Abstract
Background
Glycemic control in critically ill patients has been shown to be beneficial. In this prospective study, we evaluated the accuracy and technical feasibility of a continuous glucose monitoring system using intravascular microdialysis.
Method
Fifty patients undergoing cardiac surgery were monitored using a 4 Fr intravenous microdialysis catheter (Eirus SLC™, Dipylon Medical AB, Solna, Sweden) percutaneously placed with the tip of the catheter positioned in the superior vena cava. The catheter was connected to the Eirus™ monitoring system, and the patients were monitored for up to 48 h postoperatively in the intensive care unit (ICU). As reference, arterial blood samples were taken every hour and analyzed in a blood gas analyzer.
Results
Data were available from 48 patients. A total of 994 paired (arterial blood gas microdialysis) samples were obtained. Glucose correlation coefficient (R2) was 0.85. Using Clarke error grid analysis, 100% of the paired samples were in region AB, and 99% were in region A. Mean glucose level was 8.3 mmol/liter (149 mg/dl), mean relative difference was 0.2%, and mean absolute relative difference was 5%. A total of 99.2% of the paired samples were correct according to International Organization for Standardization (ISO) criteria. Bland-Altman analysis showed that bias ± limits of agreement were 0.02 ± 1.1 mmol/liter (0.36 ± 20 mg/dl).
Conclusions
Central venous microdialysis using the Eirus monitoring system is a highly accurate and reliable method for continuous blood glucose monitoring up to 48 h in ICU patients undergoing cardiac surgery. The system may thus be useful in critically ill ICU patients.
Keywords: critically ill patients, glucose monitoring, glycemic control, microdialysis
Introduction
Glucose control in critically ill patients has been the topic of an intense debate since the 2000s. It is a known fact that critically ill patients, both with and without diabetes, frequently develop hyperglycemia. Mechanisms underlying this stress-induced hyperglycemia include peripheral insulin resistance and increased glucose production by gluconeogenesis.1,2 With the arrival of the Leuven studies, demonstrating a substantial mortality and morbidity risk reduction with tight glycemic control (TGC) using intensive insulin therapy (IIT),3,4 the interest in glucose control in the intensive care unit (ICU) increased considerably. Further studies have investigated the benefit versus harm with IIT, and the results are diverging. However, they have failed to reproduce the same benefits as in the Leuven studies and instead have indicated adverse effects, one being increased risk of hypoglycemia.5–7 The Normoglycemia in Intensive Care Evaluation—Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study reported a significantly increased mortality in critically ill patients with IIT and more frequent episodes with severe hypoglycemia,8 possibly due to inappropriate implementation of IIT with inadequate blood glucose control. Further, a meta-analysis from 20099 including NICE-SUGAR data reported that IIT in critically ill patients results in a significantly increased risk of hypoglycemia and no overall mortality benefit but suggested that IIT may be beneficial to surgical ICU patients. It should be noted that this suggestion was questioned in a reanalysis published in 2010.10
In patients undergoing cardiac surgery, hyperglycemia is associated with increased adverse outcomes, including mortality, morbidity, and deep wound infections.11–13 Insulin infusion is associated with reduced mortality in patients with diabetes undergoing coronary artery bypass grafting.14 A systematic review declared that TGC with IIT is beneficial for patients undergoing cardiac surgery.15 It should be noted that the definition of hyperglycemia and TGC differ between studies.
Glucose control in the ICU requires frequent and correct glucose monitoring. An accurate real-time continuous glucose monitoring (CGM) system may prove to be beneficial in the management and treatment of hypoglycemia, hyperglycemia, and glucose variability. Intravascular microdialysis is a method for continuously measuring blood glucose without blood sampling, which was first experimentally described in 1996.16 This study was designed to evaluate the accuracy of this method in patients undergoing cardiac surgery.
Methods
Fifty patients undergoing elective cardiac surgery with routine clinical standards between March and July 2010 were included in the study after written informed consent from each patient. The study was approved by the Regional Ethics Committee of Stockholm. The Eirus™ microdialysis system (Dipylon Medical AB, Solna, Sweden) was used to measure blood glucose continuously. The system includes the Eirus single lumen catheter (SLC) (Dipylon Medical AB, Solna, Sweden), which is a radiopaque central venous access catheter with a microdialysis membrane close to the distal end (Figure 1). In the proximal end, there are extensions with luer connectors for inlet and outlet of perfusion fluid. The extensions are linked to the threelumen tube via a junction. Directly on the junction, there is a luer connector for the middle lumen, which is open in its distal end. The catheter body is marked in 1 cm increments, which allows determination of inserted catheter length. The effective length of the catheter is 30 cm. The outer diameter is 1.35 mm (4 Fr), except at the microdialysis chamber, where it is close to 2 mm (6 Fr). The catheter is connected to a sensor and monitor system that continuously (every second) measure and display blood glucose values using the glucose oxidase method (Figure 2).
Figure 1.
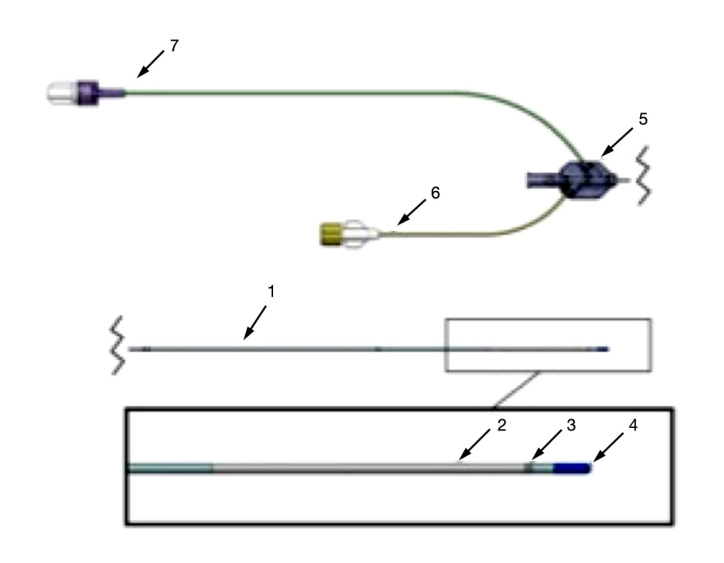
The Eirus SLC: (1) three-lumen catheter, (2) membrane, (3) marker band, (4) soft tip, (5) junction, (6) inlet, and (7) outlet.
Figure 2.
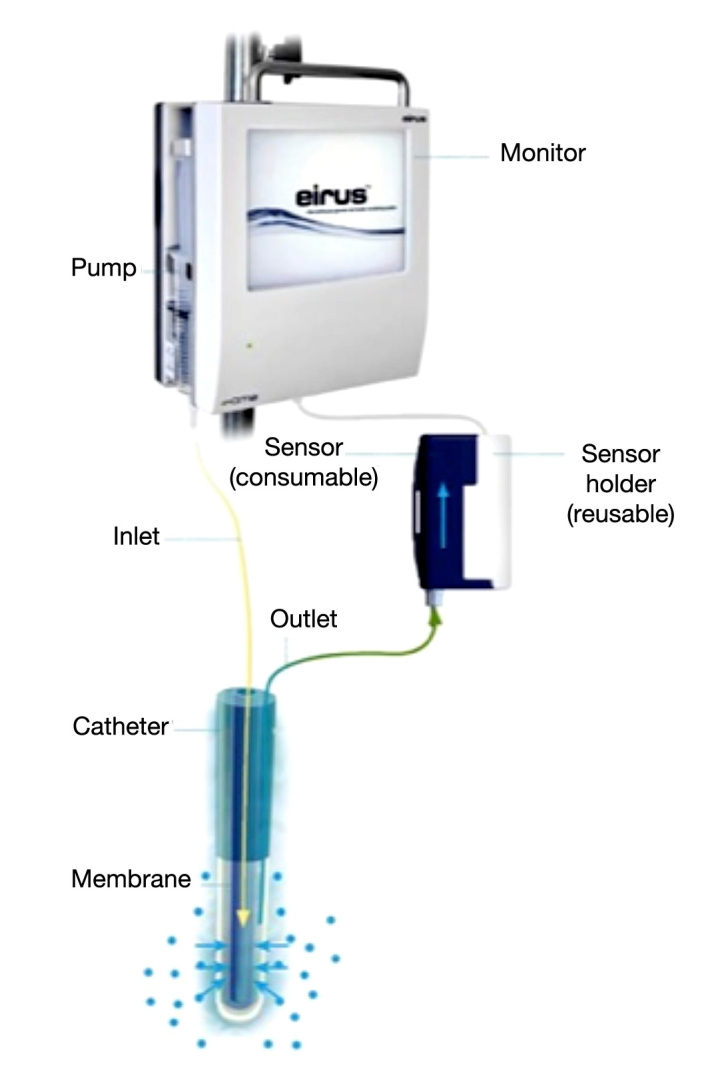
The Eirus SLC connected with the sensor and monitor. Continuous blood glucose values are displayed on the monitor screen.
The microdialysis catheter was placed preoperatively after general anesthesia through an Edwards Introflex introducer (6 Fr) in the right internal jugular vein and inserted approximately 19 cm from skin to the distal tip of the catheter to ensure placement in the vena cava superior/right atrium. All patients had at least one other central venous line, most commonly a 7 Fr triplelumen central venous catheter (CVC), through which postoperative infusions were administered. All patients received a standardized infusion of 5% glucose solution at the rate of 1 ml/kg/h during the entire ICU stay. After insertion, the microdialysis catheter was connected to the monitor and the sensor and perfusion of the system with sodium chloride was initiated (5 μl/min). Blood glucose values were displayed on the monitor after approximately 10 min, the time it takes to perfuse the system.
Upon arrival at the ICU after cardiac surgery, the monitoring of glucose values was started, with reference arterial and venous blood gas taken once every hour and analyzed in a blood gas analyzer (ABL800 FLEX, Radiometer Medical, Copenhagen, Denmark). In a previous study, we described the accuracy of arterial blood gas as a reference method compared with plasma glucose values analyzed by hospital laboratory with good results.17
Patients were monitored for up to 48 h or until discharged from the ICU (n = 49) or withdrawal of consent (n = 1). The microdialysis glucose values were calibrated using the first available arterial blood gas value and then every 8 h. The glucose values used for calibration were not included in the analysis of accuracy, which implies that seven paired arterial blood gas to microdialysis glucose values could be obtained between calibrations. The calibration time of 8 h was chosen for the convenience of the user (the nursing staff) who is used to calibrating devices once every shift (i.e., every 8 h). No consistent, systematic drift between calibrations was observed (Figure 3). The paired glucose values were corrected for the time lag (10 min) to better show the technical accuracy of the microdialysis system. No postoperative anti-coagulation was initiated the first 24 h after surgery. If the patients stayed in the ICU after 24 h, they received standard antithrombotic prophylactic medication with low molecular weight heparin.
Figure 3.
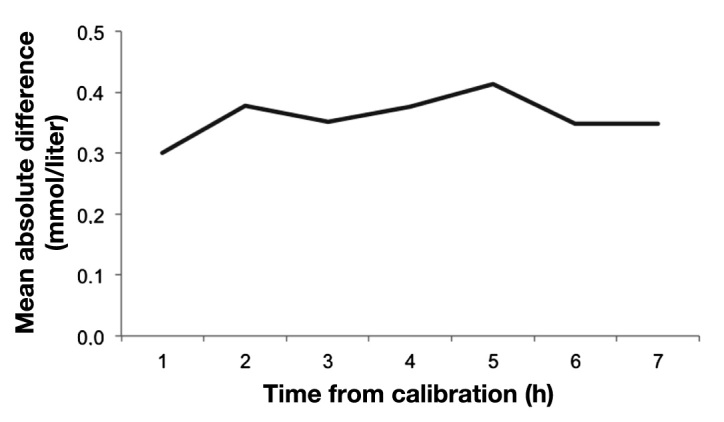
Mean absolute difference after time from calibration demonstrates the lack of systematic drift.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Correlation plots were made to calculate the correlation coefficient. Clarke error grid analysis (EGA) was made to evaluate the clinical relevance of microdialysis glucose values.18 The EGA plots display paired samples in five distinct zones of different significance. Values in zone A are within 20% of the reference value and have no clinical implications. Values in zone B exceed 20% difference from reference value but lead to appropriate clinical decisions. Values in zone C may lead to unnecessary but harmless corrections. Values in zones D and E represent overestimation of hypoglycemia (failure to detect) or underestimation of hyperglycemia that may lead to incorrect clinical actions. In short, the more values in zones A and B, the greater the clinical accuracy of the method. Glucose values were also evaluated according to the International Organization for Standardization (ISO) criteria.19 To meet this criteria, test glucose values have to be within ±20% of reference values if the reference value is >4.1 mmol/liter (74 mg/dl). If the reference value is less than 4.1 mmol/liter (74 mg/dl), the test values have to be within ±0.8 mmol/liter (14 mg/dl) 95% of the time. Bland-Altman analysis was also used to compare the bias (mean of differences) and limits of agreement (bias ± 2 SD).
Results
Data were available from 48 patients; in one patient, data were missing due to catheter damage in conjunction with mitral valve surgery, and in one patient, data were missing due to mechanical sensor failure. Patient characteristics are displayed in Table 1. Mean follow-up time was 24 h (range 13-46), and patients gave an average number of samples of 20.7 (range 8-41, median 19). All patients survived the surgical procedure. In routine cases, patients are admitted to the ICU postoperatively and discharged the day after to the postoperative ward. In this study, patients could leave the ICU on postoperative day 1 (n = 41) or postoperative day 2 (n = 7). Microdialysis glucose values were compared with arterial blood gas values, and a total of 994 paired values were obtained. Using Clarke EGA, 100% of the paired samples were in region AB and 99% in region A (Figure 4). Mean glucose level was 8.3 mmol/liter (149 mg/dl), mean relative difference [(arterial blood gas value - microdialysis value)/ arterial blood gas value] was 0.2%, and mean absolute relative difference was 5%. Bland-Altman analysis showed bias ± limits of agreement were 0.02 ± 1.1 mmol/liter (0.36 ± 20 mg/dl; Figure 5). A total of 99.2% of the paired samples were correct according to ISO criteria. No hypoglycemia was seen among the patients. In the hyperglycemic range, there was increased bias as seen in the Bland-Altman analysis. No complications were observed caused by the microdialysis catheter. There was no blood clotting of the catheters, and no loss of recovery was observed as an indicator of blood clotting.
Table 1.
Patient Characteristicsa
| Sex, male/female | 37/11 |
| Age, years | 68 ± 14 |
| Body mass index | 26.6 ± 4.4 |
| History of diabetes | 10 |
| Treated with insulin | 3 |
| Treated with oral antidiabetics | 7 |
| Surgical procedure | |
| Coronary artery bypass grafting | 25 |
| Valve surgery | 20 |
| Other | 4 |
Values are presented as mean ± SD
Figure 4.
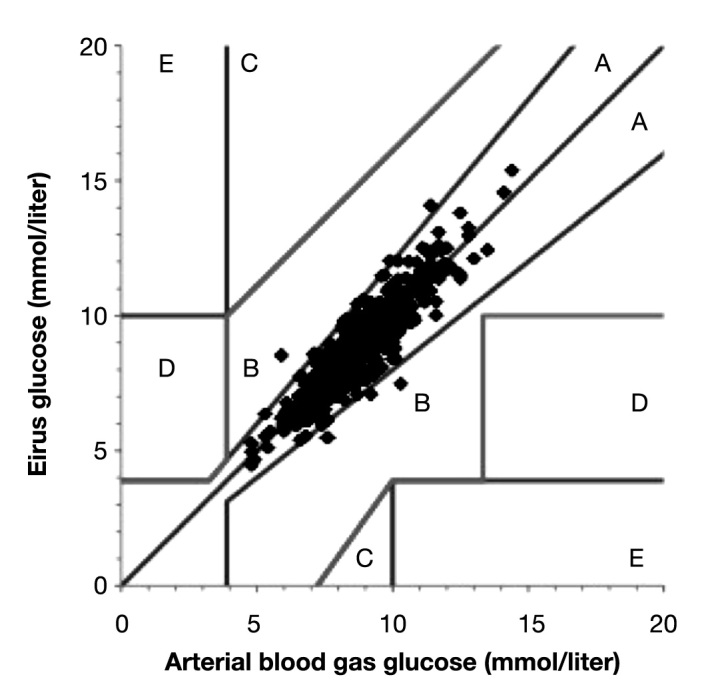
Clarke EGA comparing blood glucose measured by Eirus microdialysis and arterial blood gas.
Figure 5.
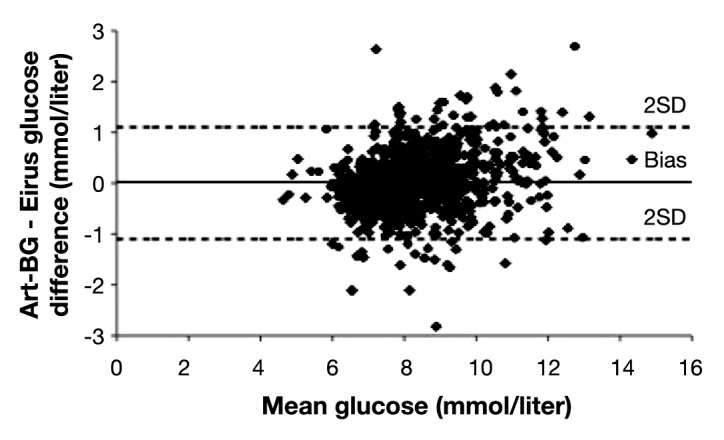
Bland-Altman analysis. The difference between microdialysis and arterial blood gas is plotted with mean blood glucose. Lines for bias and limits of agreement (± 2 SD) are shown. Art-BG, arterial blood gas.
Discussion
This study introduces the method of intravascular microdialysis for continuous measurement of blood glucose to enable improved glucose control in the ICU. Accuracy of the microdialysis system was satisfactory, with high agreement with blood gas analysis, low bias, and all paired samples within the AB zone of the Clarke error grid.
Glucose control today aims not only to avoid hyperglycemia, but also to safely detect severe hypoglycemia, which is associated with adverse outcomes in ICU patients.20–22 Studies have also discussed glucose variability23 as an independent risk factor for increased mortality in critically ill patients.24 Thus glycemic control in the ICU is important to better manage hypoglycemia, hyperglycemia, and glucose variability.
After the Leuven study was published in 2001, 3 TGC has been implemented in critical care with IIT to maintain normoglycemia. Target blood glucose levels remains controversial.25 In the original Leuven study, the target blood glucose level was 4.4-6.1 mmol/liter (79-110 mg/dl), and in the NICE-SUGAR study, the target glucose level was 4.5-6.0 mmol/liter (81-108 mg/dl). It seems to be generally accepted to try to avoid hyperglycemia in critically ill patients but not necessarily to aim for the tight glucose interval suggested by the Leuven studies. Especially, it seems diabetes patients benefit from higher glucose levels than nondiabetic patients.26
The inability to measure blood glucose accurately and with enough frequency in the ICU creates difficulties with glucose control. This can be prevented with an online real-time continuous method to measure blood glucose: a CGM system that enables the clinician to better control glucose regarding hypoglycemia, hyperglycemia, and glucose variability. It may also help reduce nurse workload.27 Several studies have evaluated different CGM systems in critically ill patients.28–30 The CGM systems used in these studies are placed subcutaneously, which may be hazardous in a critically ill patient with decreased peripheral circulation, especially if treatment with vasopressors are given, although it is also necessary to point out that some studies have shown this not to be the case.28 The Eirus microdialysis system is an intravascular method that is simple to use and now proven to be accurate in the ICU setting. The results in this study show a high accuracy between blood glucose levels measured by the microdialysis system and blood glucose levels measured conventionally by analyzing an arterial blood gas. Intravascular microdialysis may therefore provide reliable information to improve glycemic control.
The microdialysis system was placed in the superior vena cava/right atrium, in close proximity to the CVC. We did not observe any disturbances from glucose infusions administered in the CVC, probably due to the high blood flow in the superior vena cava. Given that we did not observe any hypoglycemic events, we cannot imply with certainty that the Eirus microdialysis system is accurate in hypoglycemic blood glucose levels; but we also have no reason to believe otherwise.
Another method to measure intravascular blood glucose continuously was described by Yamashita and coauthors31 in 2009, reporting the intravascular CGM system to be useful in the ICU setting. Skjaervold and coauthors32 presented another new intravascular method of CGM with good results in anesthetized pigs. Their results were in concurrence with the accuracy of the Eirus system, reporting bias ± limits of agreement of 0.01 ± 0.9 mmol/liter (0.18 ± 16 mg/dl; Eirus bias 0.02 ± 1.1 mmol/liter [0.36 ± 20 mg/dl]). A near-continuous method to measure blood glucose is described in an animal study,33 but since this method involves blood sampling every 15 min, it is our belief that the microdialysis system may be easier to use.
In this study, we have used arterial blood gas glucose values as reference values, as this is the routine method used in our clinic for glucose monitoring in critically ill patients today. However, it should be stated that the gold standard for glucose measurements is venous plasma analyzed by the hospital laboratory. We have investigated the accuracy between arterial blood gas and plasma glucose values with following results: mean difference was 0.27 mmol/liter (95% confidence interval −0.3 to 0.9 mmol/liter), and 100% of the samples were within ISO criteria and within zone A of the Clarke error grid.17 A total of 37 paired samples were obtained. Corstjens and coauthors34 found that 96.8% of paired samples were within the AB zone of the Clarke EGA (57.2% within zone A) and conclude that arterial blood gas analysis is a safe and reliable method for glucose monitoring in critically ill patients. In a similar study by Petersen and coauthors,35 arterial blood gas is also recommended for use in the ICU. Thus we believe that it is justified to use arterial blood gas as the reference method in this study.
The major limitation of this study is that no hypoglycemic episodes were observed, and the Eirus microdialysis system is therefore not satisfactorily evaluated in hypoglycemic blood glucose levels. However, the system has been tested in hypoglycemic ranges in an animal study with equal accuracy shown in this study (Liska, personal communication). Further studies using intravascular microdialysis are needed to confirm these results and especially to evaluate the accuracy in hypoglycemic glucose levels. In addition, a major disadvantage of the system is the requirement of a separate central venous line. It would be advantageous to have these two functions incorporated into the same catheter.
Conclusions
This study shows that intravascular microdialysis is an accurate, useful, and safe method for continuous measurement of glucose in critically ill patients without blood sampling for up to 48 h compared with arterial blood gas analysis. To what extent this method may improve glucose control with regard to hyperglycemia, hypoglycemia, and glucose variability remains to be further evaluated.
Acknowledgments
Registered nurses Camilla Lindell and Sofie Melin are acknowledged for their help with collecting data.
Glossary
- (CGM)
continuous glucose monitoring
- (CVC)
central venous catheter
- (EGA)
error grid analysis
- (ICU)
intensive care unit
- (IIT)
intensive insulin therapy
- (ISO)
International Organization for Standardization
- (NICE-SUGAR)
Normoglycemia in Intensive Care Evaluation—Survival Using Glucose Algorithm Regulation
- (SD)
standard deviation
- (SLC)
single lumen catheter
- (TGC)
tight glycemic control
Funding
Financial support for the complete study was obtained from the Mats Kleberg Foundation and the Signe and Olov Wallenius Foundation. Both foundations are located in Stockholm.
Disclosure
Jan Liska and Anders Franco-Cereceda are stockholders in Dipylon Medical AB, Solna, Sweden.
References
- 1.Gearhart MM, Parbhoo SK. Hyperglycemia in the critically ill patient. AACN Clin Issues. 2006;17(1):50–55. doi: 10.1097/00044067-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114(9):1187–1195. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 5.Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, Kahoul SH, Britts RJ, Sakkijha MH. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36(12):3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 6.Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 7.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 8.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 9.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrich JO, Chant C, Adhikari NK. Does intensive insulin therapy really reduce mortality in critically ill surgical patients? A reanalysis of meta-analytic data. Crit Care. 2010;14(5):324. doi: 10.1186/cc9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, Williams BA, Schrader LM, Rizza RA, McMahon MM. Intra-operative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80(7):862–866. doi: 10.4065/80.7.862. [DOI] [PubMed] [Google Scholar]
- 12.Guvener M, Pasaoglu I, Demircin M, Oc M. Perioperative hyperglycemia is a strong correlate of postoperative infection in type II diabetic patients after coronary artery bypass grafting. Endocr J. 2002;49(5):531–537. doi: 10.1507/endocrj.49.531. [DOI] [PubMed] [Google Scholar]
- 13.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–362. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 14.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 15.Haga KK, McClymont KL, Clarke S, Grounds RS, Ng KY, Glyde DW, Loveless RJ, Carter GH, Alston RP. The effect of tight glycaemic control, during and after cardiac surgery, on patient mortality and morbidity: a systematic review and meta-analysis. J Cardiothorac Surg. 2011;6:3. doi: 10.1186/1749-8090-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabenstein K, McShane AJ, McKenna MJ, Dempsey E, Keaveny TV, Freaney R. An intravascular microdialysis sampling system suitable for application in continuous biochemical monitoring of glucose and lactate. Technol Health Care. 1996;4(1):67–76. [PubMed] [Google Scholar]
- 17.Möller F, Liska J, Eidhagen F, Franco-Cereceda A. Intravascular microdialysis as a method for measuring glucose and lactate during and after cardiac surgery. J Diabetes Sci Technol. 2011;5(5):1099–1107. doi: 10.1177/193229681100500510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke WL. The original Clarke Error Grid Analysis (EGA) Diabetes Technol Ther. 2005;7(5):776–779. doi: 10.1089/dia.2005.7.776. [DOI] [PubMed] [Google Scholar]
- 19.International Organization for Standardization. ISO 15197:2003: in vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus.
- 20.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 21.Mechanick JI, Handelsman Y, Bloomgarden ZT. Hypoglycemia in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2007;10(2):193–196. doi: 10.1097/MCO.0b013e32802b7016. [DOI] [PubMed] [Google Scholar]
- 22.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85(3):217–224. doi: 10.4065/mcp.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egi M, Bellomo R. Reducing glycemic variability in intensive care unit patients: a new therapeutic target? J Diabetes Sci Technol. 2009;3(6):1302–1308. doi: 10.1177/193229680900300610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 25.Bellomo R, Egi M. What is a NICE-SUGAR for patients in the intensive care unit? Mayo Clin Proc. 2009;84(5):400–402. doi: 10.1016/S0025-6196(11)60557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36(8):2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 27.Signal M, Pretty CG, Chase JG, Le Compte A, Shaw GM. Continuous glucose monitors and the burden of tight glycemic control in critical care: can they cure the time cost? J Diabetes Sci Technol. 2010;4(3):625–635. doi: 10.1177/193229681000400317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunner R, Kitzberger R, Miehsler W, Herkner H, Madl C, Holzinger U. Accuracy and reliability of a subcutaneous continuous glucose-monitoring system in critically ill patients. Crit Care Med. 2011;39(4):659–664. doi: 10.1097/CCM.0b013e318206bf2e. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg PA, Siegel MD, Russell RR, Sherwin RS, Halickman JI, Cooper DA, Dziura JD, Inzucchi SE. Experience with the continuous glucose monitoring system in a medical intensive care unit. Diabetes Technol Ther. 2004;6(3):339–347. doi: 10.1089/152091504774198034. [DOI] [PubMed] [Google Scholar]
- 30.Holzinger U, Warszawska J, Kitzberger R, Wewalka M, Miehsler W, Herkner H, Madl C. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010;33(3):467–472. doi: 10.2337/dc09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand. 2009;53(1):66–71. doi: 10.1111/j.1399-6576.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 32.Skjaervold NK, Solligård E, Hjelme DR, Aadahl P. Continuous measurement of blood glucose: validation of a new intravascular sensor. Anesthesiology. 2011;114(1):120–125. doi: 10.1097/ALN.0b013e3181ff4187. [DOI] [PubMed] [Google Scholar]
- 33.Magarian P, Sterling B. Plasma-generating glucose monitor accuracy demonstrated in an animal model. J Diabetes Sci Technol. 2009;3(6):1411–1418. doi: 10.1177/193229680900300622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corstjens AM, Ligtenberg JJ, van der Horst IC, Spanjersberg R, Lind JS, Tulleken JE, Meertens JH, Zijlstra JG. Accuracy and feasibility of point-of-care and continuous blood glucose analysis in critically ill ICU patients. Crit Care. 2006;10(5):R135. doi: 10.1186/cc5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen JR, Graves DF, Tacker DH, Okorodudu AO, Mohammad AA, Cardenas VJ., Jr Comparison of POCT and central laboratory blood glucose results using arterial, capillary, and venous samples from MICU patients on a tight glycemic protocol. Clin Chim Acta. 2008;396(1-2):10–13. doi: 10.1016/j.cca.2008.06.010. [DOI] [PubMed] [Google Scholar]


