Abstract
Aims: Alcohol can directly impair protein synthesis in cultured myocytes as well as in in situ perfused skeletal muscle. However, alcohol in the general circulation diffuses rapidly into the central nervous system (CNS). Therefore, this study determined whether localized elevation of alcohol within the CNS is capable of decreasing muscle protein synthesis. Methods: Conscious unstrained male rats received a continuous intracerebroventricular (ICV) infusion of ethanol and skeletal muscle protein synthesis and degradation were assessed. Results: ICV alcohol decreased protein synthesis in the gastrocnemius after 6 and 24 h, compared with the time-matched controls. The reduction was equivalent for both sarcoplasmic and myofibrillar proteins and was reversible. The inhibitory effect of alcohol was not prevented by the catalase inhibitor 3-amino-1,2,4-triazole and was mimicked by ICV-administered t-butanol. The alcohol-induced decrease in muscle protein synthesis was associated with a concomitant reduction in phosphorylation of 4E-binding protein and ribosomal S6 kinase-1, suggesting impaired mammalian target of rapamycin kinase activity. ICV alcohol also impaired the ability of leucine to stimulate protein synthesis. Conversely, ICV alcohol increased muscle proteasome activity and muscle RING-finger protein-1 mRNA content. Altered muscle protein metabolism was not associated with changes in muscle mRNA content for tumor necrosis factor α, interleukin-6 or insulin-like growth factor (IGF)-I or circulating insulin or IGF-I. Conclusion: Selective elevation of alcohol within the CNS is capable of decreasing protein synthesis and increasing protein degradation in muscle in the absence of alcohol in the general circulation, thus revealing a previously unrecognized central neural mechanism, which may account for part of the inhibitory effect of ingested alcohol on muscle protein homeostasis.
INTRODUCTION
Consumption of alcohol (ethanol) decreases muscle protein synthesis and this inhibitory effect occurs in response to both acute intoxication and chronic ingestion (Preedy et al., 2001; Lang et al., 2005). Although the large majority of research in this area has been performed in rodent models, a comparable alcohol-induced decrease in muscle protein synthesis is also observed in humans (Pacy et al., 1991). It is generally accepted that alcohol impairs translational efficiency because the total ribosomal number within muscle is unaltered (Lang et al., 1999). In this regard, alcohol has been reported to decrease the phosphorylation of eukaryotic initiation factor (eIF)-4E-binding protein (4E-BP1) and ribosomal S6 kinase (S6K)-1 (Lang et al., 1999, 2000a, 2004a; Kumar et al., 2002), implicating an impairment in the kinase activity of the mammalian target of rapamycin (mTOR). These in vivo data have been confirmed and extended by in vitro studies using C2C12 myocytes that have demonstrated that alcohol can directly inhibit mTOR kinase activity (Hong-Brown et al., 2010, 2012).
Although the alcohol metabolites acetaldehyde and acetate can certainly impair protein synthesis in striated muscle when elevated to pharmacological levels (Preedy et al., 1994; Zhang and Ren, 2011), most data suggest that alcohol acts directly to impair muscle protein synthesis. For example, as already indicated, culturing muscle cells with alcohol mimics many of the protein metabolic effects observed under in vivo conditions (Hong-Brown et al., 2001, 2010, 2011, 2012). Furthermore, a direct inhibitory effect of alcohol on mTOR activity and protein synthesis has been reported in rats pretreated with the alcohol dehydrogenase inhibitor 4-methylpyrazole (Lang et al., 2004b) as well as in studies using the isolated perfused hind-limb preparation (Lang et al., 2004b). Collectively, these data suggest that alcohol directly impairs skeletal muscle protein synthesis and that its hepatic metabolism is not necessary for the development of alcoholic myopathy.
The diverse effects of alcohol on the brain have been extensively investigated and include its ability to alter various aspects of the neuroendocrine control (Ogilvie et al., 1998). Specifically, the modulation of the pituitary–adrenal and pituitary–gonadal axes by systemic alcohol is mediated in large part by its central actions at the level of the hypothalamus (Lee et al., 2004; Selvage et al., 2004a,b; Herman and Rivier, 2006; Larkin et al., 2010). Such findings are consistent with the ability of this drug to rapidly equilibrate in all body fluid compartments and within tissues, including the central nervous system (CNS) (Gill et al., 1986; Deitrich and Harris, 1996). Hence, studies where alcohol was either orally consumed or administered systemically cannot exclude the possibility that alcohol alters muscle protein balance via a centrally mediated neural mechanism. Therefore, this study determined whether localized elevation of alcohol within the CNS is capable of decreasing muscle protein synthesis and/or increasing proteolysis.
MATERIALS AND METHODS
Animals
Specific pathogen-free male Sprague–Dawley rats (300–325 g, Charles River, Cambridge, MA) were housed (3–4 rats/wire bottom cage) in a controlled environment and provided commercial diet (Harlan Teklad 2018; Indianapolis, IN) and water ad libitum for 1 week. Thereafter, the rats were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (9 mg/kg) and positioned in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). A cannula was inserted into the lateral ventricle of the brain, as described (Lang et al., 1995, 1996, 1998; Deitrich and Harris, 1996). Proper positioning of the cannula was verified by intracerebroventricular (ICV) injection of trypan blue. Additionally, sterile surgery was performed to place catheters in the left carotid artery (for collection of blood), right jugular vein (for injection of isotope) and in the stomach (for injection of alcohol or saline). A subcutaneous injection of buprenorphine (0.05 mg/kg; Hospira, Lake Forest, IL) was administered prior to the start of surgery for analgesia. The rats were housed individually in solid-bottom cages with corncob bedding, provided food and water ad libitum and allowed 7 days to recover. Only animals that regained their pre-surgical body weight (BW) were used. Protocols were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine (#97-063) and adhered to the National Institutes of Health guidelines.
Experimental protocols
Overnight-fasted rats were divided randomly into four groups, and experiments performed on unrestrained conscious rats that were not handled for 60 min prior to the start of the study. While water was available at all time, the rats did not have access to food during any experimental protocol so that metabolic differences between the groups cannot be attributed to differences in food intake among the various groups. In groups 1 and 2, rats had artificial cerebrospinal fluid (aCSF) or alcohol (100% v/v; 200 proof) administered ICV as a 5-µl bolus (over 10 min) followed by a continuous infusion (Harvard Apparatus) of 5 µl/h for various periods of time. The ICV injection of this dose of alcohol was selected on the basis of studies that have demonstrated a sustained increase in the hypothalamic–pituitary–adrenal axis (Deitrich and Harris, 1996; Selvage et al., 2004b) and the lack of neuronal damage within various brain regions using this same experimental protocol (Selvage et al., 2004b). As ICV-administered alcohol is rapidly cleared from the CNS (Friedman and Lester, 1975), a continuous infusion was initiated. Groups 3 and 4 received a slow (2 min) bolus administration of alcohol (75 mmol/kg BW; 20% w/v in saline) or sterile 0.9% saline via the implanted gastric catheter. This dose of alcohol was selected because it decreases muscle protein synthesis (Preedy et al., 1992; Lang et al., 2000a, 2004b).
At various time points, which are described in the ‘Results section’, the rats were injected IV with L-[2,3,4,5,6-3H]phenylalanine (Phe) (150 mM, 30 μCi/ml; 1 ml/100 g BW). Blood was collected from the carotid catheter 10 min after isotope injection (Vary and Lang, 2008). The soleus and gastrocnemius were freeze-clamped and powdered under liquid nitrogen. Muscles and plasma were stored at −70 C until analyzed. A portion of the powdered tissue was homogenized in ice-cold perchloric acid (PCA) and the supernatant used to estimate the incorporation of [3H]Phe into protein. The specific radioactivity was calculated by dividing the amount of radioactivity in the peak corresponding to Phe by the concentration of the amino acid in the same fraction and the rate of protein synthesis was calculated as described (Vary and Lang, 2008). Powdered gastrocnemius was used to separate the myofibrillar and sarcoplasmic proteins, according to procedures used in our laboratory (Lang et al., 2002).
To determine whether the effect of ICV-administered alcohol was direct or required metabolism, the rats were injected ICV with a non-metabolizable alcohol (i.e. t-butanol) or pretreated with an intraperitoneal injection of the catalase inhibitor 3-amino-1,2,4-triazole (1 g/kg) (Vasiliou et al., 2006; Zimatkin et al., 2006). This dose of aminotriazole effectively prevents brain metabolism of alcohol (Sanchis-Segura et al., 2005) and antagonizes a number of the central-mediated effects of alcohol (Quertemont et al., 2005).
Finally, we examined whether ICV alcohol blunts the normal anabolic effect of the amino acid leucine. The rats were surgically prepared as already mentioned and infused ICV with alcohol for 24 h.
Both the aCSF- and the alcohol-infused rats were then administered either saline (0.155 mol/l) or a maximally stimulating dose of leucine (1.35 g/kg BW) via the gastric catheter (Anthony et al., 2000; Lang et al., 2003, 2009). The rats were anesthetized and the gastrocnemius excised 30 min after leucine.
Plasma determinations
Plasma insulin, insulin-like growth factor (IGF)-I and adrenocorticotropin hormone (ACTH) concentrations were measured using commercial ELISAs (ALPCO Diagnostics, Salem, NH). The blood alcohol and glucose concentrations were determined using an Analox Instruments analyzer (Lunenburg, MA).
Western blotting
Fresh gastrocnemius was homogenized in ice-cold buffer consisting of (in mmol/l): 20 HEPES (pH 7.4), 2 EGTA, 50 sodium fluoride, 100 potassium chloride, 0.2 EDTA, 50 β-glycerophosphate, 1 DTT, 0.1 phenylmethane-sulphonylfluoride, 1 benzamidine and 0.5 sodium vanadate. The protein concentration was quantified (Bio-Rad, Hercules, CA) and equal amounts of total protein per sample were subjected to standard sodium dodecyl-sulphate-polyacrylamide gel electrophoresis. The blots were incubated with primary antibodies to phosphorylated 4E-BP-1 (Thr 37/46; Bethyl Laboratories, Montgomery, TX) and S6K-1 (T389; Cell Signaling, Beverly, MA) as well as appropriate loading controls. Blots were developed with enhanced chemiluminescence reagents (Supersignal Pico, Pierce Chemical, Rockford, IL). Dried blots were exposed to X-ray film and the film was then scanned (Microtek ScanMaker IV; Cerritos, CA) and quantified using the Scion Image 3b software (Scion Corporation, Frederick, MD).
RNA extraction and RNase protection assay
Total RNA was extracted from powdered gastrocnemius using TRI Reagent (Molecular Research Center, Cincinnati, OH). The ‘atrogenes’ Atrogin-1/Muscle Atrophy F-box (MAFbx) and muscle RING-finger protein-1 (MuRF1) as well as tumor necrosis factor α (TNFα), interleukin (IL)-6 and IGF-I mRNA contents were determined by an RNase protection assay (RPA), and the primer sequences for these mRNAs along with L32 (loading control) have been previously published (Vary et al., 2008). Blots were exposed to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, CA), visualized and analyzed using the ImageQuant software (version 5.2; Molecular Dynamics).
Proteasome activity
The gastrocnemius was homogenized in buffer containing (in mM) 25 HEPES, 5 MgCl2, 5 EDTA and 5 DTT, pH 7.5, at 4°C followed by centrifugation. The protein concentration in the supernatant was determined using a BCA Protein Assay kit (Pierce Chemical). The proteasome enzymatic activity was measured by using a Proteasome 20S Assay kit (Enzo Life Sciences, Farmingdale, New York) following the manufacturer's instructions and as described previously (Lang et al., 2012). Briefly, the protein extract from muscle was used to assess proteasome 20S activity by measuring the hydrolysis of a fluorogenic peptidyl substrate Suc-Leu-Leu-Val-Tyr-AMC (AMC: 7-amino-4-methylcoumarin).
Statistical analysis
Experimental data for each condition are summarized as means ± SE with the sample indicated in the legend to the figure or table. For statistical analysis of data from two groups, a paired t-test was performed. For experiments where the number of groups was three or more, statistical evaluation of the data was performed using ANOVA followed by the Student–Neuman–Keuls test to determine treatment effect. Differences between the groups were considered significant when P < 0.05.
RESULTS
Alcohol-induced changes in blood pressure and circulating concentrations of ACTH, insulin and glucose
The blood alcohol concentration was elevated at 1 h (355 ± 41 mg/dl) and 6 h (58 ± 8 mg/dl), but was non-detectable 24 h after intragastric alcohol. Blood alcohol was not detected at these times in rats administered alcohol via the ICV route nor at earlier time points (i.e. 5–15 min) assessed in a preliminary study.
With the exception of the decrease in mean arterial blood pressure (MABP) at 1 h after intragastric administration of alcohol, no significant change was detected in MABP between or among groups over time (Table 1). As there were no significant differences between rats infused ICV with aCSF and those administered saline intragastrically, values from these groups were combined and presented as a single ‘control’ group for MABP and other endpoints.
Table 1.
Alcohol-induced changes in blood pressure and circulating concentrations of ACTH, glucose, insulin and IGF-I
| Time post-administration (hours) |
||||
|---|---|---|---|---|
| Pre (time 0) | 1 | 6 | 24 | |
| MABP, mmHg | ||||
| i.g. Sal or ICV aCSF | 117 ± 5 | 115 ± 8 | 118 ± 7 | 114 ± 7 |
| i.g. alcohol | 119 ± 9 | 109 ± 7* | 117 ± 5 | 112 ± 5 |
| ICV alcohol | 115 ± 8 | 114 ± 6 | 115 ± 8 | 111 ± 8 |
| ACTH, pg/ml | ||||
| i.g. Sal or ICV aCSF | 49 ± 7 | 52 ± 11a | 44 ± 12 | 53 ± 9 |
| i.g. alcohol | 54 ± 8 | 531 ± 87*,b | 39 ± 22 | 49 ± 11 |
| ICV alcohol | 48 ± 12 | 355 ± 49*,c | 52 ± 23 | 44 ± 6 |
| Glucose, mmol/l | ||||
| i.g. Sal or ICV aCSF | 5.8 ± 0.2 | 5.8 ± 0.3 | 5.6 ± 0.3 | 5.2 ± 0.3* |
| i.g. alcohol | 5.7 ± 0.3 | 6.9 ± 0.4* | 5.7 ± 0.3 | 5.3 ± 0.3* |
| ICV alcohol | 5.9 ± 0.2 | 5.8 ± 0.2 | 6.0 ± 0.3 | 5.4 ± 0.4* |
| Insulin, pmol/l | ||||
| i.g. Sal or ICV aCSF | 120 ± 11 | 127 ± 9a | 124 ± 12 | 78 ± 7* |
| i.g. alcohol | 118 ± 22 | 87 ± 7*,b | 110 ± 13 | 65 ± 11* |
| ICV alcohol | 111 ± 9 | 122 ± 14a | 102 ± 28 | 66 ± 13* |
| IGF-I, ng/ml | ||||
| i.g. Sal or ICV aCSF | 688 ± 34 | 673 ± 44 | 651 ± 31a | 596 ± 48* |
| i.g. alcohol | 712 ± 47 | 683 ± 51 | 501 ± 35*,b | 484 ± 43* |
| ICV alcohol | 725 ± 39 | 689 ± 43 | 677 ± 51a | 619 ± 33* |
*P < 0.05, compared to pre-value from same group.
i.g., intragastric; Sal, saline; ICV, intracerebroventricular; aCSF, artificial cerebrospinal fluid, insulin-like growth factor (IGF)-I, adrenocorticotrophic hormone (ACTH). Rats received an i.g. injection of alcohol at a dose of 75 mmol/kg or an ICV administration of alcohol (5 µl of 100% alcohol + 5 µl/h). For each parameter, values with a different superscript letter (a,b,c) in the same column are statistically different (P < 0.05).
While both intragastric and ICV-administered alcohol increased plasma ACTH at 1 h, the increase was smaller in the latter group (Table 1). The ACTH concentrations had returned to control values at 6 h and 24 h in both alcohol-treated groups. No change in plasma ACTH was detected in control rats.
While rats injected with intragastric alcohol showed a small statistically significant increase in plasma glucose at 1 h (Table 1), rats injected with ICV alcohol did not show a hyperglycemic response. The blood glucose determined at 24 h was lower than pre-values in all the three groups. Conversely, the plasma insulin concentration decreased transiently at 1 h in rats injected with intragastric alcohol (Table 1). The insulin concentration of rats injected with ICV alcohol did not differ from control values. The insulin concentration at 24 h was decreased in all the three groups, compared with their respective pre-values.
The plasma concentration of IGF-I did not differ between the rats injected with ICV alcohol and the time-matched controls (Table 1). However, intragastric alcohol reduced IGF-I by 23% at 6 h and 17% (P < 0.07), compared with the time-matched control values. The IGF-I concentration was reduced at 24 h for all the groups, compared with their respective pre-values.
Muscle protein synthesis—effect of route of alcohol administration
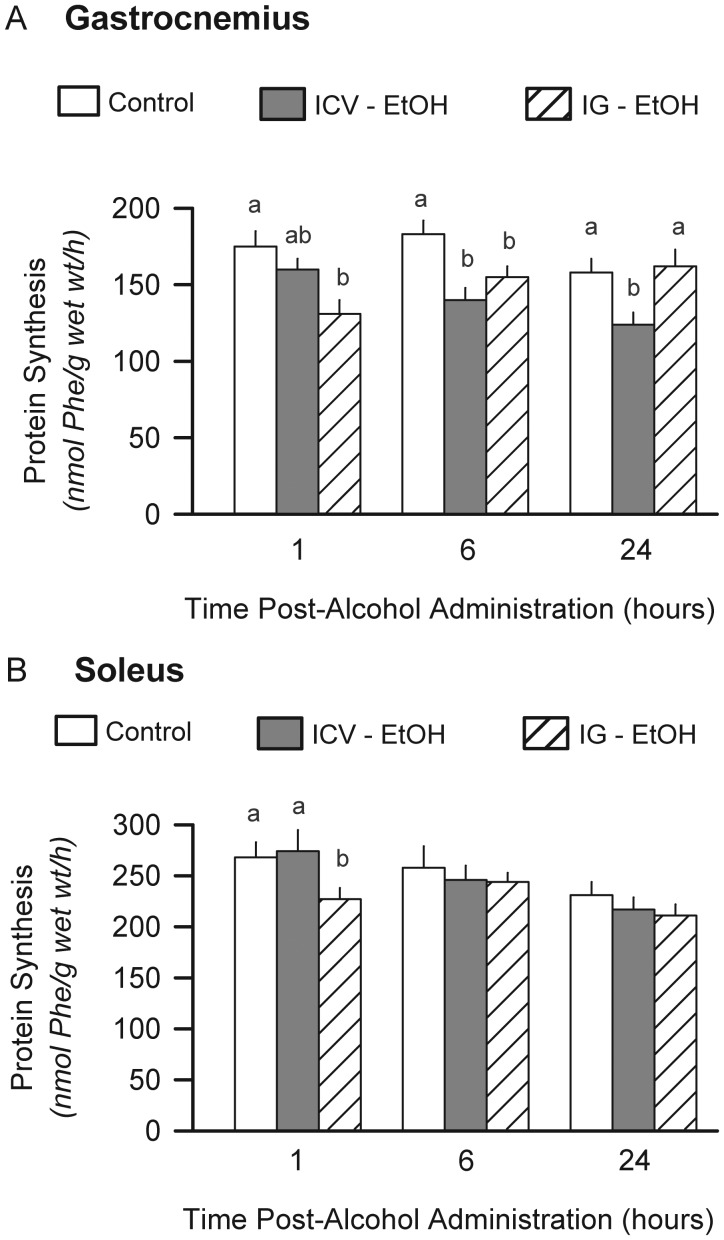
Protein synthesis was determined in fast-twitch (gastrocnemius) and slow-twitch (soleus) muscle. Intragastric alcohol decreased protein synthesis in the gastrocnemius by 25% at 1 h and by 10% at 6 h, but not at 24 h, compared with the time-matched control values (Fig. 1A). Rats infused ICV with alcohol demonstrated a 9, 23 and 21% decrease in protein synthesis in gastrocnemius at 1, 6 and 24 h, respectively. In contrast, intragastric administered alcohol only transiently decreased protein in the soleus at 1 h and there was no difference in soleus protein synthesis between the rats infused with alcohol ICV and the time-matched control values (Fig. 1B). Because ICV alcohol did not alter protein synthesis in the soleus, only the gastrocnemius was further evaluated.
Fig. 1.
Effect of alcohol on muscle protein synthesis. In vivo-determined protein synthesis was determined using 3H-phenylalanine in rats administered alcohol (ethanol; EtOH) either ICV or intragastrically (IG). Gastrocnemius (A) and soleus (B) were sampled from each group. Values are means ± SEM; n = 8–9 per group. For the same time point (e.g. 1, 6 and 24 h), values with a different letter are statistically different (P < 0.05), compared with time-matched control values (open bars). As there were no significant differences between rats infused ICV with aCSF and those administered saline IG, values from these groups were combined and presented as a single ‘control’ group for this and other endpoints.
The effect of ICV alcohol on both sarcoplasmic and myofibrillar fractions of muscle was also assessed after 24 h. ICV alcohol reduced both sarcoplasmic (control = 0.92 ± 0.06 vs ICV alcohol = 0.71 ± 0.05 nmol Phe/mg protein/h; P < 0.05) and myofibrillar (0.41 ± 0.02 vs 0.29 ± 0.02; P < 0.05) protein synthesis, compared with the time-matched control values.
We also assessed whether the decrease in muscle protein synthesis produced after an ICV infusion of alcohol was reversible. Muscle protein synthesis did not differ in rats receiving ICV alcohol for 24 h followed by a 2-h recovery period (169 ± 9 nmol/g/h) and the time-matched rats infused ICV with aCSF for 26 h (174 ± 10 nmol/g/h). However, the time-matched rats infused ICV with alcohol for 26 h still exhibited a significant decrease in muscle protein synthesis (122 ± 23 nmol/g/h; P < 0.05), compared with the other two groups.
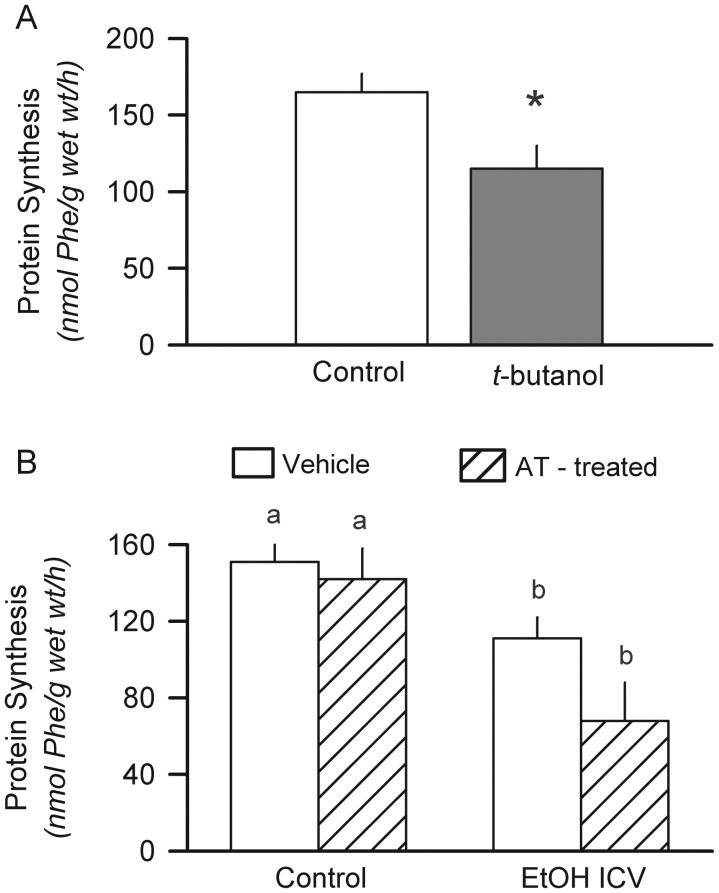
In the next study, rats were infused ICV for 6 h with an equal molar amount of t-butanol, a non-metabolizable alcohol, to investigate whether the effect of ICV-administered alcohol was direct or mediated by a metabolite. Muscle protein synthesis was decreased 30% by t-butanol, compared with the time-matched control values (Fig. 2A). Alcohol in the brain is metabolized primarily via catalase, which can be inhibited by aminotriazole (Vasiliou et al., 2006; Zimatkin et al., 2006). However, pretreatment of rats with aminotriazole did not antagonize the ability of ICV alcohol to decrease muscle protein synthesis (Fig. 2B). This dose of systemically administered aminotriazole has been previously reported to inhibit brain catalase activity and antagonize some centrally mediated psychopharmacological effects of ethanol (Pastor et al., 2004; Sanchis-Segura et al., 2005; Jamal et al., 2007).
Fig. 2.
Direct effect of ICV alcohol on muscle protein synthesis. (A) The non-metabolizable alcohol t-butanol was administered as a primed, constant infusion as described in Figure 1 for ethanol. Protein synthesis in the gastrocnemius was determined after a 6-h infusion. *P < 0.05 compared with the time-matched controls infused ICV with aCSF. Values are means ± SEM; n = 5 rats per group. (B), rats were injected intraperitoneally with the catalase inhibitor AT or vehicle and then infused ICV with alcohol or aCSF (control) for 6 h. Values are means ± SEM; n = 6–8 rats per group. Values with a different letters are statistically different (P < 0.05).
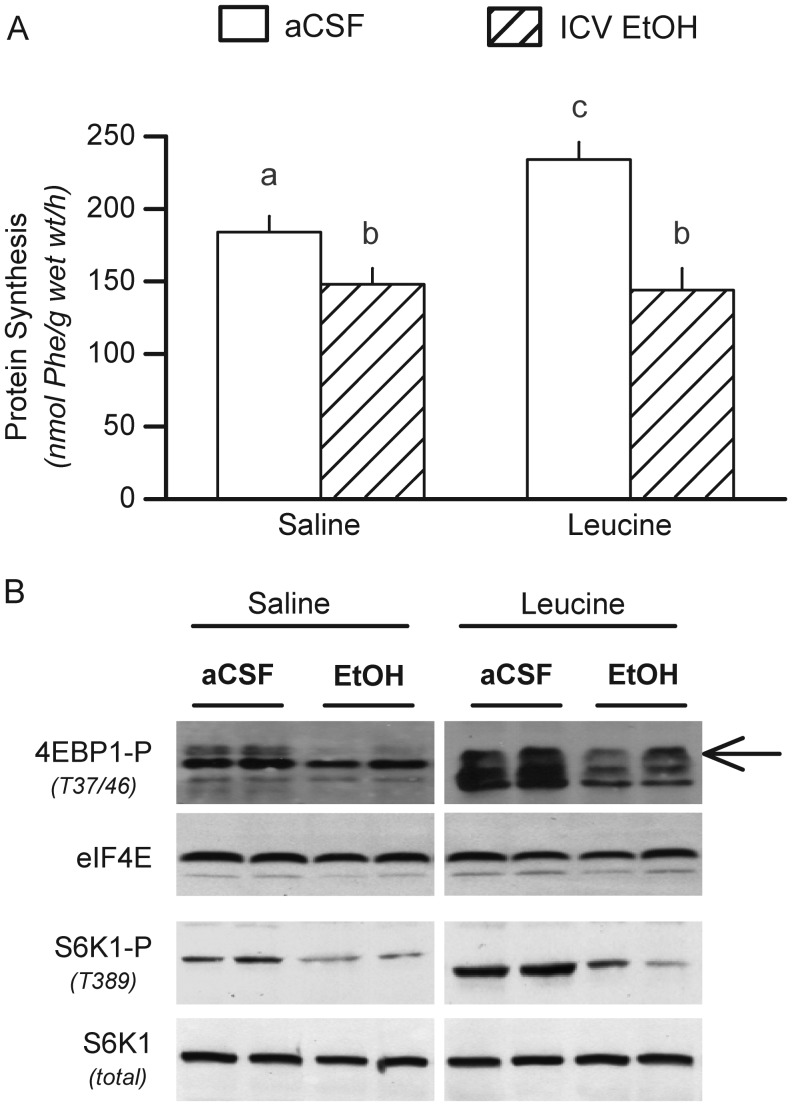
The branched-chain amino acid leucine increases muscle protein synthesis in part by increasing mTOR activity (Frost and Lang, 2011). The anabolic effect of leucine was readily apparent in control animals, but ICV alcohol prevented the normal increment (Fig. 3A). The leucine-induced increase in control rats was associated with a concomitant increase in the phosphorylation of 4E-BP1 and S6K1 (Fig. 3B). Infusion of ICV alcohol decreased basal 4E-BP1 and S6K1 phosphorylation and completely prevented the leucine-induced increase observed in control rats.
Fig. 3.
ICV infusion of alcohol impairs systemic leucine response. (A), Muscle protein synthesis in rats infused ICV with aCSF (control) or alcohol for 24 h prior to intragastric administration of saline or leucine. (B) Representative western blots for phosphorylated 4E-BP1 and S6K1 in the gastrocnemius removed from rats in the four experimental groups. Arrow to the right of blot indicates the most highly phosphorylated and active γ-isoform of 4E-BP1. Samples from saline- and leucine-treated were run on the same gel, but selected lanes were deleted for clarity and the deletion is indicated by the white line between blots. Values are means ± SEM; n = 6–8 rats per group. Values with a different letters are statistically different (P < 0.05).
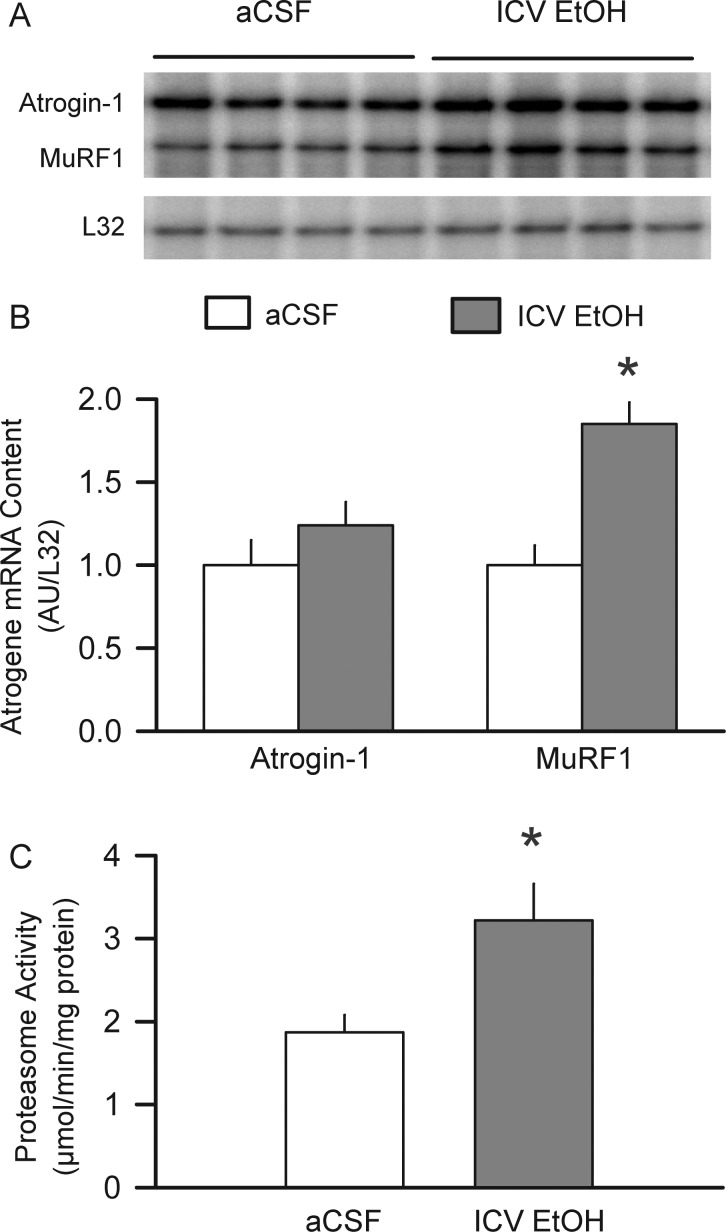
We assessed the other side of the protein balance equation by quantitating indices of protein degradation (Bodine et al., 2001). The mRNA content for the muscle-specific ubiquitin E3 ligase MuRF1, but not atrogin-1, was increased 60% in the gastrocnemius from the rats infused ICV with alcohol for 24 h (Fig. 4A and B). Moreover, ICV alcohol increased in vitro proteasome activity 85%, compared with the time-matched control values (Fig. 4C).
Fig. 4.
Direct effect of ICV-administered alcohol on indices of muscle protein degradation. (A) Representative RPA for atrogin-1 and MuRF1 mRNA in the gastrocnemius of rats infused ICV for 24 h with either aCSF or alcohol. (B) mRNA abundance was quantitated and expressed in arbitrary units (AU) as determined by densitometry and normalized to L32 mRNA. (C), 20S proteasome activity determined in rats infused ICV with aCSF or alcohol for 24 h. Values are means ± SEM; 5–6 rats per group. *P < 0.05 compared with the time-matched control (aCSF) values.
Potential muscle mediators of altered protein balance
Increases in pro-inflammatory cytokines can produce a catabolic state characterized by loss of muscle protein (Frost and Lang, 2008). However, there was no difference in the mRNA for TNFα or IL-6 in muscle from the rats infused ICV with aCSF or alcohol (data not shown). Conversely, a decrease in muscle IGF-I can lead to wasting, but the mRNA content for IGF-I was also not altered by ICV alcohol (data not shown). Also, in this regard, muscle synthesizes several IGF-binding proteins (IGFBPs), but the mRNA content for IGFBP-3, IGFBP-4 and IGFBP-5 did not differ in muscle from the control and the alcohol-infused rats (data not shown).
DISCUSSION
This study sought to determine whether localized elevation of alcohol within the CNS is capable of decreasing muscle protein synthesis and/or increasing proteolysis. Our results demonstrate that elevating alcohol selectively within the CNS is capable of decreasing gastrocnemius protein synthesis, both sarcoplasmic and myofibillar, and increasing protein degradation. Although the presence of alcohol in the local skeletal muscle environment is undoubtedly responsible for the majority of the inhibitory effect of this drug on protein synthesis (Lang et al., 2004b), our current data suggest that a previously unrecognized neural mechanism may be responsible in part for changes in muscle protein balance seen in response to systemic alcohol. The primary CNS target of this alcohol-mediated effect was not elucidated, but the neural pathway appears independent of the pituitary as ACTH was only transiently elevated. Other such neural-mediated pathways of alcohol action, independent of the presence of drug in the general circulation, have been reported for Leydig cell activity (Lee et al., 2002; Selvage et al., 2004a), the hypothalamus (Lee et al., 2004) and hyperglycemia (Erwin and Towell, 1983).
Many alcohol effects result from its metabolism and the production of either acetaldehyde and/or acetate (Jelski and Szmitkowski, 2008; Mello et al., 2008; Guo and Ren, 2010; Zhang and Ren, 2011). In contrast to the liver, where alcohol dehydrogenase is primarily responsible for alcohol metabolism, catalase and CYP2E1 account for the majority of ethanol oxidation in the rodent brain (Zimatkin et al., 2006; Zimatkin and Buben, 2007). However, treatment of rats with the known non-competitive catalase inhibitor aminotriazole (Zimatkin et al., 2006) did not prevent or ameliorate the ability of ICV alcohol to decrease protein synthesis. Moreover, ICV infusion of t-butanol, which does not undergo oxidative metabolism, also decreased muscle protein synthesis. Collectively, these data suggest that alcohol per se, as opposed to acetaldehyde or acetate, is primarily responsible for the observed neural effect on muscle protein metabolism.
The decreased muscle protein synthesis produced by the presence of systemic alcohol is associated with reduced mTOR activity (Lang et al., 2005) and an increased association of mTOR bound to the scaffold protein raptor (Lang et al., 2009; Hong-Brown et al., 2010). These data are consistent with alcohol promoting a ‘closed conformation,’ which has been posited to impair mTOR kinase activity (Kim et al., 2002). As a consequence, alcohol decreases the phosphorylation of 4E-BP1, thereby increasing its binding to eIF4E and impairing cap-dependent mRNA translation (Gingras et al., 1998). Circulating alcohol also impairs phosphorylation of S6K1 (Lang et al., 2003, 2004b, 2009), another key regulator of mRNA translation (Magnuson et al., 2012). While our current study did not examine various protein–protein interactions within the mTOR complex 1, administration of ICV alcohol reduced both 4E-BP1 and S6K1 phosphorylation under basal conditions and prevented the normal anabolic action of leucine on muscle protein synthesis and mTOR activity. This leucine resistance produced by ICV alcohol mimics that seen after systemic administration of alcohol (Lang et al., 2003; Sneddon et al., 2003).
The impact of circulating alcohol on the other side of the protein balance equation—protein degradation—is more controversial. While several studies report increased mRNA and/or protein levels for components of various proteolytic pathways (Koll et al., 2002; Vary et al., 2008; Otis and Guidot, 2009; LeCapitaine et al., 2011), others have shown that these changes are not necessarily correlated with enhanced muscle protein breakdown (Vary et al., 2008). In the current study, ICV-administered alcohol selectively increased MuRF1 mRNA, but not atrogin-1, and this increase was associated with an increase in proteasome activity. Therefore, a localized increase in alcohol within the CNS appears capable of both decreasing protein synthesis and increasing protein degradation in skeletal muscle.
Selected known physiological regulators of muscle protein synthesis and degradation were not affected by ICV-administered alcohol, and therefore, their role appears nominal in the current experimental paradigm. For example, over-production of the pro-inflammatory cytokines TNFα or IL-6 can lead to wasting (Frost and Lang, 2008). However, there was no difference in the mRNA for these cytokines in the gastrocnemius from the rats infused ICV with aCSF or alcohol. Conversely, a decrease in circulating and muscle IGF-I can lead to wasting and such a response has been observed with acute alcohol intoxication and chronic alcohol feeding (Lang et al., 2000a,b, 2004a, 2009). However, the IGF-I in blood and gastrocnemius was also not altered by ICV alcohol. Alterations in IGF-binding proteins can influence IGF-I bioavailability and systemic alcohol modulates the muscle synthesis of several IGFBPs (Lang et al., 2009). Again, the mRNA content for IGFBP-3, -4 and -5 did not differ in muscle from control- and alcohol-infused rats. Finally, the protein metabolic effects of ICV alcohol appeared independent of the prevailing insulin concentration.
In summary, our data suggest the presence of a neurally mediated pathway by which the local elevation of alcohol within the brain is sensed and this information is transmitted to fast-twitch skeletal muscle (gastrocnemius). The ability of ICV-administered alcohol to impair both basal and leucine-stimulated protein synthesis appears mediated via inhibition of mTOR. The neural pathway appears independent of a sustained activation of the hypothalamus–pituitary–adrenal axis, independent of detectable blood alcohol and not mediated by changes in many traditional mediators (e.g. inflammatory cytokines, insulin, IGF-I) of protein balance. ICV-administered alcohol also enhanced muscle protein breakdown by increasing proteasome activity, a response with differs from the effect of systemic alcohol. However, the current studies are limited by the relatively acute nature of the alcohol challenge and by the inability to selectively inhibit CNS uptake of alcohol when administered systemically. Moreover, while a previous study indicated that no neuronal damage was detected in various brain regions using this same experimental protocol (Selvage et al., 2004b), histological examination of brain tissue was not performed in the current study. However, because the inhibitory effect of ICV alcohol on muscle protein synthesis is rapidly (2 h) reversible and there was no stress-induced hyperglycemia, our data suggest that this protein metabolic response is not due to a generalized toxic response and/or tissue necrosis. The alcohol infused into the lateral ventricle of the brain would be expected to be immediately diluted by the CSF present in the ventricle, which is continuously circulating within the ventricular system, and the metabolism of the infused alcohol would further reduce the prevailing alcohol concentration within the brain. In conclusion, while the physiological importance of this neural mechanism regulating muscle protein balance remains to be fully elucidated, it seems possible that a portion of the catabolic effect of this drug can now be attributed to a secondary effect mediated by the CNS.
Funding
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R37 AA011290), to C.H.L.
References
- Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–9. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res. 1996;20:1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Towell JF. Ethanol-induced hyperglycemia mediated by the central nervous system. Pharmacol Biochem Behav. 1983;18(Suppl 1):559–63. doi: 10.1016/0091-3057(83)90236-8. [DOI] [PubMed] [Google Scholar]
- Friedman HJ, Lester D. Intraventricular ethanol and ethanol intake: a behavioral and radiographic study. Pharmacol Biochem Behav. 1975;3:393–401. doi: 10.1016/0091-3057(75)90047-7. [DOI] [PubMed] [Google Scholar]
- Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci. 2008;86:E84–93. doi: 10.2527/jas.2007-0483. [DOI] [PubMed] [Google Scholar]
- Frost RA, Lang CH. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 2011;26:83–96. doi: 10.1152/physiol.00044.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill K, France C, Amit Z. Voluntary ethanol consumption in rats: an examination of blood/brain ethanol levels and behavior. Alcohol Clin Exp Res. 1986;10:457–62. doi: 10.1111/j.1530-0277.1986.tb05124.x. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Kennedy SG, O'Leary MA, et al. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–13. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Ren J. Alcohol and acetaldehyde in public health: from marvel to menace. Int J Environ Res Public Health. 2010;7:1285–301. doi: 10.3390/ijerph7041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M, Rivier C. Activation of a neural brain-testicular pathway rapidly lowers Leydig cell levels of the steroidogenic acute regulatory protein and the peripheral-type benzodiazepine receptor while increasing levels of neuronal nitric oxide synthase. Endocrinology. 2006;147:624–33. doi: 10.1210/en.2005-0879. [DOI] [PubMed] [Google Scholar]
- Hong-Brown LQ, Frost RA, Lang CH. Alcohol impairs protein synthesis and degradation in cultured skeletal muscle cells. Alcohol Clin Exp Res. 2001;25:1373–82. [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Kazi AA, et al. Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction. J Cell Biochem. 2010;109:1172–84. doi: 10.1002/jcb.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Navaratnarajah M, et al. Alcohol-induced modulation of rictor and mTORC2 activity in C2C12 myoblasts. Alcohol Clin Exp Res. 2011;35:1445–53. doi: 10.1111/j.1530-0277.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Kazi AA, et al. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol. 2012;302:C1557–65. doi: 10.1152/ajpcell.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Uekita I, et al. Catalase mediates acetaldehyde formation in the striatum of free-moving rats. Neurotoxicology. 2007;28:1245–8. doi: 10.1016/j.neuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Jelski W, Szmitkowski M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases. Clin Chim Acta. 2008;395:1–5. doi: 10.1016/j.cca.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Koll M, Ahmed S, Mantle D, et al. Effect of acute and chronic alcohol treatment and their superimposition on lysosomal, cytoplasmic, and proteosomal protease activities in rat skeletal muscle in vivo. Metabolism. 2002;51:97–104. doi: 10.1053/meta.2002.28967. [DOI] [PubMed] [Google Scholar]
- Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E917–28. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- Lang CH, Ajmal M, Baillie AG. Neural control of glucose uptake by skeletal muscle after central administration of NMDA. Am J Physiol Regul Integr Comp Physiol. 1995;268:R492–7. doi: 10.1152/ajpregu.1995.268.2.R492. [DOI] [PubMed] [Google Scholar]
- Lang CH, Cooney R, Vary TC. Central interleukin-1 partially mediates endotoxin-induced changes in glucose metabolism. Am J Physiol Endocrinol Metab. 1996;271:E309–16. doi: 10.1152/ajpendo.1996.271.2.E309. [DOI] [PubMed] [Google Scholar]
- Lang CH, Fan J, Wojnar MM, et al. Role of central IL-1 in regulating peripheral IGF-I during endotoxemia and sepsis. Am J Physiol. 1998;274:R956–62. doi: 10.1152/ajpregu.1998.274.4.R956. [DOI] [PubMed] [Google Scholar]
- Lang CH, Wu D, Frost RA, et al. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab. 1999;277:E268–76. doi: 10.1152/ajpendo.1999.277.2.E268. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Kumar V, et al. Impaired protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4E in muscle and eIF2B in liver. Alcohol Clin Exp Res. 2000a;24:322–31. [PubMed] [Google Scholar]
- Lang CH, Liu X, Nystrom G, et al. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol. 2000b;35:148–58. doi: 10.1093/alcalc/35.2.148. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Nairn AC, et al. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336–47. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Deshpande N, et al. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab. 2003;285:E1205–15. doi: 10.1152/ajpendo.00177.2003. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Svanberg E, et al. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab. 2004a;286:E916–26. doi: 10.1152/ajpendo.00554.2003. [DOI] [PubMed] [Google Scholar]
- Lang CH, Pruznak AM, Deshpande N, et al. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res. 2004b;28:1758–67. doi: 10.1097/01.alc.0000145787.66405.59. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Summer AD, et al. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol. 2005;37:2180–95. doi: 10.1016/j.biocel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Lang CH, Pruznak AM, Nystrom GJ, et al. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: comparable effects in young and mature rats. Nutr Metab (Lond) 2009;6:4. doi: 10.1186/1743-7075-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SM, Kazi AA, Hong-Brown L, et al. Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLoS ONE. 2012;7:e38910. doi: 10.1371/journal.pone.0038910. doi:10.1371/journal.pone.0038910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JW, Binks SL, Li Y, et al. The role of oestradiol in sexually dimorphic hypothalamic-pituitary-adrena axis responses to intracerebroventricular ethanol administration in the rat. J Neuroendocrinol. 2010;22:24–32. doi: 10.1111/j.1365-2826.2009.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCapitaine NJ, Wang ZQ, Dufour JP, et al. Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. J Infect Dis. 2011;204:1246–55. doi: 10.1093/infdis/jir508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Miselis R, Rivier C. Anatomical and functional evidence for a neural hypothalamic-testicular pathway that is independent of the pituitary. Endocrinology. 2002;143:4447–54. doi: 10.1210/en.2002-220392. [DOI] [PubMed] [Google Scholar]
- Lee S, Selvage D, Hansen K, et al. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145:4470–79. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Mello T, Ceni E, Surrenti C, et al. Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol Aspects Med. 2008;29:17–21. doi: 10.1016/j.mam.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, et al. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res. 1998;22:243S–7. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- Otis JS, Guidot DM. Procysteine stimulates expression of key anabolic factors and reduces plantaris atrophy in alcohol-fed rats. Alcohol Clin Exp Res. 2009;33:1450–9. doi: 10.1111/j.1530-0277.2009.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacy PJ, Preedy VR, Peters TJ, et al. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis—a stable isotope study. Alcohol Alcohol. 1991;26:505–13. doi: 10.1093/oxfordjournals.alcalc.a045152. [DOI] [PubMed] [Google Scholar]
- Pastor R, Sanchis-Segura C, Aragon CM. Brain catalase activity inhibition as well as opioid receptor antagonism increases ethanol-induced HPA axis activation. Alcohol Clin Exp Res. 2004;28:1898–906. doi: 10.1097/01.alc.0000148107.64739.76. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Keating JW, Peters TJ. The acute effects of ethanol and acetaldehyde on rates of protein synthesis in type I and type II fibre-rich skeletal muscles of the rat. Alcohol Alcohol. 1992;27:241–51. [PubMed] [Google Scholar]
- Preedy VR, Peters TJ, Patel VB, et al. Chronic alcoholic myopathy: transcription and translational alterations. FASEB J. 1994;8:1146–51. doi: 10.1096/fasebj.8.14.7958620. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Adachi J, Ueno Y, et al. Alcoholic skeletal muscle myopathy: definitions, features, contribution of neuropathy, impact and diagnosis. Eur J Neurol. 2001;8:677–87. doi: 10.1046/j.1468-1331.2001.00303.x. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: a comprehensive review of animal studies. Prog Neurobiol. 2005;75:247–74. doi: 10.1016/j.pneurobio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Correa M, Miquel M, et al. Catalase inhibition in the Arcuate nucleus blocks ethanol effects on the locomotor activity of rats. Neurosci Lett. 2005;376:66–70. doi: 10.1016/j.neulet.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Selvage DJ, Hales DB, Rivier CL. Comparison between the influence of the systemic and central injection of alcohol on Leydig cell activity. Alcohol Clin Exp Res. 2004a;28:480–8. doi: 10.1097/01.alc.0000117839.69352.b3. [DOI] [PubMed] [Google Scholar]
- Selvage DJ, Lee SY, Parsons LH, et al. A hypothalamic-testicular neural pathway is influenced by brain catecholamines, but not testicular blood flow. Endocrinology. 2004b;145:1750–9. doi: 10.1210/en.2003-1441. [DOI] [PubMed] [Google Scholar]
- Sneddon AA, Koll M, Wallace MC, et al. Acute alcohol administration inhibits the refeeding response after starvation in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E874–82. doi: 10.1152/ajpendo.00209.2002. [DOI] [PubMed] [Google Scholar]
- Vary TC, Lang CH. Assessing effects of alcohol consumption on protein synthesis in striated muscles. Methods Mol Biol. 2008;447:343–55. doi: 10.1007/978-1-59745-242-7_22. [DOI] [PubMed] [Google Scholar]
- Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1777–89. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliou V, Ziegler TL, Bludeau P, et al. CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenet Genomics. 2006;16:51–8. doi: 10.1097/01.fpc.0000182777.95555.56. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ren J. ALDH2 in alcoholic heart diseases: molecular mechanism and clinical implications. Pharmacol Ther. 2011;132:86–95. doi: 10.1016/j.pharmthera.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimatkin SM, Buben AL. Ethanol oxidation in the living brain. Alcohol Alcohol. 2007;42:529–32. doi: 10.1093/alcalc/agm059. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, et al. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res. 2006;30:1500–5. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]