Abstract
Prospective epidemiologic data on the association between vitamin D and mortality are limited, particularly in Asian populations. Among subjects in Linxian, China, the authors aimed to test whether baseline serum 25-hydroxyvitamin D (25(OH)D) concentrations in a prospective cohort were associated with all-cause mortality and cause-specific mortality rates over 24 years of follow-up (1986–2010). Serum 25(OH)D concentrations were measured in 1,101 subjects using an immunoassay. Hazard ratios and 95% confidence intervals were calculated using Cox regression models that were adjusted for age, sex, tobacco smoking, alcohol drinking, and hypertension. The 25th, 50th, and 75th percentile concentrations of 25(OH)D were 19.6, 31.9, and 48.4 nmol/L, respectively. During follow-up, 793 subjects died, including 279 who died of cerebrovascular accident, 217 who died of cancer, and 200 cardiovascular disease deaths. All-cause mortality was not associated with 25(OH)D concentrations using continuous models (for every 15 nmol/L, hazard ratio = 1.01, 95% confidence interval: 0.97, 1.05) or quartile models (fourth vs. first quartiles, hazard ratio = 1.06, 95% confidence interval: 0.87, 1.30; P for trend = 0.731). The authors also found no association with the cause-specific mortality outcomes. Results were similar for men and women. This study showed that prediagnostic serum 25(OH)D concentrations were not associated with all-cause or cause-specific mortality rates in this Chinese population who had low levels of vitamin D.
Keywords: cancer, cardiovascular diseases, China, mortality, vitamin D
Vitamin D is a multifunctional hormone that is primarily generated in the skin after exposure to ultraviolet B radiation in sunlight. It can also be acquired from dietary supplements and some foods, including fortified dairy products, eggs, fish, liver, and a few plant foods. Vitamin D status is generally assessed by measuring circulating concentrations of 25-hydroxyvitamin D (25(OH)D) (1), which is converted into active 1,25(OH)2 vitamin D in the kidneys and other sites.
Vitamin D has been hypothesized to reduce mortality, and a recent meta-analysis of prospective cohort studies suggested that high levels of circulating 25(OH)D were associated with a decreased risk of death (2). A meta-analysis of randomized vitamin D supplementation trials suggested that vitamin D decreased mortality mainly in elderly female participants (3). The mechanisms by which vitamin D might reduce mortality are not clear, although it may decrease the risk of chronic illnesses, including common cancers, autoimmune diseases, infectious diseases, and cardiovascular diseases (1).
Because of current lifestyle and environmental factors that limit sunlight exposure, vitamin D deficiency may be increasing (4) and may be a public health problem. Thus, indicators of global health, such as mortality, in relation to vitamin D status in different populations are of great interest. Most epidemiologic studies on vitamin D and mortality have been conducted in the United States and Europe; few have been conducted in Asia. A recent meta-analysis reviewed only 1 study conducted in Asians, a cohort of postmenopausal women in Japan (5).
In the present study, we examined the relation between serum 25(OH)D and mortality in a cohort in Linxian, a semi-arid mountainous area in north-central China at 36°N latitude. This mainly rural population has some of the highest rates of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma in the world. We previously reported the vitamin D status in this cohort and found that higher serum 25(OH)D concentrations were associated with an increased risk of esophageal squamous cell carcinoma in men but not in women, and no associations were detected with gastric cardia adenocarcinoma or gastric noncardia adenocarcinoma (6). Given that greater than 75% of the cohort had an inadequate serum 25(OH)D concentration (<50 nmol/L) (7), we estimated associations with all-cause mortality and mortality due to specific causes, including the 3 leading causes of death: cerebrovascular accident, cancer (primarily upper gastrointestinal cancers), and cardiovascular disease.
MATERIALS AND METHODS
Cohort population
Subjects were selected from the cohort of all participants in the General Population Trial of Linxian, which has been described previously (8, 9). Briefly, participants were 29,584 healthy adults aged 40–69 years from 4 Linxian communes. In the spring (mid-March through May) of 1985, 1 year before the start of intervention, all participants were interviewed and given a physical examination, and each had 10 mL of blood drawn. The blood was stored on ice for 3–6 hours, separated by centrifugation, aliquoted, stored at −45°C for 3–4 days, and then stored at −85°C until used. The intervention began in March 1986 and continued through May 1991; subjects who died before the start of the intervention were excluded from the trial and this cohort. On the basis of a partial factorial design, subjects were randomly assigned to receive 1 of 7 vitamin-mineral combinations or placebo. Vitamin D was not included in any of the intervention combinations. Throughout the trial period, village doctors performed active monthly follow-up of all trial participants and recorded all deaths, causes of death, and incident cancers. Such information was also provided by local commune and county hospitals and a study team that provided free clinical and diagnostic services for patients with symptoms suggestive of esophageal or gastric cancer. After the trial, information on deaths, causes of death, and incident cancers was obtained monthly from records of the village doctors. Both during and after the trial intervention period, all causes of death were evaluated and verified by a panel of Chinese experts, as previously described (10). We obtained informed consent from participants before trial enrollment. Throughout the study, human subject protection procedures were approved by the institutional review boards of the US National Cancer Institute and the Cancer Institute of the Chinese Academy of Medical Sciences.
Selection of participants for serum 25(OH)D measurements
Individuals were selected for serum measurements from the cohort in our previous study of serum 25(OH)D concentration and risk of esophageal squamous cell carcinoma, gastric cardia adenocarcinoma, and gastric noncardia adenocarcinoma (6). Briefly, this previous analysis included a stratified random sample (subcohort) of all trial participants, without regard to cancer outcome (n = 1,105). The strata were defined by sex and 3 age categories at the start of intervention (≤50 years of age, 51–60 years of age, and >60 years of age). Of the 1,105 subjects, 1,101 (99.6%) had data on vitamin D concentrations and death; we followed them as a prospective cohort and applied age-sampling strata in all analyses for the present study.
Serum 25(OH)D laboratory analysis
Serum 25(OH)D concentrations were determined in the nutrition laboratory of the Cancer Institute, Chinese Academy of Medical Sciences as previously described (6) using the OCTEIA 25-hydroxy vitamin D enzyme immunoassay (IDS, Inc., Fountain Hills, Arizona). The coefficient of variation for quality control samples of pooled serum was 16% (6).
Statistical analysis
The serum 25(OH)D concentrations in these subjects had a skewed distribution (6), so we reported geometric means. The mean values and quantiles were calculated using known sampling weights from the General Population Trial cohort for each individual in the study; therefore, the concentrations here are estimates of the means and quantiles of the entire General Population Trial cohort. Serum 25(OH)D concentrations were analyzed as 1) a continuous variable with units standardized to 15 nmol/L (approximately the central quartile in the overall population distribution), 2) quartiles based on the cohort distribution (<19.6, 19.6–31.9, 31.9–48.3, and ≥48.4 nmol/L), and 3) predetermined, clinically defined cutpoints (<25, 25–37.4, 37.5– 49.9, 50–74.9, 75–99.9, and ≥100) (1, 11, 12). Sex-specific quartile cutpoints were used in sex-stratified analyses. We conducted additional stratified analyses for age and body mass index and tested for effect modification using interaction terms.
Subjects were followed from the date of randomization (March 1, 1986) until death or end of follow-up (December 31, 2010). None of the subjects were lost to follow-up. We examined the association of serum 25(OH)D concentrations with all deaths and 4 cause-specific categories: all cancer deaths, deaths from upper gastrointestinal cancer, deaths from cerebrovascular accidents, and deaths from cardiovascular disease. These categories have been described previously (13). In analyses of cause-specific mortality, subjects who died from all other causes were censored at time of death. We estimated hazard ratios and 95% confidence intervals using Cox proportional hazards models. All models were stratified on the age sampling strata, and continuous age was used to adjust for variation within each age stratum. We retained sex, tobacco smoking, alcohol consumption, body mass index, and hypertension data in all models because those variables were a priori potential confounders. To examine the proportionality assumption, we used models that allowed time-dependent relative risks and found no significant violations of this assumption. We tested for deviations from log linearity by adding quadratic terms to the continuous models, and no statistically significant quadratic deviations were found. All P values were 2-sided, and P < 0.05 and confidence intervals that did not overlap with 1 were considered statistically significant.
RESULTS
The characteristics of the study cohort are described in Table 1. Over approximately 24 years of follow-up, there were 793 deaths (72% of subjects), including 279 cerebrovascular accident deaths, 217 cancer deaths (including 175 upper gastrointestinal cancer deaths), and 200 cardiovascular disease deaths. Table 2 presents the serum 25(OH)D geometric means and quantiles in the population overall and stratified by total mortality and cause-specific mortality categories. Approximately 73% (814 of 1,101) of the subcohort had serum 25(OH)D concentrations generally considered inadequate for bone and overall health (<50 nmol/L) (7).
Table 1.
Characteristics of Subcohort Subjects, by Serum 25-Hydroxyvitamin D Concentration Quartiles, Linxian, China, 1986–2010
| Variable | Overall Subcohort |
Quartile of Serum 25-Hydroxyvitamin D Concentration |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (<19.6 nmol/L) |
2 (19.6–31.8 nmol/L) |
3 (31.9–48.3 nmol/L) |
4 (≥48.4 nmol/L) |
||||||||||||
| No. of Subjects | % | Mean (SD) | No. of Subjects | % | Mean (SD) | No. of Subjects | % | Mean (SD) | No. of Subjects | % | Mean (SD) | No. of Subjects | % | Mean (SD) | |
| Total | 1,101 | 254 | 278 | 262 | 307 | ||||||||||
| Male sex | 608 | 55 | 120 | 47 | 137 | 49 | 156 | 60 | 195 | 64 | |||||
| Age, years | 56.5 (7.9) | 55.8 (8.0) | 56.7 (7.8) | 56.3 (8.0) | 57.1 (7.9) | ||||||||||
| Body mass indexa | 21.9 (2.6) | 21.9 (2.7) | 21.7 (2.5) | 21.8 (2.2) | 22.0 (2.8) | ||||||||||
| Hypertension | 302 | 27 | 64 | 25 | 76 | 27 | 68 | 26 | 94 | 31 | |||||
| Cigarette smoking | 425 | 39 | 82 | 32 | 104 | 37 | 113 | 43 | 126 | 41 | |||||
| Alcohol consumption | 229 | 21 | 50 | 20 | 57 | 21 | 54 | 21 | 68 | 22 | |||||
Abbreviation: SD, standard deviation.
a Weight (kg)/height (m)2.
Table 2.
Geometric Mean Serum 25-Hydroxyvitamin D Concentrationsa in Subcohort Participants and Selected Quantiles, by Cause of Death, Linxian, China, 1986–2010
| No. of Subjects | Geometric Mean 25(OH)Db, nmol/L | Quantile of Serum 25(OHD) Concentration |
|||
|---|---|---|---|---|---|
| 25th | 50th | 75th | |||
| All subjects | 1,101 | 31.7 | 19.6 | 31.9 | 48.4 |
| Cause of death | |||||
| Total | 793 | 32.4 | 20.0 | 32.8 | 49.7 |
| Cancer | 217 | 30.7 | 18.3 | 32.7 | 48.6 |
| UGI cancer | 175 | 31.3 | 18.7 | 32.0 | 53.3 |
| Cerebrovascular | 279 | 32.8 | 20.2 | 33.9 | 49.6 |
| Cardiovascular | 200 | 32.4 | 20.3 | 31.7 | 48.7 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; UGI, upper gastrointestinal.
a Concentrations are weighted by the age and sex sampling to reflect the distributions in the full cohort.
b Serum vitamin D distributions were skewed, so geometric means are presented.
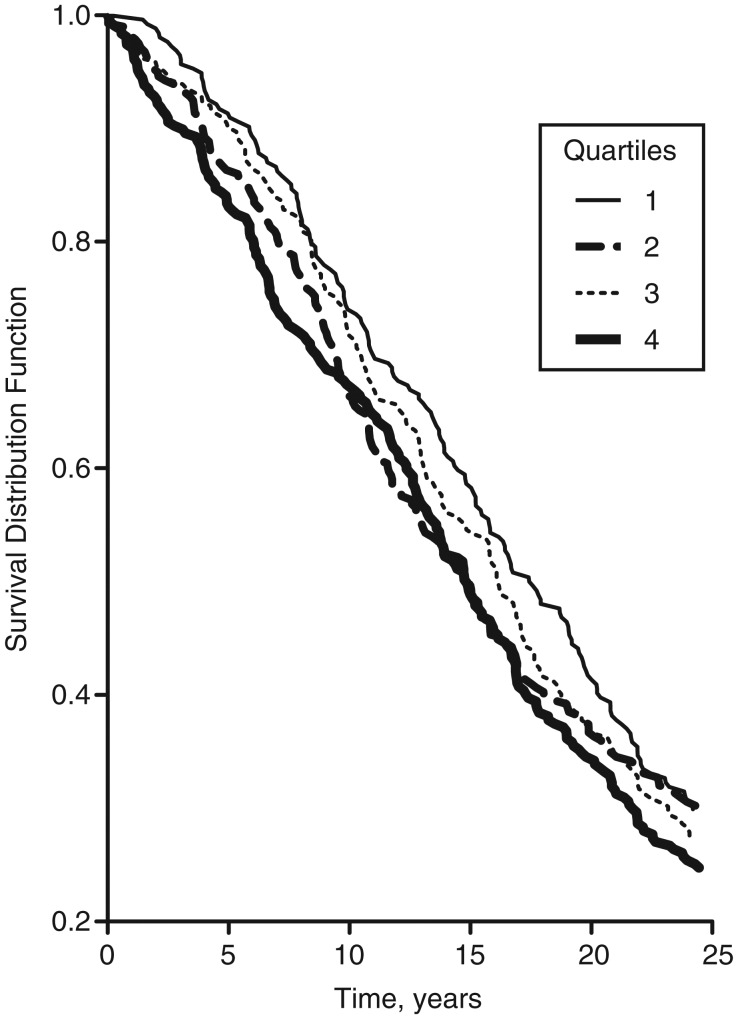
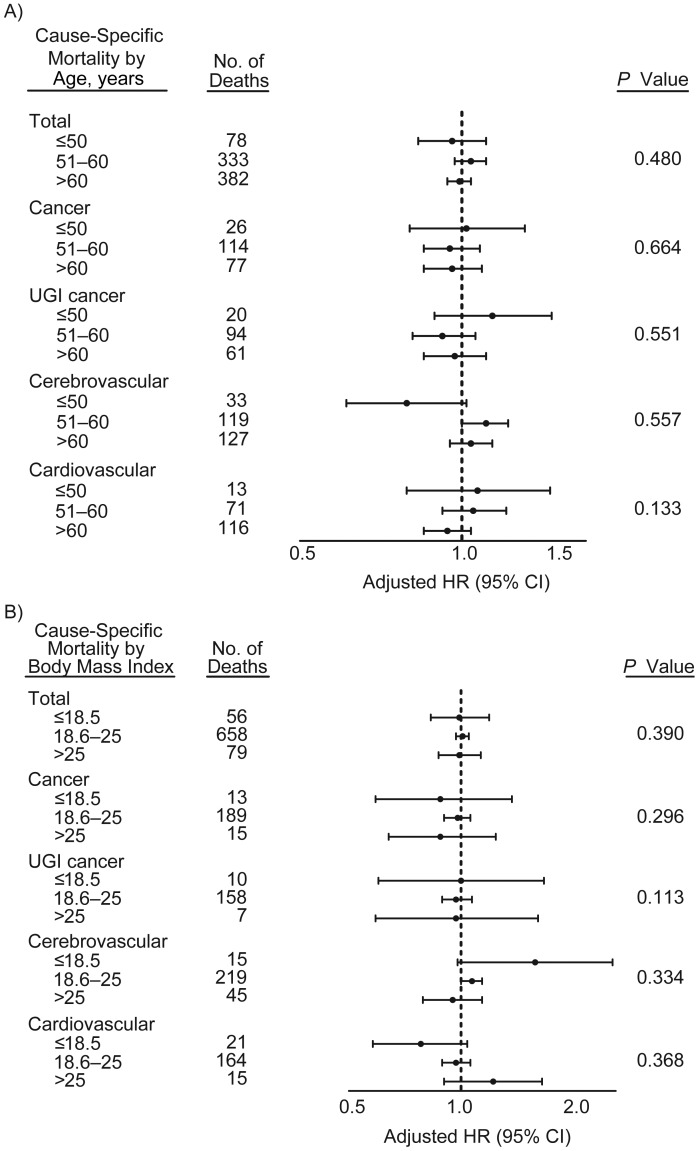
The survival curves by quartile of serum 25(OH)D concentration are presented in Figure 1 and show no differences (log-rank P = 0.215). These curves do not consider the potential impact of confounders. We present the multivariate adjusted hazard ratios and 95% confidence intervals for the association between continuous serum 25(OH)D concentration and mortality in Table 3. We found no significant associations with total mortality or any of the cause-specific mortality categories. The associations were likewise null in men and women, and no significant interactions between continuous serum 25(OH)D and sex were seen. Similarly, in stratified analyses conducted for age and body mass index, all confidence intervals overlapped, and no significant interactions were observed (Figure 2). As shown in Table 4, we found no clear association between serum 25(OH)D concentrations in quartiles with total deaths or any of the cause-specific death categories. The associations were similarly null in men and women in the quartile analysis (data not shown). Finally, we examined serum 25(OH)D concentrations in categories determined by clinically defined cutpoints and found no associations with total mortality or any of the cause-specific mortality categories (Appendix Table 1).
Figure 1.
Survival curve for each quartile of serum 25-hydroxyvitamin D concentration among subjects nested within the General Population Trial of Linxian, China, 1986–2010. Log-rank P = 0.215. Cutpoints for quartiles 1–4 were <19.6, 19.6–31.8, 31.9–48.3, and ≥48.4 nmol/L, respectively.
Table 3.
Adjusteda Hazard Ratios and 95% Confidence Intervals for the Association Between Continuous Serum 25-Hydroxyvitamin D Concentrationb and Death, Linxian, China, 1986–2010
| No. of Subjects | HR | 95% CI | P Value | P for Interaction | |
|---|---|---|---|---|---|
| Total deaths | 0.250 | ||||
| Overall | 793 | 1.01 | 0.97, 1.05 | 0.735 | |
| Men | 479 | 0.99 | 0.94, 1.04 | 0.700 | |
| Women | 314 | 1.03 | 0.97, 1.10 | 0.348 | |
| Cancer deaths | 0.183 | ||||
| Overall | 217 | 0.97 | 0.89, 1.05 | 0.406 | |
| Men | 141 | 1.00 | 0.91, 1.10 | 0.967 | |
| Women | 76 | 0.88 | 0.75, 1.03 | 0.115 | |
| UGI cancer deaths | 0.346 | ||||
| Overall | 175 | 0.97 | 0.88, 1.06 | 0.435 | |
| Men | 114 | 0.99 | 0.89, 1.10 | 0.846 | |
| Women | 61 | 0.90 | 0.75, 1.07 | 0.229 | |
| Cerebrovascular deaths | 0.701 | ||||
| Overall | 279 | 1.05 | 0.98, 1.12 | 0.141 | |
| Men | 157 | 1.04 | 0.96, 1.13 | 0.337 | |
| Women | 122 | 1.06 | 0.96, 1.17 | 0.277 | |
| Cardiovascular deaths | 0.123 | ||||
| Overall | 200 | 0.98 | 0.91, 1.06 | 0.678 | |
| Men | 119 | 0.94 | 0.85, 1.04 | 0.223 | |
| Women | 81 | 1.06 | 0.93, 1.20 | 0.399 |
Abbreviations: CI, confidence interval; HR, hazard ratio; UGI, upper gastrointestinal.
a Overall models were stratified by age and sex, with additional adjustment by separate continuous age variables for each stratum as well as sex, hypertension, tobacco smoking, body mass index, and alcohol consumption; models in women were not adjusted for smoking because less than 1% of women smoked tobacco.
b Serum 25-hydroxyvitamin D concentrations were scaled to 15 nmol/L, approximately the central quartile in the overall population distribution.
Figure 2.
Stratified analyses examining the association between serum 25-hydroxyvitamin D concentration (continuous, scaled to 15 nmol/L, approximately the central quartile in the overall population distribution) and total mortality and cause-specific mortality by (A) age and (B) body mass index (weight (kg)/height (m)2) among subjects nested within the General Population Trial of Linxian, China, 1986–2010. Models were additionally adjusted for separate continuous age and body mass index variables, as well as sex, hypertension, tobacco smoking, and alcohol consumption. P values for interaction terms are provided. CI, confidence interval; HR, hazard ratio; UGI, upper gastrointestinal.
Table 4.
Adjusteda Hazard Ratios and 95% Confidence Intervals for the Association Between Quartiles of Serum 25-Hydroxyvitamin D Concentration and Death, Linxian, China, 1986–2010
| Quartile | Total Deaths |
Cancer Deaths |
UGI Cancer Deaths |
Cerebrovascular Deaths |
Cardiovascular Deaths |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Subjects | HR | 95% CI | No. of Subjects | HR | 95% CI | No. of Subjects | HR | 95% CI | No. of Subjects | HR | 95% CI | No. of Subjects | HR | 95% CI | |
| 1 (<19.6 nmol/L) | 178 | 1.00 | Referent | 54 | 1.00 | Referent | 45 | 1.00 | Referent | 55 | 1.00 | Referent | 46 | 1.00 | Referent |
| 2 (19.6–31.8 nmol/L) | 194 | 1.09 | 0.89, 1.33 | 52 | 0.97 | 0.66, 1.42 | 44 | 0.99 | 0.65, 1.50 | 66 | 1.23 | 0.86, 1.76 | 56 | 1.20 | 0.81, 1.78 |
| 3 (31.9–48.3 nmol/L) | 190 | 0.99 | 0.80, 1.21 | 50 | 0.87 | 0.59, 1.28 | 40 | 0.83 | 0.54, 1.28 | 71 | 1.25 | 0.88, 1.79 | 46 | 0.90 | 0.59, 1.36 |
| 4 (≥48.4 nmol/L) | 231 | 1.07 | 0.88, 1.30 | 61 | 0.96 | 0.66, 1.39 | 46 | 0.87 | 0.57, 1.31 | 87 | 1.29 | 0.92, 1.82 | 52 | 0.93 | 0.62, 1.39 |
| P for trend | 0.725 | 0.902 | 0.784 | 0.472 | 0.485 | ||||||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; UGI, upper gastrointestinal.
a Models were stratified by age and sex, with additional adjustment by separate continuous age variables for each stratum as well as sex, hypertension, tobacco smoking, body mass index, and alcohol consumption.
DISCUSSION
Prospective epidemiologic data on the association between vitamin D and all-cause and cause-specific mortality risk are limited, particularly in Asian populations. In the present study, among a nested cohort of subjects from the General Population Nutrition Intervention Trial in Linxian, China, we aimed to test whether baseline serum 25(OH)D concentration was prospectively associated with all-cause mortality or mortality due to cerebrovascular accidents, all cancers, upper gastrointestinal cancers, or cardiovascular disease. We found that serum 25(OH)D concentration was not associated with all-cause mortality or any of the cause-specific mortality categories.
Two recent reviews of observational studies concluded that increasing circulating 25(OH)D levels were associated with a decrease in all-cause mortality rates (2, 14). However, previous observational and supplementation studies mostly focused on populations in the United States and Europe, and there is limited epidemiologic evidence in Asian populations. We present what is to our knowledge the first prospective study of the association between serum 25(OH)D and all-cause and cause-specific mortality risks in a Chinese population. In addition, except for 1 study conducted in Finland with 27 years of follow-up (15), all previous studies had 13 or fewer years of follow-up time, substantially less than our follow-up time of over 24 years. Ecologic studies in China have suggested that through the production of vitamin D, solar ultraviolet B radiation may be correlated with reduced mortality rates for some types of cancers (16), particularly cancers of the esophagus and stomach (17). However, we did not find associations between serum 25(OH)D concentrations and all cancer mortality or upper gastrointestinal cancer mortality in our Chinese population. Differences between our results and those of other analyses may be due to the narrow range of serum vitamin D levels and to population characteristics, so generalizability to other populations may be limited.
Although most of the Linxian population and members of this cohort are subsistence farmers (>98%) and spend large amounts of time outdoors, more than three quarters of the cohort members had inadequate levels of vitamin D by previously defined standards (18–21). The typical diet in Linxian provides little vitamin D; fatty fish and liver are rarely consumed (22), and egg consumption is also low (23). In addition, concentrations of 25(OH)D may be genetically determined to some extent (24, 25). Our blood samples were drawn in the spring, a likely nadir for serum 25(OH)D concentrations, so this single measurement may not reflect year-round exposure.
Our study has several strengths and weaknesses. We used serum 25(OH)D concentration, which is the best marker for vitamin D status, from blood collected during a single season (3 months) in 1 year. We also had a long follow-up time of more than 24 years, so reverse causation is unlikely. Over this follow-up time, we had substantial numbers of deaths and very reliable cause-of-death information (10). However, a weakness of our study is the relatively narrow distribution of serum 25(OH)D concentrations in our cohort. Furthermore, we had only a single measurement of serum 25(OH)D concentrations, and this may not adequately rank exposure status over long follow-up, although analyses stratified at the midpoint of follow-up (12 years) showed no difference in risk estimates (data not shown). In summary, we found no association between serum 25(OH)D concentrations and all-cause mortality or cause-specific mortality risks in a Chinese population with low levels of vitamin D.
ACKNOWLEDGMENTS
Author affiliations: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Shih-Wen Lin, Sanford M. Dawsey, Philip R. Taylor, Christian C. Abnet); and the Cancer Institute, Chinese Academy of Medical Sciences, Beijing, People's Republic of China (Wen Chen, Jin-Hu Fan, You-Lin Qiao).
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Conflict of interest: none declared.
Appendix Table 1.
Adjusteda Hazard Ratios and 95% Confidence Intervals for the Association Between Clinically Defined Cutpoints of Serum 25-Hydroxyvitamin D Concentration and Mortality Among Subjects Nested Within the General Population Trial of Linxian, China, 1986–2010
| Circulating 25(OH)D, nmol/L | Total Deaths |
Cancer Deaths |
UGI Cancer Deaths |
Cerebrovascular Deaths |
Cardiovascular Deaths |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Deaths | HR | 95% CI | No. of Deaths | HR | 95% CI | No. of Deaths | HR | 95% CI | No. of Deaths | HR | 95% CI | No. of Deaths | HR | 95% CI | |
| <25 | 274 | 1.00 | Referent | 75 | 1.00 | Referent | 62 | 1.00 | Referent | 95 | 1.00 | Referent | 68 | 1.00 | Referent |
| 25–37.4 | 175 | 1.08 | 0.89, 1.31 | 57 | 1.24 | 0.88, 1.76 | 47 | 1.25 | 0.85, 1.83 | 48 | 0.92 | 0.65, 1.30 | 53 | 1.31 | 0.91, 1.88 |
| 37.5– 49.9 | 130 | 0.96 | 0.78, 1.19 | 28 | 0.78 | 0.51, 1.21 | 24 | 0.81 | 0.51, 1.31 | 55 | 1.22 | 0.87, 1.70 | 32 | 0.93 | 0.61, 1.42 |
| 50–74.9 | 114 | 1.01 | 0.81, 1.27 | 35 | 1.10 | 0.73, 1.65 | 24 | 0.90 | 0.56, 1.45 | 40 | 0.97 | 0.67, 1.40 | 24 | 0.94 | 0.59, 1.50 |
| 75–99.9 | 57 | 1.00 | 0.75, 1.33 | 15 | 1.00 | 0.57, 1.75 | 11 | 0.89 | 0.47, 1.71 | 22 | 1.19 | 0.75, 1.91 | 13 | 0.89 | 0.49, 1.63 |
| ≥100 | 43 | 1.15 | 0.83, 1.59 | 7 | 0.77 | 0.35, 1.67 | 7 | 0.93 | 0.43, 2.05 | 19 | 1.60 | 0.97, 2.63 | 10 | 0.94 | 0.48, 1.84 |
| P for trend | 0.621 | 0.169 | 0.310 | 0.128 | 0.293 | ||||||||||
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; HR, hazard ratio; UGI, upper gastrointestinal.
a Models were stratified on age and sex, with additional adjustment for separate continuous age variables for each stratum, as well as sex, hypertension, tobacco smoking, body mass index, and alcohol consumption.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Zittermann A, Iodice S, Pilz S, et al. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95(1):91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- 3.Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011;(7):CD007470. doi: 10.1002/14651858.CD007470.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D Insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuroda T, Shiraki M, Tanaka S, et al. Contributions of 25-hydroxyvitamin D, co-morbidities and bone mass to mortality in Japanese postmenopausal women. Bone. 2009;44(1):168–172. doi: 10.1016/j.bone.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Dawsey SM, Qiao YL, et al. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97(1):123–128. doi: 10.1038/sj.bjc.6603834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 8.Blot WJ, Li J-Y, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85(18):1483–1491. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Taylor PR, Li JY, et al. Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann Epidemiol. 1993;3(6):577–585. doi: 10.1016/1047-2797(93)90078-i. [DOI] [PubMed] [Google Scholar]
- 10.Qiao Y-L, Dawsey SM, Kamangar F, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101(7):507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abnet CC, Qiao Y-L, Dawsey SM, et al. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol. 2005;34(2):467–474. doi: 10.1093/ije/dyh375. [DOI] [PubMed] [Google Scholar]
- 14.Scragg R. Vitamin D and public health: an overview of recent research on common diseases and mortality in adulthood. Public Health Nutr. 2011;14(9):1515–1532. doi: 10.1017/S1368980011001455. [DOI] [PubMed] [Google Scholar]
- 15.Kilkkinen A, Knekt P, Aro A, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170(8):1032–1039. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 16.Grant WB. Does solar ultraviolet irradiation affect cancer mortality rates in China? Asian Pac J Cancer Prev. 2007;8(2):236–242. [PubMed] [Google Scholar]
- 17.Chen W, Clements M, Rahman B, et al. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control. 2010;21(10):1701–1709. doi: 10.1007/s10552-010-9599-1. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 19.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 22.Zou XN, Taylor PR, Mark SD, et al. Seasonal variation of food consumption and selected nutrient intake in Linxian, a high risk area for esophageal cancer in China. Int J Vitam Nutr Res. 2002;72(6):375–382. doi: 10.1024/0300-9831.72.6.375. [DOI] [PubMed] [Google Scholar]
- 23.Tran GD, Sun X-D, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113(3):456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 24.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]