Abstract
To evaluate the utility of the QuantiFERON-TB Gold assay for monitoring latent tuberculosis treatment efficacy, the assay was performed serially for healthcare workers receiving isoniazid therapy. After 9 months of isoniazid therapy, all of these healthcare workers remained QuantiFERON-TB Gold positive, and cellular proliferation assays revealed persistently strong purified protein derivative responses. These results do not support the use of the QuantiFERON-TB Gold assay to monitor therapy.
In the United States, the QuantiFERON-TB Gold (QFT-G) assay, an interferon-γ release assay (IGRA), has been advocated for use in tuberculosis (TB) screening programs,1 including those for healthcare workers (HCWs). Standard guidelines2 recommend treatment for HCWs who meet the diagnostic criteria for having latent TB infection (LTBI). However, it is unknown whether IGRAs such as the QFT-G assay can be used as markers of response to therapy for LTBI.
Here, we evaluated whether newly hired HCWs who initially tested positive for Mycobacterium tuberculosis infection with the QFT-G assay on routine screening and who then received a standard, monitored course of LTBI therapy would subsequently test negative with the assay. Therefore, we evaluated serial QFT-G assay results before, during, and 3 months after completion of a 9-month isoniazid treatment course. In parallel, we assessed the effect of treatment on the cellular immune response to purified protein derivative in extended in vitro proliferation assays.
METHODS
Routine TB screening protocol
The Beth Israel Deaconess Medical Center Employee Occupational Health Services screens approximately 3,000 newly hired employees each year by tuberculin skin test; approximately 15% of these HCWs have positive tuberculin skin test results. QFT-G testing was introduced in 2006 for routine testing of newly hired employees. Baseline tuberculin skin testing was performed in accordance with guidelines of the Beth Israel Deaconess Medical Center Employee Occupational Health Services and the Centers for Disease Control and Prevention.2 Tuberculin skin tests (5 tuberculin U) were placed intradermally and read 48–72 hours later by experienced Employee Occupational Health Services nurses. Two-step testing was performed when indicated.2 All tuberculin skin test–positive (induration diameter greater than or equal to 10 mm) new employees had the QFT-G assay performed, typically on the day that the tuberculin skin test result was determined. Those with documented history of positive tuberculin skin test results (approximately 5% of the tuberculin skin test–positive individuals; the majority of these had a reading measured in millimeters from an outside institution) had QFT-G tests performed without tuberculin skin testing. Employees who had previously received at least 1 month of therapy for active TB or LTBI were excluded from QFT-G testing. All tuberculin skin test–positive new employees had a chest radiograph performed. QFT-G–positive employees were referred to the infectious diseases clinic for discussion of LTBI therapy.
Enrollment of participants
In the infectious diseases clinic, after deciding whether to receive 9 months of isoniazid therapy, all QFT-G–positive employees were invited to enroll in our study; enrollment commenced in November 2006. All participants signed written informed consent as approved by the Beth Israel Deaconess Medical Center Institutional Review Board.
Study protocol
Clinical data (including tuberculin skin test results, initial QFT-G test results, chest radiograph findings, and social history) for each participant were reviewed. Participants who had chosen to receive isoniazid treatment had repeat QFT-G tests performed before the initiation of isoniazid therapy (defined as baseline) and had serial QFT-G tests performed at 3, 6, 9, and 12 months after initiation of isoniazid therapy. At each time point, an extra tube of blood was collected for extended proliferation assays. In accordance with routine Employee Occupational Health Services protocol, all participants who were receiving therapy (300 mg of isoniazid and 50 mg of pyridoxine daily) presented monthly for discussion of medication compliance and symptoms, liver function testing, and new prescriptions. Participants who did not receive treatment had repeat QFT-G tests performed at the time of enrollment (baseline) and 12 months later.
QFT-G assay
Assays were performed according to the manufacturers’ recommendations.3 Accordingly, an interferon-γ concentration of 0.35 IU/mL in response to either early secretory antigenic target–6 or culture filtrate protein–10, relative to the negative control, was considered to be positive.
Extended proliferation assays
Extended proliferation assays were performed as described elsewhere,4 with use of purified protein derivative as the stimulatory antigen. In brief, human peripheral blood mononuclear cells (1 × 105 cells/well) were incubated in medium alone or in medium containing purified protein derivative (2 μg/mL). Plates were cultured for 6 days, pulsed with [methyl-3H]Thymidine for an additional 8 hours, harvested, and counted (in counts per minute).
Statistical analysis
Baseline and longitudinal data were analyzed using JMP software (version 7.0) and Microsoft Excel.
RESULTS
Participant enrollment and QFT-G testing
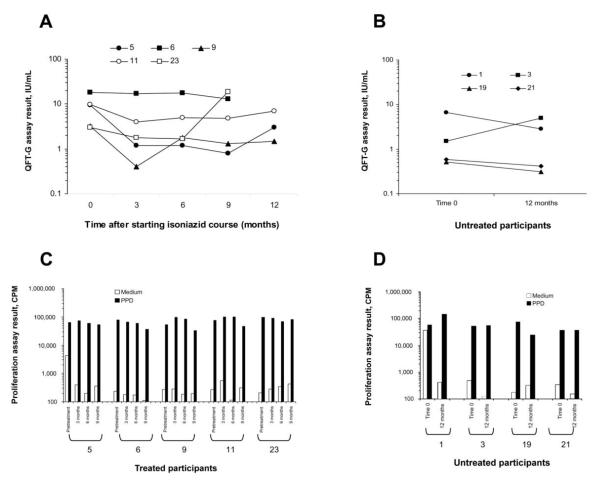
Over a 1-year period, we enrolled 28 QFT-G–positive individuals, 17 of whom chose to initiate isoniazid therapy and 11 of whom opted not to receive isoniazid. Of the 17 participants who chose to receive isoniazid therapy, 5 subsequently completed 9 full months of isoniazid therapy in addition to the entire serial testing protocol. Of the 11 participants who chose not to receive treatment, 4 had a 12-month follow-up QFT-G test performed. Therefore, we were able to fully evaluate 9 QFT-G–positive individuals who successfully completed the serial testing protocol, 5 of whom had chosen to receive isoniazid treatment and 4 of whom had chosen not to receive the treatment. Baseline clinical characteristics of the 9 participants are detailed in the Table. The 5 participants who received treatment had clearly documented tuberculin skin test readings of 15-24 mm (Table). All 5 of these participants had positive QFT-G test results at all 4 time points from baseline through the completion of the 9-month isoniazid treatment course (at 0, 3, 6, and 9 months) (Figure, panel A). Furthermore, 3 of the participants who received treatment had an additional QFT-G test performed 3 months after completing the isoniazid treatment course (at 12 months), and all of these patients remained QFT-G positive (Figure, panel A). When the absolute values of early secretory antigenic target–6 and culture filtrate protein–10 responses were examined longitudinally for each individual antigen in the 5 participants, there were no clear patterns of increase or decrease over time (data not shown).
TABLE.
Baseline Clinical Characteristics of QuantiFERON-TB Gold–Positive Participants Who Completed the Study
| Participant | Sex | Age, years |
TST result, mm |
Country of birth |
Recent immigrationa |
LTR in an area of endemicityb |
Patient carec | History of BCG vaccination |
|---|---|---|---|---|---|---|---|---|
| Received 9 months of INH treatment |
||||||||
| 5 | M | 32 | 15 | India | Yes | Yes | No | Yes |
| 6 | F | 52 | 20 | Haiti | No | Yes | No | Unknown |
| 9 | F | 40 | 24 | Haiti | No | Yes | Yes | Yes |
| 11 | M | 31 | 18 | India | No | Yes | No | Yes |
| 23 | F | 37 | 15 | India | No | Yes | Yes | Yes |
| Did not receive INH treatment |
||||||||
| 1 | M | 42 | >10d | China | No | Yes | Yes | Unknown |
| 3 | F | 39 | 25 | Philippines | No | Yes | Yes | Yes |
| 19 | F | 33 | 14 | China | No | Yes | No | Unknown |
| 21 | F | 61 | >10d | Poland | No | No | Yes | Yes |
NOTE. None of the 9 participants reported a history of or risk factors for HIV infection. Chest radiograph findings were normal for all participants. BCG, bacille Calmette-Guérin; INH, isoniazid; LTR, long-term residence; TST, tuberculin skin test.
Immigration within the previous 5 years.
Stay of at least 5 years in a country with a tuberculosis incidence rate of greater than or equal to 40 cases per 100,000 persons per year (according to World Health Organization data from 2004–2006, as summarized by the Public Health Agency of Canada).5
Indicates whether the healthcare worker had direct daily patient care responsibilities for at least 1 year (at any time), representing a potential source of tuberculosis exposure.
The TST was performed at an outside institution, and the results were reported as an induration <10 mm.
FIGURE.
Comparison of results of serial QuantiFERON-TB Gold (QFT-G) assays and proliferation assays for participants who received isoniazid treatment and those who did not receive isoniazid treatment. A, QFT-G results for the 5 treated participants (participants 5, 6, 9, 11, and 23) who completed a full 9-month course of isoniazid treatment and the entire serial testing protocol (testing at baseline and at 3, 6, and 9 months; 3 of 5 participants also had tests performed at 12 months). B, QFT-G results for the 4 untreated control subjects (participants 1, 3, 19, and 21) who had tests performed at baseline and at 12 months. For both groups, results of the QFT-G assays for each participant at the given time point are expressed as the higher of the 2 possible test results (early secretory antigenic target–6-nil or culture filtrate protein–10-nil), to depict the maximum response in the assay at the given time point. C and D, Responses of the 5 participants who received treatment (C) and the 4 participants who did not receive treatment (D) in extended (6-day) cellular proliferation assays in which peripheral blood mononuclear cells obtained from the participants at each time point (at baseline and at 3, 6, and 9 months for participants who received treatment and at baseline and at 12 months for untreated participants) were stimulated with purified protein derivative. Assay results are expressed as counts per minute (CPM) in wells containing medium alone or medium and purified protein derivative.
All participants who did not receive treatment had tests performed at enrollment and 12 months later. All 4 participants were still positive at 12 months (Figure, panel B). None of the treated or untreated participants reported any exposure to patients with active TB at any time during the serial testing protocol.
Proliferation assays
Extended proliferation assays in which purified protein derivative was used as stimulatory antigen were performed serially for all 9 participants in parallel with the QFT-G assays (Figure, panels C and D). For unknown reasons, participant 1 (who did not receive treatment) had extremely high background counts in the medium control well at baseline. Nonetheless, samples from all 9 participants strongly responded to purified protein derivative stimulation at all time points. These results support the original diagnosis of LTBI and suggest that isoniazid treatment does not affect (at least for 9–12 months) the overall immunologic reactivity to M. tuberculosis antigens.
DISCUSSION
Our goal in the present study was to examine the effect of standard LTBI treatment on QFT-G assay results in a US occupational health setting in which isoniazid treatment compliance was monitored and reexposure to active TB was not a confounding factor. Our study design reflects the fact that the QFT-G assay has been sanctioned by the Centers for Disease Control and Prevention for use in HCW screening programs.1 All 5 newly hired HCWs who initially tested positive by the QFT-G assay on routine screening and who then received 9 months of closely monitored isoniazid therapy continued to have positive QFT-G results at the time of treatment completion; 3 of these HCWs had repeat QFT-G tests performed 3 months later, and results for all 3 HCWs were still positive.
Previous studies of the effect of LTBI therapy on IGRA results6–11 have generated conflicting data, with some studies suggesting that responses to TB-specific antigens might decrease sufficiently with treatment that IGRAs might provide a useful means of monitoring treatment efficacy, and other studies suggesting that responses might not decrease sufficiently for IGRAs to be used for this purpose. However, the comparability of these studies to each other and to our study is confounded by multiple factors, including repeated exposure of individuals to active TB during and after LTBI treatment,9 differences in LTBI treatment protocols, compared with the current US standard,6–8,10,11 and differences in study populations (eg, studies of recent contacts).7,8,10,11 Our study uniquely assesses the impact of the US standard LTBI treatment course on QFT-G test results in newly hired HCWs who have received a diagnosis of LTBI by routine hospital screening protocols; the majority of these HCWs were likely to have been remotely infected.
Although our sample size is small, our results unequivocally indicate that QFT-G test results should not be used to assess the effectiveness of recent or remote treatment courses for LTBI: the majority of individuals who initially test positive by the QFT-G assay will continue to test positive after 9 months of isoniazid treatment. This result has significant implications for HCW screening programs, because it should not be assumed that HCWs who report prior LTBI therapy but who still test positive by the QFT-G assay have not received appropriate treatment in the past. Moreover, neither physicians nor patients should expect reliable changes in QFT-G test results after standard treatment. Finally, our results neither support nor refute the idea that a positive IGRA result, in the absence of treatment for LTBI, is associated with a higher risk of reactivation of LTBI, compared with a positive tuberculin skin test result alone. Only long-term follow-up of large numbers of persons will be able to address this topic.
ACKNOWLEDGMENTS
We thank the Beth Israel Deaconess Medical Center employee health team, for their efforts in organizing QuantiFERON-Gold testing, and the Beth Israel Deaconess Medical Center phlebotomy team, for their help with obtainment of blood samples.
Financial support. Pittsfield Anti-Tuberculosis Association (grant to N.R.P.) and Clinical Investigator Training Program through Beth Israel Deaconess Medical Center–Harvard/Massachusetts Institute of Technology Health Sciences and Technology, in collaboration with Pfizer and Merck (to N.R.P.). QuantiFERON-TB Gold kits were provided by Cellestis.
Footnotes
Potential conflicts of interest. E.N. reports that he has served as a consultant to Oxford Immunotech. All other authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54(RR-15):49–55. [PubMed] [Google Scholar]
- 2.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 3.QuantiFERON-TB Gold [package insert] Cellestis; Victoria, Australia: 2006. Document no. 05980000C. [Google Scholar]
- 4.Napolitano DR, Pollock N, Kashino SS, Rodrigues V, Jr, Campos-Neto A. Identification of Mycobacterium tuberculosis ornithine carboamyltransferase in urine as a possible molecular marker of active pulmonary tuberculosis. Clin Vaccine Immunol. 2008;15:638–643. doi: 10.1128/CVI.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Public Health Agency of Canada [Accessed September 1, 2008];International tuberculosis incidence rates. 2008 Mar 31; Available at: http://www.phac-aspc.gc.ca/tbpc-latb/itir_e.html.
- 6.Wilkinson KA, Kon OM, Newton SM, et al. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J Infect Dis. 2006;193:354–359. doi: 10.1086/499311. [DOI] [PubMed] [Google Scholar]
- 7.Chee CB, KhinMar KW, Gan SH, Barkham TM, Pushparani M, Wang YT. Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis–specific antigens. Am J Respir Crit Care Med. 2007;175:282–287. doi: 10.1164/rccm.200608-1109OC. [DOI] [PubMed] [Google Scholar]
- 8.Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2006;174:831–839. doi: 10.1164/rccm.200511-1783OC. [DOI] [PubMed] [Google Scholar]
- 9.Pai M, Joshi R, Dogra S, et al. Persistentlyelevated Tcellinterferon-γresponses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report. J Occup Med Toxicol. 2006;1:7. doi: 10.1186/1745-6673-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi K, Harada N, Mori T. Interferon-γ responses after isoniazid chemotherapy for latent tuberculosis. Respirology. 2008;13:468–472. doi: 10.1111/j.1440-1843.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 11.Goletti D, Parracino MP, Butera O, et al. Isoniazid prophylaxis differently modulates T-cell responses to RD1-epitopes in contacts recently exposed to Mycobacterium tuberculosis: a pilot study. Respir Res. 2007;8:5. doi: 10.1186/1465-9921-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]