Abstract
Background:
There is scarce epidemiological data on early and asymptomatic stages of chronic kidney disease (CKD) in children, especially from developing countries. In this study, we investigated the frequency of CKD stages 3-5 among general students of Isfahan (a large province of Iran), and compared the findings with those derived from the main pediatric nephrology referral center of province.
Methods:
This study was performed among 712 Isfahani school students (377 boys) aged 7-18 years, as part of the baseline survey of a national surveillance system. Blood samples were analyzed for blood urea nitrogen, creatinine, and cystatin C. Glomerular filtration rate (GFR) was calculated based on two 2009 Schwartz equations (the “updated” and the “new” equations). CKD was defined as GFR <60 ml/min/1.73 m2. Additionally, a retrospective analysis of clinical records of children with stages 3-5 CKD referred to main referral center of province from November 2001 to December 2011 was made.
Results:
The mean age of students was 12.2 ± 2.4 years. In students’ screening, the frequency of CKD was 1.3% and 1.7% based on the updated Schwartz and the new Schwartz equation, respectively. The referral center survey revealed an annual incidence of 14.5 per million age-related population (pmarp), and a prevalence of 118.8 pmarp in our province.
Conclusion:
The prevalence of asymptomatic and undetected low GFR in Iranian children is higher than what is reflected from the reports of referral centers. Simple screening programs like annual urinalysis among high-risk school students should be considered.
Keywords: Children, chronic kidney disease, cystatin C, epidemiology, Iran
INTRODUCTION
The epidemiological information on chronic kidney disease (CKD) in children is almost confined to reports derived from major tertiary care referral centers. Due to the nature of such studies, the existing epidemiological data is limited to incidence and prevalence of the terminal stages of CKD and end stage renal disease (ESRD). It has been suggested that patients with ESRD reflect only the “tip of the iceberg” of the total number of the patients with varying degrees of CKD.[1,2] At the present time, data about non-terminal stages of the disease is scarce. The scarcity of epidemiological information is even more pronounced in developing countries.[1,3] Knowledge through the epidemiology of varying stages of CKD is important for implementing effective measurements for slowing the progression of the disease in at risk individuals and allocating enough health care resources for facilitating access to renal replacement modalities. These aspects are of especial concern in low-income and middle-income countries, where the clinical and socioeconomic consequences of the disease are expected to be higher.[4,5]
Glomerular filtration rate (GFR) is generally considered the best overall indicator of kidney function.[6] Serum creatinine is the most commonly used endogenous marker for estimating GFR worldwide. In recent years, a novel endogenous marker of renal function, cystatin C, has emerged as a more sensitive marker than serum creatinine in estimating GFR.[7,8] Many equations have been proposed to estimate GFR in children based on concentration of serum creatinine and/or cystatin C. At present time, two 2009 Schwartz equations (the “updated” equation and the “new” equation) are the most popular formula for estimating GFR in children.[9] Recent studies have indicated the validity of Schwartz equations in estimating GFR in mild to severe stages of CKD.[10–13]
In this study, which to the best of our knowledge is the first of its kind, we investigated the frequency of CKD stages 3-5 among a large number of general students living in Isfahan, a large province of Iran, by estimating their GFRs using two 2009 Schwartz equations. Moreover, we investigated the medical records of the main pediatric nephrology referral center of Isfahan province for all stages 3-5 CKD patients during a ten year period. Finally, we compared the prevalence of stages 3-5 CKD in our general population with the prevalence of these stages derived from the referral center survey.
METHODS
Study area
This cross-sectional study was conducted in Isfahan, a large province located in central part of Iran. According to Iranian national census performed in 2011, the population of Isfahan was about 4,879,312 inhabitants, of whom 1,640,544 individuals were children below 18 years of age.
Population-based screening
The data used for students screening was obtained from a national surveillance program entitled “Childhood and Adolescence Surveillance and Prevent Ion of Adult Non-communicable disease” (CASPIAN study). The third phase of this nationwide school-based health survey was conducted between 2009 and 2010 among 5028 Iranian students aged 7-18 years who were selected by multistage random cluster sampling from urban and rural areas of 27 provinces of Iran. In the present paper, we describe the findings of 712 school students from Isfahan province. A detailed description about the procedure of data gathering and sample collection of CASPIAN studies has been characterized elsewhere.[14] In brief, after complete explanation of the study objectives and protocol for the students and their parents, a team of trained nurses collected demographic and clinical features of the eligible subjects, including age, sex, height, weight, and blood pressure. Blood samples were drawn from participants and transferred to Isfahan central provincial laboratory, where biochemical measurements were done. Written informed consent was obtained from parents/caregivers, and oral assent from the students before enrollment in the study. The study was approved by institutional review boards and the Research & Ethics Committee of the Faculty of Medicine, Isfahan University of Medical Sciences.
Laboratory analysis and GFR estimation
Serum creatinine and blood urea nitrogen (BUN) were measured by enzymatic methods on a Hitachi 917 auto-analyzer. Serum cystatin C was measured by the particle-enhanced immunoturbidimetric method (Dako, Glostrup, Denmark).[15] A cystatin C level of lower than 1.38 mg/l is considered as normal in general population.[16]
To calculate GFR for each individual, we applied the following equations:
The “updated” 2009 Schwartz equation:[9]
GFR(ml/ min/1.732)=0.413×Height(cm)/Serum Creatinine(mg/dl)
And the “new” 2009 Schwartz equation:[9]

According to the estimated GFR by each equation, participants were categorized as the CKD group (defined as GFR <60 ml/min/1.73 m2) or non-CKD group (defined as GFR >60 ml/min/1.73 m2). Those children with GFR lower than 60 ml/min/1.73 m2 were further subcategorized to CKD stages 3-5 based on the CKD classification described by kidney disease outcomes quality initiative (K/DOQI) guidelines.[17]
Retrospective data gathering
In addition to the population screening, we retrospectively investigated the medical records of all stages 3-5 CKD children (less than 18 years of age) who were referred to pediatric nephrology ward of St. Azahra Hospital, Isfahan, Iran from November 2001 until December 2011. St. Azahra Hospital is the main referral center for this specialty in Isfahan and has sought to register almost all CKD and ESRD children from all health caring sectors of the whole province. The inclusion criteria for case selection were: (i) Definite diagnosis of CKD defined as GFR <60 ml/min/1.73 m2 for at least 3 months (based on original Schwartz formula),[18] (ii) age at the onset of CKD below 18 years, and (iii) residing within the study area. The mean annual incidences rate of CKD was calculated as the incidence per million of the age-related population (pmarp). The prevalence rate was considered as the point prevalence on 31 December 2011, pmarp. The estimated number of population at risk was obtained from the 2011 Iranian National Census and consisted individuals who were below 18 years of age and residing within Isfahan province of Iran.
Statistical analysis
The Wilcoxon test was applied to examine the differences in estimated GFR between the equations. Agreement between equations in defining individuals as CKD or non-CKD was evaluated by weighted kappa statistics. We used Chi-square test to compare the prevalence of CKD obtained from population screening with the prevalence of CKD derived from the hospital-based survey. Statistical analyses were performed using MedCalc version 12.1.4.0 (MedCalc Software, Mariakerke, Belgium) and a P < 0.05 was considered the significance threshold.
RESULTS
Population-based screening
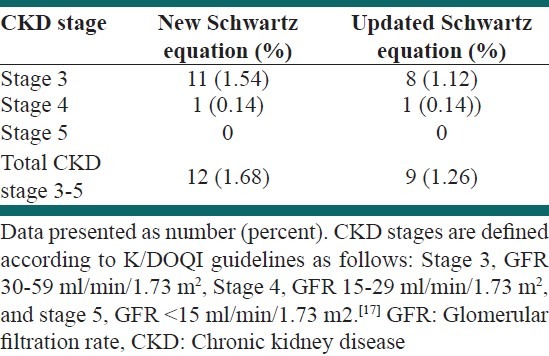
Of 712 students, 377 (52.9%) were male. The mean age was 12.2 ± 2.4 years (range 7-18), and the mean body mass index was 18.2 ± 3.8 kg/m2. Detailed demographic and clinical characteristics of the study population are presented in Table 1. Mean GFR was 99.65 ± 19.71 ml/min/1.73 m2 by new Schwartz equation and 99.66 ± 19.74 ml/min/1.73 m2 by updated Schwartz equation. The Wilcoxon test revealed no significant differences between these equations in estimating GFR (P = 0.22). The frequency of CKD was 1.7% and 1.3%, based on the new Schwartz and the updated Schwartz equations, respectively. The weighted kappa statistics revealed a very good agreement between two equations in defining CKD and non-CKD individuals (κ = 0.85; 95% confidence interval [CI] 0.69-1). Table 2 presents the prevalence of CKD stages 3-5 based on each equation.
Table 1.
Demographic and biochemical characteristics of participants
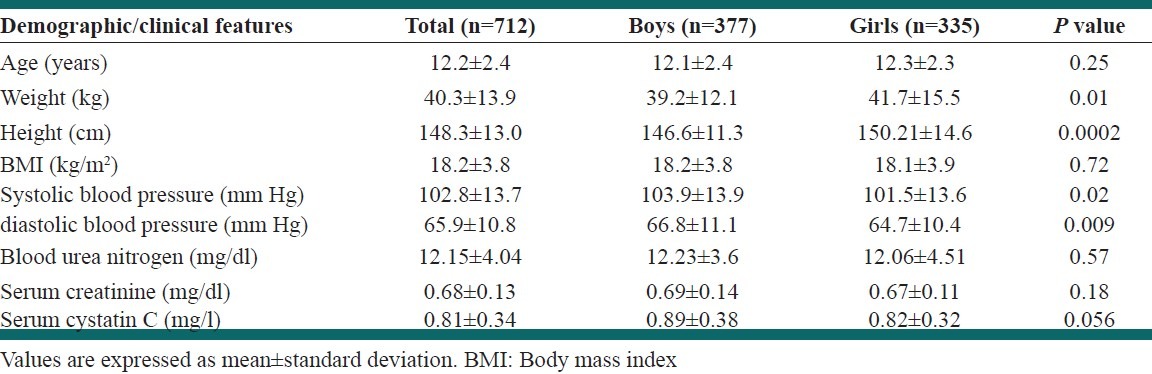
Table 2.
Prevalence of chronic kidney disease stages 3-5 in population-based screening according to each equation
Referral center survey
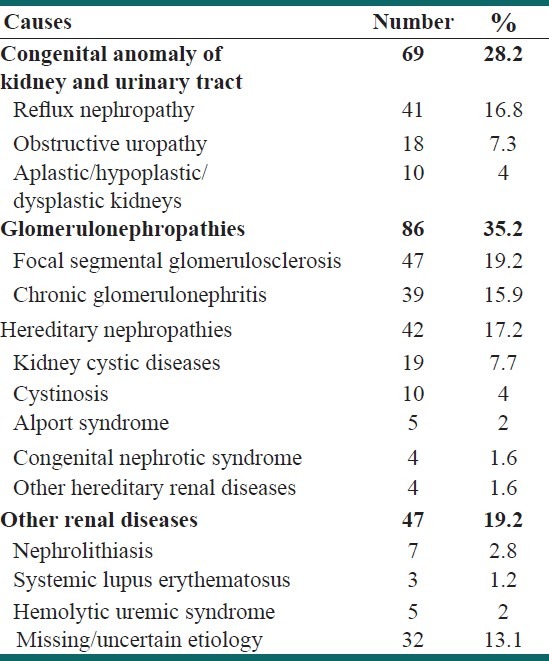
In our inspections throughout the medical records in our referral center, we could identify 244 children with CKD diagnosis who were eligible for this study. Table 3 summarizes the clinical and paraclinical features of these cases. These patients were consisted of 132 (54%) boys and 112 (46%) girls. The mean age was 11.0 ± 5.3 years (range 0-18). Glomerulonephropathies (35.2%) and congenital urological malformations (28.2%) were the most frequent primary cause of CKD in these patients. Table 4 summarizes the primary etiology of CKD among the patients. Renal replacement therapies (hemodialysis, peritoneal dialysis, and kidney transplantation) were applied for 72.3% of the children. Nearly 36% of patients had received kidney allograft, mostly from non-relative living donors (75.3%). By the end of December 2011, 49 patients (18.3%) were expired. The annual incidence of CKD was 14.5 pmarp, and the point prevalence on 31 December 2011 was 118.8 pmarp. The prevalence of stages 3-5 CKD in population screening was significantly higher that the prevalence of these stages obtained from referral center survey (P < 0.001).
Table 3.
Demographic and biochemical characteristics of chronic kidney disease individuals in referral center survey
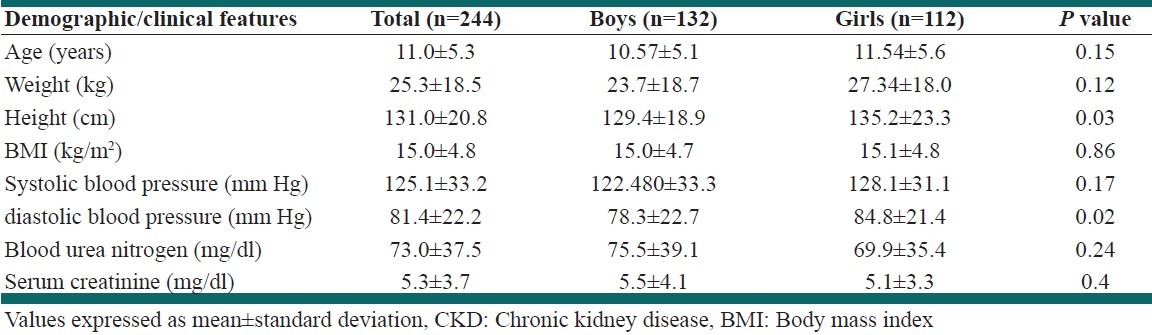
Table 4.
Primary renal causes of chronic kidney diseases in referral-center survey
DISCUSSION
This is the first study in Iran that investigated the prevalence of early stages of CKD in a general pediatric population by using cystatin C as a new endogenous marker of kidney function. Up to our knowledge, only one previous report on epidemiology of pediatric CKD in Iran exists that is limited to causes and outcome of children with ESRD[19]. No data was available about the figure of earlier and asymptomatic stages of CKD among Iranian children. The use of cystatin C as a more sensitive marker of renal function for screening reduced GFR was the strength of the present study. It is almost well-established that the concentration of serum creatinine is affected by age, sex, muscle mass and protein intake, which can result in an inaccurate GFR estimation.[20,21] On the contrary, cystatin C is independent of the mentioned factors and therefore, is a superior marker than creatinine in estimating GFR.[7,8,22] We used two 2009 Schwartz equations for estimating GFR in CKD screening. These equations are more validated than other existing GFR equations in pediatrics.[10–12] Both Schwartz equations showed a very good agreement in defining GFR <60 ml/min/1.73 m2, though, the new Schwartz equation revealed slightly a higher prevalence of CKD stages 3-5 than the updated formula (1.7% and 1.3%, respectively). The new Schwartz equation considers more demographic and biochemical variables for estimating GFR and is assumed to be more accurate in detecting true CKD individuals.[13]
In our study, the prevalence of CKD stages 3-5 obtained in population-based screening was considerably higher than the prevalence of these stages derived from the referral center-based survey. Due to the latent nature of CKD in its earlier stages, a significant proportion of patients with reduced renal function do not seek for medical care until the disease is progressed and symptoms have become much advanced. Neglecting such undetected patients with earlier stages of the disease is the main limitation of referral center-based surveys, and results in underestimating the true figure of the epidemiology of CKD stages in the population.[3] In a similar work in the adult American population, patients with earlier stages of disease exceeded those reaching ESRD by as much as 50 times.[2] In a screening survey for CKD among healthy high-school students of Kinshasa, Democratic Republic of Congo, the overall prevalence of CKD stages 3-5 was ranging from 1.5% to 2.9% depending on the method used for estimating GFR.[23] Another similar CKD screening study among Mexican children revealed a 1.7% prevalence of GFR <60 ml/min/1.73 m2.[24] These rates are much higher than the overall prevalence of CKD reports from major pediatric nephrology referral centers around the globe;[3] and show a considerably higher prevalence of undetected asymptomatic CKD in general population than what is reflected from the results of tertiary and referral centers.
The reported incidence and prevalence of CKD from hospital-based studies around the world vary with respect to ethnicities, disease definition, method of use for GFR estimation and also cultural and social features of different regions. A large prospective study in Italy, ItalKid project, revealed an incidence of 12.1 pmarp and a prevalence of 74.7 pmarp.[25] A nearly similar figure was observed in Spain with the overall incidence of 8.66 pmarp and prevalence of 71.06 pmarp.[26] An incidence of 14.3 pmarp and a prevalence of 96.1 pmarp have been reported in Serbian children.[27] Based on the results of our referral center, the incidence (14.5 pmarp) and prevalence (118.8 pmarp) of CKD was higher than the reports from Western countries, but was lower than our neighboring country, Kuwait, where the prevalence of CKD (GFR <50 ml/min/1.73 m2) in children 0-15 years was 329 pmarp.[28] The higher prevalence of childhood CKD in our region can be attributed to the high rate of consanguineous marriage that is culturally acceptable in the Middle-East.[29] Besides, some predisposing genetic risk factors may also exist in this ethnic group.[28]
The economic and clinical burden of CKD is a significant challenge for healthcare systems worldwide. The disease is accompanied by lifelong disability and imposes enormous treatment costs to the patients’ families. The socio-economic effects of the disease are greater in low and middle-income countries compared with the developed world. Renal replacement therapies are prohibitively expensive and hardly accessible in countries with limited resources. Most patients cannot afford the financial burden of the disease and withdraw treatments. In India, treatment withdrawal was the cause of death in 70% of ESRD patients.[30] Iran, as a model of a developing country, endures a tremendous burden of CKD.[31] Although great efforts have been done to make all renal replacement modalities available for all Iranian ESRD patients, these modalities are still not sufficiently available in all regions of the country.[4] It is to be noted that most of these facilities are provided for adult CKD patients, and resources for pediatric CKD are much more limited.
Given the high burden of CKD in source-limited countries, the best overall policy for health authorities can be reducing the number of affected individuals by detecting and treating kidney diseases at their early stages.[4,5] Easily implemented CKD screening programs, like an annual urinalysis for detection of hidden hematuria/proteinuria among school students can be considered. In eastern Asian countries, including Korea and Japan, all school students are mandated to have an annual urinalysis as a screening test. In Korea, a urine sample is drawn from each student, and is analyzed by a simple dipstick method for detection of proteinuria, hematuria and glucose. The suspected individuals are then referred to a pediatric nephrologist for further assessments.[32] Studies from those regions have demonstrated that such urinary screening programs were successful in detecting CKD in its early stages.[32–35] In Iran, despite the relatively high prevalence of childhood CKD, we do not have an organized CKD screening program in children. The experience of eastern Asian countries seems to be helpful for our country. However, more studies are needed to evaluate the cost-effectiveness of such programs.
The limitation of this study was using only one measurement of endogenous markers for assessing renal function for population screening. The definition of CKD stages 3-5 requires persistent GFR <60 ml/min/1.73 m2 over a period of at least 3 months.[17] However, it is cumbersome to obtain serial GFRs in such a large population with healthy renal function. The lack of serial GFR is a common limitation present in other similar studies.[23,24]
CONCLUSION
The prevalence of pediatric CKD in Iran is higher than Western countries. To reduce the enormous costs and lifelong complications of ESRD, a simple screening program using annual urinalysis and/or blood examination among high-risk children should be considered.
Footnotes
Source of Support: This work was conducted as a sub-study of a national study, and was supported by a grant from the Vice Chancellery for Research and Technology, Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Warady BA, Chadha V. Chronic kidney disease in children: The global perspective. Pediatr Nephrol. 2007;22:1999–2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 3.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–73. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahdavi-Mazdeh M. Why do we need chronic kidney disease screening and which way to go? Iran J Kidney Dis. 2010;4:275–81. [PubMed] [Google Scholar]
- 5.Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–9. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function – Measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 7.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 8.Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children – A meta-analysis. Clin Biochem. 2007;40:383–91. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staples A, LeBlond R, Watkins S, Wong C, Brandt J. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol. 2010;25:2321–6. doi: 10.1007/s00467-010-1598-7. [DOI] [PubMed] [Google Scholar]
- 11.Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L. Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol. 2011;6:552–60. doi: 10.2215/CJN.04180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmon WE. Glomerular filtration rate in children with chronic kidney disease. Clin Chem. 2009;55:400–1. doi: 10.1373/clinchem.2008.123067. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–43. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 14.Kelishadi R, Heshmat R, Motlagh ME, Majdzadeh R, Keramatian K, Qorbani M, et al. Methodology and Early Findings of the Third Survey of CASPIAN Study: A National School-based Surveillance of Students’ High Risk Behaviors. Int J Prev Med. 2012;3:394–401. [PMC free article] [PubMed] [Google Scholar]
- 15.Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindström V, et al. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994;40:1921–6. [PubMed] [Google Scholar]
- 16.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J. Reference values for cystatin C serum concentrations in children. Pediatr Nephrol. 1998;12:125–9. doi: 10.1007/s004670050419. [DOI] [PubMed] [Google Scholar]
- 17.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 18.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–63. [PubMed] [Google Scholar]
- 19.Madani K, Otoukesh H, Rastegar A, Van Why S. Chronic renal failure in Iranian children. Pediatr Nephrol. 2001;16:140–4. doi: 10.1007/s004670000522. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–90. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 21.Uzun H, Ozmen Keles M, Ataman R, Aydin S, Kalender B, Uslu E, et al. Serum cystatin C level as a potentially good marker for impaired kidney function. Clin Biochem. 2005;38:792–8. doi: 10.1016/j.clinbiochem.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Serum cystatin C as an endogenous marker of renal function in patients with mild to moderate impairment of kidney function. Nephrol Dial Transplant. 2006;21:1855–62. doi: 10.1093/ndt/gfl073. [DOI] [PubMed] [Google Scholar]
- 23.Bukabau JB, Makulo JR, Pakasa NM, Cohen EP, Lepira FB, Kayembe PK, et al. Chronic kidney disease among high school students of Kinshasa. BMC Nephrol. 2012;13:24. doi: 10.1186/1471-2369-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshy SM, Garcia-Garcia G, Pamplona JS, Renoirte-Lopez K, Perez-Cortes G, Gutierrez ML, et al. Screening for kidney disease in children on World Kidney Day in Jalisco, Mexico. Pediatr Nephrol. 2009;24:1219–25. doi: 10.1007/s00467-009-1136-7. [DOI] [PubMed] [Google Scholar]
- 25.Ardissino G, Daccò V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, et al. Epidemiology of chronic renal failure in children: Data from the ItalKid project. Pediatrics. 2003;111:e382–7. doi: 10.1542/peds.111.4.e382. [DOI] [PubMed] [Google Scholar]
- 26.Areses Trapote R, Sanahuja Ibanez MJ, Navarro M. Investigadores Centros Participantes en el REPIR II. Epidemiology of chronic kidney disease in Spanish pediatric population. REPIR II Project. Nefrologia. 2010;30:508–17. doi: 10.3265/Nefrologia.pre2010.May.10402. [DOI] [PubMed] [Google Scholar]
- 27.Peco-Antic A, Bogdanovic R, Paripovic D, Paripovic A, Kocev N, Golubovic E, et al. Epidemiology of chronic kidney disease in children in Serbia. Nephrol Dial Transplant. 2012;27:1978–84. doi: 10.1093/ndt/gfr556. [DOI] [PubMed] [Google Scholar]
- 28.Al-Eisa A, Naseef M, Al-Hamad N, Pinto R, Al-Shimeri N, Tahmaz M. Chronic renal failure in Kuwaiti children: An eight-year experience. Pediatr Nephrol. 2005;20:1781–5. doi: 10.1007/s00467-005-2000-z. [DOI] [PubMed] [Google Scholar]
- 29.Saadat M, Ansari-Lari M, Farhud DD. Consanguineous marriage in Iran. Ann Hum Biol. 2004;31:263–9. doi: 10.1080/03014460310001652211. [DOI] [PubMed] [Google Scholar]
- 30.Barsoum RS. Overview: End-stage renal disease in the developing world. Artif Organs. 2002;26:737–46. doi: 10.1046/j.1525-1594.2002.07061.x. [DOI] [PubMed] [Google Scholar]
- 31.Nafar M, Mousavi SM, Mahdavi-Mazdeh M, Pour-Reza-Gholi F, Firoozan A, Einollahi B, et al. Burden of chronic kidney disease in Iran: A screening program is of essential need. Iran J Kidney Dis. 2008;2:183–92. [PubMed] [Google Scholar]
- 32.Murakami M, Yamamoto H, Ueda Y, Murakami K, Yamauchi K. Urinary screening of elementary and junior high-school children over a 13-year period in Tokyo. Pediatr Nephrol. 1991;5:50–3. doi: 10.1007/BF00852844. [DOI] [PubMed] [Google Scholar]
- 33.Cho BS, Kim SD. School urinalysis screening in Korea. Nephrology (Carlton) 2007;12:S3–7. doi: 10.1111/j.1440-1797.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 34.Cho BS, Kim SD, Choi YM, Kang HH. School urinalysis screening in Korea: Prevalence of chronic renal disease. Pediatr Nephrol. 2001;16:1126–8. doi: 10.1007/s004670100043. [DOI] [PubMed] [Google Scholar]
- 35.Yap HK, Quek CM, Shen Q, Joshi V, Chia KS. Role of urinary screening programmes in children in the prevention of chronic kidney disease. Ann Acad Med Singapore. 2005;34:3–7. [PubMed] [Google Scholar]


