Summary
Objectives
To understand relationships between microbes in pathogenesis of acute otitis media during respiratory tract infections, we compared nasopharyngeal bacteria and respiratory viruses in symptomatic children with and without AOM.
Methods
We enrolled children (6–35 months) with acute symptoms suggestive of AOM and analyzed their nasopharyngeal samples for bacteria by culture and for 15 respiratory viruses by PCR. Non-AOM group had no abnormal otoscopic signs or only middle ear effusion, while AOM group showed middle ear effusion and acute inflammatory signs in pneumatic otoscopy along with acute symptoms.
Results
Of 505 children, the non-AOM group included 187 and the AOM group 318. One or more bacterial AOM pathogen (Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis) was detected in 78% and 96% of the non-AOM and AOM group, respectively (P < .001). Colonization with S. pneumoniae and H. influenzae, each alone, increased risk of AOM (odds ratio (OR) 2.92; 95% confidence interval (CI), .91–9.38, and 5.13; 1.36–19.50, respectively) and co-colonization with M. catarrhalis further increased risk (OR 4.36; 1.46–12.97, and 9.00; 2.05–39.49, respectively). Respiratory viruses were detected in 90% and 87% of the non-AOM and AOM group, respectively. RSV was significantly associated with risk of AOM without colonization by bacterial AOM pathogens (OR 6.50; 1.21–34.85).
Conclusions
Co-colonization by M. catarrhalis seems to increase risk of AOM and RSV may contribute to AOM pathogenesis even without nasopharyngeal bacterial colonization.
Keywords: Otitis media, Respiratory tract infections, Bacteria, Viruses, Nasopharynx, Child
Introduction
Acute otitis media is the most common bacterial infection of early childhood for which medical care is sought; over 80% of children experience at least one episode by their third birthday.1, 2, 3 Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis are the predominant bacterial AOM pathogens.4, 5 Most AOM episodes occur as a secondary bacterial infection concomitant to viral respiratory tract infection (RTI).6, 7 Respiratory viruses have been identified in middle ear fluids as co-pathogens with bacteria and even in the absence of bacterial AOM pathogens.8 Thus, AOM pathogenesis involves complex associations between bacteria and viruses.
Colonization of the nasopharynx by bacterial AOM pathogens is often considered a requisite step in the disease process.9, 10 The prevalence of colonization with these pathogens differs by age and is highest during early childhood when over 60% of children are colonized at some point in time.4, 11, 12 The proportion of children colonized by bacterial AOM pathogens is higher during AOM and RTI compared to healthy periods.9, 11 In contrast, the proportion of children colonized with non-pneumococcal alpha-hemolytic streptococci (NPAHS) is significantly lower in children with AOM compared to healthy children.9
It is important to understand relationships between bacterial AOM pathogens, commensals, and respiratory viruses during symptomatic RTI, as this is the time when the incidence of AOM is highest.1 Therefore, the aim of this study was to compare nasopharyngeal bacteria and respiratory viruses in symptomatic children with and without AOM.
Patients and methods
Study population and nasopharyngeal samples
We enrolled children aged 6–35 months with acute symptoms suggestive of AOM. At the enrollment visit, symptoms were surveyed by a structured questionnaire and children were clinically examined. The diagnostic procedures and exclusion criteria have been described in detail elsewhere.13 Written informed consent was obtained from a parent of each child before any study procedure was done. All visits were free of charge, and no compensation for participation was given. The study protocol was approved by the ethical committee of the Hospital District of Southwest Finland. This study was conducted according to the principles of Helsinki Declaration in outpatient setting from 2006 to 2009 and is part of a larger project registered at www.clinicaltrials.gov (identifier NCT00299455).
Two groups were studied. The non-AOM group had RTI without AOM at enrollment and did not develop AOM, which was confirmed by follow-up. Parents recorded daily the symptoms in a dairy and examined their child's ears with acoustic reflectometry and/or tympanometry. If parents suspected AOM, an acute visit was organized at the study clinic on any day of the week. Finally, each child in the non-AOM group was examined at the study clinic on a follow-up visit after an average of 12 days when the symptoms had already subsided. Inclusion in the AOM group required a diagnosis of AOM, which was based on three overall criteria. First, middle-ear fluid had to be detected by means of pneumatic otoscopic examination that showed at least two of the following tympanic membrane findings: bulging position, decreased or absent mobility, abnormal color or opacity not due to scarring, or air–fluid interfaces. Second, at least one of the following acute inflammatory signs in the tympanic membrane had to be present: distinct erythematous patches or streaks, or increased vascularity over full, bulging, or yellow tympanic membrane. Third, the child had to have acute symptoms.
Nasopharyngeal samples were collected with dacron swabs (Copan diagnostics, Corona, CA, USA) through the anterior nostrils from a depth of 6.5 cm on average. Microbes were released from the swab into sterilized .9% NaCl by vortexing vigorously (c. 5 s) and further agitating (c. 5 s). Volume of NaCl was .5 ml for the first 100 samples and then after 1.0 ml to increase the sample material. The sample suspension was used as fresh for bacterial cultures and for viral antigen detection. The rest of the sample suspension was frozen and thawed sample aliquots were used for molecular analyses.
Bacterial analyses
Bacterial cultures were done immediately after sampling at the study clinic. We used two non-selective plates and two selective plates to quantitate the growth and to obtain maximal recovery of different bacterial isolates because competing nasopharyngeal bacteria (e.g. M. catarrhalis) may either overgrow or restrict the growth of pneumococci. Two non-selective plates (5% sheep blood agar plate and heated blood agar (chocolate agar) plate) as well as the haemophilus selective plate (heated blood agar plate containing 300 mg/l bacitracin) were inoculated as follows: 10 μl of sample suspension was transferred and then streaked onto four quadrants. The streptococcus selective agar plate (sheep blood agar containing colistin (5 mg/l), oxolinic acid (2.5 mg/l)) was inoculated by pipetting 10 μl of suspension onto the plate and then spreading the drop over the whole plate. Plates were incubated in 5% CO2 at 37 °C and examined at 24 h and 48 h. Identification of bacterial isolates was performed by standard microbiological methods.14 For the identification of S. pneumoniae, optochin discs were used (Oxoid LTD, Basingstoke, Hampshire, England). The growth of each identified bacterial species was scored on a semi-quantitative scale from 0 to 5 as described previously.15
Viral analyses
Initially, viral antigens were detected by time-resolved fluoroimmunoassay to identify respiratory syncytial virus (RSV), adenovirus, influenza A and B virus, and parainfluenza virus types 1–3.16 For initial viral PCR detection, nucleic acids were extracted using Nuclisense easyMag automated extractor (BioMerieux, Boxtel, Netherlands). Rhinovirus (A, B, and C), enteroviruses and RSV (types A and B) were analyzed using a multiplex real-time quantitative PCR (RT-qPCR).17 Human bocavirus and human metapneumovirus were detected by separate RT-qPCR assays.18, 19 Later Seeplex RV12 ACE Detection (Seegene, Seoul, South Korea) became available and it was used primarily to extend the coverage of viral species (namely coronaviruses) and secondarily to increase sensitivity for detection of those viruses included in the antigen detection panel. Seeplex RV12 includes adenovirus, human metapneumovirus, coronavirus 229E/NL63 and OC43/HKU1, parainfluenza virus types 1–3, influenza A and B virus, RSV (types A and B), and rhinovirus (A and B). For this RV12 multiplex-PCR, nucleic acids were extracted from another aliquot of the sample suspension using MagNA Pure 96 instrument with DNA and Viral NA Small Volume kit (Roche Applied Science, Mannheim, Germany). All positive findings were regarded as true positive.
Statistical analyses
In the analyses, parainfluenza virus types 1–3 were grouped together, as well as coronaviruses (229E/NL63 and OC43/HKU1) and influenza A and B viruses. Statistical analyses were conducted using SAS version 9.2 (Cary, NC, USA) and SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Means were compared by T test and proportions by Chi-square test. Associations between covariates of interest and AOM were examined by Chi-square test. Associations between specific combinations of bacteria, viruses, and the risk of AOM were examined using logistic regression.
Results
Nasopharyngeal samples were obtained from 187 and 318 children in the non-AOM and AOM group, respectively. Detailed baseline characteristics are shown in Table 1 .
Table 1.
Characteristics of symptomatic children without acute otitis media (non-AOM group) and those diagnosed with acute otitis media (AOM group).
| Non-AOM groupa (N = 187) | AOM groupa (N = 318) | P valueb | |
|---|---|---|---|
| Demographics | |||
| Mean (range) age, mo | 16 (6–35) | 16 (6–35) | .54 |
| Age, mo, n (%) | .68 | ||
| 6–11 | 76 (41) | 117 (37) | |
| 12–23 | 77 (41) | 141 (44) | |
| 24–35 | 34 (18) | 60 (19) | |
| Male gender, n (%) | 100 (54) | 181 (57) | .45 |
| Otitis media risk factors | |||
| Recurrent otitis media in 1st degree relativesc, n (%) | 98 (54) | 187 (59) | .227 |
| Sibling(s) in the householdc, n (%) | 104 (57) | 183 (58) | .82 |
| Daycare attendancec, n (%) | 67 (36) | 172 (54) | <.001 |
| Parental smokingc, n (%) | 44 (24) | 104 (33) | .035 |
| Any breast-feedingc, d, n (%) | 180 (98) | 303 (96) | .19 |
| Current use of pacifierc, n (%) | 110 (60) | 165 (52) | .087 |
| Otitis media history | |||
| Number of previous episodes of acute otitis mediac, n (%) | .31 | ||
| 0 | 63 (34) | 94 (30) | |
| 1–3 | 83 (45) | 166 (52) | |
| ≥4 | 38 (21) | 58 (18) | |
| Vaccination history | |||
| ≥1 dose of pneumococcal conjugate vaccinec, n (%) | 4 (2) | 7 (2) | .98 |
| ≥1 dose of Haemophilus influenzae type b vaccinec, n (%) | 183 (99) | 318 (100) | .19 |
| ≥1 dose of influenza vaccinec, n (%) | 38 (21) | 41 (13) | .021 |
| Preceding symptoms | |||
| Fever, n (%) | 65 (35) | 137 (43) | .065 |
| Ear paine, n (%) | 172 (92) | 253 (80) | <.001 |
| Non-specific symptomsf, n (%) | 186 (99) | 306 (96) | .026 |
| Respiratory symptomsg, n (%) | 186 (99) | 312 (98) | .21 |
| Gastrointestinal symptomsh, n (%) | 21 (11) | 40 (13) | .653 |
Six children are included in both groups based on two different episodes separated by 2–22 weeks.
Means were compared by T test and proportions by Chi-square test.
Data missing in 3 children in non-AOM group.
Data missing in 1 child in AOM group.
Ear pain reported by parents and/or child.
Poor appetite, decreased activity, irritability, restless sleep, and/or excessive crying.
Rhinitis, nasal congestion, cough, hoarse voice, mucus vomiting, and/or conjunctivitis.
Vomiting and/or diarrhea.
Bacteria
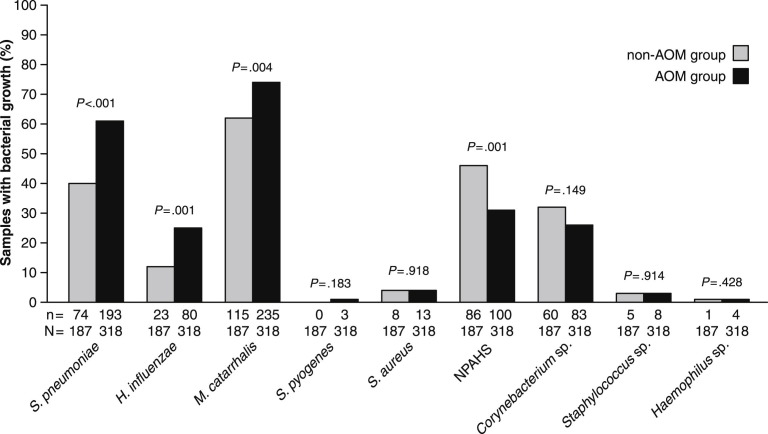
Nasopharyngeal bacterial cultures were positive for any bacteria in 185 of 187 samples in the non-AOM group (99%) and in 317 of 318 of those in the AOM group (100%). Semiquantitative scoring of the bacterial culture results did not reveal any differences between the groups (data not shown). Fig. 1 shows the detailed results of bacterial culture. At least one of the typical bacterial AOM pathogens (S. pneumoniae, H. influenzae, or M. catarrhalis) was detected in 145 (78%) and 306 (96%) samples in the non-AOM and AOM group, respectively (P < .001). Either of the two major identified commensals, i.e. NPAHS or Corynebacterium species, was identified in 108 (58%) of the non-AOM and 158 (50%) of AOM group samples (P = .079). Both NPAHS and Corynebacterium species were detected in 38 (20%) and 25 (8%) of the non-AOM and AOM group samples, respectively (P < .001).
Figure 1.
Occurrence of nasopharyngeal bacteria in children without acute otitis media (non-AOM group) and in children with acute otitis media (AOM group). NPAHS stands for non-pneumococcal alpha-hemolytic streptococci. Below the bars, n is the numerator (i.e. number of positive findings) and N is the denominator (i.e. number of samples analyzed). Proportions have been compared by Chi-square test.
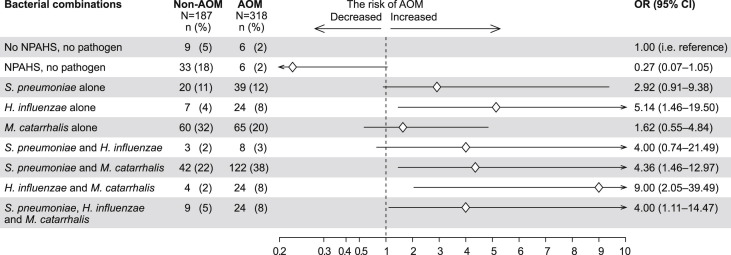
Co-occurrence of bacteria
Co-occurrence of bacterial AOM pathogens revealed bacterial interactions: the concomitant colonization of H. influenzae with S. pneumoniae as well as with M. catarrhalis was less common than expected (Fig. 2 ). The overall prevalence of colonization by S. pneumoniae and H. influenzae in 505 samples was 53% (267/505) and 20% (103/505), respectively. Thus, the expected co-colonization rate by S. pneumoniae and H. influenzae was 11% (i.e. 53% × 20%) while the observed co-colonization rate was 2% (11/505) (rate difference 9%; 95% confidence interval (CI), 6–12; P < .001). Similarly, the overall prevalence of colonization by H. influenzae and M. catarrhalis was 20% (103/505) and 69% (350/505), respectively. Thus, the expected co-colonization rate by H. influenzae and M. catarrhalis was 14% (i.e. 20% × 69%) while the observed co-colonization rate was 5.5% (28/505) (rate difference 9%; 95% CI, 5–12; P < .001). There were no differences between the expected and observed co-colonization rates regarding S. pneumoniae and M. catarrhalis or S. pneumoniae, H. influenzae and M. catarrhalis (data not shown).
Figure 2.
Occurrence of nasopharyngeal bacteria alone and in combinations in children without acute otitis media (non-AOM group) and in children with acute otitis media (AOM group). The risk of acute otitis media related to each finding according to logistic regression multivariate model. Diamonds indicate odds ratio (OR), lines 95% confidence intervals (95% CI), arrows are added when CI is beyond the scale.
In Fig. 2, the multivariate model shows that the risk of AOM was decreased when the nasopharynx was colonized by NPAHS without bacterial AOM pathogens as compared to when no NPAHS and no bacteria AOM pathogens were cultured. M. catarrhalis as a single colonizer did not significantly increase the risk of AOM. However, M. catarrhalis seemed to have a synergistic interaction with both S. pneumoniae and H. influenzae because co-colonized children had a higher risk of AOM than children colonized with S. pneumoniae or H. influenzae as single bacterial AOM pathogen. In contrast, co-colonization by S. pneumoniae and H. influenzae did not increase the risk of AOM as compared to colonization by either bacterium alone.
Respiratory viruses
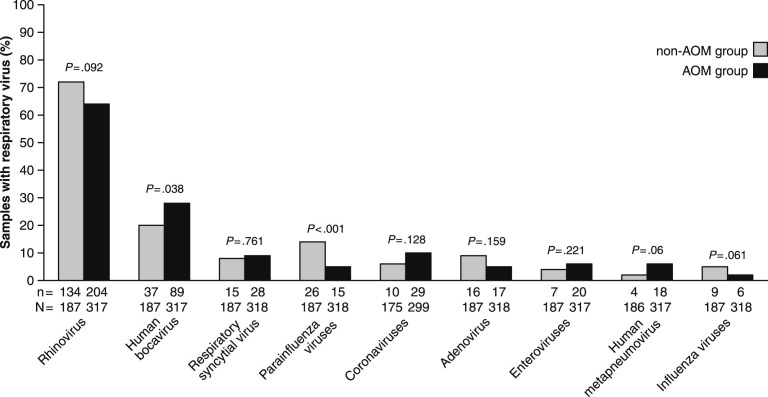
Respiratory viruses were detected in 168 of 187 non-AOM group samples (90%) and in 278 of 318 AOM group samples (87%). The most prevalent virus was rhinovirus, which was found in 72% of the samples in non-AOM group and 64% in AOM group (Fig. 3 ). Human bocavirus was less common in the non-AOM than in the AOM group. In contrast, parainfluenza viruses were found significantly more often in non-AOM than in AOM group.
Figure 3.
Occurrence of respiratory viruses in children without acute otitis media (non-AOM group) and in children with acute otitis media (AOM group). Parainfluenza virus types 1–3 are grouped together, as well as coronaviruses (229E/NL63 and OC43/HKU1) and influenza A and B viruses. Below the bars, n is the numerator (i.e. number of positive findings) and N is the denominator (i.e. number of samples analyzed). Proportions have been compared by Chi-square test.
Co-occurrence of respiratory viruses
Concurrent detection of multiple respiratory viruses was common but did not significantly differ between the non-AOM and AOM groups. One, two, three, four, and five viruses were detected in 262 (52%), 138 (27%), 38 (8%), 7 (1%), and 1 (.2%) of 505 samples, respectively. Number of viruses was not associated with the risk of AOM.
Rhinovirus, enteroviruses, and influenza viruses were equally often detected alone as with other viruses while human bocavirus, RSV, parainfluenza viruses, coronaviruses, adenovirus, and human metapneumovirus were significantly more often detected together with other viruses than alone. There was a significant negative association in the co-occurrence of rhinovirus and RSV as well as of rhinovirus and enteroviruses meaning that the co-occurrences of these viruses were observed significantly less often than expected. These negative associations did not have any effect on the risk of AOM (data not shown).
Co-occurrence of bacteria and respiratory viruses
The occurrence of respiratory viruses was not associ-ated with any of the three bacterial AOM pathogens, neither with NPAHS or Corynebacterium species. The only exception was a significant negative association between H. influenzae and parainfluenza viruses (data not shown).
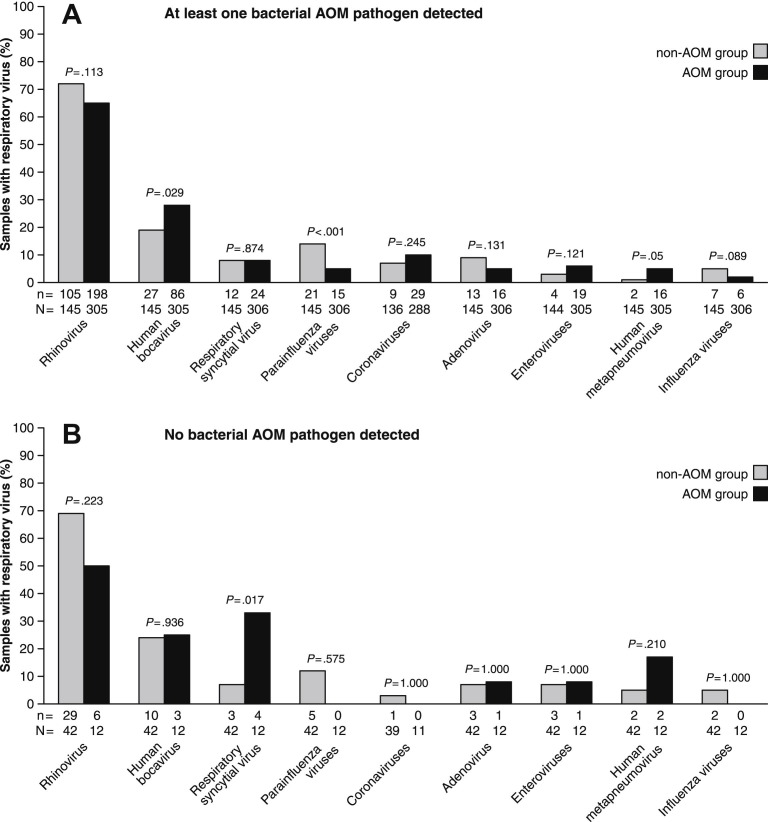
The effect of co-occurrence of bacteria and viruses on the risk of AOM was investigated by dividing the data according to whether at least one vs. no bacterial AOM pathogen was detected (Fig. 4 ). This showed that, when at least one bacterial AOM pathogen was detected, human bocavirus increased the risk of AOM as compared to no detection of human bocavirus (odds ratio (OR) 1.72; 95% CI, 1.06–2.79). In contrast, detection of parainfluenza viruses decreased the risk of AOM (OR .30; 95% CI, .15–.61). When no bacterial AOM pathogen was detected, RSV was the only virus that significantly increased the risk of AOM (OR 6.50; 95% CI, 1.21–34.85).
Figure 4.
Occurrence of respiratory viruses in children without acute otitis media (non-AOM group) and in children with acute otitis media (AOM group) when at least one of the bacterial AOM pathogen (S. pneumoniae, H. influenzae, or M. catarrhalis) is detected (panel A) and when no bacterial AOM pathogen is detected (panel B). Parainfluenza virus types 1–3 are grouped together, as well as coronaviruses (229E/NL63 and OC43/HKU1) and influenza A and B viruses. Below the bars, n is the numerator (i.e. number of positive findings) and N is the denominator (i.e. number of samples analyzed). Proportions have been compared by Chi-square test.
Discussion
Our data show that colonization by M. catarrhalis may play an important role in enhancing the risk of AOM during RTI. The risk of AOM was higher when children were co-colonized by S. pneumoniae and M. catarrhalis or H. influenzae and M. catarrhalis compared to when children were colonized by S. pneumoniae or H. influenzae alone. Our data confirm previous observations that nasopharyngeal colonization by bacterial AOM pathogens is more common in children with AOM compared to those without AOM while colonization with NPAHS is less common in children with AOM. As a new observation, we found no major differences in the detection of respiratory viruses in symptomatic children with and without AOM. However, RSV was significantly associated with the risk of AOM when no bacterial AOM pathogens were detected in the nasopharynx.
Our results confirm the previous observations that the nasopharyngeal colonization by bacterial AOM pathogens is more common in children with AOM compared to those without AOM and that M. catarrhalis is the most common colonizer in both groups.20 The importance of M. catarrhalis as an AOM pathogen has been debated,5, 21 because it has been described as less virulent than other bacterial AOM pathogens in children.5, 22 Our data indicate that the importance of M. catarrhalis may be as a co-colonizer when it significantly enhances the risk of AOM as compared to when S. pneumoniae and H. influenzae are single colonizers. Our data are supported by murine models demonstrating that the risk of AOM is highest following nasal inoculation with S. pneumoniae and M. catarrhalis together compared to S. pneumoniae alone.23 Interestingly, co-colonization and co-infection with M. catarrhalis may have implications for treatment. β-Lactamase producing M. catarrhalis can promote resistance to amoxicillin in susceptible S. pneumoniae and H. influenzae. 24, 25, 26 Virtually all strains of M. catarrhalis produce β-lactamase and are thus resistant to amoxicillin, the first line treatment for AOM.
Our data also indicate that S. pneumoniae and H. influenzae may interact competitively. These two bacteria were found together less frequently than expected by chance and their co-colonization increased the risk of AOM less than H. influenzae alone. Our data are consistent with a previous study in which colonization of S. pneumoniae was negatively associated with colonization by H. influenzae among children experiencing RTI.27
Several lines of evidence suggest that specific commensal nasopharyngeal bacteria may be protective for AOM.9, 28, 29 NPAHS interferes with nasopharyngeal colonization of S. pneumoniae.28 S. pneumoniae, H. influenzae, and M. catarrhalis are inhibited by NPAHS in vitro.30 The abundance of NPAHS is slightly higher in non-otitis prone compared to otitis prone children.29 In our study, the non-AOM group had more often NPAHS than those diagnosed with AOM although all children were acutely ill and 78% of non-AOM group was colonized by bacterial AOM pathogens. Colonization with NPAHS without bacterial AOM pathogens decreased the risk of AOM. Thus, our data give important support for the protective role of NPAHS for AOM.
Respiratory viruses could be detected in almost all children in our study and expectedly rhinovirus was the most prevalent. Detection of multiple viruses was common in general but no more common in children with AOM than in those without AOM, which is in line with previous data.7 An interesting observation was that we found no major differences in viral findings in children with and without AOM. At first sight, our observation may seem conflicting with previous studies that have repeatedly shown that the most important viral risk factor for AOM is RSV infection during which roughly 50–70% of young children develop AOM.6, 7, 8, 31, 32, 33, 34 However, the results of the previous studies have been conflicting regarding the risk of AOM related to other viruses. Chonmaitree et al. actually showed that age was more important risk factor for AOM than any of the viruses.7 Most of the previous studies have had follow-up during which the development of AOM has been studied in relation to specific viral infection. Notably, our study approach was cross-sectional and children were examined because their parents sought medical care due to suspicion of AOM. In other words, our study approach was the one where physicians encounter symptomatic children with and without AOM in daily practice. Our observation raises a question if differences in host factors play stronger role than differences between viral infections in determining which children develop AOM during RTI. Previously, Casselbrant et al. have shown that genetics determines 73% of risk of otitis media in early childhood.35 Nonetheless, our results also draw attention to RSV and suggest that RSV contributes to AOM pathogenesis even without nasopharyngeal bacterial colonization. Among children not colonized with bacterial AOM pathogens, the risk of AOM was over 6-fold higher when RSV was detected. In two previous studies, roughly half of AOM patients infected with RSV had negative bacterial culture of middle ear effusion.8, 36 These observations, including ours, show that RSV has an important role in the pathogenesis of AOM.
Our study has several strengths including the recruitment of outpatients at an otitis-prone age, careful diagnosis of AOM, and sampling from the nasopharynx rather than the anterior nose. Due to our careful follow-up we can be confident that the non-AOM group did not experience AOM directly following the RTI episode when sampling occurred. Equally important, our non-AOM group had similar symptoms, age distribution, and AOM history as the AOM group. Our extensive virology included detection of virtually all respiratory viruses except influenza C and parainfluenza type 4 viruses which we see as the reason for higher detection rates of viruses than commonly reported in studies of AOM. Furthermore, our use of selective bacterial culture plates is expected to have yielded bacteria more comprehensively than standard plates.37 Nevertheless, our study is not without limitations. These include limited sample size, which resulted in wide confidence intervals and may have limited our ability to detect otherwise significant associations. Recruitment was based on self-selection and parents were motivated to participate in our AOM treatment trial, which may have affected which viruses were detected. While we did not exclude children according to symptoms, parents whose children experienced the most severe symptoms may have been less likely to participate and on the other hand the non-AOM group may have been more symptomatic than the average child with RTI.
In summary, given the apparent importance of co-colonization in AOM pathogenesis, prevention and treatment methods targeting colonization by M. catarrhalis might also reduce AOM due to S. pneumoniae and H. influenzae. The role of RSV in pathogenesis of AOM seems to be independent of bacterial AOM pathogens.
Acknowledgements
This work was supported by the Fellowship Award of The European Society for Paediatric Infectious Diseases to A.R., by National Institute on Deafness and Communication Disorders to M.M.P. (R21DC011667), and by grants from The Foundation for Paediatric Research; The Research Funds from Specified Government Transfers; Jenny and Antti Wihuri Foundation; The Paulo Foundation; The Finnish Medical Foundation; The Maud Kuistila Memorial Foundation; The Emil Aaltonen Foundation; The National Graduate School of Clinical Investigation; The Finnish Cultural Foundation; Turku University Hospital Research Foundation. None above had any role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
References
- 1.Winther B., Alper C.M., Mandel E.M., Doyle W.J., Hendley J.O. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119:1069–1075. doi: 10.1542/peds.2006-3294. [DOI] [PubMed] [Google Scholar]
- 2.Vergison A., Dagan R., Arguedas A., Bonhoeffer J., Cohen R., Dhooge I. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10:195–203. doi: 10.1016/S1473-3099(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 3.Teele D.W., Klein J.O., Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Casey J.R., Adlowitz D.G., Pichichero M.E. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broides A., Dagan R., Greenberg D., Givon-Lavi N., Leibovitz E. Acute otitis media caused by Moraxella catarrhalis: epidemiologic and clinical characteristics. Clin Infect Dis. 2009;49:1641–1647. doi: 10.1086/647933. [DOI] [PubMed] [Google Scholar]
- 6.Ruuskanen O., Arola M., Putto-Laurila A., Mertsola J., Meurman O., Viljanen M.K. Acute otitis media and respiratory virus infections. Pediatr Infect Dis J. 1989;8:94–99. [PubMed] [Google Scholar]
- 7.Chonmaitree T., Revai K., Grady J.J., Clos A., Patel J.A., Nair S. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heikkinen T., Thint M., Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med. 1999;340:260–264. doi: 10.1056/NEJM199901283400402. [DOI] [PubMed] [Google Scholar]
- 9.Faden H., Stanievich J., Brodsky L., Bernstein J., Ogra P.L. Changes in the nasopharyngeal flora during otitis media of childhood. Pediatr Infect Dis J. 1990;9:623–626. [PubMed] [Google Scholar]
- 10.Bogaert D., De Groot R., Hermans P.W. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 11.Syrjänen R.K., Kilpi T.M., Kaijalainen T.H., Herva E.E., Takala A.K. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J Infect Dis. 2001;184:451–459. doi: 10.1086/322048. [DOI] [PubMed] [Google Scholar]
- 12.Faden H., Duffy L., Wasielewski R., Wolf J., Krystofik D., Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis. 1997:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 13.Tähtinen P.A., Laine M.K., Huovinen P., Jalava J., Ruuskanen O., Ruohola A. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med. 2011;364:116–126. doi: 10.1056/NEJMoa1007174. [DOI] [PubMed] [Google Scholar]
- 14.Murray P.R., Baron E.J., Jorgensen J.H., Pfaller M.A., Yolken R.H., editors. Manual of clinical microbiology. American Society for Microbiology Press; Washington, DC: 2003. [Google Scholar]
- 15.Vuorenoja K., Jalava J., Lindholm L., Tähtinen P.A., Laine M.K., Thorn K. Detection of Streptococcus pneumoniae carriage by the Binax NOW test with nasal and nasopharyngeal swabs in young children. Eur J Clin Microbiol Infect Dis. 2012;31:703–706. doi: 10.1007/s10096-011-1361-4. [DOI] [PubMed] [Google Scholar]
- 16.Waris M., Halonen P., Ziegler T., Nikkari S., Obert G. Time-resolved fluoroimmunoassay compared with virus isolation for rapid detection of respiratory syncytial virus in nasopharyngeal aspirates. J Clin Microbiol. 1988;26:2581–2585. doi: 10.1128/jcm.26.12.2581-2585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peltola V., Waris M., Österback R., Susi P., Ruuskanen O., Hyypiä T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–389. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 18.Koskenvuo M., Möttönen M., Waris M., Allander T., Salmi T.T., Ruuskanen O. Human bocavirus in children with acute lymphoblastic leukemia. Eur J Pediatr. 2008;167:1011–1015. doi: 10.1007/s00431-007-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heikkinen T., Österback R., Peltola V., Jartti T., Vainionpää R. Human metapneumovirus infections in children. Emerg Infect Dis. 2008;14:101–106. doi: 10.3201/eid1401.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revai K., Mamidi D., Chonmaitree T. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis. 2008;46:e34–e37. doi: 10.1086/525856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichichero M. Widening differences in acute otitis media study populations. Clin Infect Dis. 2009;49:1648–1649. doi: 10.1086/647934. [DOI] [PubMed] [Google Scholar]
- 22.Palmu A.A., Herva E., Savolainen H., Karma P., Mäkelä P.H., Kilpi T.M. Association of clinical signs and symptoms with bacterial findings in acute otitis media. Clin Infect Dis. 2004;38:234–242. doi: 10.1086/380642. [DOI] [PubMed] [Google Scholar]
- 23.Krishnamurthy A., McGrath J., Cripps A.W., Kyd J.M. The incidence of Streptococcus pneumoniae otitis media is affected by the polymicrobial environment particularly Moraxella catarrhalis in a mouse nasal colonisation model. Microbes Infect. 2009;11:545–553. doi: 10.1016/j.micinf.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Hol C., Van Dijke E.E., Verduin C.M., Verhoef J., van Dijk H. Experimental evidence for Moraxella-induced penicillin neutralization in pneumococcal pneumonia. J Infect Dis. 1994;170:1613–1616. doi: 10.1093/infdis/170.6.1613. [DOI] [PubMed] [Google Scholar]
- 25.Budhani R.K., Struthers J.K. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: investigation of the indirect pathogenic role of beta-lactamase-producing moraxellae by use of a continuous-culture biofilm system. Antimicrob Agents Chemother. 1998;42:2521–2526. doi: 10.1128/aac.42.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armbruster C.E., Hong W., Pang B., Weimer K.E., Juneau R.A., Turner J. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio. 2010;1:e00102–e00110. doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettigrew M.M., Gent J.F., Revai K., Patel J.A., Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johanson W.G., Jr., Blackstock R., Pierce A.K., Sanford J.P. The role of bacterial antagonism in pneumococcal colonization of the human pharynx. J Lab Clin Med. 1970;75:946–952. [PubMed] [Google Scholar]
- 29.Bernstein J.M., Faden H.F., Dryja D.M., Wactawski-Wende J. Micro-ecology of the nasopharyngeal bacterial flora in otitis-prone and non-otitis-prone children. Acta Otolaryngol. 1993;113:88–92. doi: 10.3109/00016489309135772. [DOI] [PubMed] [Google Scholar]
- 30.Tano K., Håkansson E.G., Holm S.E., Hellström S. Bacterial interference between pathogens in otitis media and alpha-haemolytic streptococci analysed in an in vitro model. Acta Otolaryngol. 2002;122:78–85. doi: 10.1080/00016480252775788. [DOI] [PubMed] [Google Scholar]
- 31.Henderson F.W., Collier A.M., Sanyal M.A., Watkins J.M., Fairclough D.L., Clyde W.A., Jr. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982;306:1377–1383. doi: 10.1056/NEJM198206103062301. [DOI] [PubMed] [Google Scholar]
- 32.Vesa S., Kleemola M., Blomqvist S., Takala A., Kilpi T., Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J. 2001;20:574–581. doi: 10.1097/00006454-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Pettigrew M.M., Gent J.F., Pyles R.B., Miller A.L., Nokso-Koivisto J., Chonmaitree T. Viral–bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011;49:3750–3755. doi: 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nokso-Koivisto J., Räty R., Blomqvist S., Kleemola M., Syrjänen R., Pitkäranta A. Presence of specific viruses in the middle ear fluids and respiratory secretions of young children with acute otitis media. J Med Virol. 2004;72:241–248. doi: 10.1002/jmv.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casselbrant M.L., Mandel E.M., Fall P.A., Rockette H.E., Kurs-Lasky M., Bluestone C.D. The heritability of otitis media: a twin and triplet study. JAMA. 1999;282:2125–2130. doi: 10.1001/jama.282.22.2125. [DOI] [PubMed] [Google Scholar]
- 36.Kleemola M., Nokso-Koivisto J., Herva E., Syrjänen R., Lahdenkari M., Kilpi T. Is there any specific association between respiratory viruses and bacteria in acute otitis media of young children? J Infect. 2006;52:181–187. doi: 10.1016/j.jinf.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudley S., Ashe K., Winther B., Hendley J.O. Bacterial pathogens of otitis media and sinusitis: detection in the nasopharynx with selective agar media. J Lab Clin Med. 2001;138:338–342. doi: 10.1067/mlc.2001.119311. [DOI] [PubMed] [Google Scholar]