Abstract
Objective
The purpose of this study was to characterize escitalopram population pharmacokinetics (PK)in patients treated for major depression in a cross-national, U.S.-Italian clinical trial.
Methods
Data from the two sites participating in this trial, conducted at Pittsburgh (USA) and Pisa (Italy) were utilized. Patients received 5, 10, 15, or 20 mg of escitalopram daily for a minimum of 32 weeks. Nonlinear mixed-effects modeling (NONMEM) was used to model the PK characteristics of escitalopram. One and two compartment models with various random effect implementations were evaluated during model development. Objective function values (OFV) and goodness of fit plots were used as model selection criteria. CYP2C19 genotype, age, weight, BMI, sex, race, and clinical site were evaluated as possible covariates.
Results
320 plasma concentrations from 105 Pittsburgh patients and 153 plasma concentrations from 67 Pisa patients were available for the PK model development. A one-compartmental model with linear elimination and proportional error best described the data. Apparent clearance (CL/F) and volume of distribution (V/F) for escitalopram without including any covariates in the patient population were 23.5 L/h and 884 L , respectively. CYP2C19 genotype, weight and age had a significant effect on CL/F, and patient BMI affected estimated V/F. Pisa, Italy patients had significantly lower clearances than Pittsburgh patients that disappeared after controlling for patient CYP2C19 genotype, age, and weight. Post-processed individual empirical Bayes estimates on clearance for the 172 patients show that patients without allele CYP2C19*2 or *3 (n=82) cleared escitalopram 33.7% faster than patients with heterogeneous or homogeneous *2 or *3 (*17/*2, *17/*3, *1/*2, *1/*3, *2/*2, *2/*3, and *3/*3,n=46). CL/F significantly decreased with increasing patient age. Patients younger than 30 years (n=45) cleared escitalopram 20.7% and 42.7% faster than patients aged 30-50 years (n=84) and greater than 50 years of age (n=43), respectively.
Conclusions
CYP2C19 genotype, age, and weight strongly influenced the CL/F of escitalopram. Patients with heterogeneous or homogeneous CYP2C19*2 or *3 genotype had significantly lower clearances than patients with other genotypes. CL/F significantly decreased with either increasing age or decreasing body weight. These variables may affect patient tolerance of this antidepressant and, consistent with the NIH emphasis on personalized treatment, may provide important information in the effort to tailor treatments to patients’ individual needs.
Keywords: Escitalopram, NONMEM, Pharmacokinetics, SPECTRUM study, CYP2C19 gene, pharmacogenetics
Introduction
Mood and anxiety disorders have been listed among the world’s ten most disabling illnesses by the World Health Organization. 1, 2 Escitalopram, the S-enantiomer of antidepressant citalopram, is one of the most commonly prescribed selective serotonin reuptake inhibitors (SSRI)1. It selectively binds to the primary reuptake inhibitory site of the serotonin transporter to produce its activity against both depression3-5 and anxiety disorders1, 6, 7. Escitalopram is highly selective for the serotonin transporter and approximately 30-fold more potent than R-citalopram8. Escitalopram has been shown to have efficacy and safety advantages over citalopram9-12. After oral administration, maximum plasma concentrations are reached in about 4 hours. The half life of escitalopram is 27-32 hours. Therefore, it is commonly given once daily 13, 14.
The study “Depression: The Search for Treatment-relevant Phenotypes” was a clinical trial conducted to determine the mediators and moderators of treatment response in major depression(http://clinicaltrials.gov/ct/show/NCT00073697). 15 This study was conducted at two sites, Pittsburgh (USA) and Pisa (Italy). During the study, a significant number of Pisa participants experienced intolerable side effects at the starting dosages. This was not seen in the Pittsburgh patients. One explanation could be systematic differences in the disposition (pharmacokinetics) of escitalopram in the Pisa compared to the Pittsburgh patients. More specifically, if Pisa patients cleared the escitalopram more slowly than Pittsburgh patients, this difference would lead to higher drug exposure in Pisa patients at the same dosage level. The resulting higher concentrations may result in side effects. The sampling protocol in this study provides the basis for determining individual specific exposures that can then be evaluated across the two study sites.
Population pharmacokinetic (PPK) analysis is a robust tool for obtaining pharmacokinetic information, including inter-individual variability in exposure, from large clinical trials with sparse sampling16, 17. The effect of potential covariates on drug exposure can also be evaluated using this approach. Data from this study provides the basis for determining the population PK characteristics of escitalopram in this patient population. This includes the evaluation of the impact of patient specific factors on escitalopram disposition including the CYP2C19 genotype predicted metabolizer phenotype. A better understanding of the effect of such factors has the potential to play a key role in personalizing the treatment of depression. In clinical practice, first antidepressant prescriptions are more commonly not refilled than otherwise 18, most probably because many patients have difficulty tolerating the side effects of these medications. Yet, information about the effects of a small number of variables on clearance could lead to more patient-specific prescribing practices and, in turn, to much better treatment adherence.
Other population PK analyses using these types of data have been published for citalopram19. In these reports, age and weight significantly affected the clearance19. Limited information on the population pharmacokinetics of escitalopram is available in the literature 20-22. A 50% reduction in elimination rate of escitalopram in elderly patients (≥65 years old) compared to younger healthy volunteers has been reported 21 (www.cipralex.com/images/cipralex/smpc.pdf; www.lexapro.com). Sex had no clinically significant effect on escitalopram disposition healthy volunteers14, 21. A population pharmacokinetic study in 24 patients with varying liver function showed a correlation of escitalopram clearance with CYP2C19 functional activity as measured by mephenytoin S/R excretion ratio. This study also demonstrated a relationship between body weight and the apparent volume of distribution for escitalopram22. A classical pharmacokinetic study in healthy scandinavian subjects showed a 21% reduction reduction in the AUC0-1223. No systematic PPK analysis of escitalopram has been published in patients with major depression. This study provides the opportunity to do so and to evaluate how patient specific characteristics (such as CYP2C19 phenotype, race, age, weight/BMI, clinical trial location and sex) may affect the PK of escitalopram.
The goal of this study was to develop a robust population pharmacokinetic model describing escitalopram that evaluated patient-specific characteristics and the potential contribution of these factors to the observed variability in escitalopram exposure and potentially explain the difference in susceptibility to toxicity in the Pisa versus the Pittsburgh based patients.
Subjects and Methods
Subjects and Concentration sampling
Escitalopram PK data were drawn from the study (http://clinicaltrials.gov/ct/show/NCT00073697, Depression: The search for treatment-relevant phenotypes) 15. This study was conducted at two treatment sites, Pittsburgh and Pisa, Italy. Participants were randomly assigned to a treatment sequence that began with interpersonal psychotherapy (IPT) or pharmacotherapy alone. Participants assigned to IPT who did not evidence a response at week 6 or a remission at week 12 had escitalopram added to their treatment. A total of 172 patients, aged 20-65 years old, were recruited in the study and randomly allocated to escitalopram alone or received escitalopram as an adjuctive treatment (105 patients from Pittsburgh and 67 patients from Pisa). All patients were in an episode of non-psychotic major depression defined by the DSM-IV diagnosis and were not receiving any other anti-depressant treatments. A daily dose of 5, 10, 15, or 20 mg of escitalopram was prescribed to patients for a minimum of 32 weeks. Blood samples (10 ml) for the determination of escitalopram drug concentrations were collected at weeks 4, 12, 24 and 36. 320 blood samples from 105 Pittsburgh subjects and 153 blood samples from 67 Pisa subjects were available for data analysis. The actual sample times and dates of all blood draws were recorded. Seventy-three of the Pittsburgh patients were also monitored using the Medication Event Monitoring System (MEMS) to provide dosage history timing information. All other patients provided a time of last dose.
Determination of Escitalopram Concentrations
Blood samples (10 ml) were collected by venipuncture using a tourniquet and a 21g needle placed into lavender top Vacutainer tubes containing 15 % EDTA. The blood was placed in a refrigerated tabletop centrifuge (5°C) and processed for 10 minutes at 1500g. The plasma layer was transferred into 5 ml polypropylene tubes and frozen at −70°C until analyzed.
The escitalopram concentration analysis method was developed by the Geriatric Psychopharmacology Laboratory at the University of Pittsburgh. Escitalopram was measured by reverse-phase high performance liquid chromatography (HPLC) using ultraviolet detection at a wavelength of 210 nm. Plasma was extracted using liquid-liquid extraction (ethyl acetate in heptane; 2:8, v/v) and back-extracted into 0.025 M potassium phosphate, pH 2.4. Separation was completed using a Nucleosil-100 C18 5 um HPLC column (Phenomemex, Torrance, CA), 120mm × 4.6 mm i.d. with a flow rate of 1.0 ml/minute. The assay was linear in the range of 2.5-500 ng/ml with an inter-assay variability (C.V.) of 2.9-3.93% for escitalopram.
Determination of Patient CYP2C19 Genotype
Genomic DNA was extracted from venous blood samples using the phenol chloroform method (n= 99), as well as the QIAamp 96 DNA Blood Kit (n = 125).
A total of four SNPs namely, CYP2C19*2 (rs4244285), *3(rs4986893), *17rs12248560) and a Tag SNP of *2, rs6583954 were genotyped across the CYP2C19 gene. Genotyping was performed by TaqMan assay for allelic discrimination using the Applied Biosystem Prism 7900HT instrument and analysed using the allelic discrimination end-point analysis mode of the Sequence Detection software package version 2.2 (SDS 2.2). Metabolizing status has been assigned according to the study by Rudberg et al24.
Subjects were classified according to the methods described by Rudberg et al 24 specifically: Rapid metabolizers (RM) if they were homozygous for *17 allele; Extensive metabolizers (EM) if they were either homozygous for the wildtype allele *1/*1 or were *1/*17; Intermediate metabolizers (IM) if they carried any of *1/*2, *1/*3, *17/*2 or *17/*3 genotypes; Poor metabolizers (PM) if they were homozygous for either *2 or *3 alleles.
Population Pharmacokinetic Analysis
Base model development
Nonlinear mixed effects modeling for escitalopram PPK was performed using NONMEM® (version 5.1.1, Icon, Hanover, MD). A base model without any covariates was developed initially. One and two-compartment linear mammillary PK models with first order absorption and elimination was evaluated using ADVAN2 TRANS2 and ADVAN4 TRANS4 during model development, respectively. The base model also included a statistical model where the between subject variability (BSV) and within subject variability (WSV) was described. The BSV describes the unexplained random variability in individual values of structural model parameters. It was assumed that the BSV of the PK parameters was log-normally distributed. The relationship between a PK parameter (P) and its variance could therefore be expressed as shown below25, 26:
Where, Pj was the value of PK parameter for the jth individual, PTV was the typical value of P for the population, and ηP denoted the difference between Pj and PTV, independently, which was identically distributed with a mean of zero and variance of ωP2.
The residual variability was comprised of, but not limited to, within subject variability, experimental errors, process noise and /or model misspecification. This variability was modeled using additive, proportional and combined error structures as described below25, 26:
Where yij was the jth observation in the ith individual, was the corresponding model prediction, and εij (or εij’) was a normally distributed random error with a mean of zero and a variance of σ2.
Both population characteristics and individual specific parameters were determined in this analysis. Model parameters for both the base model and the final model were estimated by the first-order conditional estimation (FOCE) with interaction method.
Final model development
The final model was developed by incorporating the effect of subject specific covariates on PK parameter estimates. Both continuous covariates (e.g., age, weight, and BMI) and discrete covariates (e.g., CYP2C19 genotype, clinical trial location, sex, and race) were tested.
The effect of continuous covariates (e.g., age, weight, and BMI) on PK parameter estimates was tested using three possible model structures. The following example illustrates the implementation of these model structures for continuous covariate on CL:
TVCL was the typical value of CL in the population; ηj was the random effect describing the difference of the jth subject from the typical population value; Cov represents the subject specific value of the continuous covariate; MedCov was the median of Cov. θCL and θCov were estimated fixed effect parameters.
The effect of a discrete binary covariate (clinical trial location and sex) on PK parameter estimates was tested as well; the following example illustrates an example of the effect of sex on CL25, 26:
For male patients, sex was equal to 0, while for female patients sex was equal to 1. θSex was an estimated fixed effect parameter for covariate sex, representing the numerical differences in the typical CL value between females and males. Other parameters (eg, TVCL, ηj, θCL, and θSex) are described previously.
Categorical variables (2C19 genotype and race) with more than two categories were assigned to each of subgroup (i.e. RM/EM=1, IM/PM=2, missing=3). An example of incorporating effect of genotype on CL estimate was shown in the following expressions: 25, 26:
Where θCL1, θCL2, and θCL3 were typical values of CL for 2C19 RM/EM, IM/PM, and missing subpopulations, respectively.
The possible relationship between individual Bayesian estimates for each parameter and the covariates was initially assessed by a graphical method using R® (version 2.6.2). Continuous covariates (e.g., age, weight, and BMI) and discrete covariates (e.g., CYP2C19 genotype, clinical trial location, sex, and race) were incorporated into each parameter in a stepwise fashion. The covariate was retained in the model if the objective function value (OFV) was decreased by 3.84 when adding one additional fixed effect parameter into model (χ2 p < 0.05 df = 1). Goodness of fit plots were used as additional model selection criteria. Additional post-processing of NONMEM® outputs were performed using SPSS (version 14.0).
Results
Patient characteristics
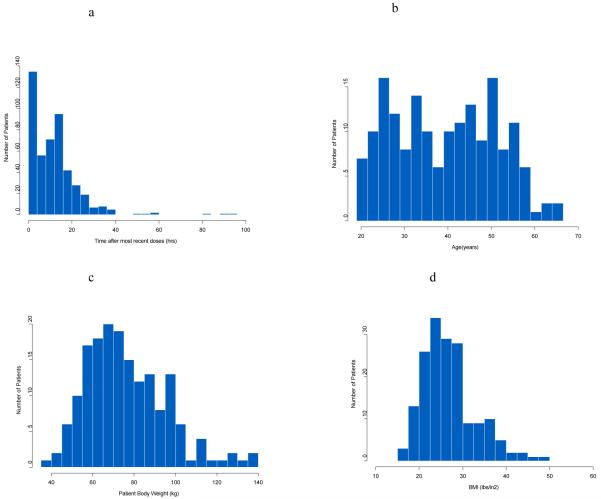
Patient demographics are summarized in Table 1. The average age of the subjects was 39.52 years with an average of body weight of 76.25 kg and average BMI of 27. The majority of subjects were white (n=161, 93%) with minority black/African American (n=8, 5%) and Asian subjects (n=3, 2%). The histogram distribution plot of the escitalopram sampling times after most recent dose is shown in Figure 1a. These sampling times were distributed in a broad time range. The distribution of sampling times provide more information to estimate population PK parameters of escitalopram compared to many population studies which only have trough sample available 27. The mean time after dose for concentration sampling the Pittsburgh patients was 11.99 hours and in the Pisa patients was 11.76 hours. Both groups had a large standard deviation around these times, specifically 11.4h for Pittsburgh and 11.8h for Pisa. Histograms of patient age, weight, and BMI are shown in Figure 1 panels b-d.
Table 1.
Patient demographics
| Demographics | Pittsburgh Patients | Pisa Patients | All patients |
|---|---|---|---|
| Number of Subjects | 105 | 67 | 172 |
| Number of Observations | 320 | 153 | 473 |
| Number of Observations for each subject | 3.048 | 2.2836 | 2.75 |
| CYP2C19 genotype | |||
| Rapid metabolizers (RM, *17/*17) | 4 | 1 | 5 |
| Extensive Metabolizers (EM, *17/*1,*1/*1) |
54 | 23 | 77 |
| Intermediate Metabolizers (IM, *1/*2, *1/*3, *17/*2, *17/*3) |
28 | 15 | 43 |
| Poor Metabolizers (PM, *2/*2, *3/*3, *2/*3) |
3 | 0 | 3 |
| Missing | 16 | 28 | 44 |
| Age, Mean Years ± SD (range) | 38.84 ± 12.05 (20.4-64.67) | 40.58 ± 11.20 (21-65) | 39.52 ± 11.73 (20.41 - 65) |
| Weight, mean kg ± SD (range) | 81.6 ± 20 ( 31.9 - 139.7) | 67.8 ± 15.2 (40-116) | 76.25 ± 19.45 ( 31.9-139.7) |
| BMI, mean lbs/in2 ± SD (range) | 28.20 ± 6.78 (15.55 - 48.26) | 24.94 ± 4.52 (16.63-37.41) | 26.93 ± 6.20 (15.55 - 48.26) |
| Sex, n (%) | |||
| Male | 47 (45) | 7 (10) | 54 (31) |
| Female | 58 (55) | 60 (90) | 118 (69) |
| Race, n (%) | |||
| White | 94 (89) | 67 (100) | 161 (93) |
| Black/African American | 8 (8) | 0 (0) | 8 (5) |
| Asian | 3 (3) | 0 (0) | 3 (2) |
Figure 1.
a: Frequency histogram showing the distribution of the sampling time after most recent doses (hrs)
b: Frequency histogram of patient age
c: Frequency histogram of patient body weight
d: Frequency histogram of patient BMI
All markers were in Hardy-Weinberg equilibrium as confirmed by Pedstats software1. DNA samples from 128 patients out of 172 were available for genotyping. Allele *3 has not been detected in our samples. According to the methods described by Rudberg et al.24, weidentified 5 RM (4 Pittsburgh vs 1 Pisa), 77 EM (54 Pittsburgh vs 23 Pisa), 43 IM (28 Pittsburgh vs 15 Pisa), and 3 PM (3 Pittsburgh vs 0 Pisa). CYP2C19 frequency for RM, EM, IM, and PM were 3.9%, 60.2%, 33.6%, and 2.3%, respectively, among all detected samples. The frequency of RM, EM, IM, and PM were 4.5%, 60.7%, 31.5%, 3.4% for the Pittsburgh site and 2.6%, 59.0%, 38.5%, 0% for the Pisa site, respectively. No DNA samples were available for genotyping for 44 patients out of 172 total. Among these 44 patients, 16 were from Pittsburgh and 28 were from Pisa.
Population pharmacokinetic modeling
A one-compartment model with linear elimination and proportional residual error best described the escitalopram pharmacokinetics in this patient population. Oral clearance (CL/F) and volume of distribution (V/F) in the patient population for escitalopram were 23.5 L/h and 884 L in base model without incorporating any patient specific covariates, respectively. Patient CYP2C19 genotype, age, and weight had a significant impact on CL/F estimates, and patient BMI significantly affects V/F estimates.
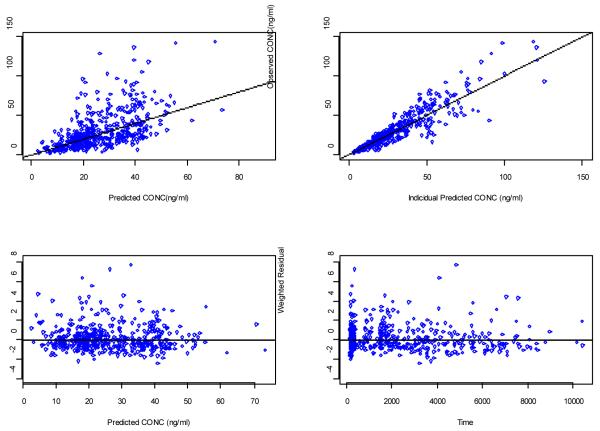
Clinical trial location was a significant covariate for CL/F in the initial univariate forward analysis. However, the effect of clinical site in CL/F disappeared after controlling for patient CYP2C19 genotype, age, and weight effects on CL/F. The process of final model development is summarized in Table 2. Figure 2 shows diagnostic plots for the final model. The scatter plots of the observed versus predicted population concentrations and observed versus predicted individual concentrations were distributed symmetrically around the line of unity. The weighted residuals were distributed symmetrically around zero. No systematic shift in residuals was evident from the plots of weighted residual versus predicted population concentrations and weighted residual versus time after dose.
Table 2.
Population pharmacokinetic model development for escitalopram
| Covariate | Model | −2LL | Δ −2LL | P value | |
|---|---|---|---|---|---|
| 1 | Base model |
2729.78 | |||
| Univariate Forward Selection | |||||
| 1~1 (CL) | CYP2C19 Genotype | M1 | 2721.40 | −8.38 | < 0.05 |
| Age (Centered Power Model) | M2 | 2724.24 | −5.54 | < 0.05 | |
| Weight (Centered Power Model) | M3 | 2725.15 | −4.64 | < 0.05 | |
| BMI (Centered Power Model) | M4 | 2729.30 | −0.49 | > 0.05 | |
| Sex | M5 | 2748.44 | 18.66 | > 0.05 | |
| Race | M6 | 2726.60 | −3.18 | > 0.05 | |
| Site (Pittsburgh vs. Pisa) | M7 | 2719.78 | −10.01 | < 0.05 | |
| 1~2 (V) | Weight (Centered Power Model) | M8 | 2729.08 | −0.70 | > 0.05 |
| BMI (Centered Power Model) | M9 | 2725.93 | −3.85 | < 0.05 | |
| Age (exponential model) | M10 | 2729.69 | −0.09 | > 0.05 | |
| Site (Pittsburgh vs. Pisa) | M11 | 2728.42 | −1.36 | > 0.05 | |
| Stepwise Backward Elimination | |||||
| 2~1 | CL(Genotype+Age+Wgt)+V(BMI) | M12 | 2707.11 | ||
| Eliminate Weight from CL | M13 | 2711.23 | 4.13 | < 0.05 | |
| Eliminate Age from CL | M14 | 2712.83 | 5.72 | < 0.05 | |
| Eliminate Genotype from CL | M15 | 2715.02 | 7.91 | < 0.05 | |
| Eliminate BMI from V | M17 | 2712.65 | 5.54 | < 0.05 | |
| 3~1 | CL(Genotype+Age+Wgt+Site)+V(BMI) | M18 | 2703.79 | −3.32 | > 0.05 |
| final model | CL(Genotype+Age+Wgt)+V(BMI) | M12 | 2707.11 | ||
Figure 2.
Diagnostic plots of final PK model. (A) Population predicted vs observed concentrations (B) Individual predicted vs observed concentrations (C) Weighted residuals versus concentration (D) weighted residuals versus time.
Estimates for the full set of population PK parameters along with the standard errors for final model are listed in Table 3. Patient genotype was initially included as a model covariate on CL/F five categories: RM (n=5) vs EM (n=77) vs IM (n=43) vs PM (n=3) vs missing (n=44). This was followed with a three category analysis by pooling RM and EM into one subpopulation (n=83), and IM and PM into one subpopulation(n=46). Both models showed that the CYP2C19 genotype is a significant covariate affecting escitalopram clearance estimates. However, breaking genotype into five categories did not improve model fit compared to the three category model. Estimated population CL/F (arising from the posterior mode of the marginal likelihood distribution for this parameter) for CYP2C19 RM/EM (n=83) and IM/PM (n=46) and the individuals missing genetic information (n=44) were 26, 19.8 , and 21.5 L/Hr, respectively.
Table 3.
Escitalopram pharmacokinetic parameter from final model
| Parameters | Final Model Estimates | SE% |
|---|---|---|
| CL for 2C19 Rapid and Extensive (L/Hr) | 26 | 7.20% |
| CL for 2C19 IM and PM (L/Hr) | 19.8 | 8.50% |
| CL for 2C19 missing (L/Hr) | 21.5 | 7.80% |
| Age on Clearance | CL1=CL0*(Age/40) −0.336 | 42.00% |
| Weight on Clearance | CL2=CL1*(Wgt/76) 0.333 | 54.10% |
| V (L) | 947 | 10.20% |
| BMI on V | V*(BMI/27) 1.11 | 49.50% |
| Ka (hr−1) | 0.8 | N/A |
| ωcl % | 48.5% | 15.10% |
| ωv % | 62.0% | 40.30% |
| ωKa % | 78.9% | 87.00% |
| ωcl,v % | 9.4% | N/A |
| ωcl,Ka % | 47.8% | N/A |
| ωV,Ka % | 81.3% | N/A |
| σ1 % | 28.9% | 8.80% |
CL, clearance; V, volume of distribution; SE, standard error; ω, coefficient of variation of inter-individual variability; σ, coefficient of variation of residual error
CL/F significantly decreased in a centered power function model as patient age increased. The age relationships are shown as: CL/F= CL0*(age/40)−0.336 L/hr. The clearance also increased with increasing weight with the following relationship:
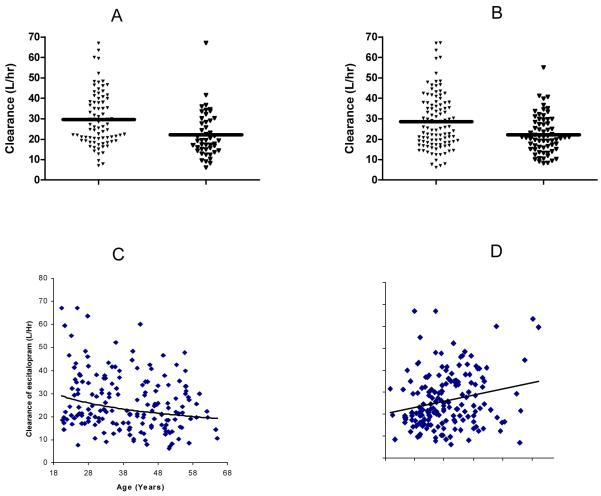
Post-processed individual empirical Bayes estimates on CL/F for the 172 patients in different subpopulations are summarized in Table 4. RM/EM cleared escitalopram 33.7% faster than IM/PM subpopulation with mean CL/F (± SD) values of 29.73 ± 13.13 and 22.23 ± 11.04, respectively (P<0.005). A one way ANOVA revealed a significant difference (P<0.005) in the empirical Bayesian estimated CL/F of escitalopram across three age groups (20~30, 30~50, 50~65 years of age with mean CL/F (± SD) values of 31.03 (±14.88) L/h, 25.71(±11.31) L/h, and 21.74 (±9.89), respectively). Patients younger than 30 years (n=45) cleared escitalopram 20.7% and 42.7% faster than patients aged 30-50 years (n=84) and 51 years of age or older (n=43), respectively. Pittsburgh patients (n=105) cleared escitalopram 28% (P<0.001) faster than patients from Pisa, Italy (n=67) with mean CL/F (± SD) values of 28.55 ± 13.54 and 22.28 ± 9.32, respectively (P<0.001).The scatter plots of the empirical Bayes estimates for CL/F (n=172) by 2C19 genotype, clinical trial location, age, weight are shown in figure 3. Patient BMI, Sex, and Race were not significant covariates affecting CL/F.
Table 4.
Post-processed individual empirical Bayes estimates on clearance
| Population | Mean Clearance (L/Hr) |
Standard Deviation (L/Hr) |
P value |
|---|---|---|---|
| Genotype information | <0.005 | ||
| Rapid and Extensive (n=82) | 29.73 | 13.13 | |
| IM and PM (n=46) | 22.23 | 11.04 | |
| Missing (n=44) | 23.41 | 10.65 | |
| Age | <0.005 | ||
| < 30 years old (n=45) | 31.03 | 14.88 | |
| 30~50 years old (n=84) | 25.71 | 11.31 | |
| 50~65 years old (n=43) | 21.74 | 9.89 | |
| Clinical Trial Location | <0.001 | ||
| Pittsburgh patients (n=105) | 28.55 | 13.54 | |
| Pisa patients (n=67) | 22.28 | 9.32 |
Figure 3.
Escitalopram clearance by (A)CYP2C19 genotype, (B) clinical trial location, (C) age and (D) weight
Estimated V/F increased in a power function relationship (V/F=V0*(BMI/27)1.11) with patient BMI with estimated exponent of 1.11. Patient body weight, age, and clinical location did not significantly affect V/F estimates.
Discussion
In this study, we successfully captured population and individual level exposure information for escitalopram in patients with major depression using sparsely sampled data. This study showed that apparent clearance of escitalopram varies nearly 10-fold in patients (n=172) with major depression, ranging from 6.24 to 67.10 L/h. CYP2C19 genotype, age, and weight were identified as significant contributors to the variability in escitalopram clearance in this patient population. This extends previous findings that showed a correlation with functional capacity of the CYP2C19 enzyme using Mephenytoin S/R enantiomer excretion ratios as a correlate of population clearance and weight as a correlate of population weight22. This study also establishes a population pharmacokinetic model that incorporates data for many more subjects (n=172) than previous models (n=24)22.
Recently, Rudberg et al 24 showed the impact of the 2C19*17 polymorphisms on escitalopram concentrations in Norwegian psychiatric patients. In these patients, individuals homozygous for the CYP2C19 *17/*17 alleles showed a 42% reduction in observed concentrations. In our study, however, no difference in clearance between CYP2C19 *17/*17 alleles (RM,n=5) and *17/*1, *1/*1 alleles (EM, n=77), and no difference between heterozygous *2 allele (IM, n=43) and homozygous *2 allele (PM, n=4) were identified. This may be attributed to the small number of patient with *17/*17 and homozygous *2/*2 alleles. However, this study showed that RM/EM cleared escitalopram 33.7% faster than IM/PM subpopulation with mean CL/F (± SD) values of 29.73 ± 13.13 and 22.23 ± 11.04, respectively (P<0.005).
A significantly lower escitalopram elimination rate (50%) in elderly patients (≥65 years old) compared to younger healthy volunteers was reported previously 21 (www.cipralex.com/images/cipralex/smpc.pdf; www.lexapro.com). This finding is confirmed in our study and extended as a continuous relationship across age. This is consistent with reports that CYP 2C19 activity decreases with increasing age 28 and is now more specifically quantified in the case of escitalopram. The CL/F significantly decreased in a centered power function model as patient age increased. Patients younger than 30 years cleared escitalopram 20.7%, and 42.7%faster than patients 30~50, and >50 years, respectively. Hence, the dose of escitalopram may need to be adjusted clinically based on patient age, especially for those over 50. This change in clearance and therefore exposure may be of particular concern in patients with panic symptoms as these individuals may be more sensitive to concentration-related side effects. A concentration related amygdala activation with acute administration of citalopram may be related to panic and anxiety symptomatology29.
Elderly patients are at highest risk of completed suicides in the first month of treatment when therapy is not fully tailored and excessive exposure may occur 30. Long-term excessive exposure in the elderly may lead to an increased risk of hyponatremia 31, GI bleed secondary to platelet-related effects 32, 33, an increased risk of falls and fragility fractures 34 as well as bradycardia 35.
Clinical trial location was a significant covariate in the initial univariate forward analysis. (Table 2). There may be several contributors to this systematic difference in elimination across site. In our study, correlation between clinical trial location (Pittsburgh vs Pisa) and 2C19 genotype was not significant. The frequencies of RM/EM and IM/PM were 65.2%, 34.8% for Pittsburgh site and 61.5%, 38.5% for Pisa site, respectively. Hence, patients from Pittsburgh are virtually indistinguishable from Pisa patients on the basis of genotype frequencies.
However, the weights and BMI values were significantly different (approximately 14 kg heavier and 3.3 BMI points larger in Pittsburgh) for the two sites. When genotype, age, and weight were incorporated as a covariate affecting clearance, the site factor no longer had a significant impact on clearance (the inclusion of clinical site after incorporating genotype, age, and weight together only improved the model fit by 3.32 objective function points (p>0.05)). This is in contrast to earlier analyses where genotype information was not available and weight was not a significant covariate on clearance (data not shown). Post-processed individual empirical Bayes estimates on CL/F, which includes the effect of genotype, age, and weight differences between locations, shows that Pittsburgh patients cleared escitalopram 28% faster than patients from Pisa, Italy. The resulting difference in exposure for a given dose may partially explain the difference in tolerability for escitalopram between Pittsburgh and Pisa patients. In this case, at a given dose, patients in Pisa were more likely to experience a higher concentration exposure. However, the difference was accounted for by differences in CYP2C19 genotype, age, and body weight. Therefore, the dosage regimen for a patient may need to be adjusted on the basis of genotype predicted phenotype, age and weight.
Sex has not been reported to exert clinically significant effect on PK parameters of escitalopram in healthy volunteers14, 21. This was confirmed in the population PK analysis described herein. Similarly, race did not have a statistically significant effect on the PK parameter estimation. However, 93% of patients in this study were white, 5% were Black/African American and 2% were Asian. It is possible that the relatively small percentage of African Americans and Asians in this study prevented the detection of any systematic difference across race.
In conclusion, apparent clearance (CL/F) and volume of distribution (V/F) for escitalopram in the patient population were 23.5 L/h and 884 L, respectively. This is consistent with preliminary population analyses reported by Areberg22. CYP 2C19 genotype, age, and weight strongly influenced the CL/F of escitalopram. Patients with CYP2C19 RM/EM cleared escitalopram significantly faster than those with 2C19 IM/PM and older patients had a significantly lower apparent clearance compared with younger patients. Patients with higher body weights cleared escitalopram faster compared to those with lower body weights. Incorporating age weight and genotype into the population PK model accounted for the majority of the variability in escitalopram exposure in this study.
Therefore, establishing a patient’s metabolizer genotype and incorporating age, weight and BMI into this assessment can better guide therapeutic decision-making with respect to the dosing strategy for escitalopram and potentially minimize excessively high exposure to this SSRI. What is of particular note for community practice is that two of these variables (age and weight) are routinely collected and require no specialized equipment or laboratory tests. Thus, physicians can readily take these variables into account when determining appropriate starting dose and upward titration schedules.
Acknowledgments
Financial Support: Sandra A. Rotman Chair in Neuropsychiatry (Pollock) ; MH65376 (PI: E. Frank) and MH30915 (PI: E. Frank); Fondazione IDEA, Italy (PI: GB Cassano) and a grant from Forrest Research Institute.
References
- 1.Thase ME. Managing depressive and anxiety disorders with escitalopram. Expert Opin Pharmacother. 2006;7:429–440. doi: 10.1517/14656566.7.4.429. [DOI] [PubMed] [Google Scholar]
- 2.Demyttenaere K, Bruffaerts R, Posada-Villa J, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. Jama. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- 3.Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63:331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- 4.Rapaport MH, Bose A, Zheng H. Escitalopram continuation treatment prevents relapse of depressive episodes. J Clin Psychiatry. 2004;65:44–49. doi: 10.4088/jcp.v65n0107. [DOI] [PubMed] [Google Scholar]
- 5.Wade A, Michael Lemming O, Bang Hedegaard K. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2002;17:95–102. doi: 10.1097/00004850-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Davidson JR, Bose A, Korotzer A, Zheng H. Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo controlled, flexible-dose study. Depress Anxiety. 2004;19:234–240. doi: 10.1002/da.10146. [DOI] [PubMed] [Google Scholar]
- 7.Stahl SM, Gergel I, Li D. Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2003;64:1322–1327. doi: 10.4088/jcp.v64n1107. [DOI] [PubMed] [Google Scholar]
- 8.Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- 9.Gorman JM, Korotzer A, Su G. Efficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: pooled analysis of placebo-controlled trials. CNS Spectr. 2002;7:40–44. doi: 10.1017/s1092852900028595. [DOI] [PubMed] [Google Scholar]
- 10.Lepola U, Wade A, Andersen HF. Do equivalent doses of escitalopram and citalopram have similar efficacy? A pooled analysis of two positive placebo-controlled studies in major depressive disorder. Int Clin Psychopharmacol. 2004;19:149–155. doi: 10.1097/00004850-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Crone CC, Gabriel GM. Treatment of anxiety and depression in transplant patients: pharmacokinetic considerations. Clin Pharmacokinet. 2004;43:361–394. doi: 10.2165/00003088-200443060-00002. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery SA, Huusom AK, Bothmer J. A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology. 2004;50:57–64. doi: 10.1159/000078225. [DOI] [PubMed] [Google Scholar]
- 13.Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46:281–290. doi: 10.2165/00003088-200746040-00002. [DOI] [PubMed] [Google Scholar]
- 14.Sogaard B, Mengel H, Rao N, Larsen F. The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J Clin Pharmacol. 2005;45:1400–1406. doi: 10.1177/0091270005280860. [DOI] [PubMed] [Google Scholar]
- 15.Frank E, Cassano GB, Rucci P, et al. Addressing the challenges of a cross-national investigation: lessons from the Pittsburgh-Pisa study of treatment-relevant phenotypes of unipolar depression. Clin Trials. 2008;5:253–261. doi: 10.1177/1740774508091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheiner LB, Rosenberg B, Marathe VV. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977;5:445–479. doi: 10.1007/BF01061728. [DOI] [PubMed] [Google Scholar]
- 17.Williams PJ, Ette EI. The role of population pharmacokinetics in drug development in light of the Food and Drug Administration’s ‘Guidance for Industry: population pharmacokinetics’. Clin Pharmacokinet. 2000;39:385–395. doi: 10.2165/00003088-200039060-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14:553–560. doi: 10.18553/jmcp.2008.14.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bies RR, Feng Y, Lotrich FE, et al. Utility of sparse concentration sampling for citalopram in elderly clinical trial subjects. J Clin Pharmacol. 2004;44:1352–1359. doi: 10.1177/0091270004269647. [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Pollock BG, Kirshner M, Frank E, Gastonguay MR, Kepple G, Feng Y, Bies RR. Effect of different dosing report methods on estimation of pharmacokinetic parameters for escitalopram. J of Clinical Pharmacology. 2007;47:1205. doi: 10.1177/0091270008327538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhillon S, Scott LJ, Plosker GL. Escitalopram: a review of its use in the management of anxiety disorders. CNS Drugs. 2006;20:763–790. doi: 10.2165/00023210-200620090-00010. [DOI] [PubMed] [Google Scholar]
- 22.Areberg J, Christophersen JS, Poulsen MN, et al. The pharmacokinetics of escitalopram in patients with hepatic impairment. Aaps J. 2006;8:E14–19. doi: 10.1208/aapsj080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenborg S, Mwinyi J, Andersson M, et al. Kinetics of omeprazole and escitalopram in relation to the CYP 2C19*17 allele in healthy subjects. European Journal of Clinical Pharmacology. 2008;64:1175–1179. doi: 10.1007/s00228-008-0529-z. [DOI] [PubMed] [Google Scholar]
- 24.Rudberg I, Mohebi B, Hermann M, et al. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther. 2008;83:322–327. doi: 10.1038/sj.clpt.6100291. [DOI] [PubMed] [Google Scholar]
- 25.Bigos KL, Pollock BG, Coley KC, et al. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48:157–165. doi: 10.1177/0091270007310385. [DOI] [PubMed] [Google Scholar]
- 26.Feng Y, Pollock BG, Ferrell RE, et al. Paroxetine: population pharmacokinetic analysis in late-life depression using sparse concentration sampling. Br J Clin Pharmacol. 2006;61:558–569. doi: 10.1111/j.1365-2125.2006.02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth BP, Gobburu JV. Considerations in analyzing single-trough concentrations using mixed-effects modeling. J Clin Pharmacol. 2003;43:1307–1315. doi: 10.1177/0091270003258670. [DOI] [PubMed] [Google Scholar]
- 28.Pollock BG, Perel JM, Kirshner M, et al. S-mephenytoin 4-hydroxylation in older Americans. Eur J Clin Pharmacol. 1991;40:609–611. doi: 10.1007/BF00279979. [DOI] [PubMed] [Google Scholar]
- 29.Bigos KL, Pollock BG, Aizenstein HJ, et al. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juurlink DN, Mamdani MM, Kopp A, Redelmeier DA. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry. 2006;163:813–821. doi: 10.1176/ajp.2006.163.5.813. [DOI] [PubMed] [Google Scholar]
- 31.Fabian TJ, Amico JA, Kroboth PD, et al. Paroxetine-induced hyponatremia in older adults: a 12-week prospective study. Arch Intern Med. 2004;164:327–332. doi: 10.1001/archinte.164.3.327. [DOI] [PubMed] [Google Scholar]
- 32.Dalton SO, Sorensen HT, Johansen C. SSRIs and upper gastrointestinal bleeding: what is known and how should it influence prescribing? CNS Drugs. 2006;20:143–151. doi: 10.2165/00023210-200620020-00005. [DOI] [PubMed] [Google Scholar]
- 33.Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol. 2000;20:137–140. doi: 10.1097/00004714-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Richards JB, Papaioannou A, Adachi JD, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- 35.Barak Y, Swartz M, Levy D, Weizman R. Age-related differences in the side effect profile of citalopram. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:545–548. doi: 10.1016/S0278-5846(03)00041-1. [DOI] [PubMed] [Google Scholar]