unit in a fasting or nonfasting state. Cannulas were placed in both antecubital veins for sampling and infusion purposes. Continuous EEG monitoring was recorded from standard cup electrodes, cap electrodes (both with an average reference montage), or subcutaneous electrodes [platinum electrodes (diameter 0.18 mm) or foramen electrodes (length 300 mm, diameter 1.1 mm, center-to-center distance 30 mm between three contact points, representing the active, ground, and reference electrode), both supplied by AD-Tech Medical, WI]. Subcutaneous electrodes were inserted over the temporal region of the brain (corresponding to the P4-T4 position) in local analgesia and connected by wires to the EEG recorder (Nervus, Taugagreining, Iceland, or g-USBamp, G-TEC, Austria). The EEG from surface recordings was extracted from the P4-T4 position. Table 2 lists the type of electrodes applied in each protocol. The EEG was sampled at 256 or 200 Hz, but downsampled to 64 Hz before analysis. Proper hardware filter settings were applied to avoid aliasing. Hypoglycemia was induced by infusion of Actrapid® with an initial infusion rate of 2–8 iU/h, depending on the plasma glucose concentration. In a few cases, hypoglycemia was induced by intra-muscular injection of Novorapid® 8 iU given with 12 mm cannula in the medial vastus muscle (both insulins supplied by NovoNordisk, Bagsvaerd, Denmark). In a few other cases, the plasma glucose level was adjusted by glucose infusion, while having a constant rate of insulin infusion (1 mU/kg/min). Plasma glucose was measured every 5 min by a Beckman Glucose Analyzer (Beckman, Palo Alto, CA) or a YSI 2300 STAT (YSI Inc., Yellow Springs, OH). The insulin/glucose infusion rate was adjusted to achieve a steady fall in plasma glucose of 4–5 mmol/liter/h or, in case of intramuscular insulin, supplemented with extra insulin according to the investigator’s decision. The hypoglycemia episode was ceased when plasma glucose was lower than 1.8 mmol/liter at two consecutive measurements, if the patient was obviously cognitively impaired, or at the demand of the patient or the investigator.
Table 1.
Patient Characteristics
| Mean ± standard deviation | Range | |
|---|---|---|
| Sex (male/female) | 22/12 | — |
| Age (years) | 51.0 ± 9.9 | 27–68 |
| Duration of diabetes (years) | 26.7 ± 14.1 | 5–53 |
| Hemoglobin A1c (%) | 7.5 ± 1.0 | 5.6–9.7 |
| Awareness status (aware/unaware)a | 6/28 | — |
| Plasma glucose at hypoglycemia (mmol/liter) | 2.1 ± 0.3 | 1.4–2.8 |
The Gold method was applied to assess the awareness status.12
Table 2.
Number of Patients, Experiment Site, and Electrode Type for Each of the Five Protocols
| Number of patients | Experiment site | Electrode type |
|---|---|---|
| 6 | Odense University Hospital | Surface cup electrodes |
| 15 | Odense University Hospital | Surface cup electrodes |
| 7 | Sydvestjysk Sygehus Esbjerg | Subcutaneous electrode |
| 4 | Sydvestjysk Sygehus Esbjerg | Subcutaneous electrode |
| 3 | Hillerød Hospital | Surface cap electrodes |
Procedures for Electroencephalogram Analysis
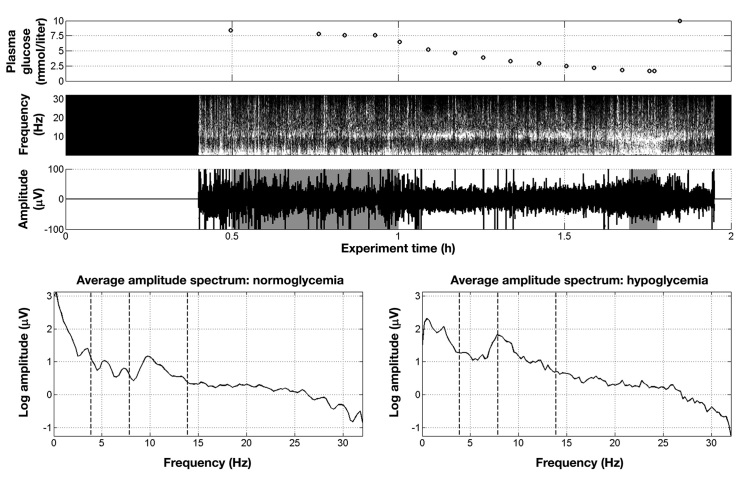
For each subject, quantitative electroencephalogram (qEEG) measures were calculated from both euglycemic and hypoglycemic EEG. Thirty minutes of consecutive EEG from the beginning of the experiment (plasma glucose > 5.0 mmol/liter) represent the euglycemic state, while 5 min of consecutive EEG at the glycemic nadir represent the hypoglycemic state (see Figure 1). The EEG signals were bandpass filtered (0.5–32 Hz). Based on visual inspection of the overall EEG patterns, the EEG signal quality was considered very good (e.g., no eye movements were recorded). Thus dedicated decontamination of the EEG signals to remove possible artifacts was not considered necessary.
Figure 1.
An example illustrating the course of an experiment of induced hypoglycemia and the selection of EEG data for quantitative analysis. The plasma glucose level declines steadily (top panel), ultimately changing the characteristics of the recorded single-channel EEG as illustrated in the spectrogram (second panel). In the third panel, the gray boxes behind the raw EEG signal indicate the parts of data representing the normoglycemic and hypoglycemic state, respectively (30 min for normoglycemic and 5 min for hypoglycemia). The bottom panels shows average amplitude spectra, which serve the basis for the qEEG measures. The dashed lines indicate the EEG band splits
For each subject, the power spectral density of the euglycemic and hypoglycemic sequences was estimated using Welch’s method applied to 4 s Hamming-windowed epochs with 50% overlap and a 0.25 Hz resolution. From these, average amplitude spectra were calculated (from the square root of the power), each of which was then subdivided into four traditional frequency bands: delta (1–3.75 Hz), theta (4–7.75 Hz), alpha (8–12.75 Hz) and beta (13–30 Hz). For each band, the absolute amplitude and the centroid frequency were calculated. The absolute amplitude was determined using a numerical integration technique applying the trapezoidal rule. The centroid frequency was defined as the center of gravity of each frequency band that subdivides the area under the spectral curve into two of equal size. Although conceptive, the EEG band split may be somewhat artificial. A dominant peak in the alpha band may be transferred to a dominant peak in the theta band by just a slight slowing of the frequency. Accordingly, the peak frequency in a unified theta and alpha band (5–12.75 Hz) was also determined. Signal processing has been conducted in Matlab 7.12.0 (R2011a).
Statistical Analysis
The fact that spectral EEG characteristics differ across subjects13 made us conduct within-patient analysis, comparing hypoglycemia versus normoglycemia changes in each qEEG measure.
Before subdividing the data to elucidate potential group differences, the total data set was analyzed, comparing EEG during normoglycemia and hypoglycemia. One-sample paired Student’s t-tests for zero mean of hypoglycemia versus normoglycemia changes were applied for normally distributed qEEG measures (centroid frequency measures and absolute amplitudes after log transformation). The nonparametric Wilcoxon signed rank test for zero median was performed on the theta–alpha peak frequency measure.
Subsequently, to investigate whether the EEG changes associated with hypoglycemia differ across subpopulations, two-sample Students t-tests were conducted, comparing the mean of the paired hypo–normo changes of different subpopulations. Similarly, the Wilcoxon rank sum test was applied for the theta–alpha peak frequency measure and in case the subpopulation split resulted in a group size of less than 10 subjects.
Results
Table 3 lists the results of the total data set analysis, which compared EEG during normoglycemia and hypoglycemia. An increase in the log amplitude of the delta, theta, and alpha band and a decrease in the alpha band centroid frequency and the peak frequency of the unified theta–alpha band constituted the most significant hypoglycemia indicators (all p < .0001). Furthermore, the centroid frequency of the delta band increased, whereas that of the beta band decreased (p < .05).
Table 3.
Analysis of Total Groupa
| qEEG measure | Normoglycemia | Hypoglycemia | Hypo–normo change | p value |
|---|---|---|---|---|
| Absolute amplitudeb | ||||
| Delta | 1.95 ± 0.52 | 2.60 ± 0.65 | 0.65 ± 0.40 | <0.0001 |
| Theta | 1.46 ± 0.48 | 2.22 ± 0.53 | 0.76 ± 0.32 | <0.0001 |
| Alpha | 1.76 ± 0.52 | 2.13 ± 0.57 | 0.37 ± 0.27 | <0.0001 |
| Beta | 2.68 ± 0.46 | 2.79 ± 0.62 | 0.10 ± 0.43 | ns |
| Centroid frequency | ||||
| Delta | 2.09 ± 0.08 | 2.14 ± 0.12 | 0.05 ± 0.13 | <0.05 |
| Theta | 5.82 ± 0.11 | 5.82 ± 0.16 | 0.01 ± 0.16 | ns |
| Alpha | 10.30 ± 0.13 | 10.13 ± 0.15 | -0.17 ± 0.18 | <0.0001 |
| Beta | 20.66 ± 0.63 | 20.39 ± 0.53 | -0.27 ± 0.71 | <0.05 |
| Peak frequency | ||||
| Theta–alpha | 9.25 | 6.50 | 1.875 | <0.0001 |
Mean and standard deviation of the qEEG measure values of the normal state, hypoglycemic state, and the paired hypoglycemia versus normoglycemia changes. A positive change means that the qEEG measure value in the hypoglycemic state exceeds that of the euglycemic state. One-sample paired t-tests for zero mean of hypoglycemia versus normoglycemia changes or Wilcoxon signed rank test for zero median lead to the associated p values.
Natural logarithm of absolute amplitude.
Table 4 shows the results of the analyses, clarifying whether hypoglycemia-related qEEG changes differ when stratified by gender, age, duration of diabetes, and diabetes awareness status. Overall, the hypoglycemia-associated EEG changes proved to be stable across subgroups (indicated by nonsignificant p values). However, a few exceptions were demonstrated. The log amplitude of the theta band increased more in female subjects than in male subjects (0.91 ± 0.34 versus 0.68 ± 0.28; p < .05). When stratified according to diabetes awareness status, the alpha and beta centroid frequency decreased more during hypoglycemia for patients with preserved aware-ness than those with impaired awareness ((p < .05 and p < .01, respectively). The beta log amplitude seemed to decrease for aware patients, whereas it increased for patients with impaired awareness (p < .05). No differences in the qEEG measures changes were observed when data were stratified according to age or duration of diabetes.
Table 4.
Analyses of Four Different Group Stratificationsa
| qEEG measure | Male (n = 22) |
Female (n = 12) |
p value | qEEG measure | Age < 50 years (n = 14) |
Age ≥ 50 years (n = 20) |
p value | |
| Absolute amplitudeb | Absolute amplitudeb | |||||||
| Delta | 0.61 ± 0.43 | 0.72 ± 0.35 | ns | Delta | 0.66 ± 0.48 | 0.64 ± 0.34 | ns | |
| Theta | 0.68 ± 0.28 | 0.91 ± 0.34 | <0.05 | Theta | 0.73 ± 0.31 | 0.78 ± 0.33 | ns | |
| Alpha | 0.36 ± 0.21 | 0.39 ± 0.37 | ns | Alpha | 0.37 ± 0.33 | 0.38 ± 0.23 | ns | |
| Beta | 0.13 ± 0.36 | 0.06 ± 0.57 | ns | Beta | 0.15 ± 0.49 | 0.07 ± 0.40 | ns | |
| Centroid frequency | Centroid frequency | |||||||
| Delta | 0.05 ± 0.14 | 0.06 ± 0.13 | ns | Delta | 0.03 ± 0.15 | 0.07 ± 0.12 | ns | |
| Theta | -0.00 ± 0.17 | 0.01 ± 0.15 | ns | Theta | -0.01 ± 0.18 | 0.02 ± 0.15 | ns | |
| Alpha | -0.18 ± 0.20 | -0.16 ± 0.13 | ns | Alpha | -0.14 ± 0.16 | -0.20 ± 0.19 | ns | |
| Beta | -0.26 ± 0.66 | -0.28 ± 0.84 | ns | Beta | -0.15 ± 0.79 | -0.35 ± 0.66 | ns | |
| Peak frequency | Peak frequency | |||||||
| Theta–alpha | -2.00 | -1.625 | ns | Theta–alpha | -2.25 | -1.625 | ns | |
| qEEG measure | dur < 20 years (n = 13) |
dur ≥ 20 years (n = 21) |
p value | qEEG measure | Unaware (n = 28) |
Aware (n = 6) |
p value | |
| Absolute amplitudeb | Absolute amplitudeb | |||||||
| Delta | 0.62 ± 0.45 | 0.67 ± 0.37 | ns | Delta | 0.53 | 0.76 | ns | |
| Theta | 0.75 ± 0.28 | 0.77 ± 0.37 | ns | Theta | 0.69 | 0.93 | ns | |
| Alpha | 0.44 ± 0.27 | 0.33 ± 0.27 | ns | Alpha | 0.37 | 0.33 | ns | |
| Beta | 0.16 ± 0.41 | 0.07 ± 0.46 | ns | Beta | 0.22 | -0.25 | <0.05 | |
| Centroid frequency | Centroid frequency | |||||||
| Delta | 0.05 ± 0.15 | 0.05 ± 0.11 | ns | Delta | 0.04 | 0.10 | ns | |
| Theta | 0.01 ± 0.14 | -0.00 ± 0.18 | ns | Theta | 0.00 | 0.08 | ns | |
| Alpha | -0.18 ± 0.19 | -0.17 ± 0.18 | ns | Alpha | -0.11 | -0.37 | <0.05 | |
| Beta | -0.34 ± 0.71 | -0.23 ± 0.73 | ns | Beta | -0.03 | -1.08 | <0.01 | |
| Peak frequency | Peak frequency | |||||||
| Theta–alpha | -2.25 | -1.75 | ns | Theta–alpha | -1.625 | -2.25 | ns | |
Mean and standard deviation of paired hypoglycemia versus normoglycemia changes (median if nonparametric). A positive change means that the qEEG measure value in the hypoglycemic state exceeds that of the euglycemic state. Two-sample t tests for equal means or Wilcoxon rank sum tests for equal medians lead to the associated p values.
Natural logarithm of absolute amplitude.
Discussion
The purpose of our ongoing studies is to develop a hypoglycemia alarm based on continuous measurements of EEG and automated real-time analysis. Iaione and Marques14 published the development of an automated algorithm using digital signal processing and artificial neural networks with the aim of developing a hypoglycemia detector system. They achieved a fair performance, but to our knowledge, the concept was not commercialized. Our aim is to develop a portable real-time hypoglycemia alarm device for T1DM patients with hypoglycemia unawareness. For such a device to be suitable for clinical use, it must fulfill a number of criteria: it must have a high sensitivity and an infrequent occurrence of false positive alarms, it must be suitable for long-term use with minimal discomfort for the patient, and, preferably, it should require little or no calibration.
One of our initial insulin-induced hypoglycemia studies demonstrated that the majority of patients develop EEG changes ahead of cognitive deterioration.5 By repeating the experiments in a subgroup of the patients, the presence of an individual glucose threshold for EEG changes was confirmed. In another study, the concept of a real-time alarm device was tested.15 We found that most patients received an alarm in proper time to take action and were able to prevent impending severe hypoglycemia by carbohydrate intake. The study was carried out both during daytime and sleep, underscoring that it was possible to differentiate the characteristic EEG sleep patterns from hypoglycemia-associated EEG changes. If an alarm device shall be used without extensive individual calibration, it is of utmost importance that the hypoglycemia-associated EEG changes are similar in all patients. A large data set is required to address this issue. Therefore, in this article, we have pooled data from different trials conducted so far. This gathering of data from different protocols, none of which is recorded for the actual purpose of this study, constitutes a weakness of the present study.
However, the hypoglycemia induction procedure was similar in all protocols. Additionally, EEG signals recorded with subcutaneous and cup/cap electrodes are comparable regarding amplitude and frequency distribution. We have conducted a number of parallel recordings confirming this during our development. Furthermore, for each patient, the normoglycemic and hypoglycemic EEG sequences were part of one continuous recording, thereby being conducted during the exact same circum-stances. Accordingly, the significant and stable changes in the qEEG variables are unlikely to be caused by differences in study procedures. If anything, it may tend to obliterate differences, underscoring the fact that qEEG changes are not dependent on procedure. We found that hypoglycemia-associated EEG changes are independent of age and duration of diabetes.
Regarding gender, a significant difference in an important hypoglycemia indicator was found since a larger increase of absolute amplitude in the theta band in female subjects compared with male subjects was demonstrated. However, taking into account the small difference as compared with the hypoglycemia versus normoglycemia, this is likely to be caused by multiple testing. In a previous study in T1DM patients conducted during euglycemic condition, it was demonstrated that female gender is associated with higher values of delta power.16 Age, age at diabetes onset, and duration of diabetes has been shown not to influence the baseline EEG in diabetes subjects.16
With respect to hypoglycemia awareness status, we found some qEEG change discrepancies across the subgroup (beta amplitude, alpha and beta centroid frequency). Bendtson and coauthors6 found that only patients with abolished glucagon release expressed hypoglycemia-related EEG changes and suggested that glucagon has a protective action on the brain during hypoglycemia. Although the awareness status of the patients was not given in that study, it may be assumed that the glucagon nonresponders were also the patients with impaired awareness of hypoglycemia. Earlier, we found that EEG changes were independent of glucagon release.5 In a study of nondiabetic patients, insulin-induced hypoglycemia was ceased either by glucose or glucagon injection. In accordance with our observation, no protective effect of glucagon on EEG changes was found.17 From the data in the present study, it is not possible to explain the EEG response difference between patients with unawareness and patients with sustained awareness. Because this difference seems independent of age, duration of diabetes, and glucagon response, unawareness and altered hypo-glycemia-associated EEG changes may share a rather common course. The hypoglycemia alarm device will be directed primarily toward patients suffering from unawareness. However, since awareness status is likely to be a dynamic rather than permanent condition, it will be necessary to look closely at potentially dynamic differences in hypoglycemia-associated EEG changes. Such is the focus of ongoing studies.
It remains controversial whether patients with diabetes have EEG aberrations as compared with nondiabetic individuals during euglycemia conditions. Previous reports have demonstrated a significant loss of fast oscillations (alpha and beta activity) in the posterior temporal regions of diabetes patients.16 In another published study, however, EEG was recorded during childhood diabetes and was repeated in adulthood 16 years later.18 The data were stratified according to the prevalence of severe hypoglycemia during childhood. This prospective study, which can be assumed to be sensitive with regard to long-term consequences of severe hypoglycemia, failed to confirm the prevalence of persistent EEG changes. Therefore, the detected EEG changes must be ascribed to the acute hypoglycemia.
Conclusions
Highly significant EEG changes occur during hypoglycemia irrespective of age, gender, duration of diabetes, and hypoglycemia awareness status. This holds promise that a general algorithm for hypoglycemia detection can be established.
Glossary
- (EEG)
electroencephalogram
- (qEEG)
quantitative electroencephalogram
- (T1DM)
type 1 diabetes mellitus
Funding
All studies were initiated and fully sponsored by Hypo-Safe A/S, Denmark.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Regan PF, 3rd, Browne-Mayers AN. Electroencephalography, frequency analysis and consciousness; a correlation during insulin-induced hypoglycemia. J Nerv Ment Dis. 1956;124(2):142–147. doi: 10.1097/00005053-195608000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Chalew SA, Sakamoto RN, McCarter R, Hanukoglu A, Kowarski AA, Matjasko J. Quantitative monitoring of brain function, vital signs, and hormonal response during acute insulin-induced hypoglycemia. J Clin Monit. 1989;5(4):229–235. doi: 10.1007/BF01618252. [DOI] [PubMed] [Google Scholar]
- 4.Pramming S, Thorsteinsson B, Stigsby B, Binder C. Glycaemic threshold for changes in electroencephalograms during hypo-glycaemia in patients with insulin dependent diabetes. Br Med J (Clin Res Ed). 1988;296(6623):665–667. doi: 10.1136/bmj.296.6623.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juhl CB, Højlund K, Elsborg R, Poulsen MK, Selmar PE, Holst JJ, Christiansen C, Beck-Nielsen H. Automated detection of hypoglycemia-induced EEG changes recorded by subcutaneous electrodes in subjects with type 1 diabetes--the brain as a biosensor. Diabetes Res Clin Pract. 2010;88(1):22–28. doi: 10.1016/j.diabres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Bendtson I, Gade J, Rosenfalck AM, Thomsen CE, Wildschiodtz G, Binder C. Nocturnal electroencephalogram registrations in type 1 (insulin-dependent) diabetic patients with hypoglycaemia. Diabetologia. 1991;34(10):750–756. doi: 10.1007/BF00401523. [DOI] [PubMed] [Google Scholar]
- 7.Bjørgaas M, Sand T, Vik T, Jorde R. Quantitative EEG during controlled hypoglycaemia in diabetic and non-diabetic children. Diabet Med. 1998;15(1):30–37. doi: 10.1002/(SICI)1096-9136(199801)15:1<30::AID-DIA526>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Hyllienmark L, Maltez J, Dandenell A, Ludvigsson J, Brismar T. EEG abnormalities with and without relation to severe hypoglycaemia in adolescents with type 1 diabetes. Diabetologia. 2005;48(3):412–419. doi: 10.1007/s00125-004-1666-2. [DOI] [PubMed] [Google Scholar]
- 9.Tamburrano G, Lala A, Locuratolo N, Leonetti F, Sbraccia P, Giaccari A, Busco S, Porcu S. Electroencephalography and visually evoked potentials during moderate hypoglycemia. J Clin Endocrinol Metab. 1988;66(6):1301–1306. doi: 10.1210/jcem-66-6-1301. [DOI] [PubMed] [Google Scholar]
- 10.Tribl G, Howorka K, Heger G, Anderer P, Thoma H, Zeitlhofer J. EEG topography during insulin-induced hypoglycemia in patients with insulin-dependent diabetes mellitus. Eur Neurol. 1996;36(5):303–309. doi: 10.1159/000117277. [DOI] [PubMed] [Google Scholar]
- 11.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350(22):2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 12.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypo-glycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17(7):697–703. doi: 10.2337/diacare.17.7.697. [DOI] [PubMed] [Google Scholar]
- 13.Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E da Silva FL eds. Electroencephalography. Basic principles, clinical applications and related fields. Baltimore: Lippincott Williams and Wilkins; 2005. pp. 167–192. [Google Scholar]
- 14.Iaione F, Marques JL. Methodology for hypoglycaemia detection based on the processing, analysis and classification of the electroencephalogram. Med Biol Eng Comput. 2005;43(4):501–507. doi: 10.1007/BF02344732. [DOI] [PubMed] [Google Scholar]
- 15.Snogdal LS, Folkestad L, Elsborg R, Remvig LS, Beck-Nielsen H, Thorsteinsson B, Jennum P, Gjerstad M, Juhl CB. Detection of hypoglycemia associated EEG changes during sleep in type 1 diabetes mellitus. Diabetes Res Clin Pract. 2012 doi: 10.1016/j.diabres.2012.04.014. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Brismar T, Hyllienmark L, Ekberg K, Johansson BL. Loss of temporal lobe beta power in young adults with type 1 diabetes mellitus. Neuroreport. 2002;13(18):2469–2473. doi: 10.1097/00001756-200212200-00019. [DOI] [PubMed] [Google Scholar]
- 17.Wilson WP, Short MJ, Deiss WP., Jr The relationship of glucagon and EEG patterns in hypoglycemia. J Psychiatr Res. 1965;3(2):99–104. doi: 10.1016/0022-3956(65)90019-1. [DOI] [PubMed] [Google Scholar]
- 18.Asvold BO, Sand T, Hestad KA, Bjørgaas MR. Quantitative EEG in type 1 diabetic adults with childhood exposure to severe hypo-glycaemia: a 16 year follow-up study. Diabetologia. 2011;54(9):2404–2408. doi: 10.1007/s00125-011-2208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]