Abstract
Background:
Metabolic syndrome is a major risk factor for coronary artery disease (CAD).
Aim:
Aim of this study was to determine the prevalence of metabolic syndrome in patients with premature myocardial infarction (before 50 years of age).
Methods:
In this case–control study, we compared 98 consecutive patients who were hospitalized in Birjand with acute first myocardial infarction before the age of 50 years and 98 age- and sex-matched healthy controls without a history of coronary artery disease. The case and control groups were categorized according to the National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III) metabolic syndrome criteria [presence of ≥3 of the following: Fasting blood glucose ≥100 mg/dL, triglyceride (TG) level ≥150 mg/dL, low high density lipoprotein (HDL; <40 mg/dL in men and <50 mg/dL in women), blood pressure ≥130/85 mm Hg, and waist circumference >102 cm in men or 88 cm in women]. The data were collected and analyzed by t-test, χ2, and logistic regression in SPSS software 11.5.
Results:
Prevalence of metabolic syndrome was significantly higher in cases than in control group (34.7% in cases, 16.3% in controls, P=0.003). All components of metabolic syndrome except high waist circumstance in the cases group were significantly higher than in control. The most common component of metabolic syndrome was high TG and the least common component was low HDL.
Conclusion:
We conclude that prevalence of metabolic syndrome in patients with premature myocardial infarction is high; high TG is the most common component of metabolic syndrome.
Keywords: Case–control study, metabolic syndrome, premature myocardial infarction
INTRODUCTION
Currently, cardiovascular diseases [coronary artery disease (CAD)] are the most common cause of mortality in the world. Myocardial infarction (MI) is the most common subtype of CAD. In addition, the prevalence of MI is increasing in the developing countries.[1] More importantly, the disease affects young men and women.[2] Most studies show that 4–10% of patients with acute myocardial infarction (AMI) are below 45 years of age.[3]
In recent years, metabolic syndrome (MetS) is introduced as one of the major risk factors for CAD. This syndrome consists of several components including abdominal obesity, hypertension, diabetes mellitus (DM), and dyslipidemia.[4] Each of these components alone raises the risk of CAD, and if several factors are present simultaneously in a person, the risk of cardiac events is significantly increased.
In persons with MetS, the risk of death from CAD is about twofold higher than normal, and their risk of MI and stroke is 3 times than the normal population.[5]
There are several definitions for MetS, but the most commonly used is that of “National Cholesterol Education Program Adult Treatment Panel (NCEP ATP) III.”[6] In a recent article published about the prevalence of MetS in Asia, the rising prevalence of MetS has been emphasized to be due to modernization and urbanization.[7]
Several studies have been conducted concerning the prevalence of MetS and MI.[8–10] The effect of ethnicity on the relationship between premature CAD and MetS has been described,[11] and Asian ethnicity was suggested to be more predisposed to MetS than other ethnicities.[12]
We therefore sought to understand the relationship between MetS and premature CAD among Iranian population in Birjand, the east of Iran.
METHODS
This case–control study was carried out during 2005–2007 in Birjand County, the east of Iran. Cases were 94 young patients aged ≤50 years who were admitted to the coronary care units of Vali-Asr Hospital, with first AMI. AMI was detected based on the presence of at least two of the following criteria:
-
a)
typical chest pain lasting for at least 30 minutes;
-
b)
at least 1 mm ST elevation in two or more contiguous leads, with subsequent evolution of the changes on electrocardiography (ECG); and
-
c)
diagnostic cardiac enzyme changes: doubling of creatine kinase with at least 10% MB fraction.
A neighborhood control was selected for each case. A person in the control group was the first person in the case neighborhood who was matched for age and sex and had not known CAD. Demographic data such as age, sex, known history of DM, hypertension, and dyslipidemia were recorded. A standardized questionnaire was used for data collection.
Blood pressure was measured twice in the supine position from right hand, using a mercury sphygmomanometer. Waist circumference (WC) was measured at the widest diameter between the xiphoid process of the sternum and the iliac crest. Two trained nurses carried out all the measurements using standardized protocols. Serum lipids and blood sugar were measured by taking a sample of 5 mL blood from the right brachial vein after 12 h overnight fasting. The blood samples were sent to central lab of Vali-Asr Hospital.
We calculated the sample size of case and control with “Estimation of Sample Size and Power for Comparing Two Proportions formula” by using the article number 13 (P1 =0.7, P2 =0.5, α=0.05, power=80%).
Definition of MetS was based on ATP III criteria (the presence of any three of the following five abnormalities):[14]
Abdominal obesity: WC in men >102 cm and WC in women >88 cm
Serum triglycerides (TG) ≥150 mg/dL or drug treatment for elevated TG
Serum high-density lipoprotein cholesterol (HDL-C) <40 mg/dL in men and <50 mg/dL in women or drug treatment for low HDL-C
Blood pressure ≥130/85 mmHg or drug treatment for elevated blood pressure (high BP)
Fasting blood sugar (FBS) ≥100 mg/dL or drug treatment for elevated blood glucose (high FBS)
The ethics review committee of Birjand University of Medical Sciences approved this study. All cases and controls signed the informed consent. We used χ2 and t-test, logistic regression for data analysis using SPSS software version 11.5 at P≤0.05. Full description of our methods is given in another article.[15]
RESULTS
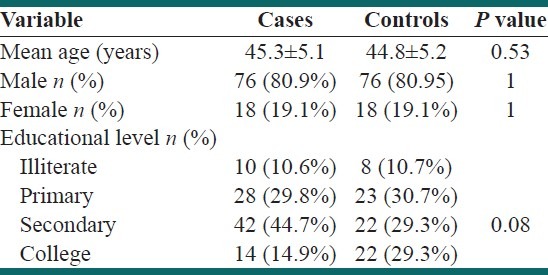
Demographic data are shown in Table 1. Mean age was 45.25±5.09 and 44.8±5.22 years for cases and controls, respectively (P=0.52). Seventy-five subjects (80.9%) in each group were males [Table 1].
Table 1.
Demographic characteristics of our subjects
The prevalence of smoking, dyslipidemia, hypertension, DM, obesity [body mass index (BMI) ≥ 30], and family history of CVD was significantly higher in the cases than in the controls [Table 2].
Table 2.
Comparison of prevalence of cardiac risk factors between cases and controls
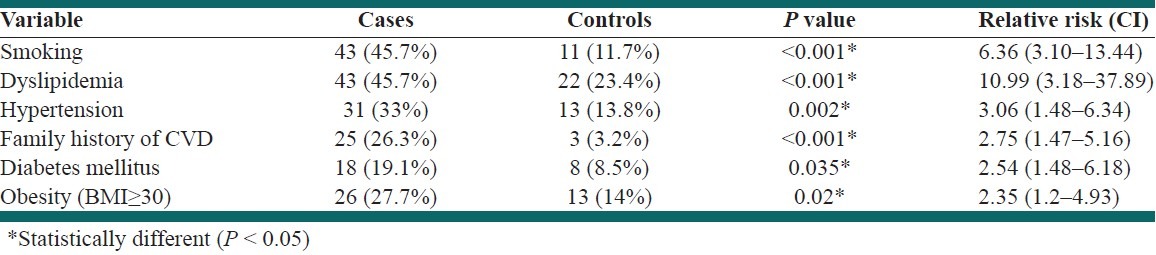
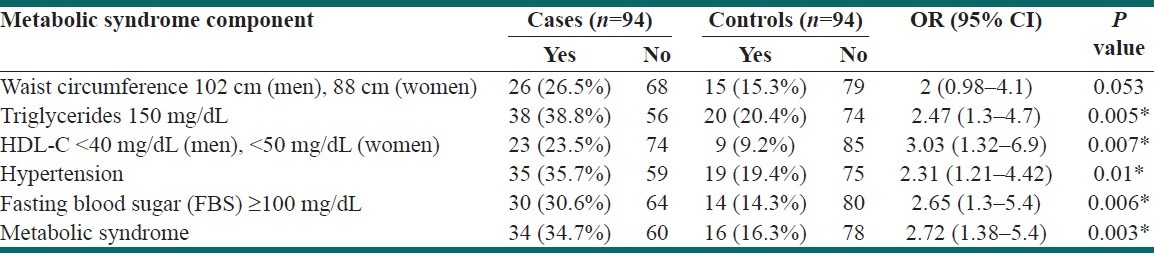
Table 3 shows the effects of various components of MetS on premature MI in our study. Prevalence of MetS in cases was significantly higher than in the control group. The effects of TG ≥150 mg/dL, low HDL, hypertension, and FBS ≥100 mg/dL were significantly higher in cases as well, but no significant difference was found in the effect of WC between the two groups in our study [Table 3].
Table 3.
The effect of various components of metabolic syndrome on premature myocardial infarction
DISCUSSION
The present study assessed the relationship between MetS and premature AMI. We found strong positive association between high TG, hypertension, high FBS, low HDL and premature MI.
Manifestations of AMI in a young adult can be disturbing for the patient and his family. In addition, long-term prognosis of these patients is poor.[16]
MetS with five main risk factors of CAD (insulin resistance, hypertension, abdominal obesity, high TG, and low HDL) undoubtedly causes accelerated atherosclerosis and CAD. In recent decades, the prevalence of MetS in the world (Europe, Asia, and the industrialized countries) is increasing.[5]
Our findings show a strong relationship between the MetS and premature MI.
Different studies have reported different prevalence rates of the MetS in patients with AMI, for example, 46% in Zeller et al.'s study,[8] 41% in Gorter et al.'s study,[17] and 20.8% in Boulon et al.'s report.[18] Chung et al. reported that 47% of patients aged 18–45 years, with AMI, had MetS.[9]
Žaliūnas et al. assessed all five components of MetS in their study. The study was conducted on 2756 patients with acute ischemic syndromes (MI and unstable angina pectoris). MetS was found in 59.5% patients.[19] Prevalence of MetS in this study was higher than that in our study, which is probably due to differences in subjects’ age in the two studies (mean age in our study 45.25±5.09, mean age in Žaliūnas et al.'s study 60.2±11.3 in males and 68.1±9.5 in females).
Other studies have shown that the prevalence of MetS increases with age.[20]
We found a strong positive association between low HDL and premature CVD. It is similar to that reported by Bajaj et al.[21]
Several mechanisms have been proposed for the protective effects of HDL against CAD.
HDL has anti-atherogenic effect due to antioxidative, antithrombotic, and anti-inflammatory effects, although the most important effect of HDL is the reverse cholesterol transport from atherosclerotic plaques to the liver before its excretion out of the body.
Our study showed an association between TG ≥150 mg/dL and premature CAD (OR=2.47).
In Zarich et al.'s study, the most common components of MetS were elevated TG and elevated BP, but the least common was high FBS.[10]
High levels of TG in the blood increase the risk of CAD. Also, high TG is often a sign of other conditions that increase the risk of heart disease as well, including obesity and MetS. Sometimes, hypertriglyceridemia is a sign of poorly controlled diabetes, hypothyroidism, liver or kidney disease. Also, the risk can be partly accounted for by a strong inverse relationship between TG level and HDL-C level.
Our study, however, suggests a positive association between hypertension (HTN) and CAD (OR=2.31).
The most common components of MetS in Žaliūnas et al.'s study in both men and women were high BP and abdominal obesity.[19] Anderson et al. found that the most common components in patients with Ischemic Heart Disease (IHD) were high BP, abdominal obesity, and low HDL-C, while elevated TG and DM were less common.[22]
In our study, abdominal obesity was not associated with increased risk of CVD.
This is inconsistent with many studies. The reason for this difference could be the small sample size in our study.
We have used the NCEP criteria (WC >102 cm in males, >88 cm in females). It is probably by using these criteria that the diagnosis of abdominal obesity is underestimated. Onat et al. compared cardiometabolic risk among Turkish and Iranian adults in their study.[23] They believe that the lower prevalence of MetS in Iranian men is due to usage of a strict criterion for abdominal obesity. They used ≥95 cm cutoff for male abdominal obesity because one-third of Turkish men with high cardiometabolic risk did not fall within the cutoff of 102 cm.[23]
Talaie et al. undertook a study to determine the optimal cut-off points of WC for predicting MetS in an Iranian population (6504 participants from three areas in central Iran were followed over 7 years). They concluded that the international recommendation WC cut-off values for the Middle East are not appropriate to Iran. The best cut-off values that fitted in the Cox regression model were 90/97 cm.[24]
Limitations of the study
Small sample size and inability to remove the effects of some risk factors like positive family history of CAD and cigarette smoking were the limitations of our study.
CONCLUSION
According to our knowledge, this is the first population-based case–control study about MetS prevalence in patients with premature MI in East Iran. Our study showed the high prevalence of MetS among patients with premature AMI. The young population of Iran is required to pay more attention to this issue. In order to to increase public awareness, prevention and treatment of MetS, It can be known as a health problem in Iran.
ACKNOWLEDGMENT
We wish to sincerely thank the Deputy of Research and Technology of Birjand University of Medical Sciences for providing financial support for this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kazemi T, Sharifzadeh Gh R. Changes in risk factors, medical careand rate ofacute myocardial infarction in Birjand (1994-2003) ARYA Journal. 2006;1:271–4. [Google Scholar]
- 2.Sharma M, Ganguly NK. Premature coronary artery disease in Indians and its associated risk factors. Vasc Health Risk Manag. 2005;1:217–25. [PMC free article] [PubMed] [Google Scholar]
- 3.Chan MY, Woo KS, Wong HB, Chia BL, Sutandar A, Tan HC. Antecedent risk factors and their control in young patients with a first myocardial infarction. Singapore Med J. 2006;47:27–30. [PubMed] [Google Scholar]
- 4.Gogia A, Agarwal PK. Metabolic syndrome. Indian J Med Sci. 2006;60:72–81. [PubMed] [Google Scholar]
- 5.Cannon CP. Mixed dyslipidemia, metabolic syndrome, diabetes mellitus, and cardiovascular disease: Clinical implications. Am J Cardiol. 2008;102:5L–9L. doi: 10.1016/j.amjcard.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Sadeghi M. The metabolic syndrome. ARYA Journal. 2006;2:1–2. [Google Scholar]
- 7.Lao XQ, Zhang YH, Wong MC, Xu YJ, Xu HF, Nie SP, et al. The prevalence of metabolic syndrome and cardiovascular risk factors in adults in southern China. BMC Public Health. 2012;12:64. doi: 10.1186/1471-2458-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeller M, Steg PG, Ravisy J, Laurent Y, Janin-Manificat L, L’Huillier I, et al. Prevalence and impact of metabolic syndrome on hospital outcomes in acute myocardial infarction. Arch Intern Med. 2005;165:1192–8. doi: 10.1001/archinte.165.10.1192. [DOI] [PubMed] [Google Scholar]
- 9.Chung EH, Curran PJ, Sivasankaran S, Chauhan MS, Gossman DE, Pyne CT, et al. Prevalence of metabolic syndrome in patients < or=45 years of age with acute myocardial nfarction having percutaneous coronary intervention. Am J Cardiol. 2007;100:1052–5. doi: 10.1016/j.amjcard.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Kazemi T, Sharifzadeh GR. Changes in risk factors, medical care and rate of acute myocardial infarction in Birjand (1994?2003) ARYA J. 2006;1:271–4. [Google Scholar]
- 11.Amin AP, Nathan S, Evans AT, Attanasio S, Mukhopadhyay E, Mehta V, et al. The effect of ethnicity on the relationship between premature coronary artery disease and traditional cardiac risk factors among uninsured young adults. Prev Cardiol. 2009;12:128–35. doi: 10.1111/j.1751-7141.2009.00025.x. Summer. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part II: Variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–64. doi: 10.1161/hc4701.099488. [DOI] [PubMed] [Google Scholar]
- 13.Ismail J, Jafar TH, Jafary FH, White F, Faruqui AM, Chaturvedi N. Risk factors for non-fatal myocardial infarction in young South Asian adults. Heart. 2004;90:259–63. doi: 10.1136/hrt.2003.013631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Executive Summary of The Third Report of The National holesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol In Adults (Adult Treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Kazemi T, Sharifzadeh GR, Zarban A, Fesharakinia A, Rezvani MR, Moezy SA. Risk factors for premature myocardial infarction: a matched case-control study. J Res Health Sci. 2011;11:77–82. [PubMed] [Google Scholar]
- 16.Christus T, Shukkur AM, Rashdan I, Koshy T, Alanbaei M, Zubaid M, et al. Coronary artery disease in patients aged 35 or less: A different beast? Heart Views. 2011;12:7–11. doi: 10.4103/1995-705X.81550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorter PM, Olijhoek JK, van der Graaf Y, Algra A, Rabelink TJ, Visseren FL. Prevalence of metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm. Atherosclerosis. 2004;173:363–9. doi: 10.1016/j.atherosclerosis.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Boulon C, Lafitte M, Richeboeuf V, Paviot B, Pradeau V, Coste P, et al. Prevalence of metabolic syndrome after acutecoronary syndrome and its prognostic significance. Am J Cardiol. 2006;98:1429–34. doi: 10.1016/j.amjcard.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Zaliūnas R, Slapikas R, Babarskiene R, Slapikiene B, Luksiene D, Milvidaite I, et al. The prevalence of the metabolic syndrome components and their combinations in men and women with acute ischemic syndromes. Medicina (Kaunas) 2008;44:521–8. [PubMed] [Google Scholar]
- 20.Sharifi F, Mousavinasab SN, Saeini M, Dinmohammadi M. Prevalence of metabolic syndrome in an adult urban population of the west of Iran. Exp Diabetes Res. 2009;2009:136501. doi: 10.1155/2009/136501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajaj S, Shamoon F, Gupta N, Parikh R, Parikh N, Debari VA, et al. Acute ST-segment elevation myocardial infarction in young adults: Who is at risk? Coron Artery Dis. 2011;22:238–44. doi: 10.1097/MCA.0b013e3283452e7f. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JL, Horne BD, Jones HU, Reyna SP, Carlquist JF, Bair TL, et al. Intermountain Heart Collaborative (IHC) Study. Which features of the metabolic syndrome predict the prevalence and clinical outcomes of angiographic coronary artery disease? Cardiology. 2004;101:185–93. doi: 10.1159/000076695. [DOI] [PubMed] [Google Scholar]
- 23.Onat A. Dynamics in cardiometabolic risk among Turkish adults: Similarities to that in Iranians? Int J Prev Med. 2011;2:56–63. [PMC free article] [PubMed] [Google Scholar]
- 24.Talaei M, Thomas GN, Marshall T, Sadeghi M, Iranipour R, Oveisgharan S, et al. Appropriate cut-off values of waist circumference to predict cardiovascular outcomes: 7-year follow-up in an Iranian population. Intern Med. 2012;51:139–46. doi: 10.2169/internalmedicine.51.6132. [DOI] [PubMed] [Google Scholar]


