Abstract
Background
There is a need for reliable methods of glucose measurement in different environmental conditions. The objective of this in vitro study was to evaluate the performance of the Enlite® Sensor when connected to either the iPro™ Continuous Glucose Monitor recording device or the Guardian® REAL-Time transmitting device, in hypobaric and hyperbaric conditions.
Methods
Sixteen sensors connected to eight iPro devices and eight Guardian REAL-Time devices were immersed in three beakers containing separate glucose concentrations: 52, 88, and 207 mg/dl (2.9, 4.9, and 11.3 mmol/liter). Two different pressure tests were conducted: a hypobaric test, corresponding to maximum 18000 ft/5500 m height, and a hyperbaric test, corresponding to maximum 100 ft/30 m depth. The linearity of the sensor signals in the different conditions was evaluated.
Results
The sensors worked continuously, and the sensor signals were collected without interruption at all pressures tested. When comparing the input signals for glucose (ISIGs) and the different glucose concentrations during altered pressure, linearity (R2) of 0.98 was found. During the hypobaric test, significant differences (p < .005) were seen when comparing the ISIGs during varying pressure at two of the glucose concentrations (52 and 207 mg/dl), whereas no difference was seen at the 88 mg/dl glucose concentration. During the hyperbaric test, no differences were found.
Conclusions
The Enlite Sensors connected to either the iPro or the Guardian REAL-Time device provided values continuously. In hyperbaric conditions, no significant differences were seen during changes in ambient pressure; however, during hypobaric conditions, the ISIG was significantly different in the low and high glucose concentrations.
Keywords: barometric pressure, continuous glucose monitoring, diabetes mellitus, glucose, sensor
Background
Physical exercise is one of the cornerstones of diabetes management.1 For subjects treated with insulin, it is important to assess plasma glucose levels in order to make proper dose adjustments. Plasma glucose is commonly self-monitored using handheld glucometers. The accuracy of glucometers varies considerably between different manufacturers, and this inaccuracy may be worsened during certain conditions such as changes of elevation and/or pressure.2–4 Such pressure reductions may occur during flight or when visiting mountains. When flying at cruising altitude, usually 30,000 to 35,000 ft (10,000 to 13,000 m), the pressure in the cabin is increased by compressors to approximately 75 kPa (0.75 atm, 8200 ft/2500 m), which is 75% of the pressure measured at sea level, 101 kPa.
Self-monitoring of blood glucose measurements are affected by altitude changes.5 The home glucometers studied underestimated the glucose levels by approximately 1–2% for each 1000 ft (300 m) of elevation after correlation for changes in temperature and humidity.4,5 Minimal effect is seen in tests based on the glucose-dehydrogenase method; larger differences are seen when using the glucose oxidase method. The lower the oxygen partial pressure (PO2), the larger the negative bias of the tests. Differences are also seen when comparing test kits from different manufacturers.5 Most glucometers use glucose oxidase methods, which depend in part on oxygen partial pressure in the sample.2
The glucose oxidase method is also used for continuous glucose monitoring (CGM) sensors. Continuous glucose monitoring is a useful tool for detecting and reducing episodes of hypoglycemia,6 and for reducing the time spent within hypoglycemic range.7 An individual with diabetes travels as frequently as everybody else, and physical activity is also advocated in all age groups.1,8 Continuous glucose monitoring is a technique used to detect9 and decrease the risk of hypoglycemia10 during physical activity and has also been shown to enable assessment of glucose levels before, during, and after underwater diving.11–13 When engaging in activities at high altitudes, there is also a need to monitor glucose levels. The pressure in the airplane cabin is 75 kPa, corresponding to the pressure at approximately 8200 ft (2500 m), even when flying at a very high altitude. As some glucometers have shown poor accuracy during various pressure conditions, there is a risk that a home glucose meter can underestimate the glucose value when used in an airplane.5 Therefore, a more reliable method to monitor glucose values in hypobaric and hyperbaric conditions is needed.
Although CGM has been used during scuba diving, it is still not fully validated for use in various pressure conditions. This present study is the first attempt to validate CGM in different glucose concentrations during conditions with both decreased (hypobaric) and increased (hyperbaric) pressure. As CGM can be carried through both in retrospective and real-time modes, our aim was to evaluate whether CGM devices worked under these conditions.
In this study, we evaluated the performance of the Enlite® subcutaneous glucose sensor (Medtronic Minimed, Inc., Fridley, MN) when connected to two different compatible devices: the iPro™ Continuous Glucose Monitor recording device (Medtronic Minimed, Inc.) and the Guardian® REAL-Time transmitting device (Medtronic Minimed, Inc.). By using the i Pro system in this study, we aimed to confirm the function of a retrospective system as shown in other studies. The Guardian REAL-Time was evaluated because we have future expectations to be able to visualize glucose levels in real-time, even during scuba diving.
Performance was studied in three different glucose concentrations and four ambient pressure conditions. The specific pressure levels were chosen to mimic high altitude (18,000 ft/5500 m), typical air cabin pressure (8200 ft/2500 m), and maximum depth (100 ft/30 m) during recreational scuba diving and the pressure corresponding to the last decompression stop according to most diving tables (10 ft/3 m).14
Methods
Pressure Chamber
The study was performed in a 15 m3 combined hypo- and hyperbaric chamber at the unit of hyperbaric medicine at Karolinska University Hospital, Stockholm, Sweden.
Prior to the in vitro tests, we evaluated the function of the in vitro test setup in hypo- and hyperbaric conditions in a pressure chamber. We also performed temperature testing to ensure that the temperature would be within acceptable limits (accepted by Medtronic, Inc.) during the tests.
Temperature and pressure recordings were performed by TrackSense® Pro (Ellab A/S, Hilleroed, Denmark). This temperature sensor has an operating temperature range of 5–40 °C with an accuracy of ± 0.1 °C. The pressure sensor has an operating pressure range of 1 mbar to 10 bar absolute, with an accuracy of 0.15% (± 1.5 kPa).
During the experiment, pressure was varied according to Figure 1. We refer to accurate pressures such as 101 kPa at sea level, 404 kPa at 30 depths, and 51 kPa at 5500 m elevation, even if these are subjected to a discrete variation due to barometric changes. The exact pressures are not reported.
Figure 1.
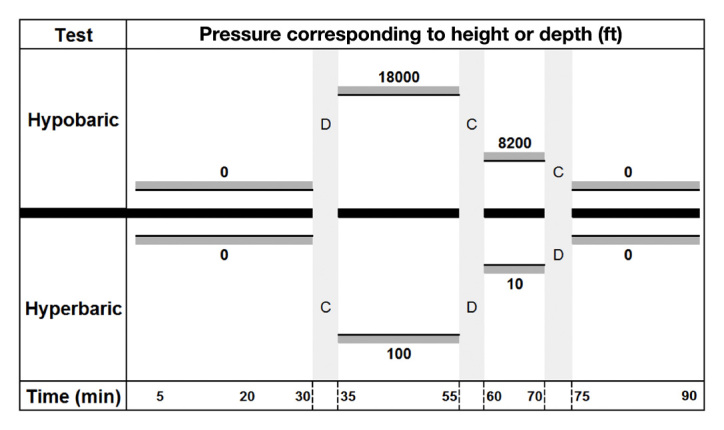
Hypobaric and hyperbaric tests illustrated. Pressure presented corresponding to altitude or depth in ft. The time is shown in min
Hypobaric test: the hypobaric test was conducted after a stabilization period of 30 min at 101 kPa (0 m, 1 atm). Chamber pressure was reduced to 51 kPa (18,000 ft/5500 m, 0.5 atm) for a period of 20 min and then increased to 75 kPa (8200 ft/2500 m, 0.75 atm) for 10 min before returning to 101 kPa (0 ft/0 m, 1.0 atm).
Hyperbaric test: the hyperbaric test was conducted after a similar stabilization period. Chamber pressure was then increased to 404 kPa (131 ft/30 m, 4.0 atm) for a period of 20 min, followed by a decompression stop at 130 kPa (10 feet/3 m, 1.3 atm) and a return to 101 kPa (0 ft/0 m, 1.0 atm).
Glucose Solutions
Different glucose concentrations were created by adding different amounts of d-glucose (Sigma) to a phosphate buffered saline solution (Sigma-Aldrich Co. LLC, St. Louis, MO).
The glucose concentration in the three solutions was controlled in duplicate samples at the Clinical Chemistry Laboratory, Karolinska University Hospital, Stockholm, Sweden (Swedish Board for Accreditation and Conformity accreditation ID number 1886). The glucose values were determined using a Beckman LX Glucose Analyzer (Beckman Coulter, Inc., Fullerton, CA). The glucose range of this instrument is 3.6–1200 mg/dl (0.2–66.7 mmol/liter). Imprecision of CVt is 1.42% when mean value is 94 mg/dl (5.2 mmol/liter) and CVt is 1.03% when mean value is 261 mg/dl (14.5 mmol/liter). The solution called “low” was found to be 52 mg/dl (2.9 mmol/liter). The “medium” concentration was found to be 88 mg/dl (4.9 mmol/liter), and the “high” concentration was 207 mg/dl (11.3 mmol/liter).
The phosphate buffered saline solution was formulated on-site and titrated to a pH of 7.4. The pH was measured by using litmus paper and correlating the color to the pH value. The pH at the beginning and end was the same—7.4 each time.
The beaker containing the sensors was placed in larger container with tepid water. This larger container was then surrounded by insulating material in the form of 10-cm-thick glass wool. Both these measures were taken in order to reduce the impact of pressure-induced air temperature changes on the glucose solutions. The holder for the 16 sensors in each beaker was provided by Medtronic and was of the same design as the holder used by Medtronic in its quality assessment work.
Continuous Glucose Monitoring
The Guardian REAL-Time transmitting device was used during the in vitro test, providing values in real-time every 5 min. The iPro recording device was used in parallel, providing values retrospectively. Twenty-four iPro units and 24 Guardian units were used; each was attached to an Enlite Sensor, and 8 sensors of each respective system were placed in three different glucose concentrations: low 52, medium 88, and high 207 mg/dl (2.9, 4.9, and 11.3 mmol/liter). Calibrations of each Guardian REAL-Time system were conducted pre- and postpressure alterations using the defined glucose concentrations in the buffer solutions.
The hypobaric and hyperbaric tests were performed as three identical sessions, one for each glucose concentration, starting with the low and ending with the high glucose concentration.
The sensor generates an input signal for glucose (ISIG) every 10 s. Input signal for glucose is defined as input signal, the current (nA) generated by the reaction at the working electrode surface, which is proportional to changes in interstitial glucose. Over a 5-min period, an average of 30 such ISIG readings are captured, filtered, and converted to a glucose reading through a proprietary algorithm. The glucose range of the Enlite Sensor is 40–400 mg/dl (2.2–22.0 mmol/liter). The linearity of the sensor signals produced by iPro and Guardian REAL-Time devices in the different glucose concentrations was evaluated during the hypobaric and hyperbaric test. The iPro and Guardian REAL-Time devices were also visually evaluated during the pressure changes, in particular, the Guardian REAL-Time devices regarding display and button function.
Data Analysis
The IBM SPSS Statistics 19 (IBM, Armonk, NY) was used for statistical analysis. The homogeneity of variance was tested by Levene’s test. The data was not normally distributed. Therefore, nonparametric statistical analysis methods were used. The independent samples t-test was used along with the Kruskal-Wallis test for comparison of the ISIGs generated in different glucose concentrations at various pressures. A p value < .05 was regarded as statistically significant.
Results
The temperature in the chamber was kept between 22 and 26 ºC during the hypobaric test and between 13 and 35 ºC during the hyperbaric test. In the beakers, the temperature was 36.0–37.6 ºC during the hypobaric test and 35.8–38.2 ºC during the hyperbaric test.
During all parts of the three sessions, 43/48 (90%) sensor signals were collected without interruption in all pressure conditions by the iPro and Guardian REAL-Time devices.
In the case of the Guardian REAL-Time device, the display shut down at a depth of 33–43 ft (10–13 m), but the sensor signal was continuously received by the monitor in spite of the display anomaly. Another pressure effect was that some of the membrane buttons were activated at pressures higher than 200 kPa, which, however, did not seem to affect the function.
During the first session at altitude and low glucose concentration, sensor failures were noted in 5/16 (31%) of the sensors; 2 of those were connected to iPro and 3 to Guardian REAL-Time devices. These sensors were then replaced. During the following two sessions when the sensors were immersed in medium and high glucose concentrations, all sensors worked continuously.
Major results are presented in Table 1 and Figure 2. Mean ISIGs in the three different glucose concentrations are shown separately for hypobaric and hyperbaric conditions. During the hyperbaric tests, no significant deviations were observed. However, during the hypobaric test, significant differences were seen. In the lower glucose concentration, significantly lower mean ISIGs were seen at both 18,000 ft (5500 m): 9.7 ± 0.8 nA [9.5–10.0, 95% confidence interval (CI)], p < .001, and at 82,00 ft (2500 m): 9.6 ± 1.3 nA (9.0–10.1, 95% CI), p < .05, when compared with the signal at sea level: 10.3 ± 0.3 nA (10.3–10.4). The altered ISIGs during hypobaric conditions in the low glucose concentration correspond to a 6–7% reduction of the ISIGs compared with sea level. In the high glucose concentration, the mean ISIGs were, on the other hand, significantly higher and more variable at both 18,000 ft (5500 m): 56.0 ± 4.4 nA (54.9–57.2, 95% CI), p < .001; and at 8200 ft (2500 m): 56.4 ± 5.7 nA (54.3–58.6, 95% CI), p < .001; compared with the signal at sea level: 50.2 ± 2.9 nA (49.6–50.8, 95% CI). This corresponds to 11% and 12% increments of the ISIGs at 18,000 ft (5500 m) and 8200 ft (2500 m), respectively.
Table 1.
Sensor Signals in Three Glucose Concentrations Shown Separately for Hypobaric and Hyperbaric Conditions
| Glucose concentration | Hypobaric test | Hyperbaric test | ||||||
|---|---|---|---|---|---|---|---|---|
| Pressure kPa |
Number of sensors | ISIG value mean ± 2SD (95% CI) | Compared with sea level, statistics | Pressure kPa |
Number of sensors | ISIG value mean ± 2SD (95% CI) | Compared with sea level, statistics | |
| 52 mg/dl 2.9 mmol/liter |
101 | 11 | 10.3 ± 0.3 (10.3 – 10.4) |
na | 101 | 16 | 11.0 ± 0.5 (10.8 – 11.1) |
na |
| 51 | 11 | 9.7 ± 0.8 9.5 – 10.0 |
p < 0.001 | 404 | 16 | 11.1 ± 0.5 (10.9 – 11.2) |
ns | |
| 75 | 11 | 9.6 ± 1.3 9.0 – 10.1 |
p < 0.05 | 130 | 16 | 11.1 ± 0.5 (10.9 – 11.3) |
ns | |
| 101 | 11 | 10.1 ± 0.3 (9.4 – 10.9) |
ns | 101 | 16 | 11.1 ± 0.2 (10.8 – 11.5) |
ns | |
| 88 mg/dl 4.9 mmol/liter |
101 | 16 | 20.2 ± 1.2 (19.9 – 20.4) |
na | 101 | 16 | 20.8 ± 1.3 (20.5 – 21.2) |
na |
| 51 | 16 | 20.2 ± 1.4 (19.8 – 20.5) |
ns | 404 | 16 | 20.7 ± 1.2 (20.4 – 21.1 |
ns | |
| 75 | 16 | 20.4 ± 1.3 (19.9 – 20.9) |
ns | 130 | 16 | 20.9 ± 1.2 (20.5 – 21.3) |
ns | |
| 101 | 16 | 20.6 ± 0.3 (20.0 – 21.3) |
ns | 101 | 16 | 21.0 ± 0.3 (20.3 – 21.6) |
ns | |
| 207 mg/dl 11.3 mmol/liter |
101 | 16 | 50.2 ± 2.9 (49.6 – 50.8) |
na | 101 | 16 | 50.4 ± 2.9 (49.7 – 51.2) |
na |
| 51 | 16 | 56.0 ± 4.4 (54.9 – 57.2) |
p < 0.001 | 404 | 16 | 50.7 ± 2.5 (50.0 – 51.3) |
ns | |
| 75 | 16 | 56.4 ± 5.7 (54.3 – 58.6) |
p < 0.001 | 130 | 16 | 51.2 ± 2.6 (50.2 – 52.2) |
ns | |
| 101 | 16 | 55.6 ± 1.4 (52.6 – 58.7) |
p < 0.001 | 101 | 16 | 51.4 ± 0.7 (50.0 – 52.9) |
p < 0.05 | |
SD, standard deviation; na, not applicable; ns, not significant.
Values are shown as mean ± 2 standard deviation and 95% CI.
Pressure corresponding to respective altitude/depth: 101 kPa: sea level, 51 kPa: 18,000 ft/5500 m, 75 kPa: 8200 ft/2500 m, 404 kPa: 100 ft/30 m, and 130 kPa: 10 ft/3 m.
Figure 2.
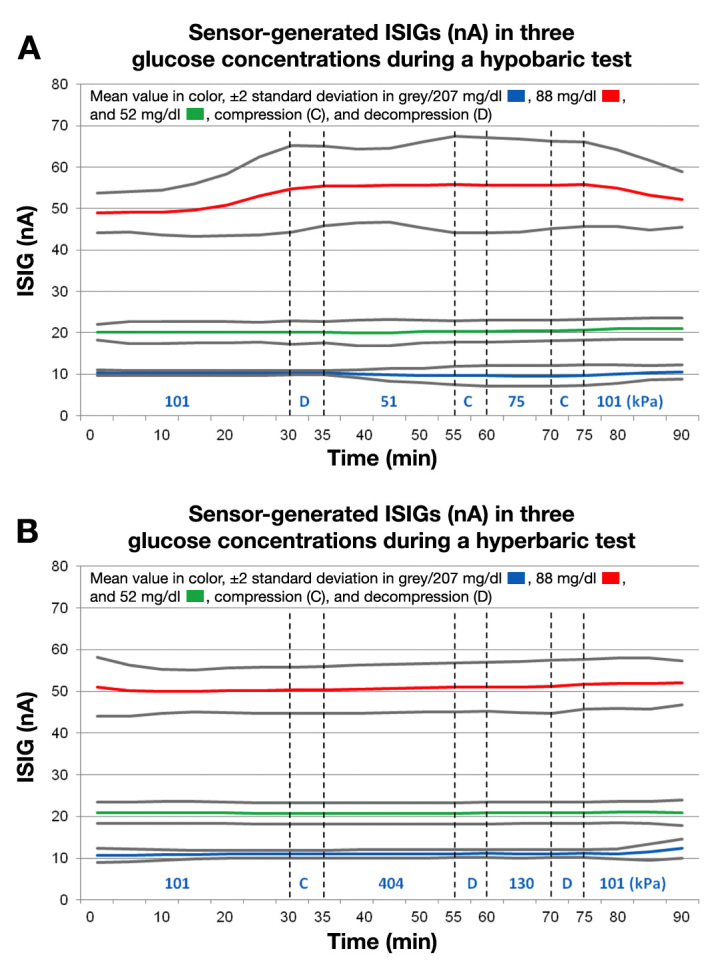
Mean ISIG (nA) values ± 2 standard deviation presented in all three glucose concentrations during the hypobaric (A) and hyperbaric (B) tests
In the medium glucose concentration, no differences were seen in the ISIGs during the hypobaric test.
When comparing the ISIGs in the different glucose concentrations during altered pressure, linearity (R2) of 0.98 was found at sea level (101 kPa), 0.98 at 18,000 ft (51 kPa), 0.97 at 8200 ft (75 kPa), 0.99 at 100-ft depth (404 kPa), and 0.99 at 10-ft depth (130 kPa). The linearities are presented in Figure 3, A–E.
Figure 3.
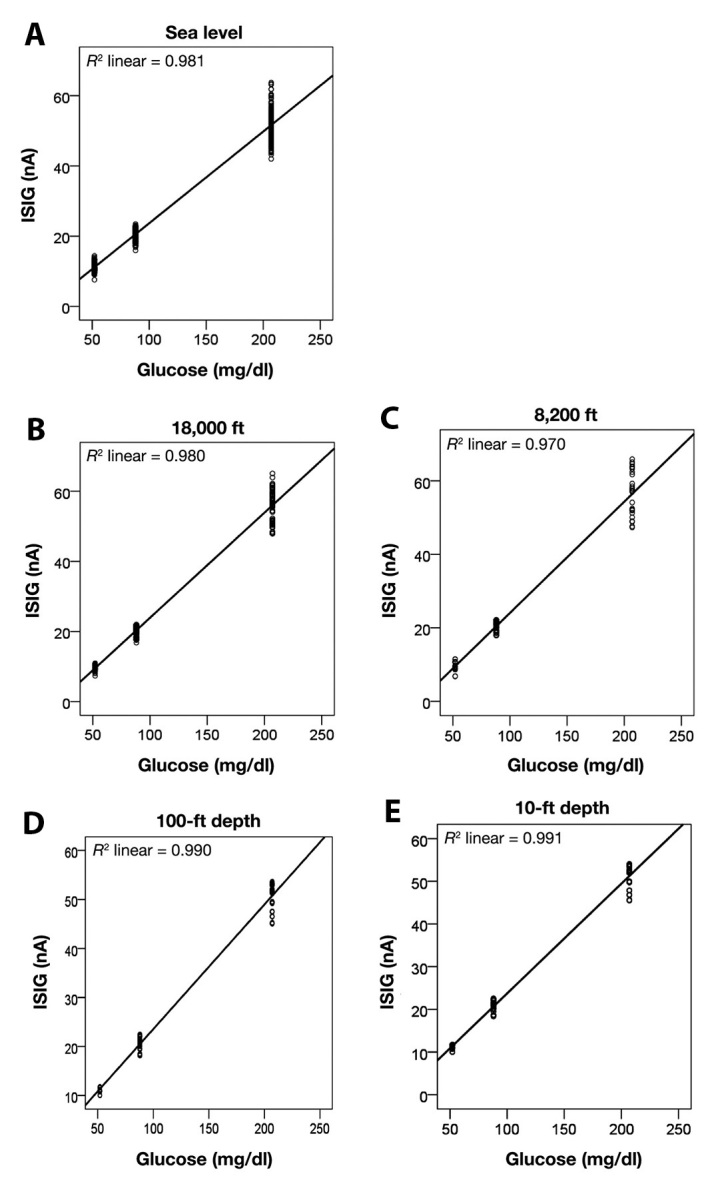
Linearity of ISIG signal vs reference glucose values in different pressure conditions where altitude or depth is expressed in ft
To get a better understanding of the disturbance of the ISIG during exposure to low ambient pressure, Figure 4 A–C presents the ISIGs generated by each sensor in the three glucose concentrations during the hypobaric test.
Figure 4.
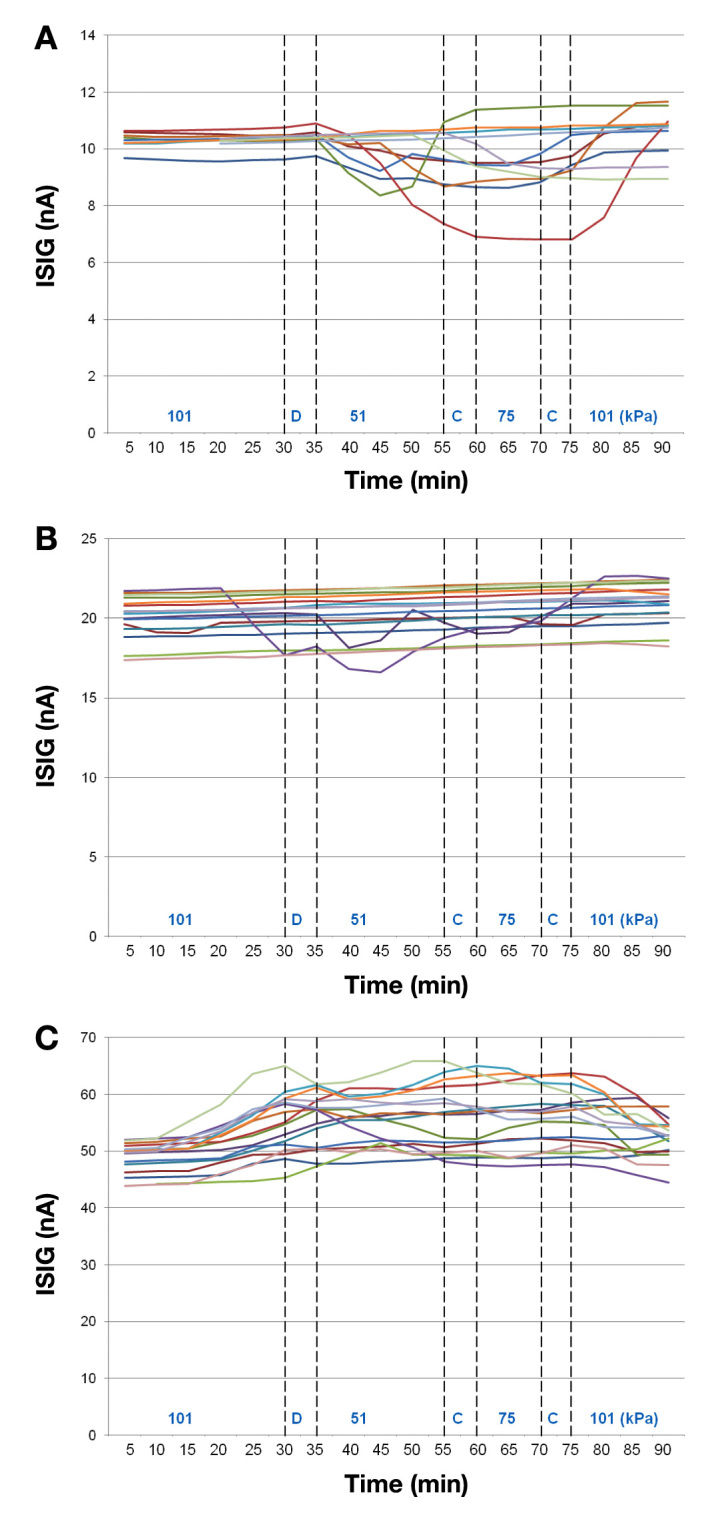
Input signal insulin glucose generated by each sensor in all three glucose concentrations: low/52 mg/dl (A), medium/88 mg/dl (B), and high/207 mg/dl (C) during the hypobaric test
Discussion
In this in vitro study, we demonstrate that Enlite Sensors together with both the retrospective (iPro) and REAL-Time devices provide reliable values during hyperbaric conditions. In hypobaric conditions, significant differences in the ISIGs were seen compared with the ones at sea level.
As with any other individual, those who have diabetes are advised to be physically active in youth15 and also as adults.1 For some individuals, this could mean exposure to different pressure conditions such as when traveling in an airplane, mounting climbing/hiking, skiing at highaltitudes, and scuba diving. Glucose monitoring is performed in some of these conditions, but several studies have shown that some blood glucose meters that use the glucose oxidase enzyme are unreliable at high altitudes.4,15,16 It has also been shown that low temperature affects the accuracy of blood glucose meters that use the enzymes glucose oxidase and glucose dehydrogenase.17 When compared with a reference value and measurements performed at high (25 °C) and low (8 °C) temperatures, some meters were shown to underestimate glucose values while other meters overestimated glucose values.
Sensors for CGM also use the glucose oxidase method. The reliability of the sensor has not been evaluated in varying pressure conditions, but CGM has been shown to be beneficial in association with scuba diving11 and has also showed good sensor function and accuracy during repeated dives.12,13 In these two studies, comparisons were made between repeated blood glucose values and sensor glucose values before and after each dive. The good results from the tests on active divers are supported by the results in this study in which we found good accuracy up to 404 kPa. The darkened display and the fact that the membrane buttons of the Guardian REAL-Time devices make it impossible to operate the unit in altered pressure, did not affect the results of this study but must be corrected if the units are used in future tests involving event marking, calibration, and read-outs from the display.
However, during the hypobaric test, we found significantly lower ISIGs in the lower glucose concentration [52 mg/dl (2.9 mmol/liter)], whereas a significantly higher signal was found in the higher glucose concentration [207 mg/dl (11.3 mmol/liter)]. The reason for this is unclear. Whether this difference is due to the sensor function or the algorithm is not known and merits further investigation. The temperature was kept stable and was therefore not the cause of deviating ISIGs. In fact, the temperature variations in the solutions were smaller in the hypobaric exposures than in the hyperbaric. The lower number of working sensors in one of the beakers (low glucose concentration and first altitude exposure) might be due to fast decompression and cable disconnection. Besides the reduced number of working sensors in the lower glucose concentration during the hypobaric test, a limitation is also that we did not measure PO2 in the glucose solutions. The glucose solution was stirred to maintain a correct glucose concentration and temperature at the sensor area. It is not known whether stirring of the glucose solution could contribute to a more rapid adjustment of the PO2 of the solution to the ambient PO2.
The fact that the hyperbaric condition gave no disturbance to the ISIGs while the hypobaric conditions did could be an indicator to an off-gassing of oxygen from the solution during exposure to 50 kPa air. The possibility that all the solutions should equilibrate to the ambient PO2, 10 kPa, is, however, most unlikely, and even so, this is a PO2 that the sensors should be made for because this is a PO2 that is seen in metabolically active tissue.
In the in vivo situation, the tissue PO2 is —thanks to the large alveolar surface in the lungs and the blood circulation—more rapidly adjusted to the ambient PO2, and when an individual is exposed to 10 kPa of inspiratory oxygen, this rapidly results in a hypoxic level in the tissues. If the chemical reaction in the sensor is sensitive to hypoxic PO2 this could result in faulty signals. More measurements with control of the solution PO2 are needed. However, the results of this in vitro study are in-line with the results we found during an in vivo test in which the accuracy was significantly better in hyperbaric compared with hypobaric conditions.18
A careful look at the individual recordings from sensors reveals two types of behavior. First, ISIGs of some individual sensors seem to deviate from the majority as seen in Figure 4A and Figure 4B, and secondly, a general deviation from true value as seen in Figure 4C. When analyzing the graph, it seems as if deviations do not happen at times when pressure has been changed, although the fact that the signals generally return to predecompression values speaks in favor of a pressure dependence. No changes in solution temperature outside the specified range were observed during the exposure. Because the ISIG current was not recorded directly online but sampled and processed by the logging unit, it might be possible that the mean value is presented on the Excel logs at a slightly different time compared with the real event due to the algorithm of the logging unit. This needs further investigation. Another factor that needs further analysis and experiments is the possible dependence on low oxygen partial pressures.
Although significant differences were found in individual ISIGs during the hypobaric test in this in vitro study, we do not want to draw any definite conclusions because the linearity of the ISIGs vs glucose concentrations in the different pressure conditions was good and the performance in the hyperbaric environment was as expected. However, further studies are necessary to evaluate the advantages and limitations of CGM registration in hyperbaric and especially hypobaric conditions.
Acknowledgments
The authors thank Ken Cooper, an employee of Medtronic Diabetes, for assistance during the study; John B. Welsh, M.D., Ph.D., an employee of Medtronic, Inc., for proofreading; and Kave Abtahi, an employee of Medtronic Sweden, for his assistance during the study.
Glossary
- (CGM)
continuous glucose monitoring
- (CI)
confidence interval
- (ISIG)
input signal for glucose
- (PO2)
partial pressure of oxygen
Funding
A grant was received from the Center of Clinical Research at the County Council of Värmland, Sweden. Medtronic MiniMed, Inc., provided the sensors, recording devices, and display units used in this study.
Disclosures
Raghavendar Gautham is an employee of Medtronic, Inc.
References
- 1.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karon BS, Boyd JC, Klee GG. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin Chem. 2010;56(7):1091–1097. doi: 10.1373/clinchem.2010.145367. Epub 2010 May 28. [DOI] [PubMed] [Google Scholar]
- 3.Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol. 2011;5(6):1621–1622. doi: 10.1177/193229681100500642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink KS, Christensen DB, Ellsworth A. Effect of high altitude on blood glucose meter performance. Diabetes Technol Ther. 2002;4(5):627–635. doi: 10.1089/152091502320798259. [DOI] [PubMed] [Google Scholar]
- 5.Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062–1070. doi: 10.1097/00003246-200105000-00038. [DOI] [PubMed] [Google Scholar]
- 6.Chico A, Vidal-Ríos P, Subirà M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care. 2003;26(4):1153–1157. doi: 10.2337/diacare.26.4.1153. [DOI] [PubMed] [Google Scholar]
- 7.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795–800. doi: 10.2337/dc10-1989. Epub 2011 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, Gutin B, Hergenroeder AC, Must A, Nixon PA, Pivarnik JM, Rowland T, Trost S, Trudeau F. Evidence based physical activity for school-age youth. J Pediatr. 2005;146(6):732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 9.Adolfsson P, Nilsson S, Lindblad B. Continuous glucose monitoring system during physical exercise in adolescents with type 1 diabetes. Acta Paediatr. 2011;100(12):1603–1609. doi: 10.1111/j.1651-2227.2011.02390.x. doi: 10.1111/j.1651-2227.2011.02390.x. Epub 2011 Oct 10. [DOI] [PubMed] [Google Scholar]
- 10.Riddell MC, Milliken J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther. 2011;13(8):819–825. doi: 10.1089/dia.2011.0052. Epub 2011 May 20. [DOI] [PubMed] [Google Scholar]
- 11.Adolfsson P, Ornhagen H, Jendle J. The benefits of continuous glucose monitoring and a glucose monitoring schedule in individuals with type 1 diabetes during recreational diving. J Diabetes Sci Technol. 2008;2(5):778–784. doi: 10.1177/193229680800200505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adolfsson P, Ornhagen H, Jendle J. Accuracy and reliability of continuous glucose monitoring in individuals with type 1 diabetes during recreational diving. Diabetes Technol Ther. 2009;11(8):493–497. doi: 10.1089/dia.2009.0017. [DOI] [PubMed] [Google Scholar]
- 13.Bonomo M, Cairoli R, Verde G, Morelli L, Moreo A, Grottaglie MD, Brambilla MC, Meneghini E, Aghemo P, Corigliano G, Marroni A. Safety of recreational scuba diving in type 1 diabetic patients: the Deep Monitoring programme. Diabetes Metab. 2009;35(2):101–107. doi: 10.1016/j.diabet.2008.08.007. Epub 2009 Feb 28. [DOI] [PubMed] [Google Scholar]
- 14.Arntzen A, Eidsvik S, Risberg J, editors. Norwegian diving- and treat- ment tables: Tables and guidelines for surface orientated diving on air and nitrox. Tables and guidelines for treatment of decompression illness. 3rd ed. Bjørndalstræ, Norway: Barotech AS; 2008. [Google Scholar]
- 15.Pecchio O, Maule S, Migliardi M, Trento M, Veglio M. Effects of exposure at an altitude of 3,000 m on performance of glucose meters. Diabetes Care. 2000;23(1):129–131. doi: 10.2337/diacare.23.1.129a. [DOI] [PubMed] [Google Scholar]
- 16.Moore K, Vizzard N, Coleman C, McMahon J, Hayes R, Thompson CJ. Extreme altitude mountaineering and type 1 diabetes; the Diabetes Federation of Ireland Kilimanjaro Expedition. Diabet Med. 2001;18(9):749–755. doi: 10.1046/j.0742-3071.2001.00568.x. [DOI] [PubMed] [Google Scholar]
- 17.Oberg D, Ostenson CG. Performance of glucose dehydrogenase-and glucose oxidase-based blood glucose meters at high altitude and low temperature. Diabetes Care. 2005;28(5):1261. doi: 10.2337/diacare.28.5.1261. [DOI] [PubMed] [Google Scholar]
- 18.Adolfsson P, Ornhagen H, Eriksson BM, Cooper K, Jendle J. Continuous glucose monitoring-a study of the enlite sensor during hypo- and hyperbaric conditions. Diabetes Technol Ther. 2012;14(6):527–532. doi: 10.1089/dia.2011.0284. Epub 2012 Mar 19. [DOI] [PubMed] [Google Scholar]


