Abstract
Low 25-hydroxyvitamin D (25(OH)D) concentrations are common among older adults and are associated with poorer physical performance and strength, but results from longitudinal studies have been inconsistent. The 25(OH)D threshold for physical performance and strength was determined, and both cross-sectional and longitudinal associations between 25(OH)D and physical performance and strength were examined, in men and women aged 71–80 years from the Health, Aging, and Body Composition Study (n = 2,641). Baseline serum 25(OH)D was measured in 1998–1999, and physical performance and strength were measured at baseline and at 2- and 4-year follow-up. Piecewise regression models were used to determine 25(OH)D thresholds. Linear regression and mixed models were used to examine cross-sectional and longitudinal associations. The 25(OH)D thresholds were 70–80 nmol/L for physical performance and 55–70 nmol/L for strength. Participants with 25(OH)D <50 nmol/L had poorer physical performance at baseline and at 2- and 4-year follow-up than participants with 25(OH)D ≥75 nmol/L (P < 0.01). Although physical performance and strength declined over 4 years of follow-up (P < 0.0001), in general, the rate of decline was not associated with baseline 25(OH)D. Older adults with low 25(OH)D concentrations had poorer physical performance over 4 years of follow-up, but low 25(OH)D concentrations were not associated with a faster rate of decline in physical performance or strength.
Keywords: aged, 25-hydroxyvitamin D, muscle strength, physical performance
It has long been recognized that vitamin D plays an important role in calcium homeostasis and bone health. More recent evidence supports a role of vitamin D in physical performance and strength through direct effects on muscular function (1, 2), as well as indirectly through its reported roles in cardiovascular disease, diabetes mellitus, hypertension, pulmonary function, and osteoarthritis (3), conditions that frequently lead to declines in physical performance and strength (4). Poor physical performance and muscle weakness have been associated with low 25-hydroxyvitamin D (25(OH)D) concentrations among older adults in cross-sectional studies (5–11). Nevertheless, longitudinal studies examining 25(OH)D and change in physical performance and strength have been inconsistent, with some studies showing greater declines in physical performance and strength and others finding no association (12–16).
The Institute of Medicine recently concluded that a 25(OH)D concentration of ≥50 nmol/L was adequate for bone (to convert to ng/mL, divide by 2.496) but that insufficient scientific evidence existed to support recommendations for outcomes unrelated to bone health (17). However, others have suggested that 25(OH)D be maintained in the range of 75–80 nmol/L or greater for health outcomes other than bone (18–20). Low 25(OH)D is common in older adults, with approximately one-third and three-fourths of community-dwelling adults aged ≥70 years having 25(OH)D concentrations of <50 nmol/L and <75 nmol/L, respectively, in the National Health and Nutrition Examination Survey (2000–2004) (21). Whether 25(OH)D ≥50 nmol/L, suggested as adequate by the Institute of Medicine for bone health, is optimal for physical performance and strength has not been tested formally.
The primary objectives of these analyses were to determine the optimal 25(OH)D thresholds for physical performance and strength and to examine the cross-sectional and longitudinal associations between 25(OH)D and physical performance and strength over 4 years of follow-up with data from the Health, Aging, and Body Composition (Health ABC) Study. Because 25(OH)D also can affect muscle function indirectly via hyperparathyroidism secondary to low 25(OH)D (22), the role of parathyroid hormone (PTH) as a potential mediator was also examined.
MATERIALS AND METHODS
Study population
Data for this analysis were from the Health ABC Study, a prospective cohort study investigating the associations between body composition, weight-related health conditions, and incident functional limitations in older adults. The Health ABC study enrolled 3,075 community-dwelling black and white men and women aged 70–79 years between April 1997 and June 1998. Participants were recruited from a random sample of white Medicare-eligible residents and from all black Medicare-eligible residents in the Pittsburgh, Pennsylvania, and Memphis, Tennessee, metropolitan areas. Participants were eligible if they reported no difficulty walking one-fourth of a mile (0.4 km), climbing up 10 steps, or performing basic activities of daily living; were free of life-threatening illness; planned to remain in the geographic area for at least 3 years; and were not enrolled in lifestyle intervention trials. All participants provided written informed consent, and all protocols were approved by the institutional review boards at both study sites.
Participants attending the year 2 clinic visit (1998–1999), when 25(OH)D was measured, served as the baseline population for this analysis (n = 2,732). Participants who lacked 25(OH)D measurements (n = 23) and those who were missing data on pertinent covariates were excluded (n = 68), for a cross-sectional analysis sample of 2,641 participants. An additional 334 participants who lacked follow-up clinic visits at either year 4 or year 6 were excluded, for a longitudinal analysis sample of 2,307 participants.
Physical performance and strength
The Short Physical Performance Battery (SPPB) was administered to assess lower-extremity physical performance (23). The SPPB consisted of standing balance tasks (side-by-side, semi-tandem, and full-tandem stands for 10 seconds each), time to complete 5 repeated chair stands, and a 6-m walk to assess usual gait speed. Each of the 3 performance measures was assigned a score ranging from 0 (inability to perform the task) to 4 (the highest level of performance), and scores were summed to create an SPPB score ranging from 0 to 12 (best). In addition, a modified physical performance battery, the Health ABC Physical Performance Battery (Health ABC PPB), was administered to minimize ceiling effects of the SPPB (24). The Health ABC PPB battery increased the holding time of the standing balance tasks to 30 seconds and added a single-leg stand and a narrow 6-m walk test of balance. Health ABC PPB scores are continuous and range from 0 to 4, with higher scores indicative of better performance. The SPPB and Health ABC PPB were administered in years 1, 4, and 6.
Usual walking speed over 20 m and walking endurance over 400 m were measured in years 2, 4, and 6 (25). The course was 20 m long and marked by cones at each end. The first part consisted of a 2-minute warm-up walk in which participants were instructed to “cover as much ground as possible.” The second part consisted of a 400-m walk, with participants instructed to “walk as quickly as possible at a pace you can maintain,” and time to complete the 400-m walk was recorded. Heart rate was monitored continuously with the Polar Pacer (model no. 61190; Woodbury, New York). Participants were excluded from the 400-m walk if they had potentially acute electrocardiographic abnormalities, blood pressure ≥200/110 mm Hg, or resting heart rate <40 or >120 beats per minute or if they reported a recent cardiac event or procedure or new or recent worsening cardiac symptoms. The walking endurance test was terminated if the participant's heart rate exceeded 135 beats per minute or if the participant was unable to continue because of pain, fatigue, or any other symptom.
Knee extensor strength was measured with an isokinetic dynamometer (Kin-Com dynamometer, model 125 AP; Chattecx Corporation, Chattanooga, Tennessee). The right leg was tested unless contraindicated by knee pain or knee replacement. Isokinetic knee strength was measured concentrically from 90° to 30° at 60° per second in 3–6 trials, with strength calculated as the mean maximal torque (nm) from the 3 best trials in years 2, 4, and 6. Participants with uncontrolled hypertension, history of brain aneurysm or stroke, bilateral knee replacement, or severe bilateral knee pain were excluded from testing. For longitudinal analyses, the same leg was tested unless contraindicated. Relative knee extensor strength was calculated by taking the ratio of knee extensor strength to kilograms of lean mass in the tested leg obtained at each clinic visit by dual-energy x-ray absorptiometry (Hologic 4500A, version 8.20a; Hologic Inc., Waltham, Massachusetts).
Grip strength (kg) was measured twice in each hand with an isometric Jamar Hydraulic Hand Dynamometer (Sammons Preston, Bolingbrook, Illinois). Grip strength was assessed in years 2, 4, and 6, and the maximum force from 2 trials for the stronger hand was used in the analyses. Participants with severe hand pain or recent surgery were excluded. For longitudinal analyses, the same hand was used unless contraindicated.
Assessment of 25(OH)D and PTH
At the year 2 clinic visit, fasting blood samples were collected in the morning after a 12-hour fast, centrifuged, and stored at −80°C. Serum 25(OH)D was measured with a 2-step radioimmunoassay (25-Hydroxyvitamin D 125I RIA Kit; DiaSorin, Inc., Stillwater, Minnesota) in a laboratory meeting the Vitamin D External Quality Assessment Scheme quality criteria. Intact PTH was measured in plasma with a 2-site immunoradiometric assay kit (N-tact PTHSP; DiaSorin). The interassay coefficients of variation for serum 25(OH)D and plasma PTH were 6.8% and 8.6%, respectively. Serum 25(OH)D was categorized as <50 nmol/L, 50–<75 nmol/L, and ≥75 nmol/L on the basis of recently recommended cutpoints (20, 26).
Potential confounders
Demographic characteristics (age, gender, race, and education), smoking status, alcohol intake, and physical activity were ascertained by interviewer-administered questionnaires. Physical activity was based on the reported time spent walking for exercise or other walking (e.g., for transportation) over the prior 7 days. Body mass index (BMI; weight (kg)/height (m)2) was calculated from measured weight and height. The season during which the blood sample was obtained was included to account for seasonal effects on 25(OH)D and PTH. Participants were asked to bring all medications and supplements they were currently taking to their clinic visit. Supplements containing more than 3 vitamin or mineral ingredients were considered multivitamins. Vitamin D-containing supplements were defined as supplements containing ≤3 ingredients, one of which was vitamin D. Depressive symptoms were measured with the 20-item Center for Epidemiologic Studies Depression Scale (27). The Modified Mini-Mental State Examination was used as an indicator of general cognitive status (28). Glomerular filtration rate was estimated from serum creatinine according to the Modification of Diet in Renal Disease formula. Knee pain, as an indicator of knee osteoarthritis, was assessed by self-report. The prevalence of diabetes, cardiovascular disease (CVD; coronary heart disease or stroke), chronic obstructive pulmonary disease (COPD), and hospitalizations in the prior year was determined with the use of algorithms based on self-report and medication use at study baseline and on adjudicated events during follow-up. Education, smoking status, depressive symptoms, cognitive function, and estimated glomerular filtration rate were measured at the baseline clinic visit; all other covariates were measured at the year 2 clinic visit when 25(OH)D was measured.
Statistical analyses
The association between 25(OH)D category (<50 nmol/L, 50–<75 nmol/L, or ≥75 nmol/L) and participant characteristics was analyzed with chi-squared tests for categorical variables and analysis of variance for continuous variables. Piecewise regression models were used to determine 25(OH)D thresholds for physical performance and strength at baseline, with adjustment for age, gender, race, site, season, BMI, and kidney function. A likelihood ratio test was used to compare the fit of the piecewise regression model with a straight-line fit to determine whether a 25(OH)D threshold existed. A test of the slopes above and below the 25(OH)D threshold from the piecewise regression models indicated whether there was a significant association between 25(OH)D and physical performance and strength. Separate intercepts and thresholds were also fitted by race, gender, season, and BMI to determine whether the 25(OH)D threshold differed by gender, race, winter season, or obesity status (BMI ≥30).
Linear regression models were used to examine the cross-sectional associations between 25(OH)D and physical performance and strength. Mixed models were used to examine the associations between 25(OH)D and change in physical performance and strength over 4 years of follow-up and to test whether change over time differed by baseline 25(OH)D by including a 25(OH)D-by-time interaction term in the model. Two-way interactions between gender and 25(OH)D and between race and 25(OH)D at baseline were tested but were not significant. Thus, physical performance and strength measures are presented for the total sample. Models were first adjusted for age, gender, race, site, education, and season. Additional models also adjusted for smoking status, alcohol intake, physical activity, BMI, kidney function, cognitive function, depressive symptoms, diabetes, CVD, COPD, knee pain, hospitalization in the past year, multivitamin use, and vitamin D supplementation. In longitudinal analyses, time-varying covariates were included for physical activity, BMI, depression, diabetes, CVD, COPD, knee pain, and hospitalizations in the past year. Finally, a model that included both 25(OH)D and PTH was examined. All analyses were conducted in SAS, version 9.1 (SAS Institute, Inc., Cary, North Carolina), and a 2-sided alpha level of 0.05 was considered significant.
RESULTS
The mean age of the study population was 74.7 years, 51% were women, and 38% were black. Participants who attended the year 2 clinic visit but were excluded from cross-sectional analyses (n = 91) were more likely to be black, from Memphis, and current smokers and to have lower cognitive function and Health ABC PPB scores (all P's < 0.05). Participants excluded from the longitudinal analyses (n = 425) were more likely to be older, male, black, from Memphis, sedentary, and current smokers and to have less than a high school education, higher BMI, lower Health ABC PPB scores, and lower 25(OH)D levels (all P's < 0.05). The descriptive characteristics of the study population by 25(OH)D are shown in Table 1. Approximately one-third of participants had 25(OH)D <50 nmol/L, and two-thirds had 25(OH)D <75 nmol/L. Women, blacks, current smokers, nondrinkers, participants who were sedentary, and those with diabetes, CVD, or COPD were more likely to have 25(OH)D <50 nmol/L. Participants with 25(OH)D <50 nmol/L also were more likely to have had 25(OH)D measured in the winter or spring; to have a higher BMI, estimated glomerular filtration rate, and PTH; and to have less than a high school education and lower cognitive function. Participants who reported taking multivitamins or vitamin D-containing supplements were more likely to have 25(OH)D ≥75 nmol/L.
Table 1.
Baseline Characteristics of Participants by 25-Hydroxyvitamin D Status, Health, Aging, and Body Composition Study, 1998–1999a
| Serum 25(OH)D Concentration |
P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Sample (n = 2,641) |
<50 nmol/L
(n = 854) |
50–<75 nmol/L(n = 924) |
≥75 nmol/L(n = 863) |
||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | ||
| Age, years | 74.7 (2.9) | 74.6 (2.9) | 74.7 (2.9) | 74.6 (2.8) | 0.59 | ||||
| Female gender | 51.1 | 56.4 | 50.0 | 46.9 | <0.0001 | ||||
| Black race | 38.5 | 64.5 | 32.9 | 18.7 | <0.0001 | ||||
| Memphis, Tennessee, site | 48.9 | 46.4 | 49.8 | 50.5 | 0.08 | ||||
| Season | |||||||||
| Winter (December–February) | 26.4 | 32.1 | 27.1 | 20.2 | <0.0001 | ||||
| Spring (March–May) | 30.2 | 32.4 | 29.0 | 29.2 | |||||
| Summer (June–August) | 17.1 | 11.8 | 18.3 | 21.0 | |||||
| Fall (September–November) | 26.3 | 23.6 | 25.6 | 29.7 | |||||
| Less than high school education | 23.2 | 32.9 | 21.1 | 15.9 | <0.0001 | ||||
| Current smokerb | 9.2 | 14.2 | 7.0 | 6.7 | <0.0001 | ||||
| Alcohol consumption | |||||||||
| None in past year | 62.4 | 68.8 | 61.9 | 56.4 | <0.0001 | ||||
| ≤7 drinks/week | 28.6 | 22.4 | 29.1 | 34.1 | |||||
| >1 drink/day | 9.1 | 8.8 | 9.0 | 9.5 | |||||
| Body mass indexc | 27.2 (4.8) | 28.6 (5.5) | 27.2 (4.5) | 25.9 (3.9) | <0.0001 | ||||
| Walking, minutes/week | |||||||||
| 0 | 39.4 | 49.5 | 36.2 | 32.8 | <0.0001 | ||||
| 1–149 | 31.7 | 30.9 | 32.4 | 31.9 | |||||
| ≥150 | 28.9 | 19.6 | 31.5 | 35.3 | |||||
| Vitamin D-containing supplement use | 10.6 | 2.7 | 11.0 | 17.8 | <0.0001 | ||||
| Multivitamin use | 35.9 | 13.5 | 37.9 | 56.0 | <0.0001 | ||||
| Cognitive functionb, 3MS score | 90.3 (8.0) | 87.8 (8.9) | 91.1 (7.4) | 91.8 (6.9) | <0.0001 | ||||
| Risk of depressionb (CES-D score ≥16) | 5.5 | 5.4 | 5.3 | 5.8 | 0.71 | ||||
| Prevalent disease | |||||||||
| Diabetes | 19.6 | 26.0 | 18.3 | 14.7 | <0.0001 | ||||
| Cardiovascular disease | 28.1 | 31.2 | 27.8 | 25.5 | 0.0092 | ||||
| COPD | 13.8 | 17.7 | 13.0 | 10.8 | <0.0001 | ||||
| Knee pain | 26.2 | 28.1 | 26.1 | 24.4 | 0.08 | ||||
| Hospitalization in past year | 13.9 | 16.5 | 11.5 | 14.0 | 0.14 | ||||
| eGFRb, mL/minute/1.73 m2 | 72.5 (16.1) | 74.8 (17.5) | 72.4 (15.9) | 70.2 (14.6) | <0.0001 | ||||
| Parathyroid hormone, pg/mL | 39.0 (26.4) | 48.2 (33.7) | 37.0 (21.6) | 32.1 (19.4) | <0.0001 | ||||
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; 3MS, Modified Mini-Mental State Examination; 25(OH)D, 25-hydroxyvitamin D; SD, standard deviation.
a The chi-squared test or analysis of variance was used to evaluate the distribution across categories of 25(OH)D.
b Smoking status, cognitive function, depressive symptoms, and eGFR were assessed in 1997–1998.
c Weight (kg)/height (m)2.
At baseline, a threshold model was tested to determine the 25(OH)D thresholds for physical performance and strength (Table 2). In general, the 25(OH)D thresholds for physical performance were approximately 70–80 nmol/L, with slightly lower 25(OH)D thresholds for strength, at 55–70 nmol/L. For 400-m gait speed, the 25(OH)D threshold differed by season of measurement and was higher when 25(OH)D was measured in the spring, summer, or fall than when 25(OH)D was measured in the winter. Otherwise, 25(OH)D thresholds did not differ significantly by race, gender, winter season, or obesity (P > 0.10 for all; data not shown). With the exception of knee extensor strength, the slopes of the association between 25(OH)D and physical performance and grip strength were all significant below the 25(OH)D thresholds (P < 0.05) but were not significant above the 25(OH)D thresholds (P > 0.15). Furthermore, the fit of the piecewise regression model was significantly better than a straight-line model fit for the physical performance measures (SPPB, Health ABC PPB, 20-m gait speed, and 400-m gait speed (for 25(OH)D measured during spring, summer, or fall); P < 0.05) but not for strength.
Table 2.
25-Hydroxyvitamin D Thresholds for Physical Performance and Muscle Strength at Baseline, Health, Aging, and Body Composition Study, 1998–1999a
| 25(OH)D Threshold, nmol/L | 95% Confidence Interval | Slope Below the Threshold per 10-nmol/L Increment of 25(OH)D, β (SE) | P for Slope Below the Threshold | Slope Above the Threshold per 10-nmol/L Increment of 25(OH)D, β (SE) | P for Slope Above the Threshold | |
|---|---|---|---|---|---|---|
| SPPB scoreb | 70.4 | 54.7, 86.1 | 0.099 (0.027) | 0.0002 | −0.021 (0.026) | 0.41 |
| Health ABC PPB scoreb | 74.9 | 60.4, 89.4 | 0.036 (0.008) | <0.0001 | −0.008 (0.010) | 0.39 |
| Gait speed, m/second | ||||||
| 20 m | 82.8 | 67.8, 97.7 | 0.014 (0.003) | <0.0001 | −0.004 (0.005) | 0.36 |
| 400 m | ||||||
| Winter season | 50.1 | 27.7, 72.4 | 0.036 (0.017) | 0.04 | 0.008 (0.006) | 0.15 |
| Not winter season | 81.5 | 61.8, 101.2 | 0.014 (0.004) | 0.0003 | −0.003 (0.006) | 0.63 |
| Knee extensor strength, nm/kg of leg lean mass | 54.8 | 27.8, 81.9 | 0.196 (0.131) | 0.14 | −0.009 (0.055) | 0.87 |
| Grip strength, kg | 69.7 | 49.7, 89.7 | 0.345 (0.130) | 0.01 | −0.114 (0.124) | 0.36 |
Abbreviations: Health ABC, Health, Aging, and Body Composition; Health ABC PPB, Health ABC Physical Performance Battery; 25(OH)D, 25-hydroxyvitamin D; SE, standard error; SPPB, Short Physical Performance Battery.
a Piecewise regression models adjusted for age, gender, race, site, season, body mass index, and kidney function.
b SPPB and Health ABC PPB scores were assessed in 1997–1998.
Cross-sectional associations between 25(OH)D and physical performance and strength are shown in Table 3. In models adjusted for demographic factors, site, and season, 25(OH)D was associated with all physical performance and strength measures. Participants with 25(OH)D <50 nmol/L had significantly poorer physical performance (P < 0.001), slower gait speed (P < 0.001), and lower knee extensor and grip strength (P < 0.05) than those with 25(OH)D ≥75 nmol/L. Among the individual physical performance battery tasks, standing balance time and gait speed were associated with 25(OH)D (P < 0.0001 for both). After further adjustment for health behaviors and chronic conditions, the associations were attenuated but remained significant for the Health ABC PPB score, 20-m and 400-m gait speed, and grip strength. Results were similar after further adjustment for PTH (data not shown).
Table 3.
Serum 25-Hydroxyvitamin D Status, Physical Performance, and Muscle Strength (Least-Squares Mean (SE)) at Baseline, Health, Aging, and Body Composition Study, 1998–1999a
| Variable and Model | No. of Participants | Serum 25(OH)D Concentration |
P Value | ||
|---|---|---|---|---|---|
| <50 nmol/L | 50–<75 nmol/L | ≥75 nmol/L | |||
| SPPB scoreb (range, 0–12) | 2,603 | ||||
| Model 1 | 9.77 (0.05)*** | 10.02 (0.05) | 10.05 (0.06) | 0.0002 | |
| Model 2 | 9.46 (0.11) | 9.60 (0.11) | 9.60 (0.11) | 0.11 | |
| Health ABC PPB scoreb (range, 0–4) | 2,546 | ||||
| Model 1 | 2.07 (0.02)*** | 2.17 (0.02) | 2.21 (0.02) | <0.0001 | |
| Model 2 | 1.95 (0.04)* | 1.99 (0.04) | 2.01 (0.04) | 0.05 | |
| 20-m gait speed, m/second | 2,607 | ||||
| Model 1 | 1.08 (0.01)*** | 1.12 (0.01)* | 1.14 (0.01) | <0.0001 | |
| Model 2 | 1.03 (0.01)*** | 1.06 (0.01) | 1.07 (0.01) | 0.003 | |
| 400-m gait speed, m/second | 1,825 | ||||
| Model 1 | 1.20 (0.01)*** | 1.25 (0.01)** | 1.28 (0.01) | <0.0001 | |
| Model 2 | 1.14 (0.02)*** | 1.17 (0.02)* | 1.19 (0.02) | 0.0005 | |
| Knee extensor strength, nm/kg of leg lean mass | 2,254 | ||||
| Model 1 | 13.43 (0.13)*** | 13.98 (0.13) | 14.07 (0.14) | 0.001 | |
| Model 2 | 12.83 (0.27) | 13.01 (0.27) | 12.91 (0.27) | 0.58 | |
| Grip strength, kg | 2,522 | ||||
| Model 1 | 30.73 (0.25)* | 31.58 (0.24) | 31.50 (0.26) | 0.02 | |
| Model 2 | 28.87 (0.51)* | 29.71 (0.50) | 29.81 (0.50) | 0.02 | |
Abbreviations: Health ABC, Health, Aging, and Body Composition; Health ABC PPB, Health ABC Physical Performance Battery; 25(OH)D, 25-hydroxyvitamin D; SE, standard error; SPPB, Short Physical Performance Battery.
* P < 0.05; **P < 0.01; ***P < 0.001 (difference from 25(OH)D ≥75 nmol/L when P < 0.05 for the overall model).
a Linear regression models adjusted for age, gender, race, education, site, and season (model 1) and for age, gender, race, education, site, season, smoking status, alcohol consumption, physical activity, body mass index, multivitamin and vitamin D-containing supplement use, kidney function, cognitive function, depressive symptoms, diabetes mellitus, cardiovascular disease, chronic obstructive pulmonary disease, knee pain, and prior hospitalization (model 2).
b SPPB and Health ABC PPB scores were assessed in 1997–1998.
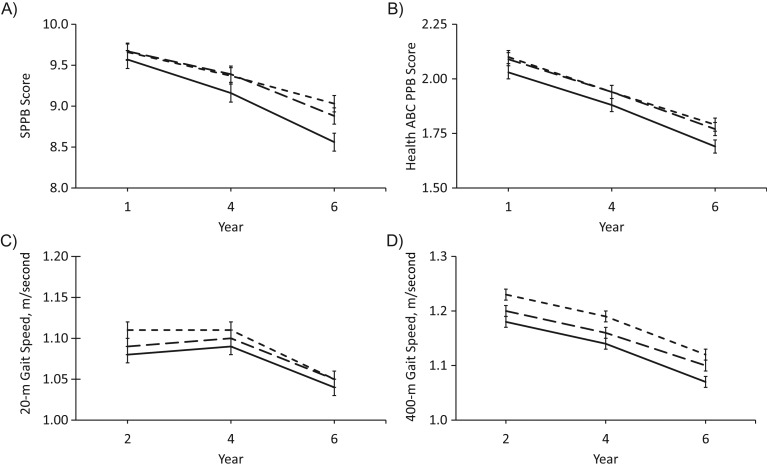
Longitudinal associations between baseline 25(OH)D and physical performance and strength over 4 years of follow-up are shown in Table 4. In models adjusted for demographic factors, site, and season, baseline 25(OH)D was associated with the SPPB score, Health ABC PPB score, and 20-m and 400-m gait speed at baseline and 2 and 4 years later. Participants with baseline 25(OH)D <50 nmol/L had poorer physical performance and slower gait speed at each time point than did those with 25(OH)D ≥75 nmol/L (P < 0.01 for all). Among the individual physical performance battery tasks, standing balance time and gait speed were associated with baseline 25(OH)D at baseline and 2 and 4 years later (P ≤ 0.0001 for all), but chair stand time was associated with baseline 25(OH)D only 2 and 4 years later (P < 0.05). After adjustment for health behaviors and chronic conditions, the association between 25(OH)D and physical performance at each time point was attenuated but, in general, remained significant for the SPPB score, Health ABC PPB score, and 400-m gait speed. With the exception of knee extensor strength at baseline (P < 0.05), baseline 25(OH)D was not associated with knee extensor or grip strength over 4 years of follow-up. Physical performance and strength declined significantly over 4 years (P < 0.0001), but, with the exception of the SPPB (25(OH)D-by-year interaction (P ≤ 0.01), change in physical performance and strength was not associated with baseline 25(OH)D (Figure 1). Results were similar after further adjustment for PTH (data not shown).
Table 4.
Serum 25-Hydroxyvitamin D Status in 1998–1999 and Physical Performance and Muscle Strength (Least-Squares Mean (SE)) Over 4 Years of Follow-up, Health, Aging, and Body Composition Studya
| Variable, Model, and Visit Yearb | No. of Participants | Serum 25(OH)D Concentration |
P Value |
||||
|---|---|---|---|---|---|---|---|
| <50 nmol/L | 50–<75 nmol/L | ≥75 nmol/L | 25(OH)D Within Year | 25(OH)D-by-Year Interaction | Overall 25(OH)D | ||
| SPPB score (range, 0–12) | |||||||
| Model 1 | |||||||
| 1 | 2,278 | 9.82 (0.06)** | 10.04 (0.05) | 10.07 (0.06) | 0.002 | 0.004 | |
| 4 | 2,195 | 9.29 (0.08)*** | 9.67 (0.07) | 9.71 (0.08) | <0.0001 | ||
| 6 | 1,956 | 8.65 (0.09)*** | 9.11 (0.08) | 9.31 (0.09) | <0.0001 | ||
| Model 2 | |||||||
| 1 | 2,278 | 9.57 (0.10) | 9.67 (0.09) | 9.66 (0.10) | 0.39 | 0.01 | |
| 4 | 2,195 | 9.16 (0.11)* | 9.39 (0.10) | 9.37 (0.10) | 0.049 | ||
| 6 | 1,956 | 8.56 (0.11)*** | 8.88 (0.11) | 9.03 (0.11) | 0.0007 | ||
| Health ABC PPB score (range, 0–4) | |||||||
| Model 1 | |||||||
| 1 | 2,231 | 2.09 (0.02)*** | 2.19 (0.02) | 2.23 (0.02) | <0.0001 | 0.77 | <0.0001 |
| 4 | 2,193 | 1.90 (0.02)*** | 2.02 (0.02) | 2.05 (0.02) | <0.0001 | ||
| 6 | 1,955 | 1.70 (0.02)*** | 1.84 (0.02) | 1.88 (0.02) | <0.0001 | ||
| Model 2 | |||||||
| 1 | 2,231 | 2.03 (0.03)* | 2.09 (0.03) | 2.10 (0.03) | 0.04 | 0.75 | <0.0001 |
| 4 | 2,193 | 1.88 (0.03) | 1.94 (0.03) | 1.94 (0.03) | 0.06 | ||
| 6 | 1,955 | 1.69 (0.03)** | 1.77 (0.03) | 1.79 (0.03) | 0.01 | ||
| 20-m gait speed, m/second | |||||||
| Model 1 | |||||||
| 2 | 2,287 | 1.09 (0.01)*** | 1.13 (0.01)* | 1.15 (0.01) | <0.0001 | 0.38 | <0.0001 |
| 4 | 2,175 | 1.09 (0.01)*** | 1.13 (0.01) | 1.15 (0.01) | <0.0001 | ||
| 6 | 1,953 | 1.03 (0.01)*** | 1.07 (0.01) | 1.08 (0.01) | 0.0006 | ||
| Model 2 | |||||||
| 2 | 2,287 | 1.08 (0.01)** | 1.09 (0.01) | 1.11 (0.01) | 0.02 | 0.47 | 0.0001 |
| 4 | 2,175 | 1.09 (0.01) | 1.10 (0.01) | 1.11 (0.01) | 0.13 | ||
| 6 | 1,953 | 1.04 (0.01) | 1.05 (0.01) | 1.05 (0.01) | 0.48 | ||
| 400-m gait speed, m/second | |||||||
| Model 1 | |||||||
| 2 | 1,640 | 1.21 (0.01)*** | 1.26 (0.01)*** | 1.30 (0.01) | <0.0001 | 0.80 | <0.0001 |
| 4 | 1,541 | 1.17 (0.01)*** | 1.21 (0.01)*** | 1.25 (0.01) | <0.0001 | ||
| 6 | 1,331 | 1.09 (0.01)*** | 1.14 (0.01)** | 1.18 (0.01) | <0.0001 | ||
| Model 2 | |||||||
| 2 | 1,640 | 1.18 (0.01)*** | 1.20 (0.01)** | 1.23 (0.01) | 0.0001 | 0.86 | <0.0001 |
| 4 | 1,541 | 1.14 (0.01)*** | 1.16 (0.01)** | 1.19 (0.01) | <0.0001 | ||
| 6 | 1,331 | 1.07 (0.01)*** | 1.10 (0.01) | 1.12 (0.01) | 0.0008 | ||
| Knee extensor strength, nm/kg of leg lean mass | |||||||
| Model 1 | |||||||
| 2 | 1,999 | 13.5 (0.1)*** | 13.9 (0.1) | 14.2 (0.1) | 0.001 | 0.21 | 0.01 |
| 4 | 1,935 | 12.6 (0.1) | 13.0 (0.1) | 13.0 (0.1) | 0.07 | ||
| 6 | 1,818 | 12.1 (0.1) | 12.4 (0.1) | 12.4 (0.1) | 0.14 | ||
| Model 2 | |||||||
| 2 | 1,999 | 13.2 (0.2) | 13.4 (0.2) | 13.4 (0.2) | 0.58 | 0.20 | 0.76 |
| 4 | 1,935 | 12.5 (0.2) | 12.5 (0.2) | 12.3 (0.2) | 0.41 | ||
| 6 | 1,818 | 11.9 (0.2) | 11.9 (0.2) | 11.8 (0.2) | 0.76 | ||
| Grip strength, kg | |||||||
| Model 1 | |||||||
| 2 | 2,205 | 31.0 (0.3) | 31.6 (0.2) | 31.7 (0.3) | 0.12 | 0.98 | 0.06 |
| 4 | 2,226 | 30.8 (0.3) | 31.6 (0.2) | 31.5 (0.3) | 0.07 | ||
| 6 | 1,971 | 29.8 (0.3) | 30.5 (0.3) | 30.5 (0.3) | 0.12 | ||
| Model 2 | |||||||
| 2 | 2,205 | 29.9 (0.4) | 30.5 (0.4) | 30.7 (0.4) | 0.08 | 0.93 | 0.09 |
| 4 | 2,226 | 30.1 (0.4) | 30.7 (0.4) | 30.8 (0.4) | 0.14 | ||
| 6 | 1,971 | 29.2 (0.4) | 29.8 (0.4) | 30.0 (0.4) | 0.16 | ||
Abbreviations: Health ABC, Health, Aging, and Body Composition; Health ABC PPB, Health ABC Physical Performance Battery; 25(OH)D, 25-hydroxyvitamin D; SE, standard error; SPPB, Short Physical Performance Battery.
* P < 0.05; **P < 0.01; ***P < 0.001 (difference from 25(OH)D ≥75 nmol/L when 25(OH)D within-year P value < 0.05).
a Mixed models adjusted for age, gender, race, education, site, and season (model 1) and for age, gender, race, education, site, season, smoking status, alcohol consumption, physical activity, body mass index, multivitamin and vitamin D-containing supplement use, kidney function, cognitive function, depressive symptoms, diabetes, cardiovascular disease, chronic obstructive pulmonary disease, knee pain, and prior hospitalization (model 2).
b Visit year: year 1, 1997–1998; year 2, 1998–1999; year 4, 2000–2001; year 6, 2002–2003.
Figure 1.
Serum 25-hydroxyvitamin D (25(OH)D) status in 1998–1999 and A) score on the Short Physical Performance Battery (SPPB), B) score on the Health ABC Physical Performance Battery (Health ABC PPB), C) 20-m gait speed, and D) 400-m gait speed over 4 years of follow-up in the Health, Aging, and Body Composition (Health ABC) Study. Least-squares mean values from mixed models adjusted for age, gender, race, education, site, season, smoking status, alcohol consumption, physical activity, body mass index, multivitamin and vitamin D-containing supplement use, kidney function, cognitive function, depressive symptoms, diabetes, cardiovascular disease, chronic obstructive pulmonary disease, knee pain, and prior hospitalization. Visit year: year 1, 1997–1998; year 2, 1998–1999; year 4, 2000–2001; year 6, 2002–2003. 25(OH)D concentration categories: solid line, <50 nmol/L; long-dashed line, 50–<75 nmol/L; short-dashed line, ≥75 nmol/L. Bars, standard error.
DISCUSSION
In this community-dwelling cohort of older, initially well-functioning white and black men and women, physical performance increased up to 25(OH)D concentrations of 70–80 nmol/L, and strength increased up to 25(OH)D concentrations of 55–70 nmol/L, which suggests that there could be different thresholds for different aspects of physical function. Furthermore, older adults with 25(OH)D <50 nmol/L at baseline had consistently poorer physical performance at baseline and at the 2- and 4-year follow-ups than those with 25(OH)D ≥75 nmol/L. Physical performance and strength declined significantly over time, but baseline 25(OH)D was, in general, not associated with the rate of decline.
Although the Institute of Medicine (17) has suggested that 25(OH)D ≥50 nmol/L is adequate for bone health, others have suggested that the optimal 25(OH)D concentration for health conditions other than bone could indeed be higher (18–20). We found that, in general, physical performance and strength increased in persons with 25(OH)D concentrations up to approximately 70–80 nmol/L, depending on the function assessed. However, persons with 25(OH)D concentrations beyond 70–80 nmol/L did not exhibit any additional functional advantage. Furthermore, the 25(OH)D threshold did not differ appreciably by gender, race, or obesity status. In the Third National Health and Nutrition Examination Survey (1988–1994), time needed to walk 8 feet (2.5 m) and complete 5 repeated chair stands decreased with increasing 25(OH)D concentration in older adults, with most of the time decrease occurring at concentrations of <40 nmol/L but with further, less dramatic time decreases occurring at 40–94 nmol/L (9). Among older women in the Rancho Bernardo Study, physical performance increased up to 25(OH)D concentrations of 80 nmol/L (16).
Previous cross-sectional studies in older adults have shown that low 25(OH)D is associated with poorer physical performance and lower strength (5–11). Nevertheless, studies examining the association between baseline 25(OH)D and change in physical performance and strength over time have been inconsistent, showing either no association with 25(OH)D (12–14) or greater declines in physical performance and strength among those with low 25(OH)D (15–16). The discrepancies among these studies could stem from variation in the measurement of 25(OH)D as well as from differences in the study population characteristics, such as the prevalence of low 25(OH)D and baseline functional status. We found that low baseline 25(OH)D was associated with poorer physical performance and lower strength at baseline and that the differences in physical performance persisted over 2 and 4 years of follow-up. However, low baseline 25(OH)D was not associated with faster declines in physical performance or strength. Similarly, in a small subset of the Women's Health Initiative clinical trial, women with 25(OH)D ≥75 nmol/L had better physical performance over 6 years of follow-up than did women with 25(OH)D <25 nmol/L, but the rate of decline in physical performance did not differ by baseline 25(OH)D (29).
Vitamin D plays an important role in skeletal muscle function through its regulation of calcium transport, uptake of inorganic phosphate for the production of energy-rich phosphate compounds, and protein synthesis in the muscle (2). In addition, the association of vitamin D with physical performance and muscle strength might be mediated by PTH. Vitamin D deficiency is a recognized cause of secondary hyperparathyroidism (22). Previous studies have shown elevated PTH to be associated with poorer physical performance and lower strength (8, 10, 30, 31). Furthermore, administration of PTH increases protein catabolism, decreases the number of type 2 muscle fibers and intracellular energy-rich phosphate compounds, and decreases mitochondrial oxygen uptake in animal models (22, 32). In the present study, 25(OH)D was associated with physical performance over 4 years of follow-up. Furthermore, the associations between 25(OH)D and physical performance were similar after inclusion of PTH in the model, which suggests independent roles of low 25(OH)D and elevated PTH.
A major strength of these analyses is the use of data from the Health ABC cohort study, a large study of well-characterized, community-dwelling older adults, which has excellent retention and a comprehensive set of relevant covariates. Nevertheless, some features of Health ABC limit the generalizability of these findings. Well-functioning participants were recruited; thus, these results may not be generalizable to the general older population. Participants who were excluded because they lacked follow-up visits had lower baseline 25(OH)D concentrations and poorer physical performance, which likely would have attenuated the observed results. Blood samples were collected in 1998–1999, when the use of individual vitamin D supplements was less common and the vitamin D content of multivitamins was low, likely resulting in lower 25(OH)D concentrations than would be found currently. Serial measures of 25(OH)D are not available in Health ABC; thus, we were unable to account for changes in 25(OH)D over time. Although it is biologically plausible that low 25(OH)D could result in poorer physical performance and lower strength, the observational nature of this study did not allow us to evaluate a causal association between 25(OH)D and physical performance and strength.
In conclusion, the 25(OH)D threshold for physical performance in this initially well-functioning, community-dwelling population of older adults was 70–80 nmol/L, with slightly lower 25(OH)D thresholds for strength of 55–70 nmol/L. These findings are consistent with recommendations for 25(OH)D concentrations of 75–80 nmol/L or higher for health- and function-related outcomes other than bone health (18–20). Furthermore, low 25(OH)D was associated with poorer physical performance over 4 years of follow-up. Although lower 25(OH)D concentrations were not associated with a greater rate of decline in physical performance, individuals with low 25(OH)D had poorer physical performance at each time point studied and thus were likely to cross the physical disability threshold earlier than individuals with higher 25(OH)D concentrations. Recently, low 25(OH)D was shown to increase the risk of incident mobility disability, a subjective measure of difficulty in walking one-fourth of a mile or up 10 steps, over 3 years of follow-up (33). Definitive trials of vitamin D supplementation in individuals with low 25(OH)D concentrations are needed to determine whether increasing 25(OH)D will improve function in older adults or slow the age-related decline in physical performance and strength to delay or prevent the onset of disability among older adults.
ACKNOWLEDGMENTS
Author affiliations: Department of Internal Medicine, Wake Forest School of Medicine, Winston-Salem, North Carolina (Denise K. Houston, M. Kyla Shea, Jeff D. Williamson, Stephen B. Kritchevsky); Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, North Carolina (Janet A. Tooze, Rebecca H. Neiberg); Department of Cancer Biology, Wake Forest School of Medicine, Winston-Salem, North Carolina (Gary G. Schwartz); Department of Foods and Nutrition, College of Family and Consumer Sciences, University of Georgia, Athens, Georgia (Dorothy B. Hausman, Mary Ann Johnson); Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania (Jane A. Cauley); Department of Medicine, School of Medicine, University of California, San Francisco, San Francisco, California (Doug C. Bauer); California Pacific Medical Center Research Institute, California Pacific Medical Center, San Francisco, California (Peggy M. Cawthon); Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, Tennessee (Frances A. Tylavsky); Department of Health Sciences, Vrije Universiteit, Amsterdam, the Netherlands (Marjolein Visser); Clinical Research Branch, National Institute on Aging, Baltimore, Maryland (Eleanor M. Simonsick); and Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging, Bethesda, Maryland (Tamara B. Harris).
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (NIA); NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grants R01 AG028050, R01 AG029364, and K01 AG030506 (to D. K. H.); National Institute of Nursing Research grant R01 NR012459; and the Wake Forest University Claude D. Pepper Older Americans Independence Center (grant P30 AG021332).
Conflict of interest: none declared.
REFERENCES
- 1.Dawson-Hughes B. Serum 25-hydroxyvitamin D and functional outcomes in the elderly. Am J Clin Nutr. 2008;88(2):537S–540S. doi: 10.1093/ajcn/88.2.537S. [DOI] [PubMed] [Google Scholar]
- 2.Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Aspects Med. 2008;29(6):407–414. doi: 10.1016/j.mam.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45(1):92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 5.Mowé M, Haug E, Bøhmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47(2):220–226. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff HA, Stahelin HB, Urscheler N, et al. Muscle strength in the elderly: its relation to vitamin D metabolites. Arch Phys Med Rehabil. 1999;80(1):54–58. doi: 10.1016/s0003-9993(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni M, Zoico E, Tosoni P, et al. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57(1):M7–M11. doi: 10.1093/gerona/57.1.m7. [DOI] [PubMed] [Google Scholar]
- 8.Dhesi JK, Bearne LM, Moniz C, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17(5):891–897. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥60 y. Am J Clin Nutr. 2004;80(3):752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 10.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62(4):440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annweiler C, Schott AM, Montero-Odasso M, et al. Cross-sectional association between serum vitamin D concentration and walking speed measured at usual and fast pace among older women: the EPIDOS study. J Bone Miner Res. 2010;25(8):1858–1866. doi: 10.1002/jbmr.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verreault R, Semba RD, Volpato S, et al. Low serum vitamin D does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50(5):912–917. doi: 10.1046/j.1532-5415.2002.50219.x. [DOI] [PubMed] [Google Scholar]
- 13.Faulkner KA, Cauley JA, Zmuda JM, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17(9):1318–1328. doi: 10.1007/s00198-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 14.Bartali B, Frongillo EA, Guralnik JM, et al. Serum micronutrient concentrations and decline in physical function among older persons. JAMA. 2008;299(3):308–315. doi: 10.1001/jama.299.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 16.Dam TT, von Mühlen D, Barrett-Connor EL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. 2009;20(5):751–760. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 18.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 19.Souberbielle JC, Body JJ, Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev. 2010;9(11):709–715. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 21.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 24.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 25.Simonsick EM, Montgomery PS, Newman AB, et al. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 26.Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 28.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 29.Michael YL, Smit E, Seguin R, et al. Serum 25-hydroxyvitamin D and physical performance in postmenopausal women. J Womens Health (Larchmt) 2011;20(11):1603–1608. doi: 10.1089/jwh.2010.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 31.Gerdhem P, Ringsberg KA, Obrant KJ, et al. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16(11):1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13(3):187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 33.Houston DK, Tooze JA, Davis CC, et al. Serum 25-hydroxyvitamin D and physical function in older adults: the Cardiovascular Health Study All Stars. J Am Geriatr Soc. 2011;59(10):1793–1801. doi: 10.1111/j.1532-5415.2011.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]