Ultrasensitive molecular diagnostic tools allow early identification of disease-related biomarkers before advanced symptoms manifest.
Abstract
Clinical decision making stands to benefit from ultrasensitive molecular diagnostic tools that identify disease-related changes in biomarker levels before advanced clinical signs and symptoms manifest.
Decades of biopharmaceutical research point toward one universal and immutable conclusion: the earlier a disease is detected, the greater the likelihood that treatments will alleviate or stabilize the disease process effectively. As the pharmaceutical industry strives to develop effective new therapies for diseases — from cancers to cardiovascular and neurodegenerative disorders to a host of metabolic, infectious, and genetic conditions — the need for earlier diagnosis to help ensure positive outcomes has come to dominate the mindsets of researchers, clinicians, patients, and third-party payers.
The development of new molecular diagnostic methods capable of detecting disease at the molecular level in blood, cerebrospinal fluid, and other body specimens is central to the emerging revolution in disease diagnosis. Using specific and targeted protein and nucleic acid (i.e., DNA and RNA) biomarkers, clinicians will be able to detect diseases and confirm diagnoses very early on, perhaps even before patients present with clinical signs and symptoms, when a disease is most amenable to successful treatment. Development of the tools and technology needed to bring molecular diagnostic tests into the clinical mainstream will have a profound effect on the discovery of novel biomarkers, which will, in turn, accelerate drug discovery research and further efforts to screen targeted populations for a variety of common medical disorders and risk factors for disease.
Clinicians and lab technicians could rely on a combination of nonquantitative, costly, and complicated molecular diagnostic techniques that detect protein or nucleic acid biomarkers separately, primarily in the reference laboratory or highly sophisticated laboratory setting. Yet, the availability of a highly sensitive, automated, cost-effective, and easy-to-use diagnostic platform that is capable of rapidly and reliably identifying both protein and nucleic acid biomarkers (often present in minute concentrations in biological specimens) could deliver the power of molecular diagnostics to both reference laboratories and community hospital laboratories.
In addition, for many disorders, biomarker discovery research — using this tool or other methods — may identify highly specific protein and nucleic acid markers that are key to targeted detection of early stage pathology. Also, molecular diagnostic tools that are capable of performing multiplexed assays — that is, tests that simultaneously detect multiple protein biomarkers and/or genetic mutations (i.e., single nucleotide polymorphisms [SNPs] of DNA and RNA) — will save time and resources and, more importantly, will allow clinicians to detect patterns of disease-related markers. Multiplexing will enable diagnoses based on a more informative assessment of panels of biomarkers that could signal the presence of, or predisposition to, a disease and would provide information on disease stage and aggressiveness that could contribute to the determination of prognosis and the course of effective patient management.
THE NEED
Medical schools do a very good job of teaching budding physicians how to detect diseases when a patient presents with easily observable classical signs and symptoms. A host of diagnostic tests — from imaging studies to laboratory-based analyte measurements to biopsies of affected tissues — are available to supplement and support clinical findings and to guide physicians through the decision-making process that leads to a diagnosis and treatment plan. Nonetheless, these diagnostic strategies often are based on discovering disease that already has caused irreversible damage to organs, tissues, and vital biochemical and physiological processes.
Alzheimer’s disease, ovarian cancer, and coronary artery disease represent three potent examples of the need for a paradigm shift in disease diagnostics. There is now potential for the detection of protein and nucleic acid biomarkers with ultrasensitive molecular diagnostic tools that identify disease-related changes in biomarker levels before they manifest with advanced clinical signs and symptoms secondary to disease progression.
Alzheimer’s disease often presents with nonspecific cognitive changes and is extremely difficult to detect in its early stages (i.e., mild cognitive impairment, prior to significant beta-amyloid plaque formation). Currently, Alzheimer’s disease can be diagnosed definitively only on postmortem examination. Promising therapies now in development have the potential to be more effective if treatment can be initiated earlier in the natural history of the disease. Such neurodegenerative diseases are likely to be more susceptible to drugs capable of slowing their progression at an early stage, thereby prolonging the time between diagnosis and the appearance of more debilitating clinical symptoms that compromise patient function and quality of life.
Researchers at Northwestern University have discovered toxic derivatives of beta-amyloid, one of a family of proteins in the plaques and neurofibrillary tangles characteristically found in the brains of individuals with Alzheimer’s disease on postmortem evaluation (Lambert 1998). Called amyloid-derived diffusible ligands (ADDLs), these derivatives are more neurotoxic than beta-amyloid itself, and they could serve as early indicators of Alzheimer’s disease. Similarly, a phosphorylated form of the tau protein, p-tau-231 — also implicated in the brain pathology that is linked to Alzheimer’s disease — may be a more sensitive diagnostic marker than the tau protein itself, as it is a precursor to tau that appears earlier in the disease course (deLeon 2002, Hampel 2004). Both these biomarkers are present in extremely low concentrations (<1 picomolar) in the cerebrospinal fluid (CSF) of patients with Alzheimer’s disease, concentrations below the levels reliably detectable with available assay technology.
The small sizes of ADDLs and p-tau-231 may allow these molecules to cross the blood-brain barrier, suggesting that it might be possible to develop an assay to detect their presence in blood. This would allow physicians to rely on a blood sample to diagnose and monitor the disease, eliminating the need for the more complicated, potentially risky, and uncomfortable procedure necessary to obtain a sample of CSF.
Even as the discovery of new biomarkers for cancer progresses at a promising pace, current assay technologies lack the limits of detection (analytical sensitivity) needed to identify these biomarkers in biological samples efficiently, reproducibly, and cost-effectively. Ovarian cancer is a good example. In nearly two thirds of women with this disease, the tumor typically is not detected until it has progressed to an advanced stage when the 5-year life expectancy is only 12 to 39 percent (Johns Hopkins 2001, 2002). Though the 5-year survival rate for all stages of ovarian cancer combined is only 35 to 38 percent, these survival rates can reach 90 to 98 percent if the diagnosis is made early in the disease course (Medline 2005).
Researchers at the U.S. Food and Drug Administration and the National Cancer Institute have reported that qualitative mass spectroscopy patterns of proteins in patients’ blood could distinguish between ovarian cancer and control samples (Petricoin 2002). Ongoing research is focusing also on inhibin, a protein that antagonizes the action of another protein, activin, and which may have a role as a potent and specific biomarker for one form of ovarian cancer (Robertson 2004).
Whereas the CA-125 protein routinely used to screen and monitor patients for ovarian cancer can signal the presence of the most common type of ovarian cancer — which is epidermal in origin and which represents about 90 percent of ovarian cancers, it is not particularly useful for detecting the 10 percent or so of granulosa cell tumors. In contrast, inhibin levels are increased in blood samples taken from postmenopausal women with granulosa cell ovarian tumors.
Diagnostic test results, combining detection of CA-125 and inhibin, have been presented that may detect 95 percent of all ovarian cancers with 95 percent specificity (Robertson 2004). More precisely, it has been reported that inhibin is almost 100 percent accurate for granulosa cell ovarian tumors, CA-125 is about 60 percent accurate for epidermal ovarian tumors, and an assay of CA-125 plus inhibin is about 90 percent accurate for epidermal ovarian tumors. Inhibin and other potential protein biomarkers for ovarian cancer (e.g., soluble epidermal growth factor receptor, Mullerian inhibitory substance) are present in extremely low concentrations in blood that cannot be measured quantitatively with current methods.
Coronary artery disease represents another diagnostic area in which ultrasensitive protein biomarkers may help guide clinical decision making. In the United States, approximately 8 million patients with chest discomfort present to the emergency department annually.
Measurements of blood levels of a highly specific protein, cardiac troponin, represent the gold standard for diagnosing an acute myocardial infarction (MI). The troponin level rises as heart muscle breaks down, but the troponin level cannot be detected as abnormal until it reaches more than 0.01 to 0.10 ng/mL, depending on the given assay. It takes 4 to 6 hours from the first cardiac symptoms for the troponin level to rise above this abnormal threshold. The ability to detect an initial burst of troponin from the myocardial cell, which appears in the blood within an hour after MI symptoms, may enable a more rapid diagnosis and subsequent treatment of MI.
Multiplexing will enable diagnoses based on a more informative assessment of biomarker panels, providing better disease prognosis and more effective patient management.
Likewise, a more sensitive troponin assay may allow clinicians to differentiate unstable angina (UA) from less dangerous forms of chest pain. Patients who are experiencing UA are at high risk of having another cardiac event within the next 30 days. Frequently, though, UA goes undetected, because troponin levels remain below the current thresholds of abnormality. These UA patients may be sent home without the administration of any form of therapy, often with fatal consequences (i.e., up to 10 to 20 percent mortality). An ultra-sensitive cardiac troponin test capable of detecting extremely low levels of troponin (e.g., limit of detection of 0.0001 ng/mL) may be useful in the diagnosis of UA and in identifying patients who require treatment and hospitalization even though their troponin level never reaches the current MI threshold.
A TECHNOLOGY PLATFORM
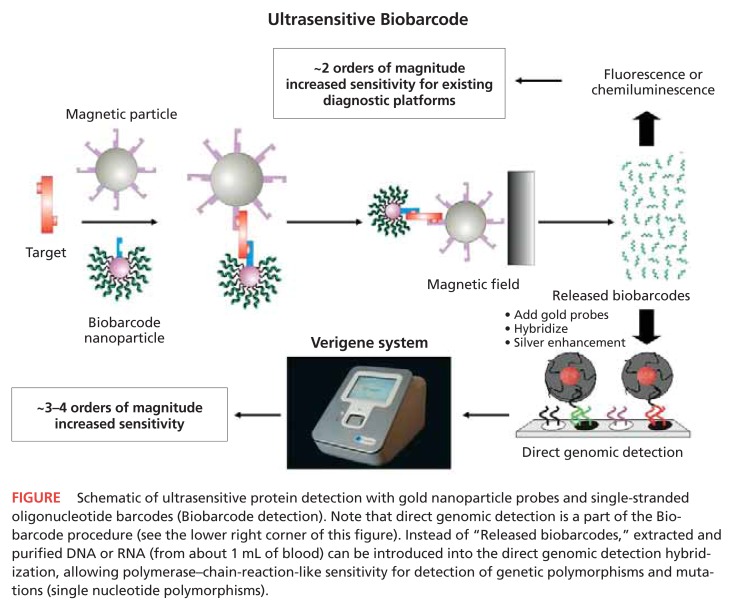
To address the challenges presented above, Nanosphere Inc. (Northbrook, Ill.) has developed a technology platform (Verigene System)1 based on the company’s laboratory-proven nanoparticle (gold) probe and Biobarcode1 technology. This system design enables ultrasensitive, multiplexed detection of both protein (Biobarcode detection) and nucleic acid (direct genomic detection) biomarkers, using enhanced signal amplification techniques (see Figure, next page).
FIGURE.
Schematic of ultrasensitive protein detection with gold nanoparticle probes and single-stranded oligonucleotide barcodes (Biobarcode detection). Note that direct genomic detection is a part of the Biobarcode procedure (see the lower right corner of this figure). Instead of “Released biobarcodes,” extracted and purified DNA or RNA (from about 1 mL of blood) can be introduced into the direct genomic detection hybridization, allowing polymerase–chain-reaction-like sensitivity for detection of genetic polymorphisms and mutations (single nucleotide polymorphisms).
The scientific basis of the technology came from two world-renowned Northwestern University professors, Chad A. Mirkin, PhD, and Robert L. Letsinger, PhD. Mirkin, a pioneer in the development of ultrasensitive and highly selective assays based on nanostructures, is director of Northwestern University’s International Institute for Nanotechnology and is one of the most influential figures in nanotechnology. Letsinger developed the chemistry behind the modern day “gene machines.” Together, these scientists developed the foundation for the technology in the Verigene System, including the processes to create the Biobarcode nanoparticles.
The combination of gold nanoparticle and Biobarcode technology enables detection of extremely low levels of proteins — far below levels that are detectable using routine enzyme-linked immunosorbent assay (ELISA), Western blot, or other currently available assay methods. The Biobarcode nanoparticle assay achieves signal amplification in two ways: 1) the multiplicity of identical barcodes (about 100 to 1,000) released as a result of each target protein molecule that is captured (Multiplexing occurs here by changing the capture antibodies and barcode sequence for each specific analyte in a panel.); and 2) a silver-enhanced optical detection method. In comparison with conventional ELISA-based diagnostic assays, Biobarcode technology is 1,000- to 10,000-fold more sensitive, with a detection limit that is in the attomolar range.
CONCLUSIONS
The need for ultrasensitive detection of biomarkers was presented above, using only three examples — Alzheimer’s disease, ovarian cancer, and coronary artery disease. Nonetheless, myriad other clinical applications exist wherein enhanced diagnostic assay sensitivity could improve the health of at-risk populations worldwide. Some general need areas include:
- Oncology
- Prostate cancer recurrence, after surgery or radiation therapy*
- Ovarian cancer*
- Other cancers (lung, pancreatic, colon, uterine, renal, bladder)
- Neurodegenerative diseases
- Alzheimer’s disease (Georganopoulou 2005)*
- Parkinson’s disease
- Other protein-folding disorders
- Cardiovascular diseases
- Myocardial ischemia (coronary artery disease)*
- Infectious diseases
- HIV*
- Transmissible spongioform encephalopathies — prion proteins*
- Variant Creutzfeldt-Jakob disease — humans
- Bovine spongiform encephalopathy — cows
- Chronic wasting disease — deer, elk
- Scrapie — sheep
- Sepsis
- Genetic abnormalities
- Down’s syndrome
- Hypercoagulability (Factor V Leiden, Factor II, methylene tetrahydrofolate reductase)*
- Cystic fibrosis*
- Cytochrome P450*
For the disease states marked with an asterisk above, Nanosphere (and perhaps other companies) either have initiated testing of feasibility or are further along in ultra-sensitive assay development. In these assay development initiatives, the nanoparticle probe strategy offers several unique advantages when compared with traditional ELISA assays (for protein detection) and polymerase–chain-reaction (PCR)-based target amplification methods (for genetic detection). The strategy eliminates the need for other, more costly and time-consuming approaches, such as PCR amplification for current genetic detection and qualitative mass spectroscopy or immuno-PCR for current protein detection.
With direct genomic detection, which represents the first commercial application of the nanoparticle probe technology, signal amplification without the need for PCR in an automated, cost-effective system will provide an economically feasible, easy-to-perform method of detecting genomic markers. The first products based on the direct genomic technology will include assays for hypercoagulability, cystic fibrosis, and cytochrome P450 enzymes (to assess drug metabolism).
Ultrasensitive detection of protein and nucleic acid biomarkers not only will enable screening for and early detection of diseases with established diagnostic biomarkers, but it will improve biomarker discovery research for both clinical diagnostic applications and drug development. It also will have a role in advancing pharmacogenomics and efforts to improve blood screening. As new biomarkers are identified and used in the clinical arena to diagnose, stage, and monitor disease, the simplicity and efficiency of ultrasensitive detection technology should make it possible for smaller, community-based hospitals to access and implement the molecular tools and strategies that are at the forefront of advances in molecular diagnosis, risk stratification of diseases, and targeted therapeutics.
Footnotes
DISCLOSURES: Gregory Shipp, MD, reports that he does not have any financial arrangements or affiliations with corporate organizations that would constitute a conflict of interest with respect to this article.
Verigene and Biobarcode are trademarks of Nanosphere.
REFERENCES
- deLeon MJ, Segal S, Tarshish CY, et al. Longitudinal cerebrospinal fluid tau load increases in mild cognitive impairment. Neurosci Let. 2002;333:183–186. doi: 10.1016/s0304-3940(02)01038-8. [DOI] [PubMed] [Google Scholar]
- Georganopoulou DG, Chang L, Nam JM, et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc Natl Acad Sci. 2005;102:2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Buerger K, Zinkowski R, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- Johns Hopkins Pathology Ovarian Cancer: Prognostic Factors. 2001. Available at: « http://ovariancancer.jhmi.edu/prognosis.cfm». Accessed March 14, 2006.
- Johns Hopkins Pathology Ovarian Cancer: Significance of Early Detection. 2002. Available at: « http://ovariancancer.jhmi.edu/earlydx.cfm» Accessed March 14, 2006.
- Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Ab1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medline Plus. Medical Encyclopedia. Ovarian cancer. Available at: «http://www.nlm.nih.gov/medlineplus/ency/article/000889.htm». Accessed March 14, 2006.
- Petricoin EF, Ardekani AM, Hitt BA, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–577. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Pruysers E, Burger HG, et al. Inhibins and ovarian cancer. Mol Cell Endocrinol. 2004;225:65–71. doi: 10.1016/j.mce.2004.02.014. [DOI] [PubMed] [Google Scholar]