Abstract
To address possible effects of heat shock protein 70 (Hsp70) on energy metabolism, we established a cell line expressing different levels of Hsp70 and evaluated changes in glucose and lactate metabolites, as well as ATP levels accordingly. In addition, activities of enzymes involved in glycolysis [phosphofructokinase (PFK) and lactate dehydrogenase (LDH)], Krebs cycle [citric synthase (CS)], and oxidative phosphorylation {NADH dehydrogenase [complex I (CI)] and ubiquinol:cytochrome-c reductase [complex III (CIII)]} were analyzed. The results show that both glucose consumption and lactate excretion were elevated significantly in cells expressing increased levels of Hsp70. Simultaneously, the activities of glycolytic enzymes PFK and LDH were increased markedly in cells overexpressing Hsp70. Activities of enzymes CI and CIII, both involved in oxidative phosphorylation, decreased upon increased expression of Hsp70. These findings were supported by nonsignificant reductions of CS activities in cells that overexpressed Hsp70, whereas intracellular ATP levels remained constant over a wide range of Hsp70 expression. In conclusion, overexpression of Hsp70 in HeLa cells results in downregulation of oxidative phosphorylation, in particular, multiprotein CIII, the main source of reactive oxygen species. In exchange, upregulation of the glycolytic pathway compensates for the homeostasis of cellular ATP supply.
Keywords: glycolysis, ROS, adaptation to heat stress, HeLa cells
during cellular evolution, eukaryotic cells have developed mechanisms to cope with environmental challenges. The expression of heat shock proteins (Hsps) is one of the essential stress reactions of cells. The 70-kD protein, Hsp70, with its indispensable role in maintaining cellular homeostasis, is the most prominent molecular chaperone and has been investigated extensively among the Hsp family (54). Its sequence homology between prokaryotic version DnaK and human is ∼60%, expressing the remarkable conservation of this protein in such a highly diverse organism (41).
A variety of environmental and cellular stress conditions are able to induce the Hsp70 response, including bacterial infections (17), isolation stress (46), immunotoxic effects (8, 31), DNA damaging through oxidative stress (23), noise overstimulation (18), or the classically defined stress response to hyperthermia (56). The characteristic role of Hsp70 is to prevent cell death by interfering with the ability of cytochrome c and apoptotic protease-activating factor 1 to recruit procaspase-9, which leads to suppression of apoptosis by blocking the assembly of a functional apoptosome (3). Many of these stress triggers have an impact on energy metabolism caused by ATP-consuming protein-protein interactions of Hsp70 serving as a molecular chaperone (48). It is performed by two functional domains of Hsp70: an ATPase domain (nucleotide-binding domain) and a substrate-binding domain, able to interact with an extended polypeptide chain (26). The protein exerts its ATP-dependent chaperoning function by binding and release of protein substrates regulated by ATP binding and hydrolysis, which occur at the N-terminal ATPase domain (9). The rate of ATP turnover is controlled by auxiliary chaperones binding to the ATPase domain and a C-terminal, 10-kDa region, which is highly conserved in all Hsp70 family members (16).
It has been proven that increased production of reactive oxygen species (ROS), caused by high-energetic metabolic rates, induces Hsp70 expression (1, 4). Furthermore, increasing concentrations of lactate or decreasing pH values provoke expression levels of Hsp70 (34). Hypoxia and ischemia conditions, which interfere with energy metabolism, have also been shown to induce a Hsp70 response (11, 22). In addition, it has been reported that physical exercise, which depends on intensified energy expenditure, can induce Hsp70 expression (29), and this exercise-induced Hsp70 response also reacts in an intensity-dependent manner (28). These observations lead to the conclusion that peak power, and not the extent of workload, determines the level of Hsp70 expression. Undoubtedly, all of these Hsp70 inducers are known to be associated with energy metabolism.
On the other hand, Hsp70 might have a profound impact on energy metabolism itself. Studies on myocardium undergoing ischemia have demonstrated that via Hsp70 induction, cellular vitality and function are better preserved with consequences for maintaining ATP levels (11, 43). Chronic stimulation of rabbit skeletal muscle leads primarily to Hsp70 expression in oxidative fibers (35). In training experiments with rats, Hsp70 expression is associated with a shift of the composition of myosin heavy chain isoforms, which determine metabolic rates (39). Taken together, these data are indicative of the fact that multifunctional Hsp70 protein expression is related to the extent of energy expenditure that cannot be distinguished from the chaperoning effects on protein metabolism in previous studies.
To date, there is no evidence that Hsp70 exerts a direct effect on energy metabolism, particularly providing sufficient amounts of ATP for power-consuming reactions. However, the cellular adaptation to stress could also be mediated by a signaling effect of Hsp70 on metabolic rates, which requires adequate levels of ATP. This study was aimed to investigate the effects of overexpression of Hsp70 on energy metabolism. To pursue this goal, we established a HeLa cell line transfected with human Hsp70 cDNA and examined its effects on glycolytic and oxidative phosphorylation pathways.
MATERIALS AND METHODS
Plasmid Construction
To construct the recombinant response plasmid pTRE2hyg-HSP70 (hyg = hygromycin), a human Hsp70 donor plasmid pAT153 containing the complete coding region of human Hsp70 cDNA (ATCC57494) was amplified by PCR using primers with flanking BamHI and HindIII restriction sites. The PCR product was subcloned in competent DH5α cells into the Tet-On expression vector pTRE2hyg, which codes for the hyg-resistance gene. The resulting recombinant response plasmid construct, pTRE2hyg-HSP70, was confirmed by appropriate restriction enzyme digestions, which corresponded to the expected sizes in a subsequent agarose gel and electrophoresis procedure (data not shown).
Cell Culture and Transfection
HeLa Tet-On cells (Cat. No. 630901, BD Biosciences Clontech, San Jose, CA) were maintained in tetracycline-free DMEM-F12 HAM, supplemented with 10% (v/v) FBS, 5 mM l-glutamine, and 100 μg/ml G418 at 37°C in a 5% CO2 environment inside six-well plates. For transfection, 5–11 μl Lipofectin reagent was mixed with 100 μl Opti-MEM I-reduced serum medium and incubated at room temperature for 30 min, while 0.7 μg recombinant pTRE2hyg-HSP70 plasmids diluted in 100 μl-reduced serum medium were incubated with 10 μl PLUS reagent for 15 min. After combining the two solutions for 15 min, the Lipofectin–DNA complex was introduced to cells and incubated in FBS-free medium for 3 h. The DNA-containing medium was then replaced by 2 ml DMEM containing 10% FBS. After incubation at 37°C for 24 h, cells were allowed to passage into petri dishes at a 1:10 dilution. The next day, antibiotic hyg was added to the final concentration, 400 μg/ml, for a further selection process. After 2 wk, hyg-resistant colonies appeared, and the large, healthy colonies were isolated and transferred into individual plates.
In preliminary experiments, each of the 3.1 × 106 stable, transfected HeLa cells was seeded in 175 cm2 cell-culture flasks and incubated with 20 ml complete growth medium containing doxycycline at different final concentrations (0, 0.001, 0.1, and 2 μg/ml) to test for optimum results with regard to a broad spectrum of Hsp70 expression. To exclude the possibility that the increase in expression of Hsp70 was a false result of a general stress response induced by changes in the doxycycline concentration, untransfected HeLa cells serving as control were cultured at identical conditions as transfected cells, i.e., medium containing increasing amounts of doxycycline. On the following day, fresh medium was added. Eight hours later, medium was collected every 2 h for a total time of 8 h, and finally, cells were harvested, counted, and shock frozen at −80°C for further analysis. All experiments were done in triplicates. For data requisition, all of the final experiments were performed in culture media containing doxycycline concentrations of 1 μg/ml.
Determination of Hsp70 Level
A part of each harvested cell line was taken for total protein determination using protein extraction buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.02% NaN3, 100 μg/ml PMSF, 1% Nonidet P-40, and 1 μg/ml aprotinin), and the total protein concentration was determined according to Lowry et al. (30). Hsp70 sample amounts were calculated from 0.3125 μg total protein using Western blots with a specific MAb directed against native, human Hsp70 protein (ADI-SPA-810, Enzo Life Sciences, Farmingdale, NY) and referenced to a series of known, standard Hsp70 protein amounts. Further details were described elsewhere (29).
Biochemical Measurements
Medium glucose and lactate analysis.
Cell culture media were collected at different time points as stated above, followed by a centrifugation step at 750 g for 5 min at 4°C. Cell-free supernatants were stored at −20°C for further analysis. Concentrations of glucose and lactate in each sample were measured electroenzymatically in replicates using a YSI 2300 STAT Plus Glucose & Lactate Analyzer (YSI Life Sciences, Yellow Springs, OH).
Determination of glycolytic enzyme activity.
Cells were resuspended in diluted PBS (1:10, v/v; 268 μM KCl, 146 μM KH2PO4, 13.7 mM NaCl, and 804 μM Na2HPO4, pH 7.4) and subjected to three freeze-thaw cycles followed by centrifugation at 2,000 g for 20 min at 4°C. Supernatants were recovered for determination of enzymatic activities. Phosphofructokinase (PFK) and lactate dehydrogenase (LDH) enzymatic activities were measured at 334 nm and 37°C in an Eppendorf spectrophotometer (Eppendorf, Hamburg, Germany) as described earlier (5).
Determination of mitochondrial enzyme activities.
Cells were resuspended in 5–10 μl of a 25-mM potassium phosphate solution (pH 7.2) containing 5 mM MgCl2 and were subjected to three cycles of freeze and thaw. After centrifugation at 2,000 g for 20 min at 4°C, crude mitochondria were stored at −80°C (50). Citrate synthase (CS) activities were measured according to Robinson et al. (42). NADH-dehydrogenase [complex I (CI)] activities were determined using the method of Birch-Machin et al. (6), and determination of ubiquinol:cytochrome-c reductase [complex III (CIII)] activity followed the oxidation of decylubiquinol with cytochrome c as the electron acceptor at 550 nm. The measurement was conducted at 30°C. Activities were expressed as an apparent first-order rate constant.
ATP Measurement
For each experiment, 10,000 cells were washed twice with ice-cold PBS (2.68 mM KCl, 1.46 mM KH2PO4, 137 mM NaCl, and 8.04 mM Na2HPO4, pH 7.4). Cell pellets were resuspended in 100 μl Tris-EDTA buffer (100 mM Tris-HCl and 4 mM EDTA, pH 7.55) and incubated for 3 min at 100°C. After the centrifugation step at 10,000 g for 2 min, supernatants were stored immediately at −80°C until further analysis. ATP contents were measured with an ATP bioluminescence assay kit (Sigma, Steinheim, Germany) performed on a luminometer (Lumat LB 9507, Berthold Technologies, Germany). The amounts of ATP were calculated from a log-log plot of a standard curve for ATP (1.56–800 nM) and expressed as nmol/mg protein.
Statistical Analysis
Each data set was averaged for multiple samples (duplicates and replicates) and expressed as a box plot representing the smallest observation, lower quartile, median, upper quartile, and largest observation. For statistical analysis, all data were logarithmized to fit the normal distribution. Comparisons among multiple groups were assessed by the Kruskal-Wallis test, and differences among groups were determined using the Mann-Whitney test. The relationship between determined parameters was analyzed using regression analysis tools, and significances were analyzed using Spearman correlation coefficient. IBM SPSS Statistics software (Armonk, NY) was used for statistical analyses and GraphPad Prism 5 (La Jolla, CA) to draw graphics. Differences of P < 0.05 were considered statistically significant.
RESULTS
Screening for Stable HeLa-Tet-on-HSP70 Cell Lines
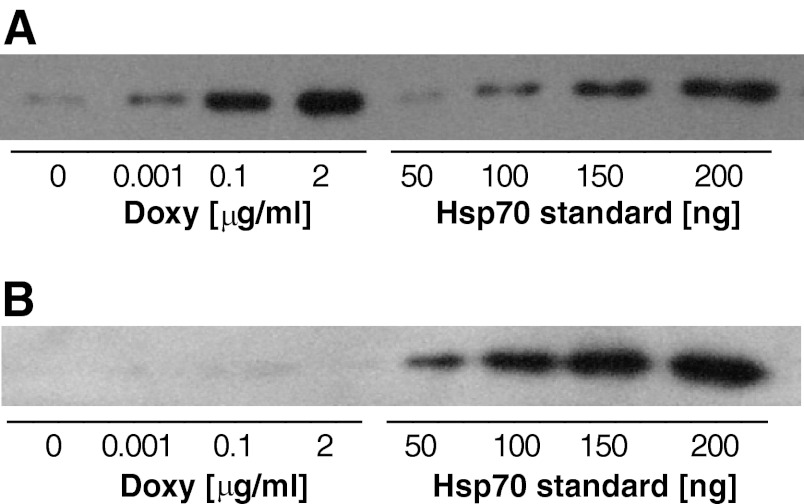
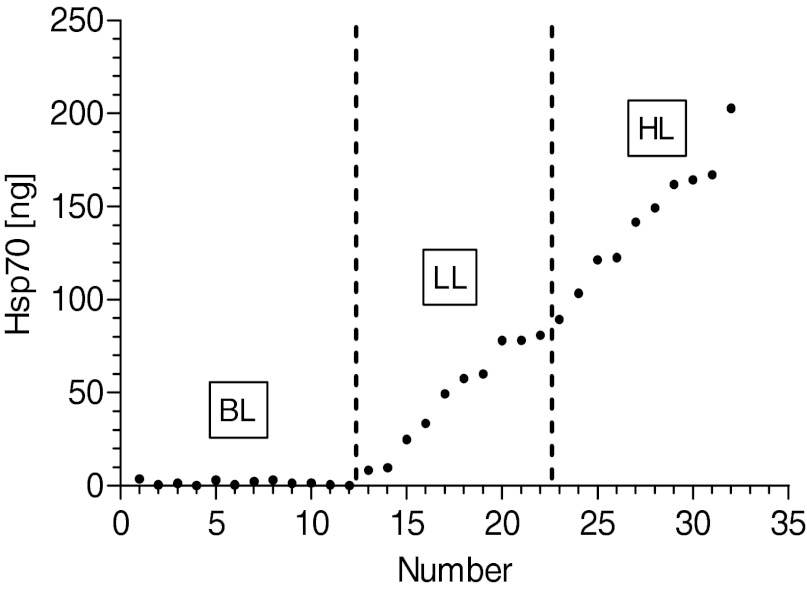
In transfected cells, incubation with doxycycline clearly induced Hsp70 expression in a dose-dependent manner (Fig. 1A), whereas untransfected cells showed very low endogenous Hsp70 levels and did not respond to diverse concentrations of doxycycline with regard to Hsp70 expression (Fig. 1B). Untransfected cells reflected baseline levels (BL) and served as negative controls. In such cells, application of doxycycline did not induce significant effects in any of the analyzed biochemical parameters. Moreover, overexpression of Hsp70 did not result in any lethal effects, as this was tested for similar vitality rates in each of the cell groups using the trypan blue test (data not shown). From a total of 20 independently transfected hyg-resistant cells, clones with low background- and high doxycycline-induced Hsp70 expression were selected and arbitrarily, gradually allocated to two different groups of expression levels (Fig. 2). Based on the doxycycline-induced Hsp70 expression levels, transfected cells were categorized into two groups representing a low level (LL) and high level (HL) of Hsp70 expression, in addition to the BL group of untransfected cells and low endogenous Hsp70 expression.
Fig. 1.
Representative immunoblots of heat shock protein 70 (Hsp70) expression in cells in the presence of distinct concentrations of doxycycline (Doxy; 0, 0.001, 0.1, and 2 μg/ml). Total protein lysates (312.5 ng) were used, followed by SDS-PAGE and immunodetection with Hsp70 MAb. A: samples from double-stable HeLa cells expressing a tetracycline-controlled transactivator protein and transfected with the plasmid pTRE2hyg-HSP70 (hyg = hygromycin). A set of known amounts of standard Hsp70 (50–200 ng) was loaded in parallel. B: samples derived from nontransfected cells treated the same way as transfected cells serving as controls.
Fig. 2.
Expression levels of Hsp70 in different categories of HeLa cells. In addition to the nontransfected baseline-level (BL) group, transfected cells were allocated arbitrarily into low-level (LL) and high-level (HL) groups according to the intensity of their Hsp70 expression. BL, n = 12; LL, n = 10; HL, n = 10.
Glucose and Lactate
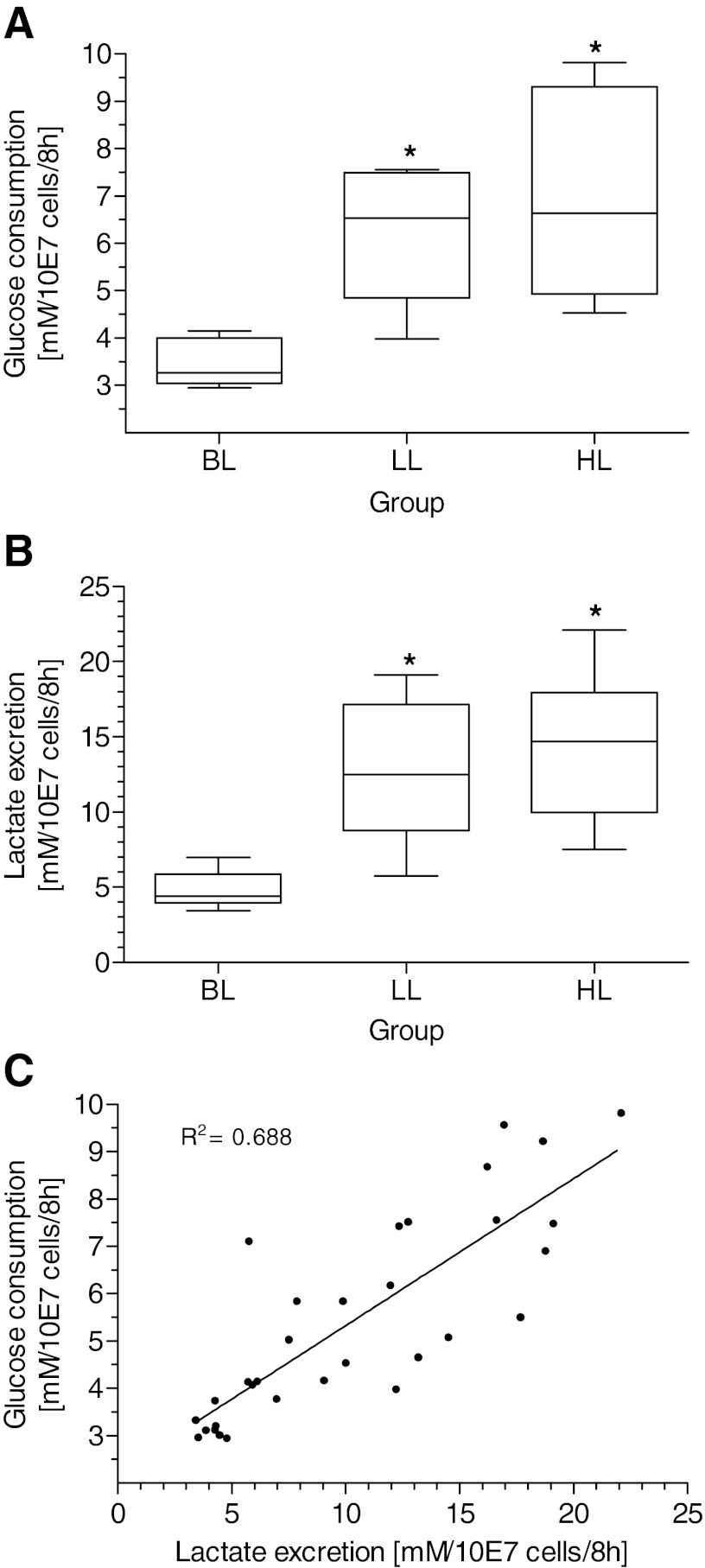
The relative contribution of the glycolysis pathway to the cellular energy supply is grossly reflected by the cellular use of substrates and excretion of metabolites. The use of glucose provided in the medium was augmented significantly in both transfected groups compared with BL, with just an increasing trend between LL and HL (Fig. 3A). These results were also reflected by the excretion of lactate into the media (Fig. 3B), with lactate excretion following the pattern of glucose according to the rate of Hsp70 expression. The correlation between glucose and lactate is highly significant (Fig. 3C). Furthermore, the arithmetic averages of glucose substrates are 48.1% for LL and 48.6% for HL vs. lactate metabolites and reflect the expected ratio of two lactate molecules/glucose composition. These findings are underlined by a diminished factor of lactate vs. glucose molecules that degrades from factor 2.0 in LL and HL to 1.3 only in the BL group. Consequently, the proportion of oxidative phosphorylation in the BL group is higher than in LL and HL, and the rate of glycolysis increases with expression of Hsp70.
Fig. 3.
Glucose consumption (A) and lactate excretion (B) of transfected cells expressing different amounts of Hsp70 and linear correlation between glucose and lactate (C). BL, cells with BL of Hsp70 (n = 12); LL, n = 10; HL, n = 10. *P < 0.05 vs. BL. Comparison among multiple groups was assessed by Kruskal-Wallis test. Differences among groups were tested by Wilcoxon test. Correlation significance was calculated using Spearman correlation coefficient (P < 0.01).
Activities of Glycolytic and Mitochondrial Enzymes
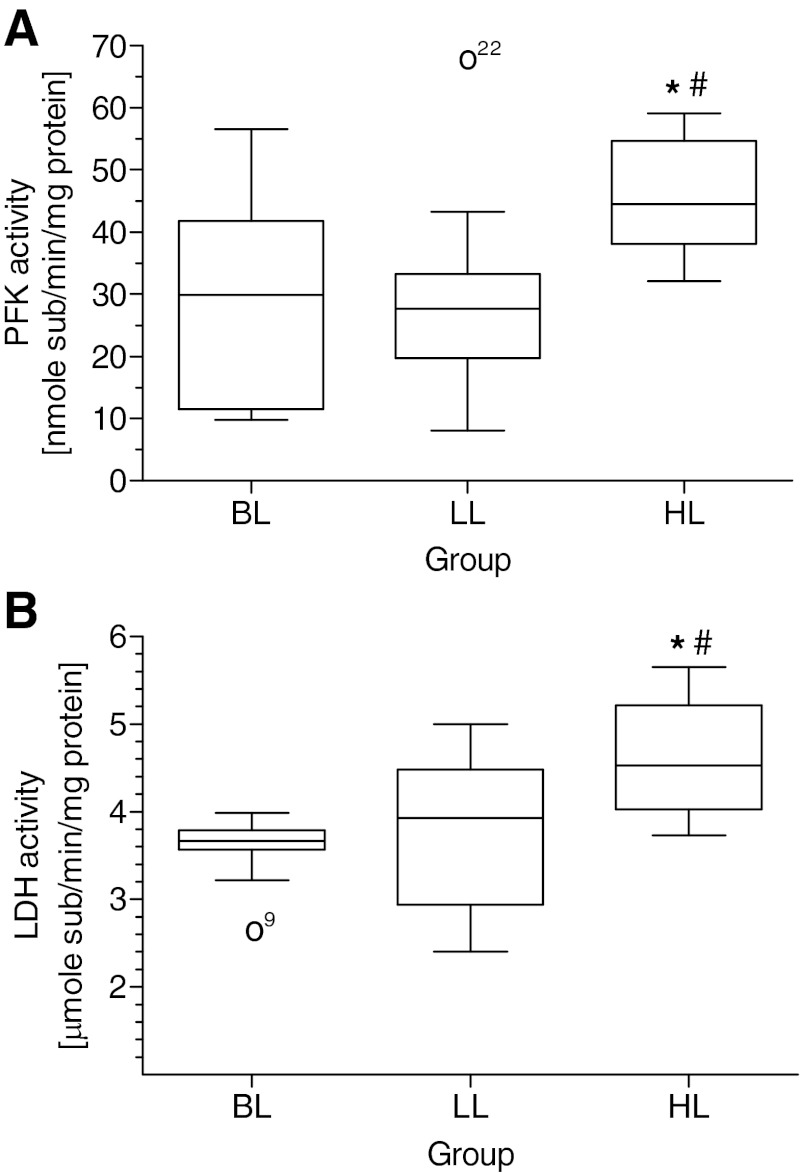
The activities of glycolytic enzymes PFK and LDH in the HL group are increased significantly vs. BL and LL and increased by trend in LL vs. BL groups, considering that the weak trend for PFK in LL is rooted in an upper-outlet value that was excluded from statistical calculations (Fig. 4, A and B). The correlations between enzymes and their corresponding substrates are statistically significant yet with poor linear R2 values (data not shown). The reasons are rooted in methodological difficulties, presenting the consumption of glucose and excretion of lactate as endpoint measurements on one hand and enzyme activities PFK and LDH as snapshot values on the other hand.
Fig. 4.
Activity of glycolytic enzymes in HeLa cells expressing different levels of Hsp70. A: activity of phosphofructokinase (PFK). B: activity of lactate dehydrogenase (LDH). BL, n = 12; LL, n = 10; HL, n = 10. *P < 0.05 vs. BL; #P < 0.05 vs. LL. For details, see Fig. 3. Open circles represent statistical outliers.
The activity of CS, a mitochondrial matrix key enzyme of all aerobic organisms and one of the best indicators for mitochondrial abundance (12), was statistically unaltered among groups yet with a decreasing trend for group LL compared with BL (Fig. 5A). Therefore, based on CS activity rates, there is no indication for newly synthesized mitochondria triggered by Hsp70 overexpression.
Fig. 5.
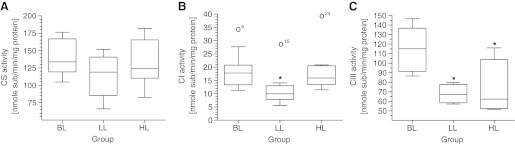
Activity of mitochondrial aerobic enzymes from HeLa cells expressing different levels of Hsp70. A: citrate synthase (CS); BL, n = 12; LL, n = 9; HL, n = 10. B: NADH dehydrogenase [complex I (CI)]; BL, n = 12; LL, n = 9; HL, n = 10. Open circles represent statistical outliers. C: ubiquinol:cytochrome-c reductase [complex III (CIII)]; BL, n = 12; LL, n = 4; HL, n = 7. For details, see Fig. 3. *P < 0.05 vs. BL.
The electron transport chain in the mitochondrion is the site of oxidative phosphorylation in eukaryotes. Its net effect is to create a gradient in charge, pH, and concentration of hydrogen ions. The NADH and succinate generated in the citric acid cycle are oxidized, providing energy to power ATP synthase. Regarding the activity of the multiprotein CI, a significant decrease in LL vs. BL and HL was visible, and HL showed a weak, decreasing trend toward BL (Fig. 5B). This activity pattern of CI is also evident for CIII, since groups LL and HL—both expressing increased levels of Hsp70—end up in significantly reduced CIII activities compared with BL (Fig. 5C).
In summary, neither of the mitochondrial protein complexes gives an indication to boost its contribution in support of ATP synthesis. Instead, the oxidative phosphorylation pathway appears downregulated.
ATP Levels
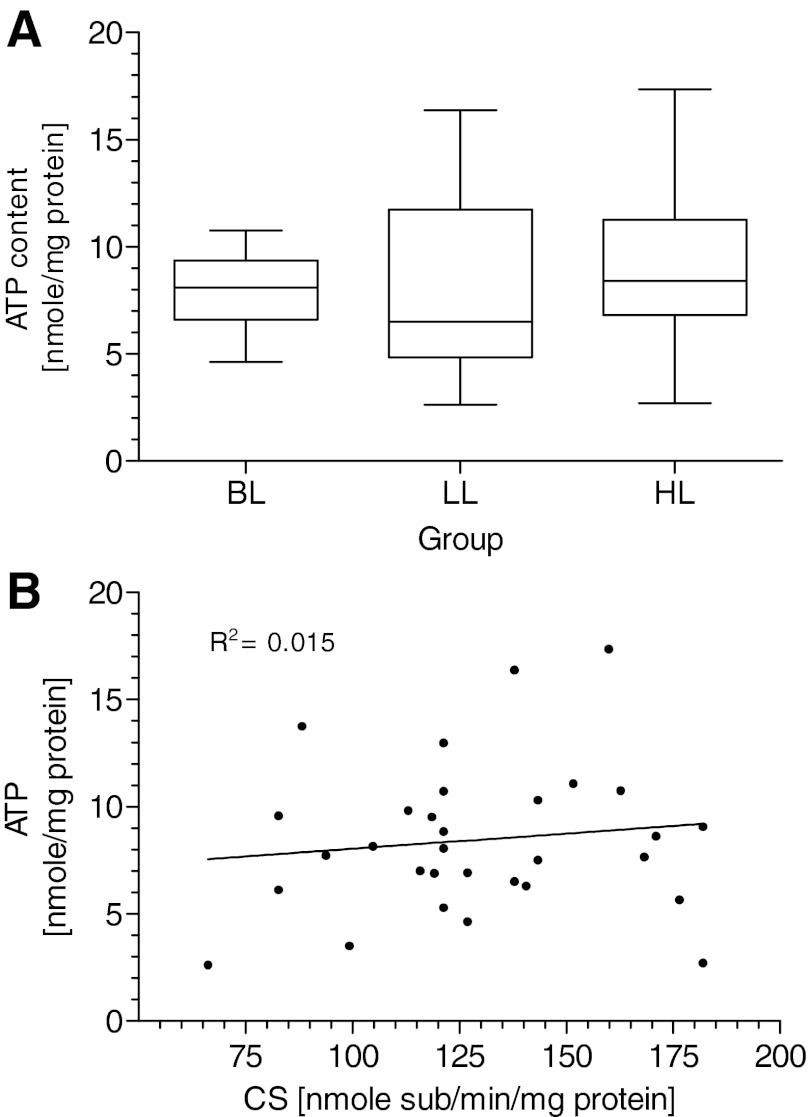
To assess the net effects of changes in glycolytic pathway and oxidative phosphorylation states on the energy status of HeLa cells, cellular ATP pools were measured as a function of increased Hsp70 levels due to doxycycline exposure (Fig. 6A). Compared with untransfected cells, there are no statistically relevant differences in cellular ATP levels between various expression levels of Hsp70. The homeostasis between ATP consumption and ATP generation is well balanced, despite an apparent decline in oxidative phosphorylation processes. This result is also supported by a nonsignificant correlation between ATP amount and CS activity reflecting mitochondrial abundance (Fig. 6B). Without further information regarding the total metabolic rate of ATP, generation, and consumption, it can be stated that in view of statistically unaffected, total cell numbers and vitality rates among different groups (data not shown), the increased glycolytic pathway is able to compensate for the decline in ATP production caused by a diminished proportion of oxidative phosphorylation that was induced by rising Hsp70 expression.
Fig. 6.
Intracellular ATP levels of transfected cells expressing different amounts of Hsp70 (A). Relationship between ATP level and CS activity (B). BL, n = 12; LL, n = 10; HL, n = 10. For details, see Fig. 3.
DISCUSSION
To date, studies have focused predominantly on the central function of Hsp70 serving as a molecular chaperone, facilitating protein synthesis in the extended sense by stabilizing and refolding denatured proteins (20, 44). Direct effects of Hsp70 on energy supply in bona fide, unstressed cells have not been investigated yet. To address this issue, we have conducted the current study using transgenic HeLa cells expressing different levels of Hsp70 upon induction with doxycycline. We demonstrate that overexpression of Hsp70 leads to a shift in energy metabolism by augmented anaerobic glycolytic activity and a concomitant, reduced contribution of oxidative phosphorylation toward energy supply. This shift does not affect the stability of intracellular ATP levels, which remain constant over a broad range of Hsp70 expression. To our knowledge, this is the first study that reports such a straight effect of Hsp70 on energy metabolism.
Intracellular ATP levels represent a critical marker for the cellular energy metabolism. Any challenge regarding the energy metabolism of a cell leads to changes in ATP levels and consequently, to ATP depletion, a fatal condition that may precede cell death (37). For instance, in the event of demanding energy requirements by ischemia, Hsp70 exerts cellular protection by preserving cellular ATP levels (11). In the current study, a moderate overexpression of Hsp70 already led to a significant increase in glucose consumption and lactate excretion that was not reflected by an oxidative phosphorylation process. Glycolytic enzyme activities mirror these findings with a shift toward high expression grades of Hsp70 in a dose-dependent manner. This way, glycolytic activities, rather than oxidative phosphorylation processes, ensure cellular ATP levels and imply a regulatory effect of Hsp70 on energy balance.
Taking into account the Hsp70 dose-dependent increase in glucose consumption, as well as lactate excretion, it seems plausible that a constant ATP range might be rooted in elevated requirements of ATP-consuming processes. Such effects could be caused by alterations in cell proliferation rates and/or upregulation of other proteins than those we analyzed, including Hsp70 itself.
Interestingly, in experienced athletes shortly after finishing an ultra-marathon race, increased mRNA levels of Hsp70 were found in mononuclear blood cells (2). Their levels remained increased significantly 2 h after the race but returned to baseline during the course of 24 h. These results in well-trained athletes were not consistent with published Hsp70 protein levels in moderately trained subjects exercising within normal limits (45). It is likely that moderately exercising subjects are going below the physiological limit that calls for cell-protective reactions. On the other hand, extremely challenged athletes need to counteract thermal stress with its potential implications—the release of cell-damaging ROS (57). Such heat-stressed cells are forced to confine the electron flow into CIII, which is the primary site for the net ROS generation with potential cell pathological alterations that include lipid peroxidation (10, 32, 33, 47). Furthermore, it seems tempting that downregulation of oxidative phosphorylation could be considered as cellular “anticipation” of thermal damage, which harms the mitochondrial membrane morphology and functional integrity (24, 51). In muscle cells, exercise per se with its developing heat stress is the primary cause associated to Hsp70 expression (38). Consequences of cellular injury might be an uncontrolled release of mitochondrial DNA and formyl peptides. These damage-associated molecular patterns are able to end in a “severe systemic inflammatory response syndrome” in humans (58).
A case report in 2010 (15) compared the sampling of cell-free plasma DNA (cfDNA) in a time-dependent manner in moderately trained men over a period of 24 h with inflammatory and muscle-damage markers. It found that cfDNA peaked immediately after exercising and went back to baseline only 1 h later. Combining both findings from Fatouros et al. (15) and Atamaniuk et al. (2), the expression pattern of Hsp70 rather reflects the model of cfDNA, showcasing a time when cells are in danger of necrotic events caused by overheating and energy depletion. In this regard, expression of Hsp70 could be an adaptation to rescue cells from activity depression of the mitochondrial respiratory chain with fatal lipid peroxidation and oxidative stress. This correlation between heat stress and mitochondrial generation of ROS was described earlier in chickens exposed to enhanced temperatures (57). Also, in highly trained athletes, the physical endurance performance is determined by the core temperature. Heat ∼40°C leads to exhaustion and immediate stop of physical activity (19, 36, 49). Interestingly, it is possible to prolong the individual endurance performance of an athlete just by relocation of the challenging activity into a hypothermal environment or an athlete's upstream residence in a cooling chamber. In either way, the endurance performance is correlated significantly to the time when the core temperature reaches a critical level that reflects heat stress (7, 25, 52).
Compared with anaerobic glycolysis, oxidative phosphorylation serves cells as a more efficient way to generate ATP. It is dependent on an integrated respiratory chain composed of polypeptide complexes located on the inner membrane of mitochondria (43). Regarding the LL group, both electron transport systems CI and CIII are downregulated significantly compared with BL, and it also applies for the HL group with CIII, whereas for CI, only a weak trend can be stated compared with BL. Moreover, there is no constant linear activity decrease visible among BL, LL, and HL. Our rationale for this observation is rooted in feedback mechanisms known from many biological regulatory circuits. For instance, after a process of adaptation, inflammatory processes in tissue cells are limited and finally, downregulated by anti-inflammatory cytokines to reach their original ground state. Such feedback circuits are crucial network motifs, ubiquitously found in many intra- and intercellular regulatory systems (14). In this regard, it seems reasonable that impacts of increased Hsp70 levels on oxidative phosphorylation are looped to limit the effect of Hsp70 to sustain a functional condition of mitochondria.
Still, it is obvious that Hsp70 shows a distinct impact on oxidative energy metabolism. In conclusion, the negative impact on oxidative phosphorylation suggests that the effects of Hsp70 on cellular ATP changes are twofold: downregulation of oxidative phosphorylation while elevating glycolytic processes to maintain the synthesis of energy carriers.
In addition, mitochondrial biogenesis contributes to changes in ATP levels. Therefore, we investigated the Hsp70-dependent activity of CS, one of the indicators of mitochondrial biogenesis (55). Comparing the CS curve with intracellular ATP levels displays a similar course that does not argue for newly synthesized mitochondria and indirectly delivers further support for an intrinsical downregulation of oxidative phosphorylation. It is supported by the decrease in number of mitochondria under heat-stress conditions, which are well attributed to direct damaging of mitochondrial morphology in conjunction with enhanced membrane permeability (40).
Without further data available, the physiological significance and effect of Hsp70 on energy metabolism remain speculative. In the current study, it was possible to achieve high transfection efficiency. Hsp70 rates in LL were ∼25-fold elevated compared with resting conditions, which seems similar to those in trained muscles of athletes (8) or in chronic ischemic disease (27). It has been reported that under appropriate cellular stress, the Hsp70 fraction can be increased up to 20% of the total cellular proteins (13) compared with 19% in LL and 40% in HL of this study. The LL group therefore mimics quite well the range of this molecular chaperone, which is inducible in vivo by heat shock. Noteworthy, with the performance of our own heat-shock experiments with untransfected HeLa cells serving as expression control for Hsp70 toward transfected cells, we found endogenous, average Hsp70 levels of 140 ng, very similar to the averaged value of 142 ng in cells that belong to the HL group (data not shown). These findings underline the physiological range of Hsp70 levels in our transfected cells comprising both groups—LL and HL. One possible explanation for these high physiological expression rates of Hsp70 might be that Hsp70 facilitates cellular adaptation because overall ATP turnover is increased under cellular stress conditions. This viewpoint is supported by studies on cancer cells in which an elevated rate of glycolysis coincides with high expression levels of Hsp70 (21). Nota bene, we compare in this study metabolic differences within the same cell line. Nevertheless, similar impacts of Hsp70 on energy metabolism in other cell types need to be shown to exclude the possibility of a “Warburg effect” with its generalized metabolic changes in tumor cells (53). Furthermore, despite a methodological weakness of our study—the lack of a proper negative control to exclude nonspecific metabolic changes due to overexpression per se—preliminary data of a German multicenter study, including our group, support the significance of our findings. Biopsy microarray data from Musculus vastus lateralis at different time points and data from liquid chromatography–mass spectrometry showed that transcription and protein levels of Hsp70, as well as gene products involved in glycolytic processes, increased in athletes after endurance exercise. Moreover, gene transcripts and proteins of oxidative phosphorylation are downregulated, demonstrating the correlation among Hsp70, glycolysis, and oxidative phosphorylation.
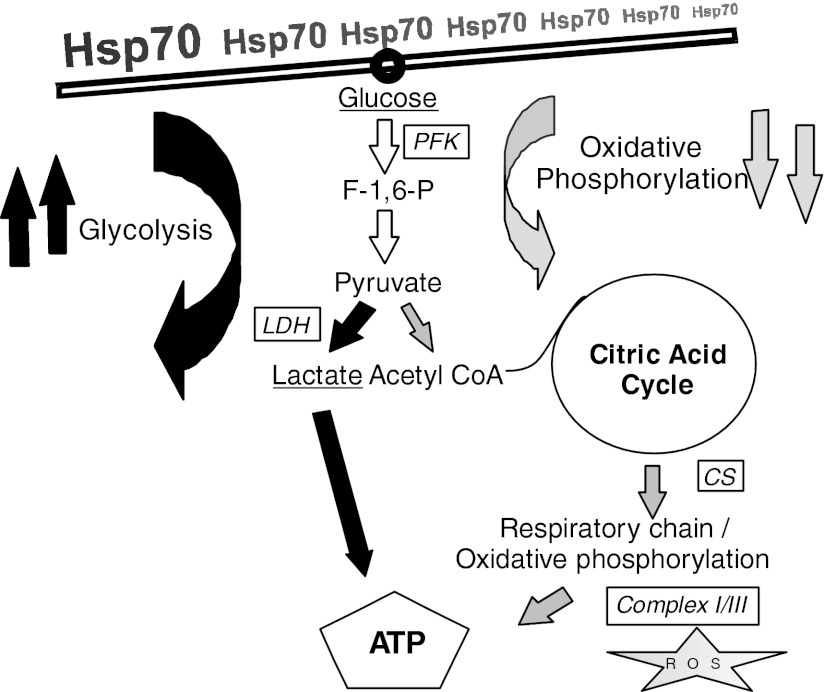
In summary, we have been able to provide proof for the context of Hsp70 with a constant supply of ATP (Fig. 7). The source of ATP derives from augmented glycolytic activity rather than from mitochondrial oxidative phosphorylation, and furthermore, Hsp70-induced biogenesis of newly synthesized mitochondria seems unlikely. The reasons for downregulation of the oxidative phosphorylation pathway have to remain speculative at this point, yet it seems plausible that thermal cellular stress causes release of ROS with lipid peroxidation and profound changes in membrane integrity when ATP is not generated. Future research has to confirm this direct impact of Hsp70 on energy metabolism in a nontumor cell line and needs to analyze the coupled downregulation of oxidative phosphorylation, which is compensated by increased glycolysis. Furthermore, we have to clarify the more detailed mechanism with regard to molecular pathways—the involved routes of signal transduction, subsequent physiological effects, and consequences of abnormal expression of Hsp70 on the integrity of cellular energy status.
Fig. 7.
Simplified flow chart of Hsp70 impact on ATP generation. High expression levels of Hsp70 in heat-shocked HeLa cells led to a shift in energy metabolism with increased anaerobic glycolysis and attenuated oxidative phosphorylation. Therefore, under challenging environmental conditions, the danger of potential, harmful release of reactive oxygen species (ROS) in mitochondria is diminished. F-1,6-P, fructose 1,6-bisphosphate; CoA, coenzyme A.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.L. and J.M.S. conception and design of research; L.W. and O.P. performed experiments; L.W., U.S., Y.L., and J.M.S. analyzed data; L.W., U.S., Y.L., and J.M.S. interpreted results of experiments; U.S. drafted manuscript and prepared figures; L.W., U.S., Y.L., and J.M.S. edited and revised manuscript; L.W., U.S., Y.L., O.P., and J.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We are deeply grateful to Dr. Immo E. Scheffler for his critical comments on this manuscript.
Present addresses of L. Wang: Dept. of Anesthesiology, Multidisciplinary Neuroprotection Laboratories, Duke Univ. Medical Center, Durham, NC 27710.
Present address of O. Prokopchuk: Chirurgische Klinik und Poliklinik, Klinikum rechts der Isar, Technical Univ. Munich, 81675 München, Germany.
REFERENCES
- 1. Arnaud CM, Joyeux M, Garrel C, Godin-Ribout D, Demenge P, Ribout C. Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Br J Pharmacol 135: 1776–1782, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atamaniuk J, Stuhlmeier KM, Vidotto C, Tschan H, Dossenbach-Glaninger A, Mueller MM. Effects of ultra-marathon on circulating DNA and mRNA expression of pro- and anti-apoptotic genes in mononuclear cells. Eur J Appl Physiol 104: 711–717, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2: 469–475, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bellmann K, Wenz A, Radons J, Burkart V, Kleemann R, Kolb H. Heat shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest 95: 2840–2845, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods. New York: Grune & Stratton, 1984 [Google Scholar]
- 6. Birch-Machin M, Jackson S, Singh Kler R, Turnbull DM. Study of skeletal muscle mitochondria dysfunction. In: Methods in Toxicology: Mitochondria Dysfunction, edited by Lash LH, Lone DP. San Diego, CA: Academic, 1993, p. 51–69 [Google Scholar]
- 7. Bogerd N, Perret C, Bogerd CP, Rossi RM, Daanen HA. The effect of pre cooling intensity on cooling efficiency and exercise performance. J Sports Sci 28: 771–779, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Brunt JJ, Khan S, Heikkila JJ. Sodium arsenite and cadmium chloride induction of proteasomal inhibition and HSP accumulation in Xenopus laevis A6 kidney epithelial cells. Comp Biochem Physiol C Toxicol Pharmacol 155: 307–317, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Chappell TG, Konforti BB, Schmid SL, Rothman JE. The ATPase core of a clathrin uncoating protein. J Biol Chem 262: 746–751, 1987 [PubMed] [Google Scholar]
- 10. Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278: 36027–36031, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Currie RW, Karmazyn M, Koc M, Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res 63: 543–549, 1988 [DOI] [PubMed] [Google Scholar]
- 12. DiMauro S, Bonilla E. Mitochondrial encephalomyopathies. In: Myology, Vol.II, edited by Engel AG, Franzini-Armstrong C. Philadelphia: McGraw Hill, 2004, p. 1623–1676 [Google Scholar]
- 13. Donati YR, Slosman DO, Polla BS. Oxidative injury and the heat shock response. Biochem Pharmacol 40: 2571–2577, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Dong CY, Shin D, Joo S, Nam Y, Cho KH. Identification of feedback loops in neural networks based on multi-step Granger causality. Bioinformatics 28: 2146–2153, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Fatouros IG, Jamurtas AZ, Nikolaidis MG, Destouni A, Michailidis Y, Vrettou C, Douroudos II, Avloniti A, Chatzinikolaou A, Taxildaris K, Kanavakis E, Papassotiriou I, Kouretas D. Time of sampling is crucial for measurement of cell-free plasma DNA following acute aseptic inflammation induced by exercise. Clin Biochem 43: 1368–1370, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J 14: 2281–2292, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gally F, Minor MN, Smith SK, Case SR, Chu HW. Heat shock factor 1 protects against lung Mycoplasma pneumoniae infection in mice. J Innate Immun 4: 59–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gong TW, Fairfield DA, Fullarton L, Dolan DF, Altschuler RA, Kohrman DC, Lomax MI. Induction of heat shock proteins by hyperthermia and noise overstimulation in Hsf1 −/− mice. J Assoc Res Otolaryngol 13: 29–37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 86: 1032–1039, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295: 1852–1858, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Jäättelä M. Escaping cell death: survival proteins in cancer. Exp Cell Res 248: 30–43, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Knowlton AA, Brecher P, Apstein CS. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest 87: 139–147, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kong W, Kuester RK, Gallegos A, Sipes IG. Induction of DNA damage in human urothelial cells by the brominated flame retardant 2,2-bis(bromomethyl)-1,3-propanediol: role of oxidative stress. Toxicology 290: 271–277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kruuv J, Glofcheski D, Cheng KH, Campbell SD, Al-Qysi HM, Nolan WT, Lepock JR. Factors influencing survival and growth of mammalian cells exposed to hypothermia. I. Effects of temperature and membrane lipid perturbers. J Cell Physiol 115: 179–185, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Lee DT, Haymes EM. Exercise duration and thermoregulatory responses after whole body precooling. J Appl Physiol 79: 1971–1976, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Liberek K, Lewandowska A, Zietkiewicz S. Chaperones in control of protein disaggregation. EMBO J 27: 328–335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Lehmann M, Baur C, Stork M, Sunder-Plassmann L, Steinacker JM. HSP70 expression in skeletal muscle of patients with peripheral arterial occlusive disease. Eur J Vasc Endovasc Surg 24: 269–273, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Lormes W, Baur C, Opitz-Gress A, Altenburg D, Lehmann M, Steinacker JM. Human skeletal muscle HSP70 response to physical training depends on exercise intensity. Int J Sports Med 21: 351–355, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Mayr S, Opitz-Gress A, Zeller C, Lormes W, Baur S, Lehmann M, Steinacker JM. Human skeletal muscle HSP70 response to training in highly trained rowers. J Appl Physiol 86: 101–104, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 31. Martini F, Fernández C, Tarazona JV, Pablos MV. Gene expression of heat shock protein 70, interleukin-1β and tumor necrosis factor α as tools to identify immunotoxic effects on Xenopus laevis: a dose-response study with benzo[a]pyrene and its degradation products. Environ Pollut 160: 28–33, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Mehlen P, Kretz-Remy C, Préville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J 15: 2695–2706, 1996 [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Narasimhan P, Swanson RA, Sagar SM, Sharp FR. Astrocyte survival and HSP70 heat shock protein induction following heat shock and acidosis. Glia 17: 147–159, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Neufer PD, Ordway GA, Hand GA, Shelton JM, Richardson JA, Benjamin IJ, Williams RS. Continuous contractile activity induces fiber type specific expression of HSP70 in skeletal muscle. Am J Physiol Cell Physiol 271: C1828–C1837, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 460: 467–485, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nieminen AL, Saylor AK, Herman B, Lemasters JJ. ATP depletion rather than mitochondrial depolarization mediates hepatocyte killing after metabolic inhibition. Am J Physiol Cell Physiol 267: C67–C74, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Noble EG, Ho R, Dzialoszynski T. Exercise is the primary factor associated with Hsp70 induction in muscle of treadmill running rats. Acta Physiol (Oxf) 187: 495–501, 2006 [DOI] [PubMed] [Google Scholar]
- 39. O'Neill DE, Noble EG. Constitutive expression of inducible Hsp70 is linked to natural shifts in skeletal muscle phenotype. Acta Physiol Scand 181: 41, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Piper PW, Millson SH, Mollapour M, Panaretou B, Siligardi G, Pearl LH, Prodromou C. Sensitivity to Hsp90-targeting drugs can arise with mutation to the Hsp90 chaperone, cochaperones and plasma membrane ATP binding cassette transporters of yeast. Eur J Biochem 270: 4689–4695, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell 40: 253–266, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Robinson JB, Brent LG, Sumegi B, Srere PA. An enzymetric approach to the study of the Krebs tricarboxylic acid cycle. In: Mitochondria: A Practical Approach, edited by Darley-Usmar VM, Rickwood D, Wilson T. Oxford, UK: IRL, 1987, p. 153–170 [Google Scholar]
- 43. Sammut IA, Jayakumar J, Latif N, Rothery S, Severs NJ, Smolenski RT, Bates TE, Yacoub MH. Heat stress contributes to the enhancement of cardiac mitochondrial complex activity. Am J Pathol 158: 1821–1831, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saris N, Holkeri H, Craven RA, Stirling CJ, Makarow M. The Hsp70 homologue Lhs1p is involved in a novel function of the yeast endoplasmic reticulum, refolding and stabilization of heat-denatured protein aggregates. J Cell Biol 137: 813–824, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shastry S, Toft DO, Joyner MJ. HSP70 and HSP90 expression in leucocytes after exercise in moderately trained humans. Acta Physiol Scand 175: 139–146, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Soleimani AF, Zulkifli I, Omar AR, Raha AR. The relationship between adrenocortical function and Hsp70 expression in socially isolated Japanese quail. Comp Biochem Physiol A Mol Integr Physiol 161: 140–144, 2012 [DOI] [PubMed] [Google Scholar]
- 47. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Stahl M, Beck J, Nassal M. Chaperones activate hepadnavirus reverse transcriptase by transiently exposing a C-proxiaml region in the terminal protein domain that contributes to epsilon RNA binding. J Virol 81: 13354–13364, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tyler CJ, Sunderland C. Cooling the neck region during exercise in the heat. J Athl Train 46: 61–68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vantieghem A, Xu Y, Assefa Z, Piette J, Vandenheede JR, Merlevede W, De Witte PA, Agostinis P. Phosphorylation of Bcl-2 in G2/M phase-arrested cells following photodynamic therapy with hypericin involves a CDK1-mediated signal and delays the onset of apoptosis. J Biol Chem 277: 37718–37731, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Vigh L, Nakamoto H, Landry J, Gomez-Munoz A, Harwood JL, Horvath I. Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann N Y Acad Sci 1113: 40–51, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Walters TJ, Ryan KL, Tate LM, Mason PA. Exercise in the heat is limited by a critical internal temperature. J Appl Physiol 89: 799–806, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Warburg O. On the origin of cancer cells. Science 123: 309–314, 1956 [DOI] [PubMed] [Google Scholar]
- 54. Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 72: 1063–1081, 1992 [DOI] [PubMed] [Google Scholar]
- 55. Williams RS, Garcia-Moll M, Mellor J, Salmons S, Harlan W. Adaptation of skeletal muscle to increased contractile activity. Expression nuclear genes encoding mitochondrial proteins. J Biol Chem 262: 2764–2767, 1987 [PubMed] [Google Scholar]
- 56. Xu X, Gupta S, Hu W, McGrath BC, Cavener DR. Hyperthermia induces the ER stress pathway. PLoS One 6: e23740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang L, Tan GY, Fu YQ, Feng JH, Zhang MH. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C Toxicol Pharmacol 151: 204–208, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]