Abstract
In recent decades, epidemiology, public health, and medical sciences have been increasingly compartmentalized into narrower disciplines. The authors recognize the value of integration of divergent scientific fields in order to create new methods, concepts, paradigms, and knowledge. Herein they describe the recent emergence of molecular pathological epidemiology (MPE), which represents an integration of population and molecular biologic science to gain insights into the etiologies, pathogenesis, evolution, and outcomes of complex multifactorial diseases. Most human diseases, including common cancers (such as breast, lung, prostate, and colorectal cancers, leukemia, and lymphoma) and other chronic diseases (such as diabetes mellitus, cardiovascular diseases, hypertension, autoimmune diseases, psychiatric diseases, and some infectious diseases), are caused by alterations in the genome, epigenome, transcriptome, proteome, metabolome, microbiome, and interactome of all of the above components. In this era of personalized medicine and personalized prevention, we need integrated science (such as MPE) which can decipher diseases at the molecular, genetic, cellular, and population levels simultaneously. The authors believe that convergence and integration of multiple disciplines should be commonplace in research and education. We need to be open-minded and flexible in designing integrated education curricula and training programs for future students, clinicians, practitioners, and investigators.
Keywords: education, public health professional; health care reform; individualized medicine; interdisciplinary communication; molecular epidemiology; pathology
Editor's note: An invited commentary on this article appears on page 668, and the authors' response appears on page 672.
Education is a crucial mission of the academic community. Excellence in research and education requires the combined efforts of many different disciplines (1, 2). As fundamental disciplines of biomedical and public health sciences, both pathology and epidemiology are fields of study of the entire spectrum of human diseases—the former focused on disease mechanisms in individual cases, the latter on patterns of disease in populations. The importance of these fields is well exemplified by the universal presence of pathology in medical school curricula and that of epidemiology in public health school curricula. Because of advances in both laboratory technologies and epidemiologic methods, pathology and epidemiology have become compartmentalized in schools of medicine and public health, respectively. By virtue of our training in both pathology and epidemiology, we can appreciate that knowledge, skills, and concepts from both fields can be integrated and synergized to advance biomedical, public health, and population sciences. In this era of personalized medicine (3), we need integrated, convergent scientific disciplines, which will enable us to decipher the characteristics of diseases simultaneously at both the individual and population levels (4–6).
The importance of integration of divergent disciplines has repeatedly been discussed (7–10). As an initial step toward such integrated scientific disciplines, our discussion is primarily focused on the integration of molecular pathology and epidemiology—that is, molecular pathological epidemiology (MPE) (4–6). This integrated field improves understanding of human diseases and may provide a model for future integrations of other subspecialties. Thus, this article will help foster an interdisciplinary integration of a wide variety of other fields in biomedical, public health, population, and social science and an establishment of hybrid disciplines.
PATHOLOGY EDUCATION IN PUBLIC HEALTH SCHOOLS
Epidemiology is a core component of public health school curricula, reflecting its pivotal role in the health sciences. However, in public health schools, most students get little, if any, opportunity to study pathology, resulting in limited understanding of disease pathogenesis. Recently, integration of pathology into epidemiologic studies has become increasingly common (4, 6, 11), because many diseases are being defined by molecular pathogenic mechanisms. As current disease classification schemes become more reflective of pathobiology (4, 6, 11), epidemiologists must appreciate the rationale behind disease classifications and subtyping in their study designs. Possibilities for pathology training include: lectures by pathologists, rotations at clinical pathology laboratories, and participation in MPE research.
EPIDEMIOLOGY EDUCATION IN PATHOLOGY AND MEDICAL SCHOOLS
Pathology is a core component of medical school curricula, reflecting its central role in medical education. In addition, training in pathology as a medical specialty occurs as a part of postgraduate medical education. Unfortunately, most pathologists and other physicians have limited knowledge of epidemiology. Education in epidemiology can provide knowledge of proper study design, data interpretation, and statistical and causal inferences, which are necessary in correlative pathology research. However, neither epidemiology nor biostatistics is a common component of pathology training (12). Only a minority of pathologists and physicians have sufficient understanding of epidemiology to apply relevant principles to their investigations. Epidemiology can provide ideas about potential etiologic factors and can teach pathologists proper study design and conduct in terms of population selection, sample size determination, statistical methods, causal inference, assessment of generalizability, and validation of findings. In our opinion, pathology training and medical school programs should be encouraged to include formal epidemiology courses or lectures, preferably as a mandatory requirement.
Substantial concerns have been raised about the validity of much of published scientific research (13–17). Published studies are often called into question for inappropriate study design, biased sample selection, inadequate sample size, inappropriate statistical methods, etc. Studies conducted by pathologists and other clinical investigators are commonly biased, because cases typically come from tertiary referral medical centers. Those common problems in study design and analysis should be considered, and the best attempts to improve study design must be made.
MPE AS AN INTERDISCIPLINARY SCIENCE
MPE is a recently established interdisciplinary and transdisciplinary field (4–6). Traditional epidemiology (including molecular epidemiology and genome-wide association studies) has the substantial limitation of treating pathogenically heterogeneous diseases (e.g., hypertension, diabetes mellitus, major depression, breast cancer) as a single entity. In contrast, from the MPE viewpoint (4–6), any human disease entity is fundamentally heterogeneous from person to person (18), just as each individual is unique. Nonetheless, by classifying disease according to its pathogenic mechanisms, we can better predict the course of a disease in a given individual (6). In fact, there exists heterogeneity of risk factors as well as heterogeneity of molecular pathogenesis in any given disease (4–6).
A growing body of literature (see Web Appendix (http://aje.oxfordjournals.org/)) supports this MPE paradigm (4–6), with evidence suggesting that carcinogenic or protective effects of lifestyle, dietary, environmental, and genetic factors differ according to specific molecular characteristics in neoplastic cells. The MPE concept is gaining widespread adoption (19–30). As Table 1 shows, MPE studies have improved our understanding of pathogenesis by demonstrating consistent links between etiologic factors and molecular subtypes of diseases (31–66). Furthermore, recent evidence suggests that host factors can interact with tumor molecular changes to modify cancer cell behavior (67–74). Thus, the MPE approach, unlike the traditional epidemiologic research design, allows insights into etiologic factors and pathogenic mechanisms.
Table 1.
Examples of Molecular Pathological Epidemiology Studies Which Have Shown Consistent Links Between Etiologic Factors and Cellular Molecular Changesa
| First Author, Year (Reference No.) | Disease (as in Traditional Epidemiology) | Study Design | Sample Size (No. of Participants) | Putative Etiologic Factor | Tumor Molecular Changes (Subtypes) | Direction of Association |
|---|---|---|---|---|---|---|
| Chen, 2007 (31) | Colorectal cancer | Case-case | 383 | MLH1 rs1800734 SNP | MLH1 methylation | Positive |
| Chen, 2007 (31) | Endometrial cancer | Case-case | 498 | MLH1 rs1800734 SNP | MLH1 methylation | Positive |
| Raptis, 2007 (32) | Colorectal cancer | Case-control | 766 cancer cases, 1,098 controls | MLH1 rs1800734 SNP | MSI | Positive |
| Samowitz, 2008 (33) | Colon cancer | Case-control | 795 cancer cases, 1,968 controls | MLH1 rs1800734 SNP | MLH1 methylation, CIMP, BRAF mutation | Positive |
| Allan, 2008 (34) | Colorectal cancer | Case-case | 1,392 | MLH1 rs1800734 SNP | MLH1 loss of expression | Positive |
| Campbell, 2009 (35) | Colon cancer | Case-control | 1,211 cancer cases, 1,972 controls | MLH1 rs1800734 SNP | MSI | Positive |
| Oyama, 2004 (36) | Colon cancer (proximal) | Case-case | 194 | 1-carbon metabolism (MTHFR rs1801131 SNP) | CDKN2A (p16) methylation | Positive |
| Curtin, 2007 (37) | Colon cancer | Case-control | 916 cancer cases, 1,972 controls | 1-carbon metabolism (MTHFR rs1801131 SNP) | CIMP | Positive |
| Jensen, 2008 (38) | Colorectal cancer | Case-case | 130 | 1-carbon metabolism (plasma homocysteine) | MSI | Positive |
| de Vogel, 2009 (39) | Colorectal cancer | Case-cohort | 373 cancer cases, 4,774 in subcohort | 1-carbon metabolism (MTHFR rs1801131 SNP) | CIMP | Null |
| Schernhammer, 2010 (40) | Colon cancer | Prospective cohort | 609 incident cancer cases, 136,056 participants | 1-carbon nutrients (folate, B vitamins), alcohol | LINE-1 hypomethylation | Lack of folate and excess alcohol are associated with increased incidence of LINE-1 hypomethylated cancer but not that of LINE-1 methylation-high cancer. |
| Hazra, 2010 (41) | Colorectal cancer | Case-case (in prospective cohort studies) | 182 | 1-carbon metabolism (MTHFR rs1801131 SNP) | CIMP | Positive |
| Van Guelpen, 2010 (42) | Colorectal cancer | Nested case-control (in prospective cohort study) | 190 cancer cases, 380 controls | 1-carbon metabolism (MTHFR rs1801131 SNP) | CIMP | Negative (inverse) |
| Curtin, 2011 (43) | Rectal cancer | Case-control | 671 cancer cases, 1,205 controls | 1-carbon metabolism (MTHFR rs1801131 SNP and folate intake) | CIMP | MTHFR rs1801131 SNP and folate intake interact to modify an association with CIMP-positive rectal cancer |
| Ogino, 2007 (44) | Colorectal cancer | Case-case (in prospective cohort studies) | 182 | MGMT rs16906252 SNP | MGMT methylation | Positive |
| Hawkins, 2009 (45) | Colorectal cancer and normal individuals (colon mucosa) | Case-case | 1,039 cancer cases, 97 normal samples from cancer patients, 20 normal mucosa samples from persons without cancer | MGMT rs16906252 SNP | MGMT methylation in cancer and normal colon mucosa | Positive |
| Candiloro, 2009 (46) | Normal individuals (peripheral blood cells) | 89 | MGMT rs16906252 SNP | MGMT methylation (in peripheral blood cells) | Positive | |
| Leng, 2011 (47) | Lung adenocarcinoma | Case-case | 179 | MGMT rs16906252 SNP | MGMT methylation | Positive |
| Kristensen, 2011 (48) | Malignant pleural mesothelioma | Case-case | 95 | MGMT rs16906252 SNP | MGMT methylation | Positive |
| Pedroni, 1999 (49) | Colorectal cancer | 78 (all synchronous cancer patients and 0 solitary tumors) | Tumor synchronicity/metachronicity | MSI | Concordant pattern of MSI status in synchronous/metachronous tumor pairs | |
| Velayos, 2005 (50) | Colorectal cancer | 110 (all synchronous/metachronous cancer patients and 0 solitary tumors) | Tumor synchronicity/metachronicity | MSI | Concordant pattern of MSI status in synchronous/metachronous tumor pairs | |
| Nosho, 2009 (51) | Colorectal cancer | Case-case (in prospective cohort studies) | 1,113 (29 synchronous cancer patients) | Tumor synchronicity | CIMP, MSI, BRAF mutation, LINE-1 hypomethylation | Positive; concordant pattern of LINE-1 hypomethylation in synchronous tumor pairs |
| Konishi, 2009 (52) | Colorectal cancer | Case-case | 97 (28 synchronous cancer patients) | Tumor synchronicity | CIMP | Positive |
| Gonzalo, 2010 (53) | Colorectal cancer | Case-case | 82 (37 synchronous cancer patients) | Tumor synchronicity/metachronicity | Methylation in MGMT, RASSF1 | Positive |
| Slattery, 2000 (54) | Colon cancer | Case-control | 1,510 cancer cases, 2,410 controls | BMI | MSI | Obesity is associated with MSS cancer but not MSI-high cancer |
| Satia, 2005 (55) | Colon cancer | Case-control | 486 cancer cases, 1,048 controls | BMI | MSI | Obesity is associated with MSS cancer but not MSI-high cancer |
| Slattery, 2007 (56) | Colon cancer | Case-control | 1,154 cancer cases, 2,401 controls | BMI | CIMP | Obesity is associated with CIMP-negative cancer but not CIMP-high cancer |
| Campbell, 2010 (57) | Colorectal cancer | Case-control | 1,250 cancer cases, 1,880 controls | BMI | MSI | Obesity is associated with MSS cancer but not MSI-high cancer |
| Sinicrope, 2010 (58) | Colon cancer | Case-case | 2,222 | BMI | MSI | Negative (inverse) |
| Kuchiba, 2012 (59) | Colorectal cancer | Prospective cohort | 536 cancer cases, 109,051 participants | BMI | FASN expression | Obesity is associated with FASN-negative cancer but not with FASN-positive cancer |
| Slattery, 2000 (54) | Colon cancer | Case-control | 1,510 cancer cases, 2,410 controls | Smoking | MSI | Smoking is associated with MSI-high cancer but not MSS cancer |
| Wu, 2001 (60) | Colon cancer | Case-case | 276 | Smoking | MSI | Positive |
| Lüchtenborg, 2005 (61) | Colorectal cancer | Case-cohort | 650 cancer cases, 2,948 in subcohort | Smoking | APC mutation | Negative (inverse) |
| Chia, 2006 (62) | Colorectal cancer | Case-control | 1,792 cancer cases, 1,501 controls | Smoking | MSI | Smoking is associated with MSI-high cancer but not MSS cancer |
| Samowitz, 2006 (63) | Colon cancer | Case-control | 1,315 cancer cases, 2,392 controls | Smoking | CIMP, BRAF mutation | Smoking is associated with CIMP-high cancer and BRAF-mutated cancer but not CIMP-negative or BRAF-wild-type cancer |
| Poynter, 2009 (64) | Colorectal cancer | Case-control | 1,564 cancer cases, 4,486 controls | Smoking | MSI | Smoking is associated with MSI-high cancer but not MSS cancer |
| Rozek, 2010 (65) | Colorectal cancer | Case-control | 1,297 cancer cases, 2,019 controls | Smoking | BRAF mutation | Positive |
| Limsui, 2010 (66) | Colorectal cancer | Prospective cohort | 540 cancer cases, 41,528 participants | Smoking | MSI, CIMP, BRAF mutation | Smoking is associated with CIMP-high cancer, MSI-high cancer, and BRAF-mutated cancer but not CIMP-negative, MSS, or BRAF-wild-type cancer |
Abbreviations: BMI, body mass index; CIMP, CpG island methylator phenotype; MSI, microsatellite instability; MSS, microsatellite stability; SNP, single nucleotide polymorphism.
a The official symbols approved by the Human Genome Organization's Gene Nomenclature Committee are used for genes and gene products (APC, BRAF, CDKN2A, FASN, MGMT, MLH1, and MTHFR).
NECESSITY FOR MPE GUIDELINES AND INTERDISCIPLINARY SCIENTISTS
MPE is a relatively new field of science, and no standard research guidelines have yet been established, as they have been for observational epidemiology (STROBE, which stands for Strengthening the Reporting of Observational Epidemiology) (16, 17, 75) and molecular epidemiology (STROBE-ME) (76). For MPE, there are specific caveats in addition to the typical limitations in observational epidemiology (6). We plan to develop international guidelines for MPE research (STROBE-MPE) as a logical extension of STROBE. To develop and implement guidelines, we need to produce more scientists with cross-disciplinary training and expertise in molecular pathology and epidemiology.
INTEGRATED EDUCATIONAL PROGRAMS IN PUBLIC HEALTH AND MEDICAL SCHOOLS
Pathology and epidemiology are inherently complementary disciplines. Both fields encompass the entire spectrum of human diseases, generate hypotheses from observations, and attempt to elucidate disease etiologies. This shared scientific framework is the foundation of the field of MPE (4, 6) and should serve as the underpinning for integrated pathology and epidemiology educational programs.
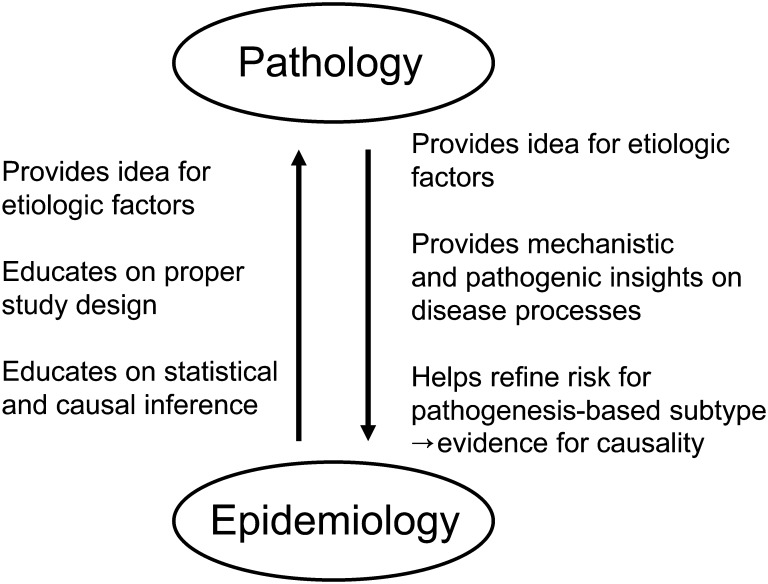
Eventually, there will be a universal collaborative relation between pathology and epidemiology (Figure 1), which will facilitate high-quality health science at the molecular, cellular, and population levels. Pathology is capable of providing detailed insights into pathogenic mechanisms and improving understanding of disease processes. In comparison, epidemiology can identify novel potential etiologic factors for pathologic processes. Pathologists also often contribute to early recognition of new exposure-disease associations, such as those among microbiota, inflammation, and cancers (77–86). Another crucial component of the discipline of epidemiology is expertise in study designs, statistical methods, and causal inference, all of which are of utmost importance in correlative clinicopathologic and translational research.
Figure 1.
Collaborative relation between pathology and epidemiology. Both pathology and epidemiology are method-based disciplines and fields of study covering the entire spectrum of human diseases. The methods of pathology and those of epidemiology can complement each other and can be synergized to create an integrated science: molecular pathological epidemiology. In the integrated interdisciplinary environment, pathologists and epidemiologists can help each other and benefit from educating each other as illustrated.
As an integrated discipline, MPE will draw on the knowledge base of pathology and epidemiology. A scientist with integrated MPE training would have the skills to consider pathogenic hypotheses, design and conduct studies, analyze data, make inferences, and validate/generalize findings in populations. This type of researcher can work well with other investigators in diverse disciplines and can “translate” between collaborators who do not share this scientific background.
For these reasons, it is desirable to establish integrated educational programs of pathology and epidemiology. In the current system, such educational opportunities will require the merger of resources held by medical schools, public health schools, and hospitals with pathology training programs. We acknowledge that dual-degree Doctor of Medicine/Master of Public Health programs exist, but they are not standardized and do not systematically offer training in epidemiology and biostatistics. We hope that institutions will adopt integrated educational programs across medical and public health schools and hospitals to meet these interdisciplinary research and educational needs.
SUBSTANTIAL ROLE OF FUNDING AGENCIES
Most biomedical and public health research projects are funded by governments or nongovernment organizations. Currently, relatively few funded projects integrate molecular pathology and epidemiology or population health science. There exists a significant knowledge gap between various etiologic factors and cellular and molecular changes that occur during disease evolution, and interdisciplinary investigations in these areas are needed. Funding agencies need to increase career development grants in order to nurture the next generation of scientists who can fully integrate the fields of molecular pathology and epidemiology.
CONCLUSIONS AND FUTURE DIRECTIONS
Over the last century, biomedical and public health sciences have been practiced in a highly compartmentalized way, typically missing the value of the perspectives gained through integration of divergent scientific fields. MPE (4–6) is an example of the integration of molecular biologic and population health science in order to gain insights into disease etiology and pathogenesis. MPE research stands to benefit both individuals and the population at large. To advance integrated MPE research, appropriate interdisciplinary educational programs are needed. This will require reforms in medical and public health education as well as postgraduate pathology training. We need to be open-minded and flexible in designing optimal education and training programs at various levels. We believe that convergence and integration of scientific disciplines should become more commonplace in the future, as MPE will prove to be a successful field.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Cancer Epidemiology Program, Dana-Farber/Harvard Cancer Center, Boston, Massachusetts (Shuji Ogino, Andrew H. Beck, Edward Giovannucci); Department of Pathology, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Shuji Ogino, Emily E. King, Danny A. Milner); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Shuji Ogino, Edward Giovannucci); Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts (Shuji Ogino); Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts (Andrew H. Beck); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Mark E. Sherman); Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, Massachusetts (Danny A. Milner); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Edward Giovannucci); and Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Edward Giovannucci).
This work was supported in part by National Institutes of Health grants (grant R01 CA151993 to Shuji Ogino, grant K23 AI072033 to Danny A. Milner, grant P01 CA87969 to Susan E. Hankinson, and grant P01 CA55075 to Walter C. Willett) and in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies did not have any role in the decision to submit the manuscript for publication or the writing of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Alberts B. Getting education right [editorial] Science. 2011;333(6045):919. doi: 10.1126/science.1212394. [DOI] [PubMed] [Google Scholar]
- 2.Sharp PA, Langer R. Research agenda. Promoting convergence in biomedical science. Science. 2011;333(6042):527. doi: 10.1126/science.1205008. [DOI] [PubMed] [Google Scholar]
- 3.Smith GD. Epidemiology, epigenetics and the ‘Gloomy Prospect’: embracing randomness in population health research and practice. Int J Epidemiol. 2011;40(3):537–562. doi: 10.1093/ije/dyr117. [DOI] [PubMed] [Google Scholar]
- 4.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102(6):365–367. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogino S, Galon J, Fuchs CS, et al. Cancer immunology—analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8(12):711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011; 60(3):397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wuchty S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science. 2007; 316(5827):1036–1039. doi: 10.1126/science.1136099. [DOI] [PubMed] [Google Scholar]
- 8.Rebbeck TR, Paskett E, Sellers TA. Fostering transdisciplinary science. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1149–1150. doi: 10.1158/1055-9965.EPI-10-0266. [DOI] [PubMed] [Google Scholar]
- 9.Stokols D, Hall KL, Taylor BK, et al. The science of team science: overview of the field and introduction to the supplement. Am J Prev Med. 2008;35(2 suppl):S77–S89. doi: 10.1016/j.amepre.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Sellers TA, Caporaso N, Lapidus S, et al. Opportunities and barriers in the age of team science: strategies for success. Cancer Causes Control. 2006;17(3):229–237. doi: 10.1007/s10552-005-0546-5. [DOI] [PubMed] [Google Scholar]
- 11.Sherman ME, Howatt W, Blows FM, et al. Molecular pathology in epidemiologic studies: a primer on key considerations. Cancer Epidemiol Biomarkers Prev. 2010;19(4):966–972. doi: 10.1158/1055-9965.EPI-10-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wade A. Fear or favour? Statistics in pathology. J Clin Pathol. 2000;53(1):16–18. doi: 10.1136/jcp.53.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. doi:10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McShane LM, Altman DG, Sauerbrei W. Identification of clinically useful cancer prognostic factors: what are we missing? J Natl Cancer Inst. 2005;97(14):1023–1025. doi: 10.1093/jnci/dji193. [DOI] [PubMed] [Google Scholar]
- 15.Kohane IS, Masys DR, Altman RB. The incidentalome: a threat to genomic medicine. JAMA. 2006;296(2):212–215. doi: 10.1001/jama.296.2.212. [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. doi:10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. doi:10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10(1):13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtin K, Slattery ML, Samowitz WS. CpG island methylation in colorectal cancer: past, present and future. Pathol Res Int. 2011;2011:902674. doi: 10.4061/2011/902674. doi:10.4061/2011/902674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes LA, Simons CC, van den Brandt PA, et al. Body size, physical activity and risk of colorectal cancer with or without the CpG island methylator phenotype (CIMP) PLoS One. 2011;6(4):e18571. doi: 10.1371/journal.pone.0018571. doi:10.1371/journal.pone.0018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyle T, Fritschi L, Heyworth J, et al. Long-term sedentary work and the risk of subsite-specific colorectal cancer. Am J Epidemiol. 2011;173(10):1183–1191. doi: 10.1093/aje/kwq513. [DOI] [PubMed] [Google Scholar]
- 22.Kelley RK, Wang G, Venook AP. Biomarker use in colorectal cancer therapy. J Natl Compr Canc Netw. 2011; 9(11):1293–1302. doi: 10.6004/jnccn.2011.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen D, Song S, Lu J, et al. Functional variants of –1318T > G and –673C > T in c-Jun promoter region associated with increased colorectal cancer risk by elevating promoter activity. Carcinogenesis. 2011;32(7):1043–1049. doi: 10.1093/carcin/bgr047. [DOI] [PubMed] [Google Scholar]
- 24.Campbell PT, Newton CC, Dehal AN, et al. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30(1):42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 25.Boyle T, Heyworth J, Bull F, et al. Timing and intensity of recreational physical activity and the risk of subsite-specific colorectal cancer. Cancer Causes Control. 2011;22(12): 1647–1658. doi: 10.1007/s10552-011-9841-5. [DOI] [PubMed] [Google Scholar]
- 26.Gehoff A, Basten O, Sprenger T, et al. Optimal lymph node harvest in rectal cancer (UICC stages II and III) after preoperative 5-FU-based radiochemotherapy. Acetone compression is a new and highly efficient method. Am J Surg Pathol. 2012;36(2):202–213. doi: 10.1097/PAS.0b013e31823fa35b. [DOI] [PubMed] [Google Scholar]
- 27.Hughes LA, Khalid-de Bakker CA, Smits KM, et al. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochim Biophys Acta. 2012; 1825(1):77–85. doi: 10.1016/j.bbcan.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Iwagami S, Baba Y, Watanabe M, et al. Pyrosequencing assay to measure LINE-1 methylation level in esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19(8):2726–2732. doi: 10.1245/s10434-011-2176-3. [DOI] [PubMed] [Google Scholar]
- 29.Esteban S, Moya P, Fernandez-Suarez A, et al. Diagnostic and prognostic utility of methylation and protein expression patterns of myopodin in colon cancer. Tumour Biol. 2012; 33(2):337–346. doi: 10.1007/s13277-012-0320-8. [DOI] [PubMed] [Google Scholar]
- 30.Begg CB. A strategy for distinguishing optimal cancer subtypes. Int J Cancer. 2011;129(4):931–937. doi: 10.1002/ijc.25714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Taylor NP, Sotamaa KM, et al. Evidence for heritable predisposition to epigenetic silencing of MLH1. Int J Cancer. 2007;120(8):1684–1688. doi: 10.1002/ijc.22406. [DOI] [PubMed] [Google Scholar]
- 32.Raptis S, Mrkonjic M, Green RC, et al. MLH1 –93G>A promoter polymorphism and the risk of microsatellite-unstable colorectal cancer. J Natl Cancer Inst. 2007; 99(6):463–474. doi: 10.1093/jnci/djk095. [DOI] [PubMed] [Google Scholar]
- 33.Samowitz WS, Curtin K, Wolff RK, et al. The MLH1 –93 G>A promoter polymorphism and genetic and epigenetic alterations in colon cancer. Genes Chromosomes Cancer. 2008;47(10):835–844. doi: 10.1002/gcc.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allan JM, Shorto J, Adlard J, et al. MLH1 –93G>A promoter polymorphism and risk of mismatch repair deficient colorectal cancer. Int J Cancer. 2008;123(10):2456–2459. doi: 10.1002/ijc.23770. [DOI] [PubMed] [Google Scholar]
- 35.Campbell PT, Curtin K, Ulrich CM, et al. Mismatch repair polymorphisms and risk of colon cancer, tumour microsatellite instability and interactions with lifestyle factors. Gut. 2009;58(5):661–667. doi: 10.1136/gut.2007.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyama K, Kawakami K, Maeda K, et al. The association between methylenetetrahydrofolate reductase polymorphism and promoter methylation in proximal colon cancer. Anticancer Res. 2004;24(2B):649–654. [PubMed] [Google Scholar]
- 37.Curtin K, Slattery ML, Ulrich CM, et al. Genetic polymorphisms in one-carbon metabolism: associations with CpG island methylator phenotype (CIMP) in colon cancer and the modifying effects of diet. Carcinogenesis. 2007; 28(8):1672–1679. doi: 10.1093/carcin/bgm089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen LH, Lindebjerg J, Cruger DG, et al. Microsatellite instability and the association with plasma homocysteine and thymidylate synthase in colorectal cancer. Cancer Invest. 2008;26(6):583–589. doi: 10.1080/07357900801970992. [DOI] [PubMed] [Google Scholar]
- 39.de Vogel S, Wouters KA, Gottschalk RW, et al. Genetic variants of methyl metabolizing enzymes and epigenetic regulators: associations with promoter CpG island hypermethylation in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3086–3096. doi: 10.1158/1055-9965.EPI-09-0289. [DOI] [PubMed] [Google Scholar]
- 40.Schernhammer ES, Giovannucci E, Kawasaki T, et al. Dietary folate, alcohol, and B vitamins in relation to LINE-1 hypomethylation in colon cancer. Gut. 2010;59(6):794–799. doi: 10.1136/gut.2009.183707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazra A, Fuchs CS, Kawasaki T, et al. Germline polymorphisms in the one-carbon metabolism pathway and DNA methylation in colorectal cancer. Cancer Causes Control. 2010;21(3):331–345. doi: 10.1007/s10552-009-9464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Guelpen B, Dahlin AM, Hultdin J, et al. One-carbon metabolism and CpG island methylator phenotype status in incident colorectal cancer: a nested case-referent study. Cancer Causes Control. 2010;21(4):557–566. doi: 10.1007/s10552-009-9484-y. [DOI] [PubMed] [Google Scholar]
- 43.Curtin K, Samowitz WS, Ulrich CM, et al. Nutrients in folate-mediated, one-carbon metabolism and the risk of rectal tumors in men and women. Nutr Cancer. 2011;63(3): 357–366. doi: 10.1080/01635581.2011.535965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogino S, Hazra A, Tranah GJ, et al. MGMT germline polymorphism is associated with somatic MGMT promoter methylation and gene silencing in colorectal cancer. Carcinogenesis. 2007;28(9):1985–1990. doi: 10.1093/carcin/bgm160. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins NJ, Lee JH, Wong JJ, et al. MGMT methylation is associated primarily with the germline C>T SNP (rs16906252) in colorectal cancer and normal colonic mucosa. Mod Pathol. 2009;22(12):1588–1599. doi: 10.1038/modpathol.2009.130. [DOI] [PubMed] [Google Scholar]
- 46.Candiloro IL, Dobrovic A. Detection of MGMT promoter methylation in normal individuals is strongly associated with the T allele of the rs16906252 MGMT promoter single nucleotide polymorphism. Cancer Prev Res (Phila Pa) 2009;2(10):862–867. doi: 10.1158/1940-6207.CAPR-09-0056. [DOI] [PubMed] [Google Scholar]
- 47.Leng S, Bernauer AM, Hong C, et al. The A/G allele of Rs16906252 predicts for MGMT methylation and is selectively silenced in premalignant lesions from smokers and in lung adenocarcinomas. Clin Cancer Res. 2011;17(7): 2014–2023. doi: 10.1158/1078-0432.CCR-10-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristensen LS, Nielsen HM, Hager H, et al. Methylation of MGMT in malignant pleural mesothelioma occurs in a subset of patients and is associated with the T allele of the rs16906252 MGMT promoter SNP. Lung Cancer. 2011; 71(2):130–136. doi: 10.1016/j.lungcan.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Pedroni M, Tamassia MG, Percesepe A, et al. Microsatellite instability in multiple colorectal tumors. Int J Cancer. 1999;81(1):1–5. doi: 10.1002/(sici)1097-0215(19990331)81:1<1::aid-ijc1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 50.Velayos FS, Lee SH, Qiu H, et al. The mechanism of microsatellite instability is different in synchronous and metachronous colorectal cancer. J Gastrointest Surg. 2005;9(3):329–335. doi: 10.1016/j.gassur.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Nosho K, Kure S, Irahara N, et al. A prospective cohort study shows unique epigenetic, genetic, and prognostic features of synchronous colorectal cancers. Gastroenterology. 2009; 137(5):1609–1620. doi: 10.1053/j.gastro.2009.08.002. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konishi K, Shen L, Jelinek J, et al. Concordant DNA methylation in synchronous colorectal carcinomas. Cancer Prev Res (Phila Pa) 2009;2(9):814–822. doi: 10.1158/1940-6207.CAPR-09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalo V, Lozano JJ, Muñoz J, et al. Aberrant gene promoter methylation associated with sporadic multiple colorectal cancer. PLoS One. 2010;5(1):e8777. doi: 10.1371/journal.pone.0008777. doi:10.1371/journal.pone.0008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slattery ML, Curtin K, Anderson K, et al. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92(22):1831–1836. doi: 10.1093/jnci/92.22.1831. [DOI] [PubMed] [Google Scholar]
- 55.Satia JA, Keku T, Galanko JA, et al. Diet, lifestyle, and genomic instability in the North Carolina colon cancer study. Cancer Epidemiol Biomarkers Prev. 2005;14(2): 429–436. doi: 10.1158/1055-9965.EPI-04-0486. [DOI] [PubMed] [Google Scholar]
- 56.Slattery ML, Curtin K, Sweeney C, et al. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer. 2007;120(3): 656–663. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
- 57.Campbell PT, Jacobs ET, Ulrich CM, et al. Case–control study of overweight, obesity, and colorectal cancer risk, overall and by tumor microsatellite instability status. J Natl Cancer Inst. 2010;102(6):391–400. doi: 10.1093/jnci/djq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sinicrope FA, Foster NR, Sargent DJ, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16(6):1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuchiba A, Morikawa T, Yamauchi M, et al. Body mass index and risk of colorectal cancer according to fatty acid synthase expression in the Nurses' Health Study. J Natl Cancer Inst. 2012;104(5):415–420. doi: 10.1093/jnci/djr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu AH, Shibata D, Yu MC, et al. Dietary heterocyclic amines and microsatellite instability in colon adenocarcinomas. Carcinogenesis. 2001;22(10):1681–1684. doi: 10.1093/carcin/22.10.1681. [DOI] [PubMed] [Google Scholar]
- 61.Lüchtenborg M, Weijenberg MP, Kampman E, et al. Cigarette smoking and colorectal cancer: APC mutations, hMLH1 expression, and GSTM1 and GSTT1 polymorphisms. Am J Epidemiol. 2005;161(9):806–815. doi: 10.1093/aje/kwi114. [DOI] [PubMed] [Google Scholar]
- 62.Chia VM, Newcomb PA, Bigler J, et al. Risk of microsatellite-unstable colorectal cancer is associated jointly with smoking and nonsteroidal anti-inflammatory drug use. Cancer Res. 2006;66(13):6877–6883. doi: 10.1158/0008-5472.CAN-06-1535. [DOI] [PubMed] [Google Scholar]
- 63.Samowitz WS, Albertsen H, Sweeney C, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98(23):1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 64.Poynter JN, Haile RW, Siegmund KD, et al. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev. 2009; 18(10):2745–2750. doi: 10.1158/1055-9965.EPI-09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rozek LS, Herron CM, Greenson JK, et al. Smoking, gender, and ethnicity predict somatic BRAF mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010; 19(3):838–843. doi: 10.1158/1055-9965.EPI-09-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102(14):1012–1022. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogino S, Nosho K, Meyerhardt JA, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26(35):5713–5720. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogino S, Nosho K, Shima K, et al. p21 expression in colon cancer and modifying effects of patient age and body mass index on prognosis. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2513–2521. doi: 10.1158/1055-9965.EPI-09-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogino S, Shima K, Nosho K, et al. A cohort study of p27 localization in colon cancer, body mass index, and patient survival. Cancer Epidemiol Biomarkers Prev. 2009;18(6): 1849–1858. doi: 10.1158/1055-9965.EPI-09-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ogino S, Nosho K, Baba Y, et al. A cohort study of STMN1 expression in colorectal cancer: body mass index and prognosis. Am J Gastroenterol. 2009;104(8): 2047–2056. doi: 10.1038/ajg.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyerhardt JA, Ogino S, Kirkner GJ, et al. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15(18):5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morikawa T, Kuchiba A, Yamauchi M, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305(16):1685–1694. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morikawa T, Kuchiba A, Liao X, et al. Tumor TP53 expression status, body mass index and prognosis in colorectal cancer. Int J Cancer. 2012;131(5):1169–1178. doi: 10.1002/ijc.26495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebrahim S, Clarke M. STROBE: new standards for reporting observational epidemiology, a chance to improve. Int J Epidemiol. 2007;36(5):946–948. doi: 10.1093/ije/dym185. [DOI] [PubMed] [Google Scholar]
- 76.Gallo V, Egger M, McCormack V, et al. STrengthening the Reporting of OBservational studies in Epidemiology—Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement. PLoS Med. 2011;8(10):e1001117. doi: 10.1371/journal.pmed.1001117. doi:10.1371/journal.pmed.1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein RA. Epigenetics—the link between infectious diseases and cancer. JAMA. 2011;305(14):1484–1485. doi: 10.1001/jama.2011.446. [DOI] [PubMed] [Google Scholar]
- 78.Nosho K, Shima K, Kure S, et al. JC virus T-antigen in colorectal cancer is associated with p53 expression and chromosomal instability, independent of CpG island methylator phenotype. Neoplasia. 2009;11(1):87–95. doi: 10.1593/neo.81188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karpinski P, Myszka A, Ramsey D, et al. Detection of viral DNA sequences in sporadic colorectal cancers in relation to CpG island methylation and methylator phenotype. Tumour Biol. 2011;32(4):653–659. doi: 10.1007/s13277-011-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61(6): 847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61(6): 794–797. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baba Y, Nosho K, Shima K, et al. PTGER2 overexpression in colorectal cancer is associated with microsatellite instability, independent of CpG island methylator phenotype. Cancer Epidemiol Biomarkers Prev. 2010;19(3):822–831. doi: 10.1158/1055-9965.EPI-09-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang D, Xia D, DuBois RN. The crosstalk of PTGS2 and EGF signaling pathways in colorectal cancer. Cancers. 2011;3(4):3894–3908. doi: 10.3390/cancers3043894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia D, Wang D, Kim SH, et al. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.