Abstract
Prepregnancy diabetes and obesity have been identified as independent risk factors for several birth defects, providing support for a mechanism that involves hyperglycemia and hyperinsulinemia in the development of malformations. Data from the National Birth Defects Prevention Study from 1997 to 2007 were used to investigate the association between the maternal dietary glycemic index (DGI) and the risk of birth defects among nondiabetic women. DGI was categorized by using spline regression models and quartile distributions. Adjusted odds ratios and 95% confidence intervals were calculated. The joint effect of DGI and obesity was also examined. Among the 53 birth defects analyzed, high DGI, categorized by spline regression, was significantly associated with encephalocele (adjusted odds ratio (aOR) = 2.68), diaphragmatic hernia (aOR = 2.58), small intestinal atresia/stenosis (aOR = 2.97) including duodenal atresia/stenosis (aOR = 2.48), and atrial septal defect (aOR = 1.37). Using quartiles to categorize DGI, the authors identified associations with cleft lip with cleft palate (aOR = 1.23) and anorectal atresia/stenosis (aOR = 1.40). The joint effect of high DGI and obesity provided evidence of a synergistic effect on the risk of selected birth defects. High DGI is associated with an increased risk of a number of birth defects under study. Obesity coupled with high DGI appears to increase the risk further for some birth defects.
Keywords: glycemic index, hyperglycemia, hyperinsulinemia, obesity
The importance of obesity and diabetes in the development of birth defects has become a substantial concern in the United States. Prepregnancy obesity and diabetes have been shown to be independent risk factors for several types of birth defects, including congenital heart defects, neural tube defects, and gastrointestinal defects (1–6). Although the mechanisms through which obesity or diabetes operates to cause abnormalities in fetal development remain unclear, it has been hypothesized that both hyperglycemia and hyperinsulinemia play a role. There is evidence of a relation between an increased risk of birth defects and increasing glycohemoglobin levels, a measure of hyperglycemia, among diabetic women (7). Furthermore, it has been reported that diabetic women with normoglycemic levels have a risk of birth defects similar to that of the general population (8–10). Although the importance of glycemic control has been identified as a recommendation in the care of pregnant diabetic women (11), the concept of whether maintaining normal blood glucose levels in pregnant, nondiabetic women reduces the risk of birth defects remains unclear. With measures of dietary glycemic intake as indicators of blood glucose, previous studies have shown associations between high dietary glycemic index and neural tube defects among nondiabetic women (12, 13), while a study of dietary glycemic load found no association with neural tube defects (14). Studies of associations between maternal glycemic intake and non-central nervous system defects have been limited (15).
Hyperinsulinemia, or insulin resistance, is known to increase with obesity and has been associated with the development of neural tube defects (16). It is thought that hyperglycemia and hyperinsulinemia in coexistence might exacerbate the effect seen compared with either one alone. Studies of preexisting and gestational diabetes and obesity and the risk of neural tube defects provide evidence for this joint effect (17, 18). Among nondiabetics, 2 studies reported findings of associations between glycemic intake and neural tube defects that were elevated among obese women (12, 19), while other studies of dietary glycemic intake reported no change in the risk of birth defects, including neural tube defects, with body mass index (13, 15).
By use of data from the National Birth Defects Prevention Study, the aims of this analysis were to investigate the association between dietary glycemic index and a number of birth defect phenotypes, among nondiabetic women, and to describe the impact of obesity on such an association.
MATERIALS AND METHODS
The National Birth Defects Prevention Study is an ongoing, multisite, case-control study that collects data on over 30 structural birth defects and several subcategories of these defects. Chromosomal abnormalities and single-gene conditions are excluded. Cases among live births, fetal deaths, and electively terminated pregnancies were identified through birth defects surveillance systems in Arkansas, California, Iowa, Georgia, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah. Controls were nonmalformed, live-born infants randomly selected from birth certificates or hospital birth records. Cases and controls with estimated dates of delivery between October 1, 1997, and December 31, 2007, were included. Maternal interviews were conducted within 2 years of the estimated date of delivery by using a computer-assisted telephone interview. Participation rates were 68.5% among cases and 64.9% among controls. Data on demographics, medication use, lifestyle behaviors, and health conditions are collected through this interview. Specific details of the National Birth Defects Prevention Study have been previously described (20). One component of the interview is the shortened Willett food frequency questionnaire. Mothers are asked to recall their average consumption of 58 different food items in the year prior to pregnancy. By use of the year prior to pregnancy, the food frequency questionnaire best captures diet in the earliest part of pregnancy and the relevant time period in which most birth defects develop. Information from the food frequency questionnaire is used to calculate several dietary measures, including dietary glycemic index (DGI) and dietary glycemic load (DGL). We calculated DGL by multiplying the glycemic index values for each reported food item by the number of daily servings and then multiplying by the carbohydrate content before summing over all reported foods. To calculate DGI, we divided the DGL by the total reported carbohydrate. DGI reflects the quality of carbohydrate consumed, while DGL is a measure of the quality and quantity of carbohydrate consumed. Previous research has suggested that the shortened food frequency questionnaire can be useful in estimating DGI, while its ability to measure DGL may not be as accurate compared with longer versions of the food frequency questionnaire. A study comparing the 2 versions of the food frequency questionnaire found a 1-unit decrease in the median value of DGI and a 23-unit decrease in the median value of DGL when using the shortened food frequency questionnaire (13). We therefore restricted our analysis to describing the association between DGI and birth defects.
Women with more than 3 missing food frequency questionnaire items and/or reporting extreme caloric intake, <500 or >3,800 kcal per day, were excluded. Additionally, women with preexisting or gestational diabetes were excluded. This analysis was restricted to homogenous birth defect groups consisting of at least 40 cases.
With regard to statistical methods, when describing the association between DGI and birth defects, we first adjusted DGI for a subject's total energy intake using the residual method (21). Cubic-restricted splines with 5 knots were used to identify cutoff values for the dichotomization of DGI for each group of birth defects included in this analysis (22). The cutpoint was identified as the DGI value that corresponded with a divergence of cases and controls at an odds ratio of 1.5. Spline regression is the preferred method for identifying cutpoints in the event that associations are present only at the extreme ends of the exposure distribution (23). In addition to using the value determined by the spline regression model, we also categorized DGI into quartiles based on the distribution among controls for each birth defect group. Control groups for orofacial clefts, glaucoma and cataract, and pulmonary valve stenosis were reduced because not all study sites ascertained these birth defects for the entire study period. The control group for hypospadias was restricted to male infants. Multivariable logistic regression models were used to calculate adjusted odds ratios and 95% confidence intervals. All regression models were adjusted for the following potential confounders on the basis of previous studies: study site, maternal age (<25, 25–29, 30–34, ≥35 years), maternal education (<12, 12, 13–15, ≥16 years), maternal race, and folic acid use during the month prior to and/or after conception. Subjects with missing data on any of the covariates were not included in the adjusted regression model, 0.64% of controls and 0.52% of cases. To understand the role of body mass index on the association between DGI and birth defects, case groups for which spline regression identified a divergence were assessed for interdependence between high DGI and obesity, defined as a body mass index of 30 kg/m2 or greater. Subjects with a DGI value less than the identified cutpoint and a body mass index of less than 30 kg/m2 were used as the reference. All analyses were performed by using SAS, version 9.1.3, statistical software (24).
RESULTS
A total of 7,505 controls and 18,964 cases among 53 case groups were included in this analysis. We excluded 647 controls and 2,325 cases with prepregnancy diabetes, gestational diabetes, or unknown information on diabetes. An additional 342 controls and 985 cases were excluded for missing nutritional data or extreme caloric intake. Those excluded for missing nutritional data were more likely to be younger than 25 years of age and non-Hispanic black, although this did not differ by case-control status. The characteristics of controls in the lowest quartile and highest quartile of DGI values are presented in Table 1. Compared with mothers in the lowest quartile of DGI, those in the highest quartile were more likely to be non-Hispanic black, to be less than 25 years of age, to have no more than 12 years of education, and to be less likely to take folic acid in the periconceptional period. DGI values also differed among study sites, with Arkansas and Iowa having more subjects in the highest quartile of DGI and California and Massachusetts having more subjects in the lowest quartile. A prepregnancy body mass index of either <18.5 or ≥30 was also associated with the highest DGI quartile.
Table 1.
Characteristics of Control Mothers by Glycemic Index Quartile, National Birth Defects Prevention Study, 1997–2007
| First Quartile,
DGI ≤49.1 |
Fourth Quartile,
DGI ≥55.6 |
|||
|---|---|---|---|---|
| No. | % | No. | % | |
| Race/ethnicity | ||||
| Non-Hispanic white | 1,004 | 53.5 | 1,195 | 63.7 |
| Non-Hispanic black | 80 | 4.3 | 389 | 20.7 |
| Hispanic | 626 | 33.4 | 216 | 11.5 |
| Other | 165 | 8.8 | 76 | 4.1 |
| Missing | 1 | 0.1 | 0 | 0.0 |
| Maternal age, years | ||||
| <25 | 453 | 24.1 | 922 | 49.1 |
| 25–29 | 512 | 27.3 | 511 | 27.2 |
| 30–34 | 545 | 29.1 | 322 | 17.2 |
| ≥35 | 366 | 19.5 | 121 | 6.4 |
| Maternal education, years | ||||
| <12 | 337 | 18.0 | 332 | 17.7 |
| 12 | 352 | 18.8 | 627 | 33.4 |
| 13–15 | 421 | 22.4 | 560 | 29.9 |
| ≥16 | 747 | 39.8 | 350 | 18.7 |
| Missing | 19 | 1.0 | 7 | 0.4 |
| Study site | ||||
| Arkansas | 107 | 5.7 | 428 | 22.8 |
| California | 282 | 15.0 | 146 | 7.8 |
| Iowa | 145 | 7.7 | 267 | 14.2 |
| Massachusetts | 331 | 17.6 | 110 | 5.9 |
| New Jersey | 154 | 8.2 | 110 | 5.9 |
| New York | 146 | 7.8 | 162 | 8.6 |
| Texas | 236 | 12.6 | 191 | 10.2 |
| CDC/Atlanta MSA | 188 | 10.0 | 205 | 10.9 |
| North Carolina | 137 | 7.3 | 144 | 7.7 |
| Utah | 150 | 8.0 | 113 | 6.0 |
| Periconceptional folic acid use | ||||
| Yes | 1,023 | 54.5 | 807 | 43.0 |
| No (none, other time) | 852 | 45.4 | 1,069 | 57.0 |
| Missing | 1 | 0.1 | 0 | 0.0 |
| Body mass indexa | ||||
| <18.5 | 82 | 4.4 | 136 | 7.2 |
| 18.5–24.9 | 956 | 51.0 | 903 | 48.1 |
| 25–29.9 | 461 | 24.6 | 434 | 23.1 |
| ≥30 | 246 | 13.1 | 378 | 20.1 |
| Missing | 131 | 7.0 | 25 | 1.3 |
Abbreviations: CDC, Centers for Disease Control and Prevention; DGI, dietary glycemic index; MSA, metropolitan statistical area.
a Body mass index: weight (kg)/height (m)2.
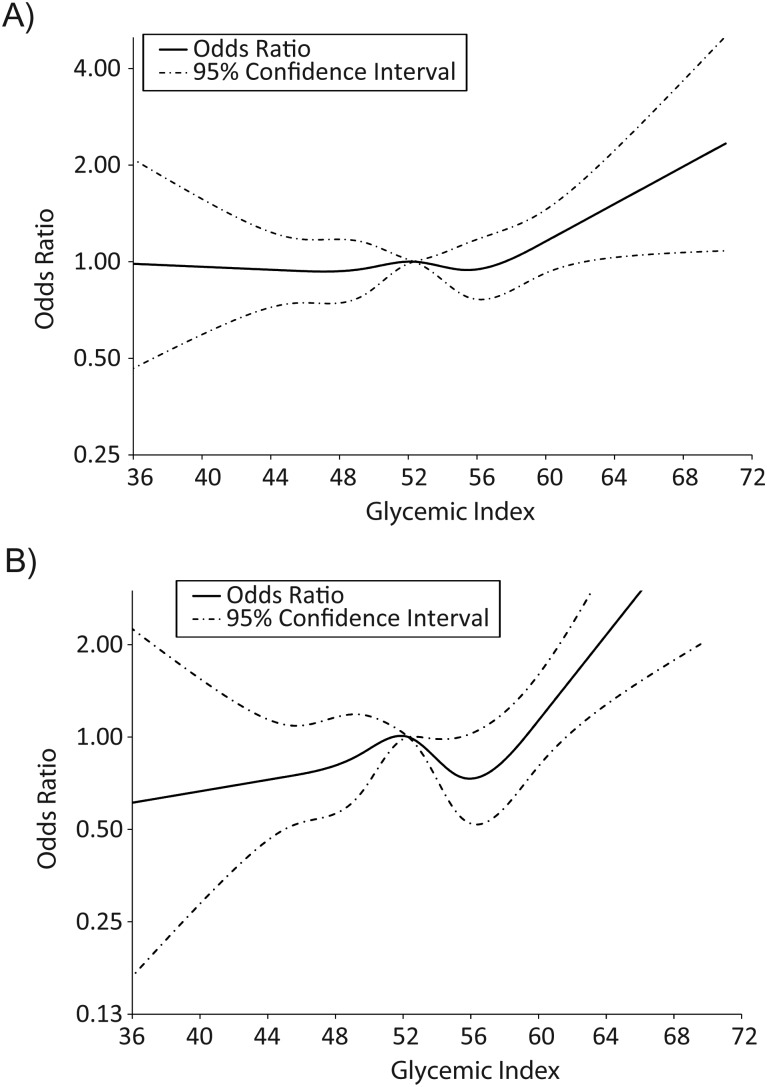
Spline regression methods were able to identify divergence points for the dichotomization of DGI values for a limited number of birth defects, 2 of which are presented in Figure 1. Out of the 6 central nervous system defects investigated, splines were successful in identifying a divergence point for 4 (Table 2). Encephalocele was the only defect significantly associated with high DGI (adjusted odds ratio (aOR) = 2.68, 95% confidence interval (CI): 1.13, 6.34). Spina bifida had an increased odds ratio, but this finding was not statistically significant (aOR = 2.15, 95% CI: 0.98, 4.72). These estimates, which dichotmomized DGI at 62 and 64, respectively, were larger than those observed when using the highest quartile categorization value of 55.6. In our analysis of DGI and obesity, the effect of high DGI alone was larger than the effect of obesity alone for 3 of the 4 central nervous system defects with spline regression cutpoints (Table 3). For encephalocele, when high DGI and obesity were coupled together, the resulting odds ratio was larger than expected given the sum of the individual effects (aOR = 6.97, 95% CI: 1.93, 25.19).
Figure 1.
Unadjusted cubic splines for glycemic index and spina bifida (A) and small intestinal atresia/stenosis (B), National Birth Defects Prevention Study, 1997–2007.
Table 2.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index and Central Nervous System Defects Using Spline Regression and Quartile Categorization, National Birth Defects Prevention Study, 1997–2007
| Defect | Spline Regression |
DGI Quartilesa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No.b | Cutoff | High DGI |
49.1–52.3 |
52.4–55.6 |
>55.6 |
|||||
| ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | |||
| Anencephaly | 384 | None | 1.17 | 0.88, 1.56 | 1.00 | 0.74, 1.36 | 0.96 | 0.70, 1.33 | ||
| Dandy-Walker Syndrome | 110 | None | 0.90 | 0.52, 1.54 | 0.88 | 0.51, 1.52 | 0.86 | 0.48, 1.54 | ||
| Encephalocele | 140 | 62 | 2.68 | 1.13, 6.34 | 2.20 | 1.26, 3.85 | 2.14 | 1.21, 3.78 | 2.64 | 1.48, 4.72 |
| Holoprosencephaly | 94 | 62 | 2.17 | 0.66, 7.15 | 2.18 | 1.16, 4.09 | 1.47 | 0.74, 2.91 | 1.95 | 0.98, 3.87 |
| Hydrocephaly | 320 | 62 | 0.91 | 0.37, 2.27 | 1.01 | 0.72, 1.41 | 0.95 | 0.68, 1.33 | 1.10 | 0.78, 1.54 |
| Spina bifida | 811 | 64 | 2.15 | 0.98, 4.72 | 1.03 | 0.84, 1.28 | 1.10 | 0.89, 1.36 | 1.14 | 0.91, 1.42 |
Abbreviations: CI, confidence interval; DGI, dietary glycemic index; OR, odds ratio.
a Reference group for quartile odds ratios is DGI <49.1.
b Number of cases in adjusted regression model.
c Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Table 3.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index, Body Mass Index, and Central Nervous System Defects Using Spline Regression, National Birth Defects Prevention Study, 1997–2007
| Defect | No.a | Cutoff | Low DGI, BMI <30 |
Low DGI, BMI ≥30 |
High DGI, BMI <30 |
High DGI, BMI ≥30 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | |||
| Encephalocele | 133 | 62 | 1.0 | Referent | 1.32 | 0.86, 2.02 | 1.84 | 0.56, 6.00 | 6.97 | 1.93, 25.19 |
| Holoprosencephaly | 89 | 62 | 1.0 | Referent | 0.96 | 0.54, 1.70 | 2.52 | 0.76, 8.36 | N/C | |
| Hydrocephaly | 306 | 62 | 1.0 | Referent | 0.99 | 0.73, 1.35 | 0.93 | 0.34, 2.57 | 0.92 | 0.12, 6.98 |
| Spina bifida | 763 | 64 | 1.0 | Referent | 1.55 | 1.29, 1.85 | 2.92 | 1.31, 6.55 | N/C | |
Abbreviations: BMI, body mass index; CI, confidence interval; DGI, dietary glycemic index; N/C, not calculable; OR, odds ratio.
a Number of cases in adjusted regression model.
b Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Among gastrointestinal and genitourinary defects, the odds of esophageal atresia with or without tracheoesophageal fistula and anorectal atresia/stenosis, which was significant, were 32% and 40% higher in the highest quartile of DGI compared with the lowest quartile, respectively (Table 4). By using slightly higher values of 62 and 61 identified through the spline regression, small intestinal atresia/stenosis and duodenal atresia/stenosis were significantly associated with high DGI. The odds of biliary atresia increased with each quartile of DGI resulting in an aOR of 1.69 (95% CI: 0.95, 2.98) among those in the highest quartile. Bladder exstrophy had an aOR of 4.10 (95% CI: 0.93, 18.07) but was based on only 2 cases with a DGI value above the cutoff of 62. Obesity coupled with high DGI had a larger positive association with small intestinal atresia and duodenal atresia than having a high DGI and not being obese (Table 5).
Table 4.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index and Gastrointestinal and Genitourinary Defects Using Spline Regression and Quartile Categorization, National Birth Defects Prevention Study, 1997–2007
| Defect | Spline Regression |
DGI Quartilesa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No.b | Cutoff | High DGI |
49.1–52.3 |
52.4–55.6 |
>55.6 |
|||||
| ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | |||
| Esophageal atresia | 463 | None | 1.10 | 0.85, 1.43 | 0.98 | 0.74, 1.30 | 1.32 | 0.99, 1.76 | ||
| Small intestinal atresia/stenosis | 303 | 62 | 2.97 | 1.59, 5.56 | 1.06 | 0.76, 1.48 | 1.21 | 0.87, 1.70 | 1.22 | 0.85, 1.75 |
| Duodenal atresia/stenosis | 134 | 61 | 2.48 | 1.21, 5.07 | 1.21 | 0.75, 1.95 | 0.87 | 0.52, 1.46 | 1.07 | 0.63, 1.82 |
| Anorectal atresia/stenosis | 661 | None | 1.05 | 0.83, 1.33 | 1.18 | 0.93, 1.49 | 1.40 | 1.10, 1.79 | ||
| Cloacal exstrophy | 59 | None | 0.87 | 0.43, 1.78 | 0.72 | 0.34, 1.54 | 0.77 | 0.35, 1.68 | ||
| Biliary atresia | 115 | None | 0.96 | 0.53, 1.75 | 1.22 | 0.69, 2.16 | 1.69 | 0.95, 2.98 | ||
| Bladder exstrophy | 47 | 62 | 4.10 | 0.93, 18.07 | 1.78 | 0.75, 4.24 | 1.22 | 0.47, 3.17 | 1.71 | 0.66, 4.46 |
| Renal agenesis | 105 | 58 | 1.02 | 0.57, 1.82 | 0.95 | 0.52, 1.75 | 1.33 | 0.75, 2.35 | 1.20 | 0.66, 2.19 |
| 49.1–52.2 | 52.3–55.7 |
>55.7 |
||||||||
| ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | |||||
| Hypospadias | 1,471 | None | 1.05 | 0.88, 1.25 | 1.03 | 0.86, 1.23 | 0.93 | 0.76, 1.13 | ||
Abbreviations: CI, confidence interval; DGI, dietary glycemic index; OR, odds ratio.
a Reference group for quartile odds ratios is DGI <49.1.
b Number of cases in adjusted regression model.
c Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Table 5.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index, Body Mass Index, and Gastrointestinal and Genitourinary Defects Using Spline Regression, National Birth Defects Prevention Study, 1997–2007
| Defect | No.a | Cutoff | Low DGI, BMI <30 |
Low DGI, BMI ≥30 |
High DGI, BMI <30 |
High DGI, BMI ≥30 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | |||
| Small intestinal atresia/stenosis | 290 | 62 | 1.0 | Referent | 1.43 | 1.06, 1.92 | 2.90 | 1.42, 5.93 | 4.65 | 1.32, 16.41 |
| Duodenal atresia/stenosis | 127 | 61 | 1.0 | Referent | 0.94 | 0.57, 1.54 | 1.93 | 0.82, 4.55 | 3.43 | 0.78, 15.10 |
| Bladder exstrophy | 46 | 62 | 1.0 | Referent | 0.84 | 0.35, 2.01 | 5.07 | 1.13, 22.69 | N/C |
|
| Renal agenesis | 97 | 58 | 1.0 | Referent | 1.48 | 0.88, 2.48 | 1.17 | 0.62, 2.21 | 0.86 | 0.20, 3.60 |
Abbreviations: BMI, body mass index; CI, confidence interval; DGI, dietary glycemic index; N/C, not calculable; OR, odds ratio.
a Number of cases in adjusted regression model.
b Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
The majority of congenital heart defects were not significantly associated with quartiles of DGI (Table 6). The odds of aortic stenosis and heterotaxia with a congenital heart defect increased with each quartile of DGI resulting in an aOR of 1.29 (95% CI: 0.91, 1.82) and 1.55 (95% CI: 0.99, 2.41) among those in the highest quartile of DGI, respectively, but remained nonsignificant. Furthermore, the spline regression model was not useful in identifying a point of divergence for the majority of congenital heart defects. With spline regression cutoffs, secundum atrial septal defects were significantly associated with high DGI. The effect of obesity and high DGI followed a similar trend, with obesity alone having little to no effect, high DGI alone having a larger effect, and obesity coupled with high DGI having the greatest effect (Table 7).
Table 6.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index and Congenital Heart Defects Using Spline Regression and Quartile Categorization, National Birth Defects Prevention Study, 1997–2007
| Defect | Spline Regression |
DGI Quartilesa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No.b | Cutoff | High DGI |
49.1–52.3 |
52.4–55.6 |
>55.6 |
|||||
| ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | |||
| Conotruncal defects | ||||||||||
| Truncus arteriosus | 66 | None | 1.06 | 0.54, 2.11 | 0.90 | 0.44, 1.86 | 0.78 | 0.36, 1.69 | ||
| Tetralogy of Fallot | 732 | None | 1.17 | 0.95, 1.45 | 1.06 | 0.84, 1.32 | 1.01 | 0.80, 1.29 | ||
| d-TGA | 510 | None | 1.09 | 0.85, 1.41 | 0.95 | 0.73, 1.24 | 1.13 | 0.85, 1.49 | ||
| DORV/TGA | 109 | 60 | 1.58 | 0.74, 3.36 | 0.79 | 0.47, 1.33 | 0.57 | 0.32, 1.02 | 0.88 | 0.50, 1.54 |
| VSD, conoventricular | 100 | None | 1.30 | 0.77, 2.18 | 0.84 | 0.47, 1.51 | 0.58 | 0.29, 1.15 | ||
| Anomalous pulmonary venous return | ||||||||||
| Total APVR | 193 | None | 1.05 | 0.70, 1.58 | 1.27 | 0.85, 1.89 | 0.97 | 0.61, 1.53 | ||
| Partial APVR | 42 | None | 0.89 | 0.40, 1.97 | 0.40 | 0.15, 1.04 | 0.42 | 0.16, 1.07 | ||
| Left ventricular outflow tract defects | ||||||||||
| Hypoplastic left heart syndrome | 392 | None | 1.11 | 0.83, 1.49 | 1.00 | 0.74, 1.35 | 1.16 | 0.84, 1.58 | ||
| Coarctation of the aorta | 692 | None | 0.92 | 0.74, 1.14 | 0.90 | 0.72, 1.12 | 0.82 | 0.65, 1.05 | ||
| Aortic stenosis | 302 | None | 0.97 | 0.70, 1.36 | 0.98 | 0.69, 1.38 | 1.29 | 0.91, 1.82 | ||
| Septal defects | ||||||||||
| VSD, perimembranous | 1296 | None | 1.08 | 0.92, 1.28 | 1.07 | 0.90, 1.27 | 0.93 | 0.77, 1.12 | ||
| Atrial septal defect, secundum | 1501 | 61 | 1.37 | 1.01, 1.85 | 1.04 | 0.88, 1.23 | 1.00 | 0.84, 1.18 | 1.10 | 0.93, 1.31 |
| Single ventricle | 213 | 61 | 1.90 | 0.98, 3.70 | 1.65 | 1.09, 2.49 | 1.49 | 0.97, 2.29 | 1.84 | 1.18, 2.85 |
| Atrioventricular septal defect | 213 | 63 | 1.69 | 0.52, 5.52 | 1.24 | 0.83, 1.87 | 1.03 | 0.67, 1.57 | 1.21 | 0.79, 1.86 |
| Pulmonary atresia | 158 | None | 1.30 | 0.83, 2.03 | 1.02 | 0.63, 1.65 | 1.50 | 0.92, 2.44 | ||
| Ebstein anomaly | 111 | None | 1.03 | 0.61, 1.75 | 1.06 | 0.62, 1.81 | 0.89 | 0.49, 1.61 | ||
| Tricuspid atresia | 105 | None | 1.29 | 0.74, 2.26 | 1.12 | 0.62, 2.00 | 1.27 | 0.69, 2.33 | ||
| Heterotaxia with CHD | 213 | None | 1.42 | 0.94, 2.14 | 1.54 | 1.02, 2.33 | 1.55 | 0.99, 2.41 | ||
| Heterotaxia without CHD | 42 | None | 1.36 | 0.58, 3.19 | 1.06 | 0.42, 2.67 | 1.38 | 0.53, 3.60 | ||
|
49.1–52.3 |
52.4–55.7 |
>55.7 |
||||||||
| ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | |||||
| Pulmonary valve stenosis | 924 | 64 | 1.60 | 0.73, 3.52 | 1.19 | 0.98, 1.46 | 1.09 | 0.88, 1.34 | 1.06 | 0.85, 1.32 |
Abbreviations: APVR, anomalous pulmonary venous return; CHD, congenital heart defect; CI, confidence interval; DGI, dietary glycemic index; DORV/TGA, double outlet right ventricle with transposition of great arteries; d-TGA, dextro-transposition of the great arteries; OR, odds ratio; VSD, ventricular septal defect.
a Reference group for quartile odds ratios is DGI <49.1.
b Number of cases in adjusted regression model.
c Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Table 7.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index, Body Mass Index, and Congenital Heart Defects Using Spline Regression, National Birth Defects Prevention Study, 1997–2007
| Defect | No.a | Cutoff | Low DGI, BMI <30 |
Low DGI, BMI ≥30 |
High DGI, BMI <30 |
High DGI, BMI ≥30 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | |||
| DORV/TGA | 99 | 60 | 1.0 | Referent | 0.89 | 0.50, 1.56 | 1.22 | 0.48, 3.12 | 3.21 | 0.95, 10.88 |
| Atrial septal defect, secundum | 1,432 | 61 | 1.0 | Referent | 1.18 | 1.02, 1.37 | 1.36 | 0.97, 1.92 | 1.78 | 0.94, 3.41 |
| Single ventricle | 202 | 61 | 1.0 | Referent | 1.01 | 0.68, 1.49 | 1.82 | 0.87, 3.81 | 2.38 | 0.55, 10.25 |
| Atrioventricular septal defect | 209 | 63 | 1.0 | Referent | 0.65 | 0.42, 0.99 | 2.12 | 0.64, 6.97 | N/C | |
| Pulmonary valve stenosis | 884 | 64 | 1.0 | Referent | 1.30 | 1.09, 1.56 | 2.18 | 0.97, 4.90 | N/C | |
Abbreviations: BMI, body mass index; CI, confidence interval; DGI, dietary glycemic index; DORV/TGA, double outlet right ventricle with transposition of great arteries; N/C, not calculable; OR, odds ratio.
a Number of cases in adjusted regression model.
b Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Other birth defects significantly associated with high DGI include transverse limb deficiency, diaphragmatic hernia, and gastroschisis (Table 8). Obesity paired with a high DGI resulted in a lower odds ratio for gastroschisis than that for nonobese women with a high DGI (aOR = 0.57, 95% CI: 0.30, 1.09 and aOR = 1.33, 95% CI: 1.05, 1.68, respectively) (Table 9). Cleft lip with cleft palate is another birth defect significantly associated with high DGI (Table 10). The odds of cleft lip with cleft palate are increased among obese and nonobese women with a high DGI compared with nonobese women with a low DGI, although neither association was statistically significant (Table 11).
Table 8.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index and Musculoskeletal Defects Using Spline Regression and Quartile Categorization, National Birth Defects Prevention Study, 1997–2007
| Defect | Spline Regression |
DGI Quartilesa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No.b | Cutoff | High DGI |
49.1–52.3 |
52.4–55.6 |
>55.6 |
|||||
| ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | |||
| Limb deficiencies | ||||||||||
| Longitudinal | 289 | 64 | 1.48 | 0.35, 6.29 | 1.05 | 0.75, 1.47 | 0.90 | 0.63, 1.28 | 1.06 | 0.74, 1.52 |
| Preaxial | 169 | 60 | 1.31 | 0.69, 2.48 | 1.20 | 0.77, 1.86 | 0.83 | 0.52, 1.35 | 1.25 | 0.78, 1.99 |
| Transverse | 457 | None | 1.31 | 1.00, 1.72 | 1.30 | 0.98, 1.72 | 1.38 | 1.02, 1.86 | ||
| Intercalary | 32 | 59 | 1.64 | 0.59, 4.53 | 2.08 | 0.71, 6.05 | 0.68 | 0.18, 2.61 | 1.67 | 0.54, 5.16 |
| Amniotic band syndrome | 228 | None | 0.86 | 0.57, 1.29 | 1.20 | 0.82, 1.75 | 1.05 | 0.69, 1.57 | ||
| Diaphragmatic hernia | 560 | 64 | 2.58 | 1.06, 6.27 | 1.27 | 1.00, 1.63 | 1.06 | 0.81, 1.38 | 1.38 | 1.06, 1.80 |
| Sacral agenesis | 45 | None | 1.49 | 0.61, 3.69 | 1.61 | 0.65, 3.98 | 1.34 | 0.51, 3.52 | ||
| Omphalocele | 282 | None | 1.13 | 0.80, 1.61 | 1.08 | 0.76, 1.55 | 1.16 | 0.80, 1.69 | ||
| Gastroschisis | 872 | 59 | 1.43 | 1.14, 1.79 | 1.30 | 1.02, 1.65 | 1.35 | 1.07, 1.71 | 1.52 | 1.20, 1.93 |
Abbreviations: CI, confidence interval; DGI, dietary glycemic index; OR, odds ratio.
a Reference group for quartile odds ratios is DGI <49.1.
b Number of cases in adjusted regression model.
c Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Table 9.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index, Body Mass Index, and Musculoskeletal Defects Using Spline Regression, National Birth Defects Prevention Study, 1997–2007
| Defect | No.a | Cutoff | Low DGI, BMI <30 |
Low DGI, BMI ≥30 |
High DGI, BMI <30 |
High DGI, BMI ≥30 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | |||
| Longitudinal limb deficiency | 279 | 64 | 1.0 | Referent | 1.15 | 0.84, 1.57 | 1.00 | 0.13, 7.51 | 3.50 | 0.41, 29.78 |
| Preaxial limb deficiency | 164 | 60 | 1.0 | Referent | 1.31 | 0.88, 1.94 | 1.55 | 0.79, 3.04 | 0.70 | 0.10, 5.18 |
| Intercalary limb deficiency | 31 | 59 | 1.0 | Referent | 0.40 | 0.09, 1.72 | 1.08 | 0.31, 3.77 | 3.10 | 0.68, 14.22 |
| Diaphragmatic hernia | 537 | 64 | 1.0 | Referent | 1.13 | 0.90, 1.44 | 2.71 | 1.02, 7.20 | 2.46 | 0.29, 20.62 |
| Gastroschisis | 851 | 59 | 1.0 | Referent | 0.24 | 0.17, 0.35 | 1.33 | 1.05, 1.68 | 0.57 | 0.30, 1.09 |
Abbreviations: BMI, body mass index; CI, confidence interval; DGI, dietary glycemic index; OR, odds ratio.
a Number of cases in adjusted regression model.
b Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Table 10.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index and Craniofacial Defects Using Spline Regression and Quartile Categorization, National Birth Defects Prevention Study, 1997–2007
| Defect | Spline Regression |
DGI Quartilesa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No.b | Cutoff | High DGI |
49.1–52.3 |
52.4–55.6 |
>55.6 |
|||||
| ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | |||
| Anopthalmia/micropthalmia | 148 | 59 | 1.17 | 0.67, 2.06 | 0.79 | 0.49, 1.28 | 0.92 | 0.57, 1.48 | 1.01 | 0.62, 1.65 |
| Anotia/microtia | 402 | None | 1.10 | 0.85, 1.43 | 0.90 | 0.68, 1.20 | 0.70 | 0.50, 0.98 | ||
| Choanal atresia | 101 | None | 1.17 | 0.67, 2.04 | 1.02 | 0.56, 1.84 | 1.49 | 0.82, 2.70 | ||
| Craniosynostosis | 929 | None | 1.07 | 0.88, 1.30 | 1.20 | 0.98, 1.46 | 1.17 | 0.94, 1.46 | ||
| 49.1–52.3 | 52.4–55.7 | >55.7 | ||||||||
| ORc | 95% CI |
ORc |
95% CI | ORc | 95% CI | |||||
| Cleft lip | 697 | None | 1.00 | 0.80, 1.26 | 1.17 | 0.93, 1.47 | 1.22 | 0.96, 1.56 | ||
| Cleft palate | 1,021 | None | 1.07 | 0.89, 1.29 | 0.98 | 0.80, 1.19 | 1.10 | 0.90, 1.35 | ||
| Cleft lip with cleft palate | 1267 | 65 | 1.74 | 0.62, 4.86 | 1.07 | 0.89, 1.27 | 1.12 | 0.94, 1.34 | 1.23 | 1.03, 1.48 |
| 48.9–52.0 | 52.1–55.5 | >55.5 | ||||||||
| ORc | 95% CI | ORc | 95% CI | ORc | 95% CI | |||||
| Glaucoma | 118 | 62 | 1.91 | 0.58, 6.34 | 1.14 | 0.65, 2.00 | 1.19 | 0.68, 2.09 | 1.51 | 0.85, 2.69 |
| Cataract | 224 | None | 1.13 | 0.77, 1.67 | 1.11 | 0.75, 1.64 | 1.03 | 0.67, 1.57 | ||
Abbreviations: CI, confidence interval; DGI, dietary glycemic index; OR, odds ratio.
a Reference group for quartile odds ratios is DGI <49.1. Reference group for glaucoma and cataract is DGI <48.9.
b Number of cases in adjusted regression model.
c Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
Table 11.
Adjusted Odds Ratios and 95% Confidence Intervals for Dietary Glycemic Index, Body Mass Index, and Craniofacial Defects Using Spline Regression, National Birth Defects Prevention Study, 1997–2007
| Defect | No.a | Cutoff | Low DGI, BMI <30 |
Low DGI, BMI ≥30 |
High DGI, BMI <30 |
High DGI, BMI ≥30 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | ORb | 95% CI | ORb | 95% CI | ORb | 95% CI | |||
| Anopthalmia/micropthalmia | 142 | 59 | 1.0 | Referent | 1.26 | 0.82, 1.96 | 1.01 | 0.52, 2.00 | 1.97 | 0.77, 5.07 |
| Cleft lip with cleft palate | 1,203 | 65 | 1.0 | Referent | 1.18 | 1.01, 1.38 | 1.74 | 0.55, 5.45 | 2.00 | 0.20, 20.57 |
| Glaucoma | 110 | 62 | 1.0 | Referent | 1.53 | 0.96, 2.41 | 2.90 | 0.86, 9.75 | N/C | |
Abbreviations: BMI, body mass index; CI, confidence interval; DGI, dietary glycemic index; N/C, not calculable; OR, odds ratio.
a Number of cases in adjusted regression model.
b Odds ratios adjusted for site, maternal age, maternal education, maternal race, and folic acid use in the month prior to or after conception.
DISCUSSION
High levels of DGI were associated with selected birth defects among infants of nondiabetic women. These birth defects were not limited to one specific organ system or defect category but instead included neural tube defects, gastrointestinal defects, and musculoskeletal defects. However, high DGI levels did not seem to be associated with various heart defect phenotypes. For the majority of studied birth defects, a DGI value for the divergence between cases and controls was not identified by the spline regression methods. Birth defects for which a divergence was not identified may be less sensitive to maternal blood glucose levels during their development, where an extremely high intake may have an effect and the observed range of the data did not include such extreme values. It is also possible that the development of these birth defects may be unaffected by glucose levels.
In a comparison of previous studies on glycemic index and neural tube defects, we found larger associations between high DGI and encephalocele (13) and spina bifida (13, 19). For encephalocele, we reported an aOR of 2.7 (95% CI: 1.1, 6.3) compared with 1.9 (95% CI: 1.0, 3.8) and, for spina bifida, we reported an aOR of 2.2 (95% CI: 1.0, 4.7) compared with 1.5 (95% CI: 1.1, 2.2) and 0.9 (95% CI: 0.6, 1.4). These results may be explained by the larger values of 62 and 64 determined by splines to categorize high DGI in the present study, compared with 60 and 53 in the previous studies. It is possible that using smaller values for the dichotomization of DGI would have resulted in smaller odds ratios. In addition to neural tube defects, several gastrointestinal defects were associated with high DGI. Although a cutpoint was not identified for anorectal defects using splines, the association among those in the highest quartile of DGI is 1.4 (95% CI: 1.1, 1.8), similar to that of 1.5 (95% CI: 1.0, 2.5) previously published when using a cutpoint value of ≥60 (15). Our findings regarding amniotic bands, craniosynostosis, gastroschisis, and hypospadias are different from those in the previous study (15). We report near-null findings for amniotic bands and hypospadias compared with previously reported odds ratios of 3.0 (95% CI: 1.1, 8.1) and 1.9 (95% CI: 0.7, 5.1), respectively. For craniosynostosis, we report a smaller odds ratio of 1.2 (95% CI: 0.9, 1.5) compared with 1.8 (95% CI: 1.0, 3.2), while for gastroschisis we report an odds ratio of 1.4 (95% CI: 1.1, 1.8) compared with a reduced association of 0.4 (95% CI: 0.0, 3.4) previously observed. These inconsistencies may be explained by discrepancies in the categorization of DGI. Although the point estimates in the present study differ from those in the study by Yazdy et al. (15), they frequently lie within the reported confidence intervals, indicating that these differences are not significant.
An increase in the risk of birth defects among diabetics with poor glycemic control during pregnancy compared with diabetics with normoglycemic levels and an increase in the risk of birth defects among nondiabetic women with high DGI compared with those with low DGI provide evidence for the role for hyperglycemia in abnormal development. It has been hypothesized that hyperinsulinemia in the presence of hyperglycemia might intensify the effect on the risk of birth defects compared with hyperglycemia alone (16). Because high glycemic index foods increase the demand for insulin, those with insulin resistance would be expected to have worsened effects (25). Previous findings provide evidence for higher levels of insulin resistance among overweight and obese patients (26, 27). In comparison with the obesity literature, this report shows similar associations between high DGI and spina bifida, diaphragmatic hernia, small intestinal atresia, anorectal atresia, craniosynostosis, and atrial septal defect, providing support for a common mechanism (1, 13). Using obesity as a proxy for insulin resistance, we present findings on the interdependence of high DGI and obesity. Specifically, high DGI coupled with obesity most strongly increased the association with encephalocele, small intestinal atresia/stenosis, and longitudinal limb deficiency. Although obesity alone slightly increased the odds ratio and high DGI alone increased the odds ratio more so than obesity alone, when high DGI was coupled with obesity the odds ratio increased further, often in a synergistic fashion. Gastroschisis was one exception to this trend. High DGI levels increased the odds of gastroschisis when the woman was nonobese, but when paired with obesity the reported association moved to the opposite side of the null value becoming protective, indicating that high DGI coupled with obesity decreased the risk of gastroschisis. Protective associations of obesity on gastroschisis have been previously reported (3, 28). Because of concerns about effect measure modification by maternal age, we also investigated this association stratified by maternal age. The association between DGI and obesity with gastroschisis was similar among mothers younger than 25 and those 25 years or older (data not shown).
Strengths of the National Birth Defects Prevention Study include specific case definitions and classification protocols, a large population-based sample, and data on a spectrum of birth defects. The specific case definitions and involvement of a clinical geneticist in the classification of birth defects enhance the homogeneity of case groups. The large sample size, which included cases among live births, fetal deaths, and elective terminations, allowed for this study to look at several different structural birth defects and subgroups that previous studies have been hindered by due to small numbers. An additional strength was the use of spline regression to identify points of DGI divergence between case groups and control groups. The spline regression often yielded a higher cutoff value than the highest quartile, suggesting that the effect was present in the highest levels of exposure that was not captured by the fourth quartile. Because it is likely that the effect of DGI differs on the basis of the specific birth defect, separate spline regression models were used for each birth defect group to determine cutpoints (23). Finally, DGI was adjusted for total caloric intake by using the residual method to reduce variation between subjects caused by overreporting or underreporting of intake from a food frequency questionnaire (29). Multivariable logistic regression allowed for the adjustment for several potential confounders including maternal age, education, and folic acid use, reducing the likelihood that observed estimates were due to confounding.
This study had some limitations. A major limitation was the use of self-reported data on dietary intake. Dietary assessment relied on responses from a food frequency questionnaire that was administered within 2 years after the estimated date of delivery. The food frequency questionnaire assessed average consumption of foods over the year prior to pregnancy, which likely introduced misclassification of the exposure due to inaccurate reporting. The nature of this misclassification would be expected to be nondifferential, which may have biased results toward the null when exposure was categorized as a dichotomous variable. In the event that misclassification was differential, with controls underreporting foods that lead to a high DGI, the observed associations would be biased away from the null. Although the odds ratios reported would be biased away from the null, the results of a sensitivity analysis showed that, when corrected for differential misclassification, an increase in risk still remains. Additionally, the utilization of the shortened food frequency questionnaire likely underestimated values of DGI. It has been suggested that the shortened food frequency questionnaire is a poor measure of DGL, which is a measure of quantity and quality of carbohydrates in the diet. Validation studies have reported poor agreement between food records and even longer versions of the food frequency questionnaire for glycemic load (30). Because of this limitation, we did not investigate the association between DGL and birth defects in this analysis. The spline regression method uses only the observed data to determine the divergence between cases and controls. It is possible that a divergence for certain birth defect groups was not identified within the range of glycemic index values in this study but that it may exist at a higher value. Furthermore, splines frequently identified values with few observations above that point, leading to unstable estimates. Another possible limitation was the use of self-reported height and weight, and thereby body mass index, as a measure of obesity. It is likely that obese women underreport their weight (31). This systematic underreporting would have led to a smaller number of women in the obese category and reduced the power to observe a potential joint effect between high DGI and obesity for several case groups. This study included over 50 specific birth defect types, raising the issue of multiple comparisons.
To our knowledge, this is the second study to report findings of maternal DGI in relation to a sizable number of birth defect phenotypes. Compared with previous research conducted by Yazdy et al. (15), this study expanded the number of birth defects groups, improved on the quality of birth defect classification, and investigated the potentially important interdependence between DGI and obesity. In nondiabetic women, dietary intake of foods that influence DGI appears to contribute to several birth defect phenotypes. Future work in this area will need to overcome limitations regarding assessment of DGI. For example, utilization of biologic samples would enhance our understanding of the role of hyperglycemia and hyperinsulinemia in the development of birth defects.
ACKOWLEDGMENTS
Author affiliations: Slone Epidemiology Center, Boston University, Boston, Massachusetts (Samantha E. Parker, Martha M. Werler, Mahsa M. Yazdy); Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, Palo Alto, California (Gary M. Shaw); and the Massachusetts Department of Public Health, Boston, Massachusetts (Marlene Anderka).
This work was supported by the Centers for Disease Control and Prevention (Centers for Excellence Awards U50/CCU925286 (California) and U01/DD000493 (Massachusetts)). Funds for part of the nutrient database work were provided by grant DK56350 from the National Institutes of Health to the Nutrition Epidemiology Core, Clinical Nutrition Research Center, Department of Nutrition, University of North Carolina at Chapel Hill.
The authors thank the Maternal Child and Adolescent Health Division, California Department of Public Health, for providing surveillance data from California.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the California Department of Public Health.
Conflict of interest: none declared.
REFERENCES
- 1.Gilboa SM, Correa A, Botto LD, et al. Association between prepregnancy body mass index and congenital heart defects. Am J Obstet Gynecol. 2010;202(1):51.e1–51.e10. doi: 10.1016/j.ajog.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237.e1–237.e9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waller DK, Shaw GM, Rasmussen SA, et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161(8):745–750. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- 4.Watkins ML, Rasmussen SA, Honein MA, et al. Maternal obesity and risk for birth defects. Pediatrics. 2003;111(5 part 2):1152–1158. [PubMed] [Google Scholar]
- 5.Sheffield JS, Butler-Koster EL, Casey BM, et al. Maternal diabetes mellitus and infant malformations. Obstet Gynecol. 2002;100(5 part 1):925–930. doi: 10.1016/s0029-7844(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 6.Casson I, Clarke C, Howard C, et al. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ. 1997;315(7103):275–278. doi: 10.1136/bmj.315.7103.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzmiller J, Buchanan T, Kjos S, et al. Pre-conception care of diabetes, congenital malformations, and spontaneous abortions. Diabetes Care. 1996;19(5):514–541. doi: 10.2337/diacare.19.5.514. [DOI] [PubMed] [Google Scholar]
- 8.Temple RC, Aldridge VJ, Murphy HR. Prepregnancy care and pregnancy outcomes in women with type 1 diabetes. Diabetes Care. 2006;29(8):1744–1749. doi: 10.2337/dc05-2265. [DOI] [PubMed] [Google Scholar]
- 9.Dunne F, Brydon P, Smith K, et al. Pregnancy in women with type 2 diabetes: 12 years outcome data 1990-2002. Diabetic Med. 2003;20(9):734–738. doi: 10.1046/j.1464-5491.2003.01017.x. [DOI] [PubMed] [Google Scholar]
- 10.Fuhrmann K, Reiher H, Semmler K, et al. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983;6(3):219–223. doi: 10.2337/diacare.6.3.219. [DOI] [PubMed] [Google Scholar]
- 11.Kitzmiller JL, Wallerstein R, Correa A, et al. Preconception care for women with diabetes and prevention of major congenital malformations. Birth Defects Res A Clin Mol Teratol. 2010;88(10):791–803. doi: 10.1002/bdra.20734. [DOI] [PubMed] [Google Scholar]
- 12.Shaw GM, Quach T, Nelson V, et al. Neural tube defects associated with maternal periconceptional dietary intake of simple sugars and glycemic index. Am J Clin Nutr. 2003;78(5):972–978. doi: 10.1093/ajcn/78.5.972. [DOI] [PubMed] [Google Scholar]
- 13.Yazdy MM, Liu S, Mitchell AA, et al. Maternal dietary glycemic intake and the risk of neural tube defects. Am J Epidemiol. 2010;171(4):407–414. doi: 10.1093/aje/kwp395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw GM, Carmichael SL, Laurent C, et al. Periconceptional glycemic load and intake of sugars and their associations with neural tube defects in offspring. Paediatr Perinat Epidemiol. 2008;22(6):514–519. doi: 10.1111/j.1365-3016.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 15.Yazdy MM, Mitchell AA, Liu S, et al. Maternal dietary glycaemic intake during pregnancy and the risk of birth defects. Paediatr Perinat Epidemiol. 2011;25(4):340–346. doi: 10.1111/j.1365-3016.2011.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendricks KA, Nuno OM, Suarez L, et al. Effects of hyperinsulinemia and obesity on risk of neural tube defects among Mexican. Americans. Epidemiology. 2001;12(6):630–635. doi: 10.1097/00001648-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JL, Waller DK, Canfield MA, et al. Maternal obesity, gestational diabetes, and central nervous system birth defects. Epidemiology. 2005;16(1):87–92. doi: 10.1097/01.ede.0000147122.97061.bb. [DOI] [PubMed] [Google Scholar]
- 18.Moore LL, Singer MR, Bradlee ML, et al. A prospective study of the risk of congenital defects associated with maternal obesity and diabetes mellitus. Epidemiology. 2000;11(6):689–694. doi: 10.1097/00001648-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael SL, Yang W, Shaw GM. Periconceptional nutrient intakes and risks of neural tube defects in California. Birth Defects Res A Clin Mol Teratol. 2010;88(8):670–678. doi: 10.1002/bdra.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analysis. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE, Harrell F. New York, NY: Springer; 2001. Regression Modeling Strategies. [Google Scholar]
- 23.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 24.SAS Institute, Inc. Cary, NC: SAS Institute, Inc; 2011. Base SAS® 9.3 Procedures Guide. [Google Scholar]
- 25.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002;76(suppl):274S–280S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin T, Allison G, Abbasi F, et al. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004;53(4):495–499. doi: 10.1016/j.metabol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Ferrannini E, Natali A, Bell P, et al. Insulin resistance and hypersecretion in obesity. J Clin Invest. 1997;100(5):1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam PK, Torfs CP, Brand RJ. A low prepregnancy body mass index is a risk factor for an offspring with gastroschisis. Epidemiology. 1999;10(6):717–721. [PubMed] [Google Scholar]
- 29.Willett W. 2nd. New York, NY: Oxford University Press; 1998. Nutritional Epidemiology. [Google Scholar]
- 30.Barclay AW, Flood VM, Brand-Miller JC, et al. Validity of carbohydrate, glycaemic index and glycaemic load data obtained using a semi-quantitative food-frequency questionnaire. Public Health Nutr. 2008;11(6):573–580. doi: 10.1017/S1368980007001103. [DOI] [PubMed] [Google Scholar]
- 31.Braam L, Ocke MC, Bueno-de-Mesquita HB, et al. Determinants of obesity-related underreporting of energy intake. Am J Epidemiol. 1998;147(11):1081–1086. doi: 10.1093/oxfordjournals.aje.a009402. [DOI] [PubMed] [Google Scholar]