Abstract
Background
Diabetes care is not presently available, accessible, or affordable to people living in rural areas in developing countries, such as India. The Chunampet Rural Diabetes Prevention Project (CRDPP) was conceived with the aim of implementing comprehensive diabetes screening, prevention, and treatment using a combination of telemedicine and personalized care in rural India.
Methods
This project was undertaken in a cluster of 42 villages in and around the Chunampet village in the state of Tamil Nadu in southern India. A telemedicine van was used to screen for diabetes and its complications using retinal photography, Doppler imaging, biothesiometry, and electrocardiography using standardized techniques. A rural diabetes center was set up to provide basic diabetes care.
Results
Of the total 27,014 adult population living in 42 villages, 23,380 (86.5%) were screened for diabetes, of which 1138 (4.9%) had diabetes and 3410 (14.6%) had prediabetes. A total of 1001 diabetes subjects were screened for complications (response rate of 88.0%). Diabetic retinopathy was detected in 18.2%, neuropathy in 30.9%, microalbuminuria in 24.3%, peripheral vascular disease in 7.3%, and coronary artery disease in 10.8%. The mean hemoglobin A1c levels among the diabetes subjects in the whole community decreased from 9.3 ± 2.6% to 8.5 ± 2.4% within 1 year. Less than 5% of patients needed referral for further management to the tertiary diabetes hospital in Chennai.
Conclusions
The Chunampet Rural Diabetes Prevention Project is a successful model for screening and for delivery of diabetes health care and prevention to underserved rural areas in developing countries such as India.
Keywords: Asian Indians, diabetes, prevention, rural, south Asians, telemedicine
Introduction
According to the World Health Report 2004, non-communicable diseases (NCDs) account for nearly 60% of the 56.5 million deaths each year and 47% of the global burden of disease. Due to rapid demographic and epidemiological transition, NCDs are rapidly overtaking infectious and deficiency diseases as the major cause of mortality and morbidity even in developing countries.1,2
The Indian Council of Medical Research–India Diabetes (ICMR-INDIAB) Study estimates that 62.4 million personswith diabetes lived in India in 2011.3 The International Diabetes Federation projects that an estimated 101 million will have diabetes in India in 2030.4 Asian Indians have increased insulin resistance 5 and increased susceptibility to diabetes despite lower body mass index (BMI).6,7 In large metropolitan cities in India (Mumbai, Delhi, Calcutta, Chennai, Bangalore, and Hyderabad), the prevalence of diabetes among adults (aged ≥20 years) ranges from 8–18%.8–17 The prevalence of diabetes has been reported to be low in rural areas,18 but studies suggest that it is rapidly increasing even in rural areas.3,19,20 Indeed, in some states in India such as Kerala, the prevalence of diabetes in rural areas already exceeds that reported in urban areas,21 which is similar to the situation seen in developed countries of the world.22 Unfortunately, more than half the people with diabetes remain undiagnosed23 and even among known diabetes patients, less than one third have their diabetes under good control.24 There is also evidence to show that poverty and poor access to care, coupled with low education, are linked to a high rate of diabetes-related complications.25,26
Unfortunately, the vast majority of India’s population (70%) lives in rural areas.27 Lack of awareness due to illiteracy, lack of physicians and paramedical staff trained in diabetes, limited access to health care due to problems with transport and infrastructure, and unaffordability due to poverty are some of the major obstacles to delivering diabetes health care to rural areas. Screening for diabetes is seldom done in rural areas, resulting in a much greater burden of undiagnosed diabetes in rural areas.3 This could potentially lead to higher rates of diabetes-related complications due to delayed diagnosis and/or improper treatment. Hence, there is an urgent need for screening programs for diabetes in rural areas in India and in other developing countries. With this objective in mind, The Chunampet Rural Diabetes Prevention Project (CRDPP) was developed to provide mass screening and diabetes health care and prevention to rural India through a combination of telemedicine and personalized care.
Methods
The CRDPP was established in March 2006 with the aim of implementing preventive and therapeutic diabetes services by empowering local people. The project, supported by the World Diabetes Foundation (Denmark), and the Indian Space Research Organization (ISRO) in Bangalore, India, was undertaken in a cluster of 42 villages in and around Chunampet in the Chithamur block in the Kancheepuram district of Tamil Nadu state in southern India, with a total population of 43,158 people (refer to http://www.mdrf.in/telemedicine.html for a map of the study area).
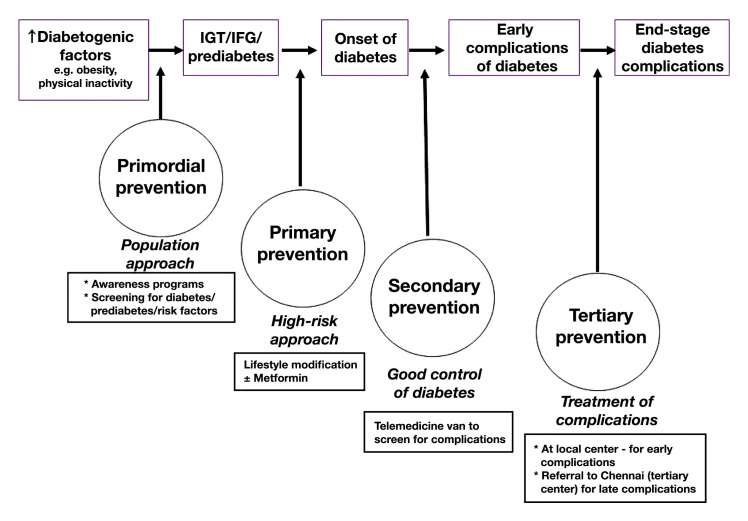
The project offers services at the following levels of prevention (see also Figure 1):
-
1.
Primordial: to screen for and reduce risk factors for diabetes
-
2.
Primary: to prevent diabetes in those identified with prediabetes
-
3.
Secondary: to prevent complications in those already diagnosed with diabetes by good control of diabetes
-
4.
Tertiary: to offer treatment to those who already have some complications and to prevent progression to the end stage of these complications.
Figure 1.
Levels of prevention provided in the model
Approaches to prevention and therapy often overlap; therefore all levels are important and complementary. Primordial and primary prevention contribute to improving the health of the whole population, while secondary and tertiary prevention focus on people with diabetes. The project was approved by the Institutional Ethical Committee of Madras Diabetes Research Foundation in Chennai, India, and written informed consent was obtained from all study subjects.
A baseline survey was conducted before implementing the project to identify the demographic characteristics of the population. A door-to-door diabetes screening was carried out in all selected villages using a structured questionnaire, which included details on demographic and socioeconomic characteristics, health behavior, knowledge of diabetes and its complications, health status and medical history of the subjects and their families, perception of health, diet, and physical activity. The questionnaire was pretested and validated using a pilot study on 100 subjects.
Anthropometric measurements including weight, height, and waist circumference were obtained using standardized techniques.28 Body mass index was calculated using the following formula: weight (kg)/height (m2). Blood pressure was recorded in the sitting position in the right arm to the nearest 1 mm Hg using the electronic Omron™ Blood Pressure Monitor (Omron Corporation, Kyoto, Japan). Two readings were taken 5 min apart, and the mean of the two was taken as the final blood pressure reading.
The structured questionnaire helped to identify those subjects with self-reported diabetes (diagnosed by a physician and on antidiabetic drugs). In all others, the initial screening comprised a fasting capillary blood glucose test using a hand-held glucose monitor (One Touch® Ultra,® LifeScan, Milpitas, CA). Those who had fasting capillary blood glucose (CBG) ≥126 mg/dl (≥7 mmol/liter) were diagnosed as having diabetes,29 and they were then subjected to a 75 g oral glucose tolerance test (OGTT) (using venous blood) to confirm the diagnosis. Those confirmed to have diabetes by OGTT and all self-reported diabetes subjects underwent screening for diabetes-related complications.
Prediabetes was diagnosed if the subject had impaired fasting glucose, i.e., fasting CBG ≥100 mg/dl (5.6 mmol/liter) and <126 mg/dl (<7 mmol/liter) or impaired glucose tolerance [2 h post- ≥140 mg/dl (≥ 7.8 mmol/liter) but <200 mg/dl (< 11.1 mmol/liter) and fasting value <126 mg/dl (< 7 mmol/liter)].29
Telemedicine Services and Screening for Complications of Diabetes
A fully equipped mobile telemedicine van fitted with all equipment necessary to screen for diabetes and its complications were donated by the World Diabetes Foundation, Denmark (Figure 2). One of the major problems faced by the rural population is the lack of facilities in rural areas for both the screening and treatment of diabetes complications. This means that patients have to travel long distances to the nearest city for these services. Use of a mobile unit with appropriate equipment, trained technicians, and satellite technology helped us to bridge this gap and ensure increased accessibility to diabetes health services in these rural areas. The infrastructure in the telemedicine van included a digital retinal camera, a slit lamp, computerized electrocardiogram (ECG), Doppler imaging, and biothesiometry. The telemedicine van also included videoconferencing equipment with a television screen, facilities for blood sampling, air conditioning, a generator for uninterrupted power supply, computers, a laser printer, and basic furniture. Information from the telemedicine van was transmitted to the tertiary care center in Chennai through the Very Small Aperture Terminal satellite connectivity kindly provided by the ISRO, Bangalore, India.
Figure 2.
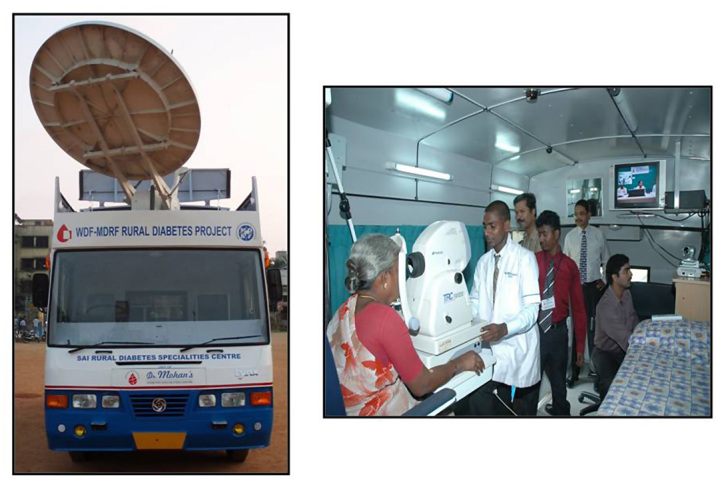
Telemedicine van and its facilities
Screening for retinopathy was done using four-field stereo color retinal photography (Zeiss FF 450 plus camera) by trained and certified photographers. Photographs were graded by an ophthalmologist using the Early Treatment Diabetic Retinopathy Study (ETDRS) grading system.30 Briefly, according to the ETDRS grading, level 10 represents no retinopathy; level ≥20, nonproliferative DR (NPDR); and level ≥60, proliferative DR (PDR). Diabetic macular edema (DME) was defined as retinal thickening at or within 1 disc diameter of the center of the macula or the presence of definite hard exudates. Sight-threatening DR (STDR) or severe DR was defined as the presence of NPDR with DME and PDR.30 To screen for neuropathy, the Bio-Thesiometer by Bio-Medical Instrument Company (Newbury, Ohio) was used to assess the vibratory perception threshold (VPT) of the great toes in a standardized fashion. Neuropathy was diagnosed if the VPT of the great toe exceeded mean +2 standard deviation (SD) of a healthy nondiabetic study population aged 20–45 years (cut point ≥20V).31
To assess coronary artery disease (CAD), a resting 12-lead ECG was performed using the Myocard R electrocardiograph (Marks Electronics, Chennai, India). Coronary artery disease was diagnosed based on a medical history of CAD and/or Minnesota coding of ECGs.32 Doppler studies were performed to screen for peripheral vascular disease (PVD), which included recording of pressure tracings using the KODY Vaslab Machine (Kody Medical Electronics, Ltd., Chennai, India). The ankle/brachial pressure index ratio was calculated in every subject. Peripheral vascular disease was defined as ankle-brachial index < .9.33
Urine samples were collected after an overnight fast. Microalbumin concentration was measured using an immuno-turbidometric assay (Hitachi 902 Autoanalyzer, Roche Diagnostics, Mannheim, Germany). Microalbuminuria was diagnosed if the albumin excretion was between 30–299 µg/mg of creatinine.34
Serum creatinine was measured using Jaffe’s method (Beckman Coulter AU 2700/480 Autoanalyzer). Renal insufficiency was defined as a serum creatinine >1.2 mg/dl for women and >1.3 mg/dl for men.35
Training of Staff
Unlike urban areas where a qualified or trained paramedical staff is usually available, it is extremely difficult to find qualified people in rural areas. India’s National Rural Health Mission provides for local community workers to be trained in health-related work. Hence, Village Health Workers (VHWs) who have a basic high school qualification can be gainfully employed. We therefore decided to recruit unemployed, young men and women locally, as this not only provided employment for the local people but also helped to “buy in” to the community for implementation of such programs. We recruited 15 VHWs and gave them intensive training in all aspects of the study for a period of 15 days, with further retraining at 6 monthly intervals. The technicians were trained to collect venous blood samples in the telemedicine van and to take digital retinal photographs, echocardiograms, Doppler images, and biothesiometry measurements for assessment of the various diabetes complications.
Primordial Prevention
Primordial prevention was provided to the whole population in the form of mass- scale diabetes awareness programs. This part of the program used VHWs to increase awareness and to screen all subjects in the community. Various modalities were used to spread diabetes awareness, such as family and self-help groups, the performing arts, peer-group support, and one-on-one sessions. Self-help groups were trained in the preparation of low-cost recipes for balanced nutrition using locally sourced food including fruits and vegetables. Information, education and communication materials were also distributed regarding adopting a healthy lifestyle by increasing physical activity, avoiding tobacco, smoking cessation, reducing weight where appropriate, continuing the traditional diet pattern, and cultivating healthier crops. In keeping with local traditions, puppet shows, skits on diabetes, awareness programs for teachers, and diet exhibitions on healthy nutrition for school children and pregnant women were presented. On occasions such as World Diabetes Day, walks for children and special programs on healthy living were organized. Finally, farmers and housewives were taught to grow nutritious vegetables.
Primary Prevention
All subjects who were identified with prediabetes during screening were given detailed lifestyle-modification advice (diet, exercise, and weight reduction whenever appropriate). The advice included going for a brisk walk for a minimum of 30 minutes every day; practicing yoga and meditation to control and manage stress, as this was culturally appropriate; consuming a diet rich in vegetables, especially greens; reducing consumption of polished white rice; increasing the intake of whole grains and legumes; reducing saturated fat; and limiting salt intake to 5 grams per day. Those who tested normal were given standard advice regarding healthy living.
Secondary Prevention
A rural diabetes center was set up in Chunampet to offer follow-up care to the subjects with self-reported diabetes and to those identified through the screening program to have newly detected diabetes. These subjects are monitored for development of complications in the rural diabetes center, which was established to provide a diagnostic and treatment facility. This center is manned by physicians and paramedical staff who were recruited locally but trained at the main hospital in Chennai. The team comprises a diabetologist (a local physician recruited and trained in diabetes) and dietitians and technicians who provide support services for those diagnosed with diabetes. Podiatry and preventive foot services and treatment of minor foot problems are also provided at the rural center. Serious foot complications that require surgical intervention or hospitalization are referred to the diabetes hospital in Chennai for further treatment.
Screening for eye complications is done with the help of a retinal camera. Retinal images are sent via satellite to Chennai. Ophthalmologists at the diabetes hospital in Chennai review the photographs and are available for teleconsultations 3 days a week at an appointed time.
Glycemic control of self-reported diabetes subjects is assessed using hemoglobin A1c. Hemoglobin A1c is estimated by high-pressure liquid chromatography using the variant machine by Bio-Rad (Hercules, CA). It is measured at baseline and in a repeat visit after 1 year. Electrocardiograms are also performed and are interpreted by the onsite diabetologist, who is available for consultations at the local center 6 days a week.
Low-cost-generic antidiabetic drugs (both tablets and insulin) are available at the local diabetes center. While entire screening services (for both diabetes and its complications) and teleconsultations are free, patients pay subsidized charges for follow-up treatment and also pay for their medications. This helps to ensure sustainability of the project.
Tertiary prevention
Tertiary prevention refers to treatment of those who have already developed any degree of diabetes complications, with the aim of preventing progression to end- stage complications and providing rehabilitation measures whenever necessary. Subjects with diabetes in an early stage of complications were treated at the local center in Chunampet, while patients with DR requiring laser treatment or patients with advanced diabetic foot, heart, or kidney complications requiring further treatment or surgical treatment were referred to the diabetes hospital in Chennai for which free transport was provided.
Statistical Analysis
Statistical analyses are performed using the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL) for Windows™ version 10.0 software (Microsoft Corporation, Redmond, WA). Values are expressed as means ±SD. The Chi-square test is used to compare proportions. A p value < .05 is considered significant.
Result
A total of 108 public awareness campaigns were conducted over a 4 year period (March 2006 to February 2010) in various locations covering all 42 villages. Locations included general public places, schools, and small-scale industries such as flour mills and poultry farms and religious places of worship, including churches, mosques, and temples. Awareness camps were conducted on both weekdays and weekends in the local language, Tamil. On special occasions such as World Diabetes Day and World Health Day, the campaign was intensified with multiple events, including diabetes awareness lectures, walks, quiz programs, cooking demonstrations, and diet exhibitions (Table 1).
Table 1.
Awareness Activities Conducted in the Chunampet Rural Diabetes Prevention Project
| Types of Activity | Number of Activities |
|---|---|
Lecture
Total lectures |
108 |
| Puppet show | 12 |
| Skit | 8 |
| Quiz program | 9 |
| Awareness walk | 6 |
| Cooking demonstration | 11 |
| Diet exhibition | 11 |
| Total, all activities | 165 |
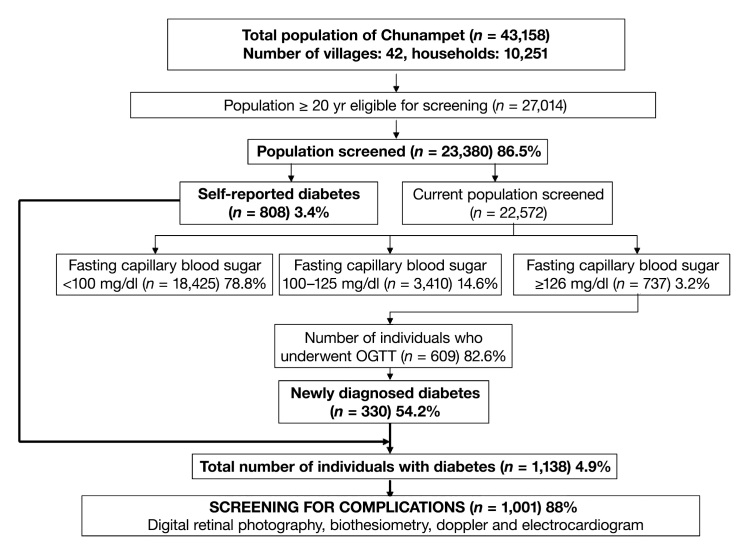
Of the total 43,158 people in the Chunampet cluster of villages, 27,014 were adults (aged ≥ 20 years) and they were considered eligible for the screening. Of these, 23,380 subjects (86.5%) (10,326 males and 13,054 females) participated in the screening program. Mean age of the study population was 41 ±15 years, mean BMI was 21.4 ±4.1 kg/m2, mean systolic blood pressure was 126 ±36 mm Hg, mean diastolic blood pressure was 79 ±13 mm Hg, and mean waist circumference was 73.5 ±10.9 cm.
Figure 3 shows the results of the screening program. Briefly, of the 23,380 subjects screened for diabetes, a total of 1138 subjects (4.9%) had diabetes (self-reported diabetes, n = 808; newly diagnosed diabetes, n = 330) while 3410 (14.6%) had prediabetes.
Figure 3.
Flowchart of Chunampet Rural Diabetes Prevention Project
All diabetes subjects were referred to the rural clinic and monitored for glycemic control. The mean HbA1c among the self-reported diabetes subjects at baseline was 9.3 ±2.6%, and this decreased to 8.5 ±2.4% (p < .001) at the end of 1 year of treatment.
Table 2 shows the prevalence of diabetes complications among known and newly detected diabetes subjects. The prevalence of DR, neuropathy, microalbuminuria, renal insufficiency, PVD, and CAD was 18.2%, 30.9%, 24.3%, 3.2%, 7.3%, and 10.8%, respectively. The prevalence of sight-threatening forms of retinopathy, PDR and DME, was 1.4% and 7.1%, respectively.
Table 2.
Prevalence of Diabetes Complications Among the Rural Population
| Complication | Overall n (%) (n = 1001) |
Known Diabetes n (%) (n = 744) |
Newly Diagnosed Diabetes n (%) (n = 257) |
p Value |
|---|---|---|---|---|
| DR | 182 (18.2%) | 165 (22.2%) | 17 (6.6%) | <.001 |
| Mild NPDR | 91 (9.1%) | 78 (10.5%) | 13 (5.1%) | <.001a |
| Moderate NPDR | 59 (5.9%) | 57 (7.7%) | 2 (0.8%) | |
| Severe NPDR | 18 (1.8%) | 16 (2.2%) | 2 (0.8%) | |
| PDR | 14 (1.4%) | 14 (1.9%) | 0 (0%) | |
| DME | 71 (7.1%) | 69 (9.3%) | 2 (0.8%) | <.001 |
| Diabetic neuropathy | 309 (30.9%) | 265 (35.6%) | 44 (17.1%) | <.001 |
| Microalbuminuria | 243 (24.3%) | 211 (28.4%) | 32 (12.4 %) | <.001 |
| Renal insufficiency | 32 (3.2%) | 27 (3.6%) | 5 (1.9%) | .180 |
| PVD | 73 (7.3%) | 61 (8.2%) | 12 (4.7%) | .070 |
| CAD | 108 (10.8%) | 83 (11.2%) | 25 (9.7%) | .562 |
Trend Chi square: 44.97
Figure 4 shows the prevalence of complications in relation to duration of diabetes. With increasing duration of diabetes, the prevalence of all complications increased significantly (p for trend, p < .05). Even among subjects with diabetes <1 year duration, the prevalence of complications ranged from 4.9% for PVD to 16.4% for neuropathy.
Figure 4.
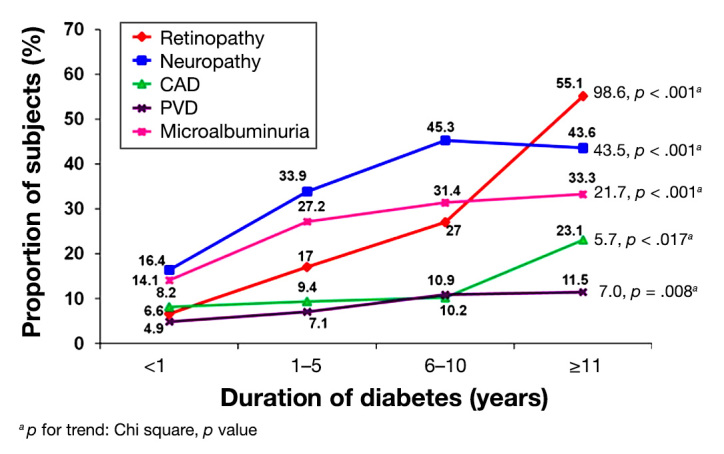
Prevalence of diabetes complications in relation to duration of diabetes
Only 49/1001 (4.9%) of the diabetes patients screened for complications had severe DR that required laser photo-coagulation. Also, only 32/1001 (3.2%) of the diabetes subjects screened had severe foot complications requiring referral to the hospital in Chennai.
Discussion
The CRDPP, to our knowledge, is the first comprehensive project that provides diabetes prevention and health care at all levels (primordial, primary, secondary, and tertiary) in a rural area of a developing country. The model uses a combination of innovative tools such as telemedicine in addition to using locally available talent and personnel, thus providing employment to local people. The project shows that in a relatively short period of time and with limited resources, a significant impact can be made in a large community in terms of mass screening for diabetes, increased awareness of diabetes, prevention at various levels, improved HbA1c levels, and provision of health care for various complications of diabetes. This is significant because all accomplishments were achieved in remote rural areas where no such facilities had previously existed.
The overall prevalence of diabetes in this study is 4.9% (Figure 3). This is lower than the prevalence of diabetes in rural Tamil Nadu according to the ICMR–INDIAB Study (8.0%). The reason for this difference may be that capillary glucose was tested first and only in those who were diagnosed with diabetes based on fasting capillary glucose, i.e., if the blood glucose level was greater than 126 mg/dl; an OGTT was performed. There might have been many individuals with fasting capillary blood sugar in the range of 100–125 mg/dl or <100 mg/dl who could have shown diabetes had they been subjected to an OGTT. We have previously shown that the prevalence of diabetes based on OGTT done in the entire population is much higher than that based on fasting glucose alone.36 This may explain the lower prevalence of diabetes in this study.
The high prevalence of prediabetes in this rural population is worrisome, as this implies a huge population at risk of developing diabetes in the near future. Based on fasting capillary blood glucose, the ratio of newly diagnosed to known diabetes was 1:0.9, which is similar to the results reported from the ICMR–INDIAB Study.3 This study reported that in four states, newly detected diabetes cases outnumbered individuals with known diabetes, except in Tamilnadu (Maharashtra-1:2.9, Chandigarh-1:1.7, and Jharkhand-1:3.3). This may be due to the periodic screening done in this state.
This article reports on the prevalence of diabetes complications in rural Tamilnadu using telemedicine and is an example of how screening for diabetes complications can be carried out effectively in remote rural areas in India. In a study done in a rural South Indian population using teleophthalmology, DR was reported to be 19%, which is similar to our findings.37 Rani and coauthors38 showed that the prevalence of DR was 17.6% among self-reported diabetes subjects in a rural area, which is lower than the results obtained in our study.
In a study conducted in rural Goa, the prevalence of diabetes complications, such as neuropathy, coronary heart disease, retinopathy, and PVD, were reported to be 60%, 32.3%, 15.4%, and 11.5%, respectively. All complications except DR were higher in rural Goa as compared to our study population. A significant, rising trend in the prevalence of all diabetes complications with advancing duration was also observed, which is similar to the results in our study.39
The strengths of our present study are two-fold: (1) the subjects studied represent the rural population in a cluster of 42 villages in which the sample size is large (n = 23,380) and the response rate is good (86.5%); and (2) this study is the first of its kind to look at the prevalence of diabetes complications in rural India using telemedicine. However, there are some limitations. First, the cross-sectional nature of the design does not allow for cause–effect relationships. Second, we could not conduct glucose tolerance testing on the entire population.
In developing countries, such as India, while more than 70% of the population lives in rural areas,27 80% of the doctors practice in urban areas.40 This mismatch is unlikely to be solved in the foreseeable future because doctors are unwilling to reside in less-developed rural areas. Thus, novel methods must be developed to provide health care, in general, and, in particular, diabetes prevention and health care, which is accessible, affordable, and acceptable to rural people in developing countries.
The CRDPP has been successful due to the support of and collaboration among multiple stakeholders: the philanthropist who donated land, those who constructed our building, the World Diabetes Foundation, which provided a fully equipped van and initial salary support for 4 years, and the ISRO, which provided satellite support for the telemedicine facilities. The staff of the project as well as its local partner organizations in Chunampet, such as the National Agro Foundation, developed an excellent partnership with the members of the community, and this ensured their continued support to these activities. Although the full benefits of this project in terms of reducing the complications of diabetes may take many years to be realized, the system of engaging the community has contributed enormously to the success of the project. The project has helped to increase the awareness of diabetes among approximately 50,000 people. There are plans to extend the CRDPP to neighboring villages to reach 200,000 people in 3 years through a public–private partnership and with the support of the Government of Tamil Nadu.
Although the CRDPP has been in place for just 4 years, several tangible benefits are already visible. The project has provided empowerment for rural people. Trained self-help groups have helped in educating the communities about healthier eating habits. Setting up a local diabetes center, along with an effective referral system, has dramatically reduced the number of patients who need to travel to specialized centers in urban areas for treatment, thus improving compliance rates. This has directly translated to improved control of diabetes in the whole community, with a reduction of HbA1c by nearly 1% within 1 year using low-cost generic drugs in the entire cohort of people with diabetes in the 42 villages. Because less than 5% of patients needed referral to the diabetes center in Chennai for further treatment, such as laser photocoagulation or diabetes foot surgery, this shows that 95% of the health problems of diabetes can be managed locally. This has helped considerably in reducing the costs of transportation and expensive treatment in the city and in preventing the loss of daily wages.
In conclusion, the Chunampet Rural Diabetes Prevention model is not only suitable for prevention and control of diabetes but also reduces the burden of other chronic NCDs such as hypertension and CAD in low-resource rural settings. Telemedicine helps to effectively use the services of trained specialists such as ophthalmologists, who are simply not available in the rural areas of developing countries such as India. In subsequent publications, we will discuss the cost effectiveness of this model and the factors that ensure its long-term sustainability.
Acknowledgments
We acknowledge the support of the World Diabetes Foundation, Denmark, in carrying out this project, the National Agro Foundation for helping us to establish the link with the community, and the Indian Space Research Organization, Bangalore, India, for providing satellite connection and telemedicine equipment. We thank Mr. C. Ramakrishna for donating the land for a rural center for diabetes in Chunampet. We thank the community leaders and the participants for their cooperation, the village health workers for helping to reach the community, and Mr. N. Sivakumar, the project coordinator for this project.
Glossary
- (ABPI)
ankle/brachial pressure index
- (BMI)
body mass index
- (CAD)
coronary artery disease
- (CBG)
capillary blood glucose
- (CRDPP)
Chunampet Rural Diabetes Prevention Project
- (DME)
diabetic macular edema
- (DR)
diabetic retinopathy
- (ECG)
electrocardiogram
- (ETDRS)
Early Treatment Diabetic Retinopathy Study
- (HbA1c)
hemoglobin A1c
- (ICMR-INDIAB)
Indian Council of Medical Research–India Diabetes
- (ISRO)
Indian Space Research Organization
- (NCD)
noncommunicable disease
- (NGO)
National Agro Foundation
- (NPDR)
nonproliferative diabetic retinopathy
- (OGTT)
oral glucose tolerance test
- (PDR)
proliferative diabetic retinopathy
- (PVD)
peripheral vascular disease
- (SD)
standard deviation
- (STDR)
sight-threatening diabetic retinopathy
- (VHW)
Village Health Workers
- (VPT)
vibratory perception threshold
- (WDF)
World Diabetes Foundation
Funding
The CRDPP was supported by a grant from the World Diabetes Foundation, Denmark, and the satellite link for telemedicine services was donated by the Indian Space Research Organization, Bangalore, India. Land was donated by Mr. C. Ramakrishna to establish a rural center for diabetes in Chunampet, India.
References
- 1.Zargar AH, Wani AI, Masoodi SR, Laway BA, Bashir MI. Mortality in diabetes mellitus – data from a developing region of the world. Diabetes Res Clin Pract. 1999;43:67–74. doi: 10.1016/s0168-8227(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 2.Mohan V, Shanthirani CS, Deepa M, Deepa R, Unnikrishnan RI, Datta M. Mortality rates due to diabetes in a selected urban south Indian population – The Chennai Urban Population Study (CUPS-16) J Assoc Physicians India. 2006;54:113–117. [PubMed] [Google Scholar]
- 3.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, Bhansali A, Joshi SR, Joshi PP, Yajnik CS, Dhandhania VK, Nath LM, Das AK, Rao PV, Madhu SV, Shukla DK, Kaur T, Priya M, Nirmal E, Parvathi SJ, Subhashini S, Subashini R, Ali MK, Mohan V. Prevalence of diabetes and prediabetes (impaired fasting glucose or/and impaired glucose tolerance) in rural and urban India: Phase 1 results of the Indian Council of Medical Research-INdiaDIABetes (INDIAB) study. Diabetologia. 2011;54:3022–3027. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 4.Unwin N, Whiting D, Guariguata L, Ghyoot G, Gan D, editors. International Diabetes Federation, Diabetes Atlas. Fifth Edition, International Diabetes Federation, Brussels, Belgium. 2011,11–74. [Google Scholar]
- 5.Sharp PS, Mohan V, Levy JC, Mather HM, Kohner EM. Insulin resistance in patients of Asian Indian and European origin with non-insulin dependent diabetes. Horm Metab Res. 1987;19:84–85. doi: 10.1055/s-2007-1011745. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A, Snehalatha C, Viswanathan V, Viswanatha M, Haffner SM. Risk of noninsulin dependent diabetes mellitus conferred by obesity and central adiposity in different ethnic groups: a comparative analysis between Asian Indians, Mexican Americans and Whites. Diabetes Res Clin Pract. 1997;36:121–125. doi: 10.1016/s0168-8227(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 7.Chandalia M, Abate N, Garg A, Stray-Gunderson J, Grundy SM. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:2329–2335. doi: 10.1210/jcem.84.7.5817. [DOI] [PubMed] [Google Scholar]
- 8.Iyer SR, Iyer RR, Upasani SV, Baitule MN. Diabetes mellitus in Dombivli–an urban population study. J Assoc Physicians India. 2001;49:713–716. [PubMed] [Google Scholar]
- 9.Misra A, Pandey RM, Devi JR, Sharma R, Vikram NK, Khanna N. High prevalence of diabetes, obesity and dyslipidaemia in urban slum population in northern India. Int J Obes Relat Metab Disord. 2001;25:1722–1729. doi: 10.1038/sj.ijo.0801748. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakaran D, Shah P, Chaturvedi V, Ramakrishnan L, Manhapra A, Reddy KS. Cardiovascular risk factor prevalence among men in a large industry of northern India. Natl Med J India. 2005;18:59–65. [PubMed] [Google Scholar]
- 11.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, Rao PV, Yajnik CS, Prasanna Kumar KM, Nair JD. Diabetes Epidemiology Study Group in India (DESI). High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001;44:1094–1101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran A, Snehalatha C, Latha E, Vijay V, Viswanathan M. Rising prevalence of NIDDM in urban population of India. Diabetologia. 1997;40:232–237. doi: 10.1007/s001250050668. [DOI] [PubMed] [Google Scholar]
- 13.Mohan V, Shanthirani CS, Deepa R. Glucose intolerance (diabetes and IGT) in a selected South Indian population with special reference to family history, obesity and lifestyle factors–The Chennai Urban Population Study (CUPS 14) J Assoc Physicians India. 2003;51:771–777. [PubMed] [Google Scholar]
- 14.Ramachandran A, Snehalatha C, Baskar AD, Mary S, Kumar CK, Selvam S, Catherine S, Vijay V. Temporal changes in prevalence of diabetes and impaired glucose tolerance associated with lifestyle transition occurring in the rural population in India. Diabetologia. 2004;47:860–865. doi: 10.1007/s00125-004-1387-6. [DOI] [PubMed] [Google Scholar]
- 15.Mohan V, Deepa M, Deepa R, Shanthirani CS, Farooq S, Ganesan A, Datta M. Secular trends in the prevalence of diabetes and glucose tolerance in urban South India–the Chennai Urban Rural Epidemiology Study (CURES-17) Diabetologia. 2006;49:1175–1178. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High Prevalence of Diabetes and Cardiovascular Risk Factors Associated with Urbanization in India. Diabetes Care. 2008;31:893–898. doi: 10.2337/dc07-1207. [DOI] [PubMed] [Google Scholar]
- 17.Bai PV, Krishnaswami CV, Chellamariappan M. Prevalence and incidence of type-2 diabetes and impaired glucose tolerance in a selected Indian urban population. J Assoc Physicians India. 1999;47:1060–1064. [PubMed] [Google Scholar]
- 18.Kodali VRR, Alberti KGMM. Diabetes mellitus and hypertension among rural-rural migrants in South India. Ecol Food Nutr. 1995;33(3):149–161. [Google Scholar]
- 19.Deo SS, Zantye A, Mokal R, Mithbawkar S, Rane S, Thakur K. To identify the risk factors for high prevalence of diabetes and impaired glucose tolerance in Indian rural population. Int J Diab Dev Countries. 2006;26:19–23. [Google Scholar]
- 20.Chow CK, Raju PK, Raju R, Reddy KS, Cardona M, Celermajer DS, Neal BC. The prevalence and management of diabetes in rural India. Diabetes Care. 2006;29:1717–1718. doi: 10.2337/dc06-0621. [DOI] [PubMed] [Google Scholar]
- 21.Mohan V, Mathur P, Deepa R, Deepa M, Shukla DK, Menon GR, Anand K, Desai NG, Joshi PP, Mahanta J, Thankappan KR, Shah B. Urban rural differences in prevalence of self-reported diabetes in India–The WHO-ICMR Indian NCD risk factor surveillance. Diab Res Clin Pract. 2008;80:159–168. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Green A, Hirsch CN, Pramming SK. The changing world demography of type 2 diabetes. Diabetes Metab Res Rev. 2003;19:3–7. doi: 10.1002/dmrr.340. [DOI] [PubMed] [Google Scholar]
- 23.Deepa M, Deepa R, Shanthirani CS, Datta M, Unwin NC, Kapur A, Mohan V. Awareness and Knowledge of Diabetes in Chennai–The Chennai Urban Rural Epidemiology Study (CURES-9) J Assoc Physicians India. 2005;53:283–287. [PubMed] [Google Scholar]
- 24.Joshi SR, Das AK, Vijay VJ, Mohan V. Challenges in Diabetes Care in India: Sheer Numbers, Lack of Awareness and Inadequate Control. J Assoc Physicians India. 2008;56:443–450. [PubMed] [Google Scholar]
- 25.Ramachandran A, Snehalatha C, Vijay V, King H. Impact of poverty on the prevalence of diabetes and its complications in urban southern India. Diabet Med 2002. 2002;19:130–135. doi: 10.1046/j.1464-5491.2002.00656.x. [DOI] [PubMed] [Google Scholar]
- 26.Kapur A, Bjork S, Nair J, Kelkar S, Ramachandran A. Socio-economic determinants of the cost of diabetes in India. Diabetes Voice. 2004;49:18–21. [Google Scholar]
- 27. Census of India. Rural Urban Distribution of Population. Office of the Registrar General and Census Commissioner, India. Available from: http://censusindia.gov.in/2011-prov-results/paper2/data_files/india/Rural_Urban_2011.pdf. Accessed May 7, 2012.
- 28.Lee RD, Nieman DC. “Anthropometry”. Nutritional Assessment 2nd Edition, McGraw Hill College, Boston; 1996:249–61.
- 29. World Health Organization: Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF Consultation (2006). World Health Organization, International Diabetes Federation. Geneva, pp 39.
- 30.Early Treatment of Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs––an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 31.Pradeepa R, Rema M, Vignesh J, Deepa M, Deepa R, Mohan V. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: the Chennai Urban Rural Epidemiology Study (CURES-55) Diabet Med. 2008;25:407–412. doi: 10.1111/j.1464-5491.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- 32.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular survey methods. 2nd ed. Geneva: World Health Organization; 1982. [PubMed] [Google Scholar]
- 33.Premalatha G, Shanthirani S, Deepa R, Markovitz J, Mohan V. Prevalence and risk factors of peripheral vascular disease in a selected South Indian population. The Chennai Urban Population Study (CUPS) Diabetes Care. 2000;23:1295–1300. doi: 10.2337/diacare.23.9.1295. [DOI] [PubMed] [Google Scholar]
- 34.Varghese A, Deepa R, Rema M, Mohan V. Prevalence of micro-albuminuria in type 2 diabetes mellitus at a diabetes center in Southern India. Postgraduate Med J. 2001;77:399–402. doi: 10.1136/pmj.77.908.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss G, Bondar R, Buzzelli D. Kinetic Enzymatic Method for Determining Serum Creatinine. Clin Chem. 1975;21:1422–1426. [PubMed] [Google Scholar]
- 36.The DECODE-DECODA Study Group on behalf of the European Diabetes Epidemiology Group and the International Diabetes Epidemiology Group. Age, body mass index and Type 2 diabetes–associations modified by ethnicity. Diabetologia. 2003;46:1063–1070. doi: 10.1007/s00125-003-1158-9. [DOI] [PubMed] [Google Scholar]
- 37.Raman R, Mahajan S, Padmaja RK, Agarwal S, Gnanamoorthy P, Paul PG, Krishna MS, Kumaramanickavel G, Sharma T. Tele-health program for diabetic retinopathy in rural South India: a pilot study. E-Health. Int. J. 2005;2:13–18. [Google Scholar]
- 38.Rani PK, Raman R, Chandrakantan A, Pal SS, Perumal GM, Sharma T. Risk factors for diabetic retinopathy in self-reported rural population with diabetes. J. Postgrad. Med. 2009;55:92–96. doi: 10.4103/0022-3859.48787. [DOI] [PubMed] [Google Scholar]
- 39.Vaz NC, Ferreira A, Kulkarni M, Vaz FS, Pinto N. Prevalence of diabetic complications in rural Goa, India. Indian J Community Med. 2011;36:283–286. doi: 10.4103/0970-0218.91330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rathi S. Public Health needs modified strategy. Healthline. 2010;1:49–52. [Google Scholar]