Abstract
The Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) was initiated in 2004 to investigate the relation between individual-level estimates of long-term air pollution exposure and the progression of subclinical atherosclerosis and the incidence of cardiovascular disease (CVD). MESA Air builds on a multicenter, community-based US study of CVD, supplementing that study with additional participants, outcome measurements, and state-of-the-art air pollution exposure assessments of fine particulate matter, oxides of nitrogen, and black carbon. More than 7,000 participants aged 45–84 years are being followed for over 10 years for the identification and characterization of CVD events, including acute myocardial infarction and other coronary artery disease, stroke, peripheral artery disease, and congestive heart failure; cardiac procedures; and mortality. Subcohorts undergo baseline and follow-up measurements of coronary artery calcium using computed tomography and carotid artery intima-medial wall thickness using ultrasonography. This cohort provides vast exposure heterogeneity in ranges currently experienced and permitted in most developed nations, and the air monitoring and modeling methods employed will provide individual estimates of exposure that incorporate residence-specific infiltration characteristics and participant-specific time-activity patterns. The overarching study aim is to understand and reduce uncertainty in health effect estimation regarding long-term exposure to air pollution and CVD.
Keywords: air pollution, atherosclerosis, cardiovascular diseases, environmental exposure, epidemiologic methods, particulate matter
Current scientific evidence can be interpreted as consistent with a causal relation between long-term exposure to ambient air pollutants and cardiovascular disease (CVD) (1). Investigators in several cohort studies have reported associations between long-term exposure to fine particulate matter air pollution (particulate matter less than 2.5 µm in diameter (PM2.5)) and risk of cardiovascular mortality, coronary heart disease events, and stroke (2–9).
Ambient PM2.5 may lead to increased CVD risk through development of atherosclerosis (10). Cross-sectional studies of the extent of atherosclerosis and air pollution exposure are somewhat mixed, though most have shown a positive association (11–15). One recent study of atherosclerosis risk found a positive association between ambient air pollution levels and increasing common carotid intima-medial wall thickness (IMT) (16); however, this study was limited by a small sample size, lack of population-based sampling, and short follow-up. Research on long-term exposure to particulate air pollution and atherosclerotic disease in humans has been limited by measurement error and potential misclassification in exposure assessment, as no exposure monitoring has been done in these studies to enhance the participant-specific characterization of fine-scale spatial differences in concentrations. Further, prior studies have not considered estimates of pollution infiltration into participants' residences or individual patterns of time-activity. These studies were also limited by cross-sectional designs and were based on projects not designed to study both CVD and air pollution exposures.
Understanding the relation between exposure to ambient (i.e., outdoor) particulate matter and development of CVD is a high priority for the Environmental Protection Agency (EPA). Recently, the EPA challenged researchers to implement a new study specifically to reduce uncertainty in estimating the concentration-response function for the relation between air pollution exposure and atherosclerosis (17). The result was the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air), which leverages data from the National Heart, Lung, and Blood Institute's Multi-Ethnic Study of Atherosclerosis (MESA).
MESA Air uses this unusual dedication of resources to combine state-of-the-art cardiovascular epidemiology (employing validated measures of subclinical atherosclerosis) with state-of-the-art exposure estimation based on cohort-specific air pollution monitoring and modeling. MESA Air prospectively assesses the relation between individual-level estimates of long-term air pollution exposure and both the progression of subclinical atherosclerosis and the incidence of CVD over a 10-year period. Recognizing the importance of characterizing fine-scale variation in pollutant concentrations within metropolitan areas, MESA Air assigns an individual-level temporally resolved estimate of ambient pollution exposure to each study participant. These estimates incorporate exposure variation due to participant-specific differences in ambient concentrations, pollutant infiltration into residences, and time-activity patterns.
MATERIALS AND METHODS
Study population
Most participants were recruited into MESA Air from the parent MESA study; others were recruited from another large MESA ancillary study (MESA Family). Additional participants were recruited specifically for MESA Air. The parent MESA study was initiated in 1999 to investigate the prevalence, correlates, and progression of subclinical CVD in a multicity, multiethnic, population-based cohort (18). MESA included 6,814 participants from 6 US communities: Baltimore City and County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota. Participants were aged 45–84 years at enrollment, with an approximately equal gender ratio, and were free of recognized CVD at baseline. Four ethnic/racial groups were targeted for inclusion, and the recruitment protocol required overlapping ethnic groups among communities. The MESA cohort is 39% white, 28% African-American, 22% Hispanic, and 12% Chinese-American.
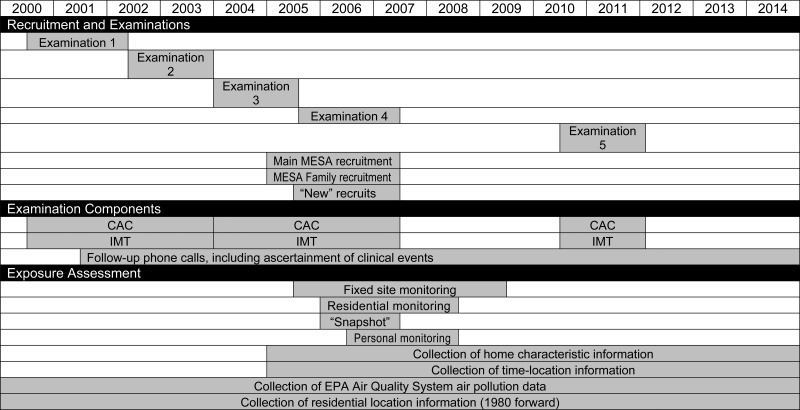
Baseline measurements (July 2000–July 2002; examination 1) for participants in the parent MESA cohort included coronary artery calcium (CAC); flow-mediated brachial artery dilation, carotid IMT, magnetic resonance imaging, and electrocardiography; standard CVD risk factors; sociodemographic factors; lifestyle habits; and psychosocial factors. Subsequent examinations, which included repetitions of certain baseline measurements as well as new measures, were scheduled at approximately adjacent 2-year intervals (Figure 1). The fifth MESA examination began in April 2010 and ended in February 2012. During the end of the third examination and throughout the fourth examination, MESA participants were approached for enrollment into MESA Air; 93% agreed to participate (Table 1).
Figure 1.
Proposed timeline for completion of the primary components of the Multi-Ethnic Study of Atherosclerosis and Air Pollution, 2004–2014. (CAC, coronary artery calcium; EPA, Environmental Protection Agency; IMT, intima-medial thickness; MESA, Multi-Ethnic Study of Atherosclerosis).
Table 1.
Initiala Enrollment in the Multi-Ethnic Study of Atherosclerosis and Air Pollution During MESA Examinations 3 and 4 (2004–2007), by Recruitment Source
| MESA Study Center | MESA |
MESA Family Study |
New Recruits |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| No. of Participants | % of Those Eligible | No. of Participants | % of Those Eligible | No. of Participants | % of Goal | No. of Participants | % of Those Eligible | |
| Baltimore, Maryland | 787 | 88 | 50 | 52 | 837 | 85 | ||
| Chicago, Illinois | 992 | 95 | 146 | 76 | 1,138 | 92 | ||
| Los Angeles, California | 1,014 | 97 | 19 | 86 | 157 | 79 | 1,190 | 94 |
| New York, New York | 963 | 98 | 145 | 95 | 100 | 100 | 1,208 | 98 |
| St. Paul, Minnesota | 876 | 95 | 86 | 81 | 962 | 93 | ||
| Forsyth County, North Carolina | 846 | 87 | 45 | 71 | 891 | 86 | ||
| Total | 5,478 | 93 | 491 | 78 | 257 | 86 | 6,226 | 91 |
Abbreviation: MESA, Multi-Ethnic Study of Atherosclerosis.
a Additional enrollment took place during MESA examination 5 (2010–2012).
The second recruitment source was the ancillary MESA Family study, which investigates genetic aspects of subclinical CVD in African-American and Hispanic-American participants, most of whom are siblings of MESA study participants but some of whom were recruited as de novo sibling pairs. MESA Family recruitment was coincident with the beginning of MESA Air, and these participants underwent the same assessments of subclinical CVD as the main MESA cohort members. The addition of MESA Family participants into MESA Air was designed to increase the size of the cohort for follow-up of clinical events. Further, while MESA Family was originally designed to be cross-sectional, MESA Family participants recruited into the MESA Air cohort are now being followed through 2014 for clinical events. MESA Family participants who were CVD-free at the time of enrollment, lived within 100 miles (160 km) of the MESA clinics, and were not planning to move within 5 years were approached; 84% of those eligible agreed to participate (see Table 1).
Third, to capitalize on exposure heterogeneity in the vicinity of 2 existing MESA population communities, new participants were also recruited from 2 areas in the Los Angeles Basin (coastal Los Angeles County and Riverside County, California) and 1 area in the New York City region (Rockland County, New York). Coastal Los Angeles represents an upwind location relative to the city center and was selected to represent lower PM2.5 concentrations within the basin. Riverside County represents a downwind location relative to the urban center and an area with high pollutant concentrations. Rockland County is upwind of New York City, reflecting regional scale pollution similar to that of northern Manhattan and southern Bronx without the urban contribution. Table 2 shows the wide range of exposure characteristics in study communities.
Table 2.
Relative Air Pollution Exposure Characteristics of Study Communities at the Time of Recruitment, Multi-Ethnic Study of Atherosclerosis and Air Pollution, 2005–2007
| Los Angeles, California | Coastal Los Angeles County, Californiaa | Riverside County, Californiaa | St. Paul, Minnesota | Chicago, Illinois | Manhattan/ The Bronx, New York City | Rockland County, New Yorka | Baltimore, Maryland | Forsyth County, North Carolina | |
|---|---|---|---|---|---|---|---|---|---|
| PM2.5b | High | Medium | Very high | Low | Medium | Medium | Low | Medium | Medium |
| PM10 | High | Medium | Very high | Medium | High | High | Medium | Medium | Low |
| Carbon monoxide | High | Medium | High | Medium | Medium | High | Low | Low | Medium |
| Nitrogen dioxide | High | High | Very high | Low | Medium | High | Low | Low | Low |
| Ozone | High | Medium | Very high | Low | Medium | Medium | Medium | Medium | Very high |
| Sulfur dioxide | Low | Low | Low | Medium | Medium | High | High | High | Medium |
| Urban contribution | +c | + | + | −d | + | + | − | − | − |
| Long-range transport | − | − | − | + | + | + | + | + | + |
Abbreviations: PM2.5, particulate matter less than 2.5 µm in diameter; PM10, particulate matter less than 10 µm in diameter.
a Area of new recruitment.
b For PM2.5, low ≈ 12 µg/m3, medium ≈ 16 µg/m3, high ≈ 20 µg/m3, and very high ≈ 24 µg/m3 (annual averages).
c A plus sign (+) indicates an expected influence from either an urban contribution or long-range transport.
d A minus sign (–) indicates that this influence is not expected.
Like MESA, new recruitment into MESA Air was community-based, with an emphasis on balancing recruitment across census blocks, ethnicity, and gender. The sampling frame contained information based on the 2000 US Census and proceeded along geographic boundaries. The sampling unit was the household, with a goal of including no more than 2 eligible participants from different households per census block. Initial contact was made by telephone or door-to-door. Telephone recruitment was based on a list built from the specified geographic boundaries. For door-to-door recruitment, geographically eligible census blocks were identified, and interviewers were provided with hybrid satellite and street maps of eligible areas overlaid with census block boundaries. Initial contact included a screening questionnaire and household enumeration of all eligible persons. The recruitment goal was 100 participants from each of the 3 areas of new recruitment.
Table 3 summarizes participant characteristics at the time of their baseline examination (2000–2002 for the main MESA participants and 2005–2007 for participants from other sources). Newly recruited participants were selected to have the same concurrent age range as the main MESA participants, so they appear older when data are summarized by recruitment date; this also explains the somewhat higher extent of subclinical atherosclerosis among these persons. Participants recruited from the MESA Family study were younger than other participants, explaining their lower baseline extent of subclinical atherosclerosis.
Table 3.
Characteristics of MESA Participants, MESA Family Participants, and MESA Air New Recruits at the Time of Recruitmenta
| Recruitment Source |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MESA (n = 6,814) |
MESA Family Study (n = 491) |
New Recruits (n = 257) |
Total (n = 7,562) |
|||||||||
| No. | % | Median (SD) | No. | % | Median (SD) | No. | % | Median (SD) | No. | % | Median (SD) | |
| Gender | ||||||||||||
| Female | 3,601 | 53 | 295 | 60 | 140 | 54 | 4,036 | 53 | ||||
| Male | 3,213 | 47 | 196 | 40 | 117 | 46 | 3,526 | 47 | ||||
| Race/ethnicity | ||||||||||||
| White | 2,622 | 38 | 0 | 0 | 163 | 63 | 2,785 | 37 | ||||
| African-American | 1,893 | 28 | 241 | 49 | 41 | 16 | 2,175 | 29 | ||||
| Hispanic | 1,496 | 22 | 250 | 51 | 51 | 20 | 1,797 | 24 | ||||
| Chinese | 803 | 12 | 0 | 0 | 2 | 1 | 805 | 11 | ||||
| Age, yearsb | ||||||||||||
| ≤59 | 2,915 | 43 | 328 | 67 | 88 | 34 | 3,331 | 44 | ||||
| 60–69 | 2,075 | 30 | 123 | 25 | 112 | 43 | 2,310 | 31 | ||||
| 70–79 | 1,536 | 23 | 34 | 7 | 41 | 16 | 1,611 | 21 | ||||
| ≥80 | 288 | 4 | 6 | 1 | 16 | 7 | 310 | 4 | ||||
| Socioeconomic status | ||||||||||||
| High school education or more | 5,566 | 82 | 363 | 74 | 225 | 88 | 6,154 | 81 | ||||
| Currently married | 4,119 | 61 | 254 | 52 | 139 | 54 | 4,512 | 60 | ||||
| Family income ≥$30,000/yearc | 4,090 | 63 | 279 | 58 | 165 | 65 | 4,534 | 62 | ||||
| Smoking behavior | ||||||||||||
| Nonsmoker | 3,418 | 50 | 266 | 54 | 120 | 47 | 3,804 | 50 | ||||
| Former smoker | 2,487 | 36 | 139 | 28 | 120 | 47 | 2,746 | 36 | ||||
| Current smoker | 887 | 13 | 85 | 17 | 17 | 7 | 989 | 13 | ||||
| Health status | ||||||||||||
| Body mass indexd | 27.6 (5.5) | 28.7 (6.4) | 28.1 (6.2) | 27.7 (5.6) | ||||||||
| Diabetes mellituse | 859 | 13 | 84 | 17 | 46 | 18 | 989 | 13 | ||||
| Hypertensionf | 3,058 | 45 | 224 | 46 | 105 | 41 | 3,387 | 45 | ||||
| Seated systolic blood pressure, mm Hg | 124 (21) | 121 (19) | 119 (20) | 123 (21) | ||||||||
| Seated diastolic blood pressure, mm Hg | 72 (10) | 73 (11) | 68 (10) | 72 (10) | ||||||||
| Subclinical atherosclerosis | ||||||||||||
| Agatston score >0 | 3,398 | 50 | 175 | 36 | 159 | 62 | 3,732 | 49 | ||||
| Agatston score, Hounsfield unitsg | 88 (553) | 52 (406) | 89 (528) | 85 (546) | ||||||||
| Mean right common carotid far wall intima-medial thickness, mm | 0.70 (0.23) | 0.67 (0.17) | 0.77 (0.17) | 0.70 (0.23) | ||||||||
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; MESA Air, Multi-Ethnic Study of Atherosclerosis and Air Pollution; SD, standard deviation.
a MESA recruitment occurred during examination 1 from 2000 to 2002, while recruitment of new participants into MESA Air occurred between 2005 and 2007. Recruitment into MESA Family occurred between 2004 and 2006.
b New participants recruited into MESA Air were matched to the age distribution of the MESA cohort and thus will appear older in this table because of the later date that recruitment represents.
c Some participants (n = 283) did not provide data on family income.
d Weight (kg)/height (m)2.
e Defined either as treated diabetes or fasting glucose concentration >125 mg/dL.
f Based on the 1997 criteria of the Joint National Committee on Prevention, Detection, and Treatment of High Blood Pressure (50).
g Restricted to nonzero scores (n = 3,732).
Table 4 shows characteristics, by PM2.5 exposure quintile, of the subset of participants (7,014 of 7,562) who explicitly consented to the use of their address data (and thus to generation of exposure estimates at their homes) either in the ancillary MESA Neighborhood study (19) or as part of MESA Air recruitment. MESA participants continue to be offered the opportunity to consent to the use of their address data. The exposure prediction approach is described below in the “Exposure assessment” section.
Table 4.
Characteristics of Participantsa at the Time of Recruitment into the MESA Air Study (2005–2007), by Quintile of Predicted Residential Outdoor Annual Average PM2.5 Level in 2007
| Quintile of Exposure, μg/m3 of PM2.5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8.76–12.64 |
12.65–13.68 |
13.69–14.45 |
14.46–15.35 |
15.36–20.78 |
||||||
| % | Median (SD) | % | Median (SD) | % | Median (SD) | % | Median (SD) | % | Median (SD) | |
| Gender | ||||||||||
| Male | 49 | 47 | 45 | 44 | 46 | |||||
| Female | 51 | 53 | 55 | 56 | 54 | |||||
| Race/ethnicity | ||||||||||
| White | 53 | 44 | 34 | 31 | 23 | |||||
| African-American | 7 | 37 | 42 | 46 | 11 | |||||
| Hispanic | 38 | 13 | 18 | 18 | 32 | |||||
| Chinese | 2 | 6 | 6 | 6 | 33 | |||||
| Age, yearsb | ||||||||||
| ≤59 | 37 | 31 | 31 | 30 | 27 | |||||
| 60–69 | 31 | 29 | 30 | 27 | 28 | |||||
| 70–79 | 24 | 29 | 27 | 28 | 32 | |||||
| ≥80 | 9 | 11 | 11 | 15 | 14 | |||||
| Socioeconomic status | ||||||||||
| High school education or more | 85 | 89 | 85 | 84 | 70 | |||||
| Currently married | 62 | 66 | 62 | 51 | 64 | |||||
| Family income ≥$30,000/year | 68 | 75 | 66 | 63 | 46 | |||||
| Smoking behavior | ||||||||||
| Nonsmoker | 41 | 48 | 46 | 44 | 53 | |||||
| Former smoker | 47 | 43 | 43 | 45 | 39 | |||||
| Current smoker | 12 | 9 | 11 | 11 | 8 | |||||
| Health status | ||||||||||
| Body mass indexc | 28.5 (5.7) | 27.9 (5.6) | 27.9 (5.7) | 27.7 (5.7) | 26.4 (5.7) | |||||
| Diabetes mellitusd | 15 | 18 | 17 | 18 | 19 | |||||
| Hypertensione | 44 | 54 | 55 | 56 | 52 | |||||
| Seated systolic blood pressure, mm Hg | 119 (21) | 120 (20) | 122 (20) | 121 (20) | 123 (22) | |||||
| Seated diastolic blood pressure, mm Hg | 69 (10) | 70 (10) | 70 (10) | 70 (10) | 69 (10) | |||||
| Subclinical atherosclerosis | ||||||||||
| Agatston score >0 | 60 | 55 | 56 | 56 | 60 | |||||
| Agatston score, Hounsfield unitsf | 118 (663) | 110 (765) | 111 (656) | 106 (658) | 88 (510) | |||||
| Mean right common carotid far wall intima-medial thickness, mm | 0.67 (0.22) | 0.68 (0.18) | 0.69 (0.21) | 0.71 (0.19) | 0.68 (0.22) | |||||
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; MESA Air, Multi-Ethnic Study of Atherosclerosis and Air Pollution; PM2.5, particulate matter less than 2.5 µm in diameter; SD, standard deviation.
a Both the MESA Air and MESA Neighborhood studies acquired consent for use of residential address data. This table represents those participants who consented to the use of their addresses through one of these studies and had complete residential history data for the year 2007. (Address history for the year 2007 was used to classify exposure groups for this table; however, the study has complete residential history data from 1980 onward.) The consent process is ongoing; additional participants were added at MESA examination 5 (2010–2012).
b Age is normalized to 2007.
c Weight (kg)/height (m)2.
d Defined either as treated diabetes or fasting glucose concentration >125 mg/dL.
e Based on the 1997 criteria of the Joint National Committee on Prevention, Detection, and Treatment of High Blood Pressure (50).
f Restricted to nonzero scores (n = 2,623).
Racial/ethnic distributions differed across exposure categories, reflecting an element of the initial MESA study design: Not all field centers recruited participants from all ethnic groups. For example, Chinese participants were only recruited in Chicago and Los Angeles, the cities with the highest PM2.5 exposure. Lower educational attainment and income were associated with higher exposures, potentially reflecting correlated residential characteristics. Gender distribution, marital status, and median blood pressure did not differ by exposure quintile.
Outcome assessment
MESA Air is a prospective study, with a follow-up period for planned analysis of approximately 10 years for most cohort members and 5 years for participants from MESA Family and participants newly recruited for MESA Air. MESA Air staff contact each participant every 9–12 months to detect changes in health status, hospitalizations, nursing home admissions, or newly diagnosed CVD events. They document possible events through review and abstraction of medical records, death certificates, autopsy reports, interviews with participants, and, for out-of-hospital deaths, interviews with or questionnaires administered to physicians, relatives, or friends. At least 2 physicians review case materials for each event, and data for myocardial infarction events are analyzed by computer algorithm using standardized criteria to assign a final classification. Event endpoints include acute myocardial infarction, angina, congestive heart failure, peripheral artery disease, and stroke or transient ischemic attack. Silent myocardial infarctions are identified on the basis of electrocardiogram results (18).
For subclinical outcomes, we selected a random sample of 50% of the main MESA cohort members and all new recruits (n ≈ 3,600) to receive follow-up CAC and IMT measurements during examination 5. All of these MESA participants had undergone baseline CAC and carotid IMT measurements during examination 1, while new recruits to MESA Air underwent these measurements in examination 4. CAC measures were obtained by either electron beam computed tomography or multidetector computed tomography and were read at a central reading center. IMT measurements in MESA Air are derived from high-resolution B-mode ultrasound using Logiq 700 ultrasound machines (General Electric Medical Systems, Waukesha, Wisconsin) imaging the right and left common carotid and internal carotid arteries. Semiautomated image analysis performed at the central reading center provides data on wall thickness for the common and internal carotid arteries and the presence and characteristics of atherosclerotic plaque.
In addition to CVD outcome data, the MESA study and its ancillary projects allow access to a rich set of data on environmental covariates and phenotypic information (Table 5). The MESA Occupation study (20) provides occupational codes for participants and linkage to the physical, chemical, and psychological characteristics of their work. The MESA Neighborhood study (19) provides residential history information from 1980 forward and neighborhood-level characteristics such as safety, walkability, food availability, and land use. Other available measures include cardiac magnetic resonance imaging (21), retinal photography (MESA Eye (22)), spirometry and quantitative measures of emphysema on partial and full lung computed tomography scans (MESA Lung (23–25)), and genetic data, including genome-wide single nucleotide polymorphism assessment, information on DNA methylation, and gene expression (MESA SHARe (Single-Nucleotide Polymorphism Health Association Resource) (26) and MESA Epigenomics).
Table 5.
Selected Environmental Covariates and Phenotypic Data Available Through the Multi-Ethnic Study of Atherosclerosis and Its Ancillary Studies
| Source Study (Reference No.) | Available Data |
|---|---|
| MESA (18) | Demographic factors (education, income, smoking history, alcohol intake) |
| Anthropometric factors | |
| Diet | |
| Physical activity | |
| Medications | |
| Psychosocial data (anxiety, depression, social support) | |
| MESA Neighborhood (19) | Residential history |
| Neighborhood characteristics | |
| MESA Occupation (20) | Census occupation code |
| MESA Lung (23–25) | Secondhand tobacco smoke (urinary cotinine levels) |
| Occupational exposure to gases, vapors, fumes, and tobacco smoke |
Abbreviation: MESA, Multi-Ethnic Study of Atherosclerosis.
Exposure assessment
In MESA Air, we will strive for accurate estimates of each study participant's exposure to ambient-source air pollutants—with particular emphasis on PM2.5 and gaseous copollutants such as oxides of nitrogen—over the 10-year study period. Data sources for modeling efforts include monitors deployed by MESA Air at stationary locations, at participants' homes, and as part of traffic-gradient and community-based “snapshot” monitoring campaigns. Cohen et al. (27) have described the monitoring in detail; during 4 years of exposure monitoring, more than 7,420 monitors were deployed by MESA Air throughout the 6 MESA cities and the 3 additional areas of new recruitment. All monitors collected cumulative, time-integrated data over 2-week periods. Samples were analyzed for PM2.5, light-absorbing carbon, oxides of nitrogen, nitrogen dioxide, nitric oxide, ozone, sulfur dioxide, and trace elements. In funded ancillary studies, investigators analyzed levels of elemental and organic carbon, endotoxin, particulate matter between 2.5 µm and 10 µm in diameter, and particulate matter less than 10 µm in diameter at a subset of locations. In addition to MESA Air monitoring, our exposure assessment incorporates air pollution data from EPA-operated Air Quality System monitors, geographic data such as roadway density and land use, and dispersion model outputs. Combined, these data will permit characterization of seasonal and shorter-term time trends, key sources of spatial variability within the study communities, and underlying spatial and spatiotemporal correlation.
Sampson et al. (28) described a first iteration of the hierarchical spatiotemporal model developed to estimate outdoor PM2.5 concentrations at each participant's residence, using a multistep pragmatic estimation procedure. This model integrates an extensive database of geographic covariates in a spatiotemporal generalization of universal kriging to predict spatial variation in long-term average concentrations, seasonal trends, and 2-week average concentrations. The framework includes sufficiently complex spatiotemporal interactions accounting for variation in seasonal patterns at different locations and accommodating missing data. Figure 2 shows the range of annual average ambient outdoor PM2.5 concentration estimates generated from this model at MESA Air participants' homes in 2000, incorporating residential relocations.
Figure 2.
Prediction of annual average ambient concentrations of particulate matter less than 2.5 µm in diameter (PM2.5) at participants' homes for 2007, by region, Multi-Ethnic Study of Atherosclerosis and Air Pollution. In this box-and-whisker plot, the central bars indicate the median values and the lower and upper ends of the rectangular boxes indicate the 25th and 75th percentiles, respectively. The top and bottom ends of the “whiskers” indicate the 10th and 90th percentiles, respectively, and the circles and asterisks indicate outliers greater than 1.5 and 3 interquartile ranges from the ends of the boxes, respectively. (CA, California; IL, Illinois; MD, Maryland; MN, Minnesota; NC, North Carolina; NY, New York).
Szpiro et al. (29) presented a similar model for predicting residential concentrations of oxides of nitrogen, including a unified estimation approach based on efficient maximum likelihood calculations, the framework to be employed in the final outdoor concentration estimates for each pollutant. This method differs from the model presented by Sampson et al. (28) in estimation approach and treatment of spatiotemporal residuals, but both approaches incorporate a suite of geographic information system-based covariates, account for a complex spatiotemporal correlation structure, and can accommodate spatiotemporally misaligned observations and substantial missing data.
For PM2.5, the principal pollutant under study, we will further estimate the fraction of the outdoor concentration that infiltrates indoors and is encountered by subjects. Estimates of residence-level infiltration efficiency will be based on meteorology and residence-specific characteristics captured by questionnaire and predicted by season- and community-specific regression; personal time-activity records provide individual-level information on the fraction of time spent outdoors (30).
Approximately 5% of all participant homes in each community have paired data from indoor-outdoor sampling of fine particulate sulfur levels. Particulate sulfur, which typically has no indoor sources, is used as a tracer to estimate the infiltration behavior of PM2.5 (31–37). Infiltration efficiency estimates are predicted in season-specific regression by factors including outdoor temperature, building type, building age, presence of double-pane/storm windows, number of days with open windows, use of air conditioning, and use of an indoor air cleaner. Infiltration estimates are possible because all MESA Air participants complete the MESA Air questionnaire at recruitment and as necessary during the periodic follow-up calls, for determination of these home characteristics. Data collected through these questionnaires can be compared with direct observations made by technicians in the subset of homes visited for infiltration monitoring.
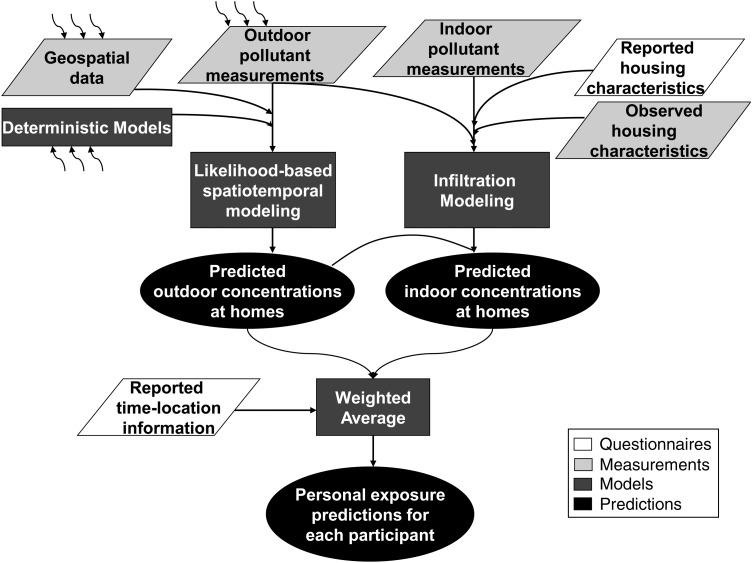
The MESA Air questionnaire also collects time-location information. Participants specify the amounts of time spent at home, at work, at other locations, and in transit and identify locations other than their residences where they spend time. Time-location information will then be used to weight individual exposure estimates according to time spent indoors (represented by the infiltrated concentrations) and time spent outdoors (represented by the ambient concentrations predicted at the participants' homes). Figure 3 shows the planned integration of all exposure monitoring and modeling outputs into a final, individual-level prediction. Predictions are created for each 2-week period of follow-up, permitting time-varying time-location information to be incorporated.
Figure 3.
Integration of exposure modeling outputs to obtain personal exposure predictions for each participant in the Multi-Ethnic Study of Atherosclerosis and Air Pollution, 2004–2014. Parallelograms indicate input data, rectangles indicate statistical modeling processes, and ovals represent products. The curved arrows indicate that multiple external data sources are included as inputs to a given data set or process.
To aid in the understanding of features of measurement error in these estimates, 12–20 participants per community participated in personal monitoring. These participants were outfitted with air monitoring equipment during a 2-week period in two distinct seasons and completed a time-location log with half-hour resolution. Personal air monitoring results will be compared with the modeled exposure estimates, and detailed time-location logs completed during the personal monitoring efforts will be compared with time-location information collected during administration of the MESA Air questionnaire.
Health analysis
MESA Air includes 2 primary health outcomes: 1) progression of subclinical atherosclerosis, measured by both IMT and CAC, and 2) incidence of clinical CVD events. We hypothesize that there will be positive associations between these outcomes and long-term cumulative, individual-level exposures to ambient-source PM2.5 and traffic-related pollution. We will consider effect modification by age, race/ethnicity, gender, obesity, diabetes, educational status, use of lipid-lowering medications, and specific classes of antihypertensive agents. The potential for confounding by socioeconomic factors and the role of social and neighborhood physical environmental factors to serve as either confounders or effect modifiers will also be considered.
Because our sample-size estimates relied on a combination of within-community and between-community variation in exposure, our primary hypothesis testing does not permit us to routinely include an adjustment for clinic site, since doing so would remove the between-community variation and hence dramatically reduce our statistical power. However, the clinic location may also be a good surrogate for potential uncontrolled confounding factors, so in every case we will evaluate the impact of this adjustment in a sensitivity analysis. For outcomes for which we are unable to adequately resolve our concerns regarding clinic-specific factors that could bias the outcome (e.g., CAC, in which different scanner models were used at different clinics), we will include appropriate adjustments (e.g., a scanner variable) in our primary analyses.
We plan to use a linear hierarchical model with subject-specific time-varying exposure that can be partitioned separately into cross-sectional, longitudinal, and transient (or measurement-specific) effects. Modeling will be conducted in 2 stages. First will be an interim analysis of progression in the main MESA cohort between examinations 1 and 3 (approximately 5 years of progression). The second will be a final analysis conducted after examination 5, permitting 10 years of follow-up. The interim analysis will employ a mixed-effects model with error components assumed to be normally distributed with subject- and community-specific random effects; the final analysis will incorporate a measurement error adjustment (38).
Based on the progression of IMT observed between examinations 1 and 2 (39), we estimate that we will have 80% power to observe an overall effect size of 0.0019 mm/year per μg/m3 of PM2.5. Splitting this into between- and within-city exposure variation, we estimate 80% power to detect a between-city effect of 0.0022 mm/year and a within-city effect of 0.0035 mm/year, per μg/m3 of PM2.5. These changes are comparable to 6% and 9% of the effect of lovastatin (40), the most robustly and consistently associated modifier of IMT progression.
For CAC, assuming a baseline rate of change of 0.074 Agatston units per year, we estimate that we will have 80% power to detect an association of 0.00038 change in log(CAC + 25) per μg/m3 of PM2.5 per year, an effect less than 2% of the observed effect of smoking in this cohort. These estimates use measures of change in CAC between examinations 1 and 4 from MESA participants and are based on Agatston units for quantification of calcium levels, although this thresholded scoring system ignores atherosclerosis information in the lower end of the distribution (approximately half of the MESA Air cohort had an Agatston score of 0 at baseline). Since we have developed an alternative method of quantifying CAC from computed tomography scans, with data throughout the distribution, these calculations probably underestimate power using the new score. While Agatston score is a successful clinical risk predictor for events, it is suboptimal as a continuous measure of subclinical disease in epidemiologic studies.
Analysis of the relation between long-term exposure to ambient air pollution and clinical events will be based on a time-dependent proportional hazards model. The power to detect clinical events (myocardial infarction, stroke, or CVD death) in this cohort will depend on the length of follow-up, due both to increasing time for events but also to increasing event rates as the population ages; the median age of the cohort in 2013 will be 75 years. On the basis of baseline events, we anticipate 300 events per 10,000 person-years over the follow-up period, and given the estimated exposures, we anticipate 80% power to detect a relative risk of 1.5 per 10 µg/m3 for all clinical events in the MESA Air cohort over a 10-year follow-up period. This falls well within the effect estimates observed in our analysis of the Women's Health Initiative Observational Study (within-city variation relative risk = 1.64 per 10 µg/m3; overall variation relative risk = 1.24 per 10 µg/m3) (4). Detection of smaller effects will require additional follow-up time beyond that currently planned.
For all outcomes, we will compare the performance of simpler exposure metrics (e.g., nearest monitor values and distance-to-roadway variables) with the final individual-level predictions—those generated on the basis of the outdoor estimates produced by our hierarchical spatiotemporal model—to assess the added value of the more complex models. Additional sensitivity analyses will explore contributions from each MESA Air exposure design component (e.g., infiltration analysis and exposure weighting using time-location information).
DISCUSSION
MESA Air is designed to provide the most advanced approach feasible regarding a critical public health issue. CVD, especially atherosclerotic disease, is the leading cause of premature death and disease in developed countries (41). According to the Office of Management and Budget's 2007 report to Congress on the costs and benefits of federal regulations, the largest estimated benefit of all federal regulations in the United States is attributable to the reduction in public exposure to PM2.5 following the passage of the Clean Air Act, due almost entirely to a reduction in mortality (42). Despite mounting evidence regarding the relation between long-term exposure to ambient air pollutants and CVD, significant questions remain. The concentration-response relation has not been fully defined, and the mechanisms that underlie this relation are uncertain.
To our knowledge, MESA Air will be the first fully prospective cohort study with prespecified outcomes to investigate the relation between air pollution and CVD using detailed individual-level health and covariate data and prospectively designed individual-level exposure estimation of PM2.5 and gaseous copollutants. Numerous approaches have been used previously to predict spatially varying outdoor concentrations of ambient air pollutants. The coarsest of these is metropolitan area-wide annual averages, whereby each study participant living within a single metropolitan area is assigned the same exposure (2, 3). Another simple technique, adding a component of intra-area variation, has been to assign each participant the concentration measured by the nearest Air Quality System monitor (4). Beyond these are basic modeling approaches that integrate multiple data sources, such as spatial interpolation, dispersion modeling, and land-use regression. More sophisticated approaches have included hybrid designs combining these simpler methods, and more recently, spatiotemporal models have been developed (43). All previous population exposure assessment focused on outdoor spatial variation and stopped short of predicting individual exposures (44, 45).
MESA Air will employ 3 methods for estimating long-term average ambient pollutant concentrations at each participant's home address. First, analyses will include a “nearest monitor” assessment, for comparability across studies and analyses. Second, we developed a “pragmatic” spatiotemporal model, using preliminary data and a more practical method, while our final modeling effort will provide refined estimates from a likelihood-based hierarchical spatiotemporal model. Third, in addition to home outdoor pollutant exposures, our exposure assessment model for PM2.5 will estimate the amounts of outdoor pollutants that infiltrate indoors. We will further incorporate time-activity information to create a weighted average exposure derived from indoor and outdoor PM2.5 concentrations. With rich study-specific monitoring, including indoor and outdoor samples at participants' homes and personal-level time-activity data, the exposure assignment of MESA Air will substantially improve upon approaches found in the epidemiology literature to date.
The range of exposures under study is extremely relevant: The annual average levels observed are largely at or below regulatory levels (46–49). Both the clinical and subclinical health measures included in this study reflect current epidemiologic methods and reflect multiple pathways and different levels of severity within the same participant. The multiethnic study design will improve the generalizability of the results. Sufficient populations with key putative risk factors are included, and genetic information provided by MESA and the MESA Air ancillary studies may illuminate possible mechanistic pathways.
Beyond the focus on its primary aims, MESA Air has proven to be a successful platform for ancillary studies, leveraging the EPA's investment to support other research endeavors. To date, there are more than a dozen funded ancillary studies and planned collaborations between MESA Air and both internal and external investigators. Air pollutant concentrations will be characterized by MESA Air investigators on both the local scale (e.g., due to traffic) and the regional scale, and ancillary studies funded by the Health Effects Institute and the EPA will allow further exploration of health effects by source type and particle size and in a “multipollutant” context. Ancillary studies funded by the National Institutes of Health will deepen our analysis of health effects, including an emphasis on cardiovascular structure and function and new techniques to account for measurement error in analyses.
The primary MESA Air health analyses await conclusion of the fifth MESA examination in 2012. This examination marks more than 10 years of participant follow-up, and the health analyses conducted then will include long-term, individual-level estimates of exposure and longitudinal progression of subclinical CVD. This study is generalizable to middle-aged and older people at risk for developing CVD, and this rich cohort will not only provide new insight into the relation between exposure to air pollutants and the development of CVD but also allow us to evaluate the impact of improvements in exposure assessment on estimation of concentration-effect relations. As such, the results of this study are expected to inform public policy designed to reduce the public health effects of exposure to ambient air pollution.
ACKNOWLEDGMENTS
Author affiliations: Department of Environmental and Occupational Health Sciences, School of Public Health, University of Washington, Seattle, Washington (Joel D. Kaufman, Martin A. Cohen, Cynthia L. Curl, Sally Lee-Jane Liu, Lianne Sheppard); Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Joel D. Kaufman, David S. Siscovick); Department of Medicine, School of Medicine, University of Washington, Seattle, Washington (Joel D. Kaufman, David S. Siscovick); Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan (Sara D. Adar, Ana V. Diez Roux); Faculty of Health Sciences, Simon Fraser University, Vancouver, British Columbia, Canada (Ryan W. Allen); Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, New York (R. Graham Barr); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (R. Graham Barr); Division of Cardiology, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California (Matthew J. Budoff); Division of Public Health Sciences, School of Medicine, Wake Forest University, Winston-Salem, North Carolina (Gregory L. Burke); UCLA Research Center, University of California, Los Angeles, Alhambra, California (Adrian M. Casillas); Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Martha L. Daviglus); Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (David R. Jacobs, Jr.); Collaborative Health Studies Coordinating Center, Department of Biostatistics, School of Public Health and Community Medicine, University of Washington, Seattle, Washington (Richard A. Kronmal, Thomas Lumley); Department of Civil and Environmental Engineering, College of Engineering, University of Washington, Seattle, Washington (Timothy V. Larson); Department of Environmental Health Sciences, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Ana Navas-Acien); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Ana Navas-Acien); Tufts University Medical Center, Brookline, Massachusetts (Daniel H. O'Leary); Cedars-Sinai Medical Center, Los Angeles, California (Jerome I. Rotter); Department of Statistics, College of Arts and Sciences, University of Washington, Seattle, Washington (Paul D. Sampson); Department of Biostatistics, School of Public Health, University of Washington, Seattle, Washington (Lianne Sheppard, Adam A. Szpiro); Division of Cardiovascular Medicine, School of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin (James H. Stein); and Colchester Research Facility, Department of Pathology, College of Medicine, University of Vermont, Colchester, Vermont (Russell P. Tracy).
The Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) is conducted and supported by the Environmental Protection Agency through grant RD831697 to the University of Washington. The Multi-Ethnic Study of Atherosclerosis (MESA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the MESA investigators. Support is provided by grants and contracts N01 HC-95159 through N01-HC-95169 and RR-024156. The MESA Family study is conducted and supported by the NHLBI in collaboration with the MESA investigators. Support is provided by grants and contracts R01HL071051, R01HL071205, R01HL071250 through R01HL0712502, R01HL071251, R01HL071252, R01HL071258, and R01HL071259.
Conflict of interest: none declared.
REFERENCES
- 1.Brook RD, Rajagopalan S, Pope CA, III, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Dockery DW, Pope CA, III, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA, III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 5.Hoek G, Brunekreef B, Goldbohm S, et al. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet. 2002;360(9341):1203–1209. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- 6.Ostro B, Lipsett M, Reynolds P, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect. 2010;118(3):363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehring U, Heinrich J, Krämer U, et al. Long-term exposure to ambient air pollution and cardiopulmonary mortality in women. Epidemiology. 2006;17(5):545–551. doi: 10.1097/01.ede.0000224541.38258.87. [DOI] [PubMed] [Google Scholar]
- 8.Rosenlund M, Berglind N, Pershagen G, et al. Long-term exposure to urban air pollution and myocardial infarction. Epidemiology. 2006;17(4):383–390. doi: 10.1097/01.ede.0000219722.25569.0f. [DOI] [PubMed] [Google Scholar]
- 9.Puett RC, Hart JE, Yanosky JD, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses' Health Study. Environ Health Perspect. 2009;117(11):1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman JD. Does air pollution accelerate progression of atherosclerosis? J Am Coll Cardiol. 2010;56(22):1809–1811. doi: 10.1016/j.jacc.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diez Roux AV, Auchincloss AH, Franklin TG, et al. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167(6):667–675. doi: 10.1093/aje/kwm359. [DOI] [PubMed] [Google Scholar]
- 12.Künzli N, Jerrett M, Mack WJ, et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113(2):201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenters V, Uiterwaal CS, Beelen R, et al. Long-term exposure to air pollution and vascular damage in young adults. Epidemiology. 2010;21(4):512–520. doi: 10.1097/EDE.0b013e3181dec3a7. [DOI] [PubMed] [Google Scholar]
- 14.Bauer M, Moebus S, Möhlenkamp S, et al. Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol. 2010;56(22):1803–1808. doi: 10.1016/j.jacc.2010.04.065. [DOI] [PubMed] [Google Scholar]
- 15.Allen RW, Criqui MH, Diez Roux AV, et al. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20(2):254–264. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Künzli N, Jerrett M, Garcia-Esteban R, et al. Ambient air pollution and the progression of atherosclerosis in adults. PLoS ONE. 2010;5(2):e9096. doi: 10.1371/journal.pone.0009096. ( doi:10.1371/journal.pone.0009096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Environmental Protection Agency. Epidemiologic Research on Health Effects of Long-term Exposure to Ambient Particulate Matter and Other Air Pollutants. Washington, DC: Environmental Protection Agency; 2003. [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Diez Roux AV, Evenson KR, McGinn AP, et al. Availability of recreational resources and physical activity in adults. Am J Public Health. 2007;97(3):493–499. doi: 10.2105/AJPH.2006.087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujishiro K, Diez Roux AV, Landsbergis P, et al. Associations of occupation, job control and job demands with intima-media thickness: the Multi-Ethnic Study of Atherosclerosis (MESA) Occup Environ Med. 2011;68(5):319–326. doi: 10.1136/oem.2010.055582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natori S, Lai S, Finn JP, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 suppl 2):S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology. 2006;113(3):373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT scans—the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hankinson JL, Kawut SM, Shahar E, et al. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) lung study. Chest. 2010;137(1):138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen-Torvik LJ, Guo X, Bowden DW, et al. Fasting glucose GWAS candidate region analysis across ethnic groups in the Multiethnic Study of Atherosclerosis (MESA) Genet Epidemiol. 2012;36(4):384–391. doi: 10.1002/gepi.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Sci Technol. 2009;43(13):4687–4693. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson PD, Szpiro AA, Sheppard L, et al. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ. 2011;45(36):6593–6606. [Google Scholar]
- 29.Szpiro AA, Sampson PD, Sheppard L, et al. Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics. 2010;21(6):606–631. doi: 10.1002/env.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen RW, Adar SD, Avol E, et al. Modeling the residential infiltration of outdoor PM2.5 in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Health Perspect. 2012;120(6):824–830. doi: 10.1289/ehp.1104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen R, Larson T, Sheppard L, et al. Use of real-time light scattering data to estimate the contribution of infiltrated and indoor-generated particles to indoor air. Environ Sci Technol. 2003;37(16):3484–3492. doi: 10.1021/es021007e. [DOI] [PubMed] [Google Scholar]
- 32.Allen R, Wallace L, Larson T, et al. Evaluation of the recursive model approach for estimating particulate matter infiltration efficiencies using continuous light scattering data. J Expo Sci Environ Epidemiol. 2007;17(5):468–477. doi: 10.1038/sj.jes.7500539. [DOI] [PubMed] [Google Scholar]
- 33.Wilson WE, Mage DT, Grant LD. Estimating separately personal exposure to ambient and nonambient particulate matter for epidemiology and risk assessment: why and how. J Air Waste Manag Assoc. 2000;50(7):1167–1183. doi: 10.1080/10473289.2000.10464164. [DOI] [PubMed] [Google Scholar]
- 34.Ozkaynak H, Xue J, Spengler J, et al. Personal exposure to airborne particles and metals: results from the Particle TEAM study in Riverside, California. J Expo Anal Environ Epidemiol. 1996;6(1):57–78. [PubMed] [Google Scholar]
- 35.Ebelt ST, Petkau AJ, Vedal S, et al. Exposure of chronic obstructive pulmonary disease patients to particulate matter: relationships between personal and ambient air concentrations. J Air Waste Manag Assoc. 2000;50(7):1081–1094. doi: 10.1080/10473289.2000.10464166. [DOI] [PubMed] [Google Scholar]
- 36.Sarnat JA, Long CM, Koutrakis P, et al. Using sulfur as a tracer of outdoor fine particulate matter. Environ Sci Technol. 2002;36(24):5305–5314. doi: 10.1021/es025796b. [DOI] [PubMed] [Google Scholar]
- 37.Stieb D, Brook J, Broder I, et al. Personal exposure of adults with cardiorespiratory disease to particulate acid and sulfate in Saint John, New Brunswick, Canada. Appl Occup Environ Hyg. 1998;13(6):461–468. [Google Scholar]
- 38.Szpiro AA, Sheppard L, Lumley T. Efficient measurement error correction with spatially misaligned data. Biostatistics. 2011;12(4):610–623. doi: 10.1093/biostatistics/kxq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polak JF, Pencina MJ, O'Leary DH, et al. Common carotid artery intima-media thickness progression as a predictor of stroke in Multi-Ethnic Study of Atherosclerosis. Stroke. 2011;42(11):3017–3021. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodis HN, Mack WJ, Barth J. Carotid intima-media thickness as a surrogate end point for coronary artery disease. Circulation. 1996;94(9):2311–2312. [PubMed] [Google Scholar]
- 41.World Health Organization. The World Health Report 2004—Changing History. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 42.Office of Management and Budget, Executive Office of the President. 2007 Report to Congress on the Benefits and Costs of Federal Regulations and Unfunded Mandates on State, Local, and Tribal Entities. Washington, DC: Office of Management and Budget; 2007. [Google Scholar]
- 43.Paciorek CJ, Yanosky JD, Puett RC, et al. Practical large-scale spatio-temporal modeling of particulate matter concentrations. Ann Appl Stat. 2009;3(1):370–397. [Google Scholar]
- 44.Hoek G, Fischer P, Van Den Brandt P, et al. Estimation of long-term average exposure to outdoor air pollution for a cohort study on mortality. J Expo Anal Environ Epidemiol. 2001;11(6):459–469. doi: 10.1038/sj.jea.7500189. [DOI] [PubMed] [Google Scholar]
- 45.Hoek G, Meliefste K, Cyrys J, et al. Spatial variability of fine particle concentrations in three European areas. Atmos Environ. 2002;36(25):4077–4088. [Google Scholar]
- 46.Environmental Protection Agency. National Ambient Air Quality Standards (NAAQS) Washington, DC: Environmental Protection Agency; 2010. (http://www.epa.gov/oar/criteria.html. ). (Accessed November 1, 2011) [Google Scholar]
- 47.Health Canada. Regulations Related to Health and Air Quality. Ottawa, Ontario, Canada: Health Canada; 2010. National Ambient Air Quality Objectives (NAAQOs) (http://www.hc-sc.gc.ca/ewh-semt/air/out-ext/reg-eng.php. ) (Accessed November 1, 2011) [Google Scholar]
- 48.Environment Directorate-General of the European Commission. Air Quality Standards. Brussels, Belgium: European Commission; 2010. (http://ec.europa.eu/environment/air/quality/standards.htm. ). (Accessed November 1, 2011) [Google Scholar]
- 49.World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide—Global Update 2005—Summary of Risk Assessment. Geneva, Switzerland: World Health Organization; 2005; [Google Scholar]
- 50.Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]