Abstract
The therapeutic effects of a controlled parasitic nematode infection on the course of inflammatory bowel disease (IBD) have been demonstrated in both animal and human models. However, the inability of individual well-characterized nematode proteins to recreate these beneficial effects has limited the application of component immunotherapy to human disease. The nematodes that cause chronic human lymphatic filariasis, Brugia malayi and Wuchereria bancrofti, are among the parasites that induce immune suppression. Filarial lymphatic pathology has been shown to involve NF-κB pathway-dependent production of vascular endothelial growth factor (VEGF), and stimulation of VEGF expression has also been reported by interleukin 8 (IL-8) via NF-κB pathways. Previously, we have shown that the filarial asparaginyl-tRNA synthetase (rBmAsnRS) interacts with IL-8 receptors using a combination of extracellular loops that differ from those bound by IL-8. To test the hypothesis that rBmAsnRS might induce an anti-inflammatory effect in vivo, we studied the effects of rBmAsnRS in an established murine colitis model using T-cell transfer mice. T-cell transfer colitis mice treated intraperitoneally with 100 μg of rBmAsnRS four times over 2 weeks showed resolution of cellular infiltration in the colonic mucosa, along with induction of a CD8+ cellular response. In addition, rBmAsnRS induced a rise in IL-10 production from CD3+ and lipopolysaccharide (LPS)- and cytosine phosphate guanosine (CPG)-stimulated splenic cells. In summary, this work demonstrates a novel anti-inflammatory nematode protein, supports the hygiene hypothesis, and supports continued refinement of alternative immunotherapies for treatment of IBD.
INTRODUCTION
The therapeutic effects of a controlled parasitic nematode infection on the course of inflammatory bowel disease (IBD) and other autoimmune diseases have been recognized for many years in animal models (1–5) and occasionally in humans (6). However, the inability of purified nematode proteins individually or in combination to reproduce these beneficial effects has limited component application of the hygiene hypothesis to clinical disease management (7). The hygiene hypothesis is the long-held belief that early life exposure to microbial antigens reduces the risk of allergic or autoimmune disease later in life through mechanisms resulting in a relative immune tolerance. The exact mechanism(s) by which specific nematode molecules exert these effects is unclear (8, 9). Thus, research to clarify the specific mechanisms by which nematode-derived molecules exert beneficial effects in IBD models is fundamental to the future clinical application of such novel complementary or alternative immunotherapies in humans.
Lymphatic filariasis in humans is caused primarily by three species of filarial nematodes, Brugia malayi, Brugia timori, and Wuchereria bancrofti. Filaria have often been referred to as “masters of regulation” because of their profound effect on host immunity, which includes parasite antigen-specific CD4+ T-cell hyporesponsiveness and interleukin 10 (IL-10) responses (10). In the subset of persons with lymphatic filariasis who develop chronic lymphatic pathology (elephantiasis), the immune response to filarial infection culminates in the development of lymph vessel hyperplasia and lymphangiogenesis. Toll-like receptor-mediated responses have been implicated in the mechanisms by which parasite molecules cause chronic lymphatic pathology, via mitogen-activated protein kinase (MAPK) and NF-κB-dependent regulation of angiogenic factors (11, 12). The transcription factor NF-κB is also a well-known regulator of the proinflammatory chemokine IL-8, which has been shown to elicit either a direct or indirect angiogenic response (13, 14).
We have reported previously that a filarial nematode protein, the cytoplasmic asparaginyl-tRNA synthetase (rBmAsnRS), is a highly expressed excretory/secretory protein, produced at 10 times the amount of any other filarial tRNA synthetase, and had the ability to block IL-8 receptors on human and murine cells by using a combination of extracellular receptor loops different from those utilized by IL-8 (15). Activation of MAPK and signal transduction via IL-8 receptors occurs with both rBmAsnRS and native IL-8 ligands (15). Determination of the atomic structure of the rBmAsnRS revealed a novel protein fold that mimics key residues of IL-8 that are critical for binding to its G protein-coupled receptors (16, 17). Thus, when it was first observed that a nematode AsnRS induced chemotaxis of cells that express IL-8 receptors, a proinflammatory role for this molecule was assumed. However, the evolution of proinflammatory molecules by filarial parasites seemed counterintuitive given the propensity of filaria to “evade” immune attack in infected persons and circulate freely in the lymphatics, blood, or subcutaneous tissues (8, 10). Thus, the chemotactic properties of AsnRS observed in vitro were reconsidered to be an overinterpretation of an in vitro test and not necessarily representative of in vivo outcomes.
The hypothesis was tested that rBmAsnRS might induce a beneficial, anti-inflammatory effect in vivo using an established mouse colitis model. Beneficial effects could be mediated in part by chemokine receptor desensitization or unknown means but consistent with the overarching mechanisms by which parasites establish chronic, nonlethal infections in their hosts. Human immature dendritic cells (iDCs) are key antigen-presenting cells which express abundant IL-8 receptors that are lost as the cell matures and can be generated in vitro from peripheral blood mononuclear cells. In addition, dysregulation of DCs has been implicated in the pathogenesis of human IBD (18) and colitis in Rag mice. However, mice are innately immune to infection with B. malayi, whereas humans are not. Therefore, a pilot study was done to examine possible effects on gene expression in human iDCs stimulated by rBmAsnRS and compared to those induced by IL-8 using the Affymetrix 3PRIME IVT ID chip (54,614 genes) and an NF-κB-specific pathway microarray. We theorized that if rBmAsnRS treatment elicited effects on human gene expression that were distinct from the IL-8 response, then this would be supportive of the idea that rBmAsnRS might be beneficial in humans with IBD.
MATERIALS AND METHODS
Production of endotoxin-free rBmAsnRS.
A cDNA encoding the entire wild-type cytoplasmic AsnRS (548 amino acids) was expressed and purified using a pET28A expression system as previously described (15–17). The construct yielded soluble recombinant protein of the expected molecular mass, which was then purified as an endotoxin-free reagent (<0.001 IU/μg). Enzymatically active 63-kDa endotoxin-free rBmAsnRS was purified by using magnesium sulfate precipitation followed by sequential rounds of size selection, nickel affinity, and anion-exchange chromatography and final adsorption using Endotrap (Hygiene Biotech Company, Ltd.).
Mice, induction of transfer colitis, and rBmAsnRS treatment.
The effect of rBmAsnRS was investigated in a well-established murine model of colitis. Rag-1 mice on a C57BL/6 background (Jax 002096) were purchased from JAX laboratories, Maine. Mice were housed under specific-pathogen-free (SPF) conditions at the University of Iowa ACU. For reconstitution of Rag-1, C57BL/6 wild-type (WT) genetically compatible mice were purchased from NCI, Frederick, MD. T-cell preparations from spleens of C57BL/6 mice were prepared by negative selection using two cycles of antibody and magnetic beads coated with sheep anti-rat IgG antibody. We reconstituted the Rag-1 mice by intraperitoneal (i.p.) injection of 5 × 106 cells in 1 ml of phosphate-buffered saline (PBS). Three days after reconstitution, mice were treated with piroxicam (P5654; Sigma) (80 mg/250 g of ground food [T 7913; Harlan Teklad, Madison, WI]), generating a highly reproducible colitis that persists after piroxicam is stopped (19). Two days after piroxicam treatment was finished and colitis was well established, mice were treated with intraperitoneal injections of endotoxin-free rBmAsnRS (100 μg) or control (phosphate-buffered saline, vehicle only) given every 3 days for a total of four doses (Fig. 1). After this treatment, mice were killed by CO2 inhalation, and organs were removed aseptically for culture and histology.
Fig 1.
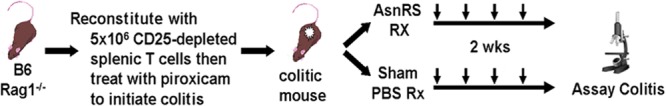
Treatment protocol using the T-cell transfer colitis model.
Isolation of colons for histology and cells for cytokine analysis and flow cytometry.
Animals were euthanized 3 days after the completion of the treatment protocol. Colons from the ileocecal valve to the mid-descending colon were opened longitudinally and rolled up onto a glass rod. The tissue was fixed in 4% neutral buffered formalin and processed for histology sectioning and hematoxylin and eosin (H&E) differential staining. The inflammation was scored on a scale of 0 to 4 using published criteria (1), where grade 0 is no change from normal tissues. Grade 4 shows lesions involving most of the intestinal section, with transmural inflammation composed mostly of lymphocytes and some neutrophils; severe prominent thickening of mucosal, submucosal, and muscle layers; mucus depletion; frequent crypt abscesses; and ulcerations. Colons were isolated for both immunological and histological studies. Cecum and ileal lamina propria mononuclear cells, mesenteric lymphocytes, and splenocytes also were isolated for immunologic assays as previously described (1).
Spleen and mesenteric lymph node single-cell preparations.
Single-cell suspension from spleens and mesenteric lymph nodes were prepared in RPMI 1640-complete medium with 10% fetal calf serum (FCS), 2 mM l-glutamine, 25 mM HEPES buffer, 5 × 10−5 M 2-β-mercaptoethanol, 1 mM sodium pyruvate, 100 μ/ml penicillin, 100 mg/ml streptomycin, and 5 mg/ml gentamicin, all purchased from GIBCO, Invitrogen, Grand Island, NY. Mesenteric lymph node cells were isolated from healthy C57BL/6 (NCI) mice. Cells were cultured (5 × 105 cells/well) in RPMI 1640-based medium for 48 h. Some cultures were stimulated with LPS (Sigma, St. Louis, MO) used at 100 ng/ml and cytosine phosphate guanosine (CPG; 1826; Coley Pharmaceutical Group, Wellesley, MA) used at 0.6 μg/ml. Anti-CD3 antibody for T-cell activation was used at 1 μg/ml and prepared by using soups from hybridomas grown in serum-free medium and then precipitated by ammonium sulfate followed by dialysis and filter sterilization. Supernatants were assayed for cytokine content by enzyme-linked immunosorbent assay (ELISA).
Interleukin ELISAs.
Cytokines were measured by sandwich ELISA. For IL-17, ELISA plates were coated with anti-IL-17 monoclonal capture antibody MAB 721 from R&D Systems, Inc., Minneapolis, MN. A biotinylated secondary antibody used for detection was BAF-421, also purchased from R&D. Recombinant IL-17, purchased from R&D Systems, was utilized as the standard. For gamma interferon (IFN-γ), determinations were done using XMG 1.2 hybridoma monoclonal antibody to capture and R4-6A2-biotinylated antibody from eBioscience (number 13-7312-85) to detect. For IL-4, determinations were done using 11B11 to capture and BVD6 biotinylated in our laboratory to detect. For IL-10, determinations were done by using JES-2A5 (DNAX) hybridoma monoclonal antibody for capture and a biotinylated secondary BAF417 from R&D Systems for detection. The lower level of detection was 30 pg/ml for all ELISAs.
Flow cytometry.
Staining for surface markers for flow cytometry analysis was done by a panel of labeled antibodies according to the manufacturer's recommendations as follows. Cells were adjusted to 107/ml in FACS buffer (Hanks' balanced salt solution [HBSS] containing 1% FCS). We added a saturating concentration (1 μg of labeled antibody/106 cells) of anti-CD4-fluorescein isothiocyanate antibody (FITC; eBioscience number 11[004[85), anti-CD8-phycoerythrin (PE) antibody (BD Pharmingen number 553033), Mac-1-PE (BD Pharmingen number 01715A), B220-FITC (R&D Systems number FAB1217F), and CTLA4-PE (eBioscience number 12-1522-82) to stain surface markers. After incubation on ice for 30 min, cells were washed in FACS buffer and fixed with perm/fix solution from Pharmingen, San Diego, CA; washed in perm/wash buffer; and resuspended in 1% FCS in PBS. Cell acquisition was done by a FACScan flow cytometer, and analysis was done by using CellQuest Pro software in the flow cytometry facility at the University of Iowa.
Production of immature dendritic cells from peripheral blood mononuclear cells for gene expression studies.
The monocyte cell population was isolated from healthy donor buffy coats without identifiers obtained from the Blood Center of Wisconsin under an exempt human subject's protocol. Mononuclear cells were purified by Ficoll-Histopaque 1.077 g/ml (Sigma-Aldrich) density gradient centrifugation followed by isolation with anti-CD14-MicroBeads according to the magnetic-activating cell sorting (MACS) manufacturer's protocol (Miltenyi Biotech). The purity of the CD14+ monocyte cell population confirmed by flow cytometry was >99%. Isolated peripheral monocyte cell populations were resuspended in RPMI 1460 medium supplemented with 100 U/ml of penicillin-streptomycin and 10% (vol/vol) of FCS (HyClone) and seeded in 6-well plates at 4 × 106 to 5 × 106 cells/well. The monocytes were stimulated for 72 h with 50 ng/ml of rhIL-4 and rhGM-CSF (PeproTech Inc.) to obtain an immature dendritic cell (iDC) population as previously described (20).
To examine the effect of rBmAsnRS on iDC gene expression, three different groups of iDCs (106 cells) were treated with either 1 μg/ml rBmAsnRS, IL-8, or medium control in the presence of 50 ng/ml of rhIL4 and rhGM-SCF. After stimulation for 72 h, total RNA was extracted from each treatment group using Qiagen spin columns. Two different human microarray systems were used to study the relative effects of rBmAsnRS versus IL-8 on gene expression in iDCs: (i) a 114-gene mini-array specific for the human NF-κB pathway (GE Superarray) and (ii) the full Affymetrix 3PRIME IVT ID chip (54,614 genes). The top 500 upregulated and downregulated genes were assigned to signal transduction pathways using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (16), version 6.7 (http://david.abcc.ncifcrf.gov/).
Nucleotide sequence accession number.
Full gene expression data from the Affymetrix microarray experiments was deposited with the National Center for Biotechnology Information database, Gene Expression Omnibus (GEO), with the accession number GSE39999 (http://www.ncbi.nlm.nih.gov/geo/query).
RESULTS
Effects of rBmAsnRS on colonic inflammation.
In the T-cell transfer colitis model, gross anatomy of rBmAsnRS-treated colons was not completely normal but was markedly less edematous and significantly improved compared to diseased (control) colons (Fig. 2). Histological examination of colons from rBmAsnRS-treated mice demonstrated a resolution of cellular infiltrates seen in the lamina propria of inflamed colons (Fig. 3) associated with restoration of normal thickness, mucous-forming goblet cells, and crypt formation. Flow cytometric studies of splenic, mesenteric lymph node, and lamina propria cells isolated from immunized mice revealed that rBmAsnRS treatment increased the number of CD8+ T cells in the lamina propria compartment, with a corresponding increase in CD4+ cells in the spleen (Table 1). Cytokine profiles of rBmAsnRS-treated T-cell transfer mouse cells isolated from spleen, mesenteric lymph nodes, and lamina propria demonstrated a significant drop in IFN-γ and interleukin 17 (IL-17) levels, along with a corresponding rise in levels of IL-4 and IL-10 (Table 2).
Fig 2.
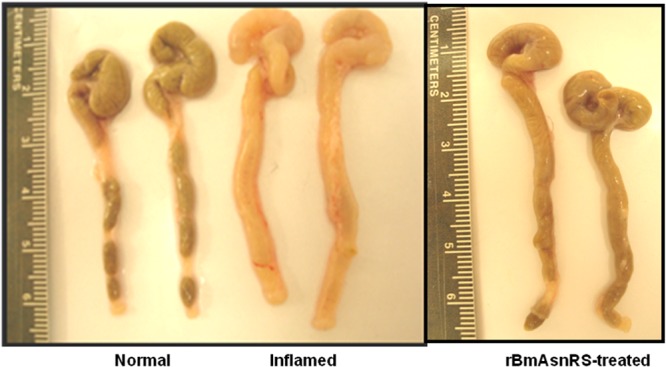
Gross appearance of negative-control, positive-control, and rBmAsnRS-treated colons of T-cell transfer mice.
Fig 3.
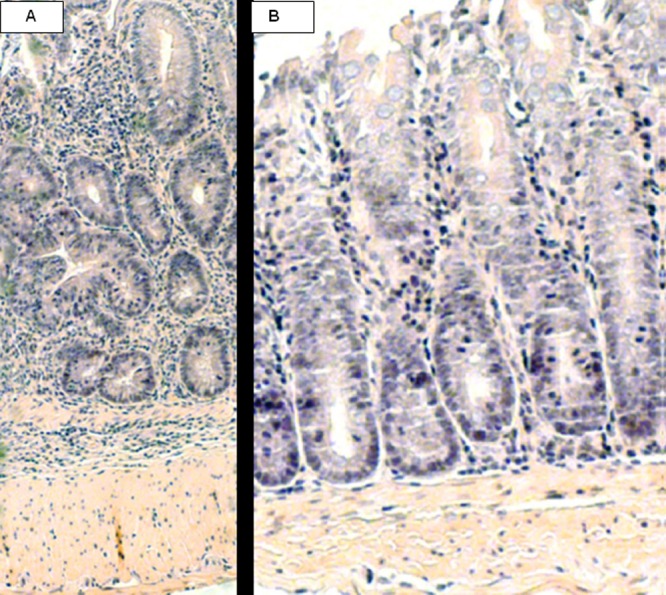
Histological sections of mouse Rag1−/− colons (A; control, positive colitis) and BmAsnRS-treated colons (B). AsnRS-treated colons show resolution of cellular infiltration in the lamina propria submucosa even at high magnification. Inflammatory score: A = 3.67 ± 0.21 (n = 6 high power fields [hpf]) and B = 1.67 ± 0.33 (n = 12 high power fields [hpf]). P = 0.0010. Histological inflammation criteria include multiple indicators of transmural inflammation, epithelial hyperplasia, erosions, goblet cell mucus depletion, crypt abscesses, and ulceration (1).
Table 1.
Flow cytometry results in control and rBmAsnRS-treated T-cell transfer colitis mice that demonstrate histological improvement in colitis
| Site | Mice | Result for cell subseta |
|||
|---|---|---|---|---|---|
| CD4+ | CD 8+ | B220+ | Mac1+ | ||
| Spleen | Untreated (PBS) | 22.9 ± 0.2 | 4.1 ± 0.2 | 7.7 ± 0.7 | 42.7 ± 0.9 |
| rBmAsnRS | 36.4 ± 1.4* | 5.6 ± 1.6 | 7.9 ± 0.3 | 36.1 ± 1.6* | |
| Mesenteric lymph nodes | Untreated | 49.3 ± 1.9 | 5.8 ± 0.2 | 6.9 ± 0.8 | 7.5 ± 0.6 |
| rBmAsnRS | 53.9 ± 3.4 | 8.9 ± 2.4 | 8.6 ± 0.4 | 12.5 ± 2.1 | |
| Lamina propria lymphocytes | Untreated | 57.4 ± 0.9 | 8.9 ± 0.4 | 4.6 ± 1.4 | 4.1 ± 0.9 |
| rBmAsnRS | 36.5 ± 2.4* | 35.1 ± 6.2* | 5.9 ± 0.7 | 2.5 ± 0.08 | |
*, statistically significant values (P < 0.05) using Student's t test.
Table 2.
Cytokine analysis from cells isolated from rBmAsnRS-treated versus untreated T-cell transfer colitis mice
| Cytokine analysis | Baseline and stimulated cytokine levela |
||||
|---|---|---|---|---|---|
| Cells | CD3+ | LPS | CPG | ||
| Spleen | |||||
| IFN-γ | Untreated | 0.045 ± 0.017 | 2.251 ± 0.03 | 0.657 ± 0.066 | 0.614 ± 0.101 |
| rBmAsnRS | 0.014 ± 0.01 | 2.086 ± 0.179 | 0.386 ± 0.043* | 0.304 ± 0.064 | |
| IL-17 | Untreated | BD | 2.102 ± 0.021 | 0.72 ± 0.12 | BD |
| rBmAsnRS | BD | 1.556 ± 0.27 | 0.641 ± 0.053 | BD | |
| IL-4 | Untreated | BD | 0.664 ± 0.032 | 0.322 ± 0.01 | 0.343 ± 0.041 |
| rBmAsnRS | BD | 0.707 ± 0.022 | 0.387 ± 0.041 | 0.473 ± 0.066 | |
| IL-10 | Untreated | 0.242 ± 0.06 | 0.271 ± 0.039 | 1.951 ± 0.05 | 1.562 ± 0.128 |
| rBmAsnRS | 0.122 ± 0.013 | 0.898 ± 0.031* | 2.966 ± 0.03* | 3.019 ± 0.025* | |
| Mesenteric lymph nodes | |||||
| IFN-γ | Untreated | 0.399 ± 0.01 | 3.009 ± 0.083 | 0.706 ± 0.097 | 1.836 ± 0.079 |
| rBmAsnRS | 0.16 ± 0.076 | 2.698 ± 0.065 | 0.357 ± 0.037 | 1.749 ± 0.132 | |
| IL-17 | Untreated | BD | 5.549 ± 0.164 | 1.170 ± 0.03 | 0.953 ± 0.122 |
| rBmAsnRS | 0.087 ± 0.01* | 4.434 ± 0.144* | 0.256 ± 0.041* | 0.246 ± 0.052* | |
| IL-4 | Untreated | BD | 0.573 ± 0.028 | BD | 0.173 ± 0.048 |
| rBmAsnRS | BD | 0.818 ± 0.042* | 0.174 ± 0.01* | 0.518 ± 0.045* | |
| IL-10 | Untreated | BD | 1.394 ± 0.027 | BD | 0.539 ± 0.011 |
| rBmAsnRS | 0.135 ± 0.062 | 2.271 ± 0.033* | 2.23 ± 0.07* | 1.252 ± 0.018* | |
| Lamina propria lymphocytes | |||||
| IFN-γ | Untreated | 0.072 ± 0.018 | 1.158 ± 0.042 | BD | BD |
| rBmAsnRS | 0.039 ± 0.01 | 0.78 ± 0.013* | BD | BD | |
| IL-17 | Untreated | BD | 1.842 ± 0.036 | 0.74 ± 0.03 | 0.62 ± 0.12 |
| rBmAsnRS | BD | 1.545 ± 0.049 | 0.618 ± 0.03 | 0.42 ± 0.16 | |
| IL-4 | Untreated | BD | 0.154 ± 0.012 | BD | BD |
| rBmAsnRS | BD | 0.376 ± 0.01* | BD | BD | |
| IL-10 | Untreated | BD | 0.228 ± 0.05 | BD | BD |
| rBmAsnRS | BD | 0.367 ± 0.03 | BD | 0.129 ± 0.03* | |
*, statistically significant values (P < 0.05) using Student's t test. BD signifies below detectable limits of the assay.
Gene expression in the NF-κB pathway induced by rBmAsnRS-treated human immature dendritic cells.
Using an NF-κB pathway-specific mini-microarray (114 genes), the effect of rBmAsnRS on human gene expression in iDCs is distinct from the effect of IL-8 (see Fig. S1 in the supplemental material) and included differential effects on IL-8 expression. Using the Affymetrix 3PRIME IVT ID human gene chip, expression of the top 500 up- and downregulated genes in human iDCs was mapped to Kyoto Encyclopedia of Genes and Genomes (KEGG) signal transduction pathways using DAVID (21). rBmAsnRS demonstrated recruitment of multiple signal transduction pathways linked to chemokine signaling, MAPK signaling, Toll-like receptor signaling, and natural killer (NK) cell-mediated cytotoxicity. rBmAsnRS induced upregulation of IL-10 and IL-22 receptors (see Fig. S2 to S5 in the supplemental material).
DISCUSSION
This study is the first report of a novel nematode protein, rBmAsnRS, that induces recovery from established murine T-cell transfer colitis. However, we do not wish to imply that any single nematode molecule could ever be expected to recreate all the immunological complexities of a natural filarial infection. First, this report focuses on describing the in vivo response to intraperitoneal treatment with a nematode-derived protein that is known to interact with IL-8 receptors using an alternative mechanism to effect signal transduction and gene expression—a putative helminth-derived chemokine receptor antagonist. Second, because murine and human immune systems are known to differ in many important ways in the context of B. malayi infection, our observation of the differential effects of rBmAsnRS and IL-8 on signal transduction in the human NF-κB pathway suggests that we may also observe anti-inflammatory effects if rBmAsnRS were administered to humans. Third, we have shown previously that the immunologically active domain of rBmAsnRS is present in an 80-amino-acid amino-terminal domain of the enzyme which is attached by a flexible linker region (33 amino acids) to a large catalytic site domain (438 amino acids), and the biologically active form of rBmAsnRS is a 126-kDa homodimer (15–17). This tremendous size difference between the two molecules that bind IL-8 receptors could be one reason for the differential effects on gene expression.
Drugs that that block select chemokine receptors have an accepted role in prevention or treatment of a wide spectrum of human conditions, including HIV infection, breast and prostate cancer, malignant melanomas, bone marrow transplantation, and organ tissue graft rejection (22, 23). In our study, we observed that the therapeutic effect of rBmAsnRS in the T-cell transfer murine colitis model also corresponded with an apparent shift from a Th1/Th17 cytokine pattern toward a Th2/ regulatory T-cell (Treg) pattern, which is consistent with the immunological effects of some whole parasitic helminth infections in humans (8). While whole parasites and crude parasite extracts have been shown clearly to elicit potent immunostimulatory effects, much fewer purified/recombinant helminth molecules have been clearly demonstrated to elicit strong, well-characterized immunological responses (9). The effects of rBmAsnRS observed herein share some features reported in the Heligmosomoides polygyrus murine colitis model (1, 24), the IL-10 response to the filarial protease inhibitor cystatin (4), the Toll-like receptor response to filarial glycoprotein ES-62 (25), the effects of Schistosoma mansoni egg glycoprotein Omega-1 (26), as well as the protective effects of the rodent filaria Litomosoides sigmoidontis in the nonobese diabetic mouse model (27). Except for the H. polygyrus studies (1, 24), the effect of these other molecules has not been reported in animal models of colitis. The effects of many filarial proteins have been reported previously to require intact IL-10 pathways (8, 10), and Metenou et al. have reported that filarial infections may have expanded adaptive T regulatory but not classical Th2 cells (28). This suggests that immune regulation induced by rBmAsnRS requires intact IL-10 circuitry. However, this theory is not supported by the fact that a live nematode, H. polygyrus, can induce a therapeutic effect in IL-10 knockout mice (1, 24). Thus, it is possible that that there are different mechanisms involved in the H. polygyrus and rBmAsnRS models, yet both result in clinical improvement in colitis.
We speculate that the therapeutic response to rBmAsnRS seen in the T-cell transfer murine colitis model is relevant to therapy of human disease, because the in vivo CD8+ response in mice and in vitro gene expression data in human iDCs have some parallels with beneficial immune responses, as reported previously in the scientific literature. Many insights into the pathogenesis of human IBD have been learned from mouse models of colitis (19). However, given the major differences between mice and humans in response to challenges with B. malayi antigens, it is difficult to anticipate a therapeutic response to rBmAsnRS in humans without some evidence of a differential effect on human cells. We believe that our human iDC gene expression studies provide an encouraging linkage to potential utility in humans. CD8+ CD122+ Tregs and CD4+ Tregs cooperatively prevent and cure mice with CD4+ cell-induced colitis (29). In transgenic colitis mice, protection from colonic tumors was associated with increased accumulation of cytotoxic CD8+ and natural killer T cells in peritumoral areas (30). CD8+ T-cell deficiency has been suggested as part of the unifying hypothesis for autoimmunity (31). Highly suppressive adaptive CD8+ CD25+ FOXP3+ regulatory T cells have been generated by continuous antigen stimulation (32). In humans, analogous CD8+ T-cell transcriptional signatures have been observed that predict prognosis in ulcerative colitis and Crohn's disease (33). Our data in human iDCs indicate that NK cell-mediated cytotoxicity pathways are activated by rBmAsnRS (see Fig. S2 in the supplemental material). Thus, in future work, the effects of rBmAsnRS on the induction of Treg cells should be studied in order to better define the mechanism of action of rBmAsnRS.
The effects of rBmAsnRS in the T-cell transfer murine colitis model share some superficial features of the response to whole filarial nematode infection in humans with chronic filarial lymphedema (11, 12), where there is an association between Toll-like receptor signaling and the development of chronic lymphatic pathology (see Fig. S3 to S5 in the supplemental material). Live B. malayi larvae have been reported to cause alternate activation of human monocytes that are coated with filarial antigens via downregulation of TLR3, -5, and -7 (34). Other immunological features of persons with chronic lymphatic filarial pathology include Toll-like receptor 2-, NF-κB-, and MAPK-dependent regulation of growth factors, including the vascular endothelial growth factor (VEGF) family. With the exception of upregulation of IL-22 receptors, the extensive gene expression studies of a human undergoing many months of Trichuris therapy for his ulcerative colitis (6) do not bear any resemblance to our study in which human iDCs were stimulated for only 72 h.
rBmAsnRS is not the only eukaryotic tRNA synthetase to exhibit chemokine receptor-specific activity. Several human cytoplasmic tRNA synthetases, including the human AsnRS, have been shown to exhibit unexpected chemokine receptor-specific activities in the context of human autoimmune diseases. The human histidyl-tRNA synthetase (HisRS) activates cells that express the chemokine receptor, CCR5, and the human AsnRS activates CCR3 receptors (20). A by-product of leukocyte elastase cleavage of the human tyrosine tRNA synthetase (TyrRS) produces a domain with both IL-8 and anti-angiogenesis effects (35). Interestingly, independent characterization of the secretome of another human parasite, Schistosoma japonicum, revealed that its AsnRS was among the top 100 most abundant secreted proteins identifiable by mass spectrometry (36). In unpublished work, we have shown that recombinant SjAsnRS has potent chemoattractant properties for human monocytes, but they do not appear to be mediated by IL-8, CCR5, or CCR3 receptors (M. Kron and O. M. Zack-Howard, unpublished data). Thus, a pattern seems to be emerging that some eukaryotic tRNA synthetases indeed may act as “physiocrines” (aTyr Pharmaceuticals, San Diego, CA), which are defined as a class of endogenous proteins that function as extracellular signaling molecules in a variety of physiologic settings (37).
In conclusion, our data provide a specific example of the hygiene hypothesis through treatment with a single purified nematode molecule, the filarial rBmAsnRS. The beneficial effects of rBmAsnRS observed herein also support the continued refinement of helminth-mediated immunomodulatory therapy for human IBD or other inflammatory diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank Martin Hessner, Department of Human and Molecular Genetics, Medical College of Wisconsin, for assistance with microarray experiments and for helpful comments.
This work was supported by grants from the U.S. Veterans Administration Health Center (D.E., A.M.) and the U.S. National Institutes of Health (M.A.K.) National Institutes of Allergy and Infectious Diseases, grant AI053877.
The murine experiments were approved by the animal care committee of the University of Iowa.
Footnotes
Published ahead of print 19 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00594-12.
REFERENCES
- 1. Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Weinstock JV. 2004. Heligomosoides polygyrus inhibits established colitis in IL-10 deficient mice. Eur. J. Immunol. 34:2690–2698 [DOI] [PubMed] [Google Scholar]
- 2. Elliott DE, Weinstock JV. 2012. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann. N. Y. Acad. Sci. 1247:83–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ince MN, Elliott DE, Setiawan T, Metwahli A. 2009. Role of T cell TGF-beta signaling in intestinal cytokine response and helminthic immune modulation. Eur. J. Immunol. 39:1870–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, Hamann A, Hamelmann E, Lucious R, Hartmann S. 2008. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J. Immunol. 180:4265–4277 [DOI] [PubMed] [Google Scholar]
- 5. Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Roojen N, Fallon PG. 2007. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 178:4557–4566 [DOI] [PubMed] [Google Scholar]
- 6. Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P. 2010. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci. Transl. Med. 2:1–11 [DOI] [PubMed] [Google Scholar]
- 7. Weinstock JV, Elliott DE. 2009. Helminths and the IBD hygiene hypothesis. Inflamm. Bowel Dis. 15:128–133 [DOI] [PubMed] [Google Scholar]
- 8. Allen J, Maizels R. 2011. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 11:375–388 [DOI] [PubMed] [Google Scholar]
- 9. Freitas TC, Pearce EJ. 2010. Growth factors and chemotactic factors from parasitic helminths: molecular evidence for roles in host-parasite interactions versus parasite development. Int. J. Parasitol. 40:761–773 [DOI] [PubMed] [Google Scholar]
- 10. Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 201:89–116 [DOI] [PubMed] [Google Scholar]
- 11. Babu S, Anuradha R, Kumar NP, George PJ, Kumaraswami V, Nutman TB. 2012. Toll-like receptor- and filarial antigen-mediated, mitogen-activated protein kinase- and NF-κ B-dependent regulation of angiogenic growth factors in filarial lymphatic pathology. Infect. Immun. 80:2509–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Venugopal PG, Nutman TB, Semnani RT. 2009. Activation and regulation of Toll-like receptors (TLRs) by helminth parasites. Immunol. Res. 43:252–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elliott CL, Allport VC, Loudon Wu JAGD, Bennett PR. 2001. Nuclear factor-kappa B is essential for up regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol. Hum. Reprod. 7:787–790 [DOI] [PubMed] [Google Scholar]
- 14. Martin D, Galistero R, Gutkind JS. 2009. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGF2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 284:6038–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramirez BL, Howard OM, Dong HF, Edamatsu T, Gao P, Haertlein M, Kron MA. 2006. Brugia malayi asparaginyl-tRNA synthetase induces chemotaxis of human leukocytes and activates G-protein coupled receptors CXCR1 and CXCR2. J. Infect. Dis. 193:1164–1171 [DOI] [PubMed] [Google Scholar]
- 16. Crepin T, Peterson F, Haertlein M, Jensen D, Wang C, Cusack S, Kron M. 2011. A hybrid model of the complete Brugia malayi cytoplasmic asparaginyl-tRNA synthetase. J. Mol. Biol. 405:1056–1069 [DOI] [PubMed] [Google Scholar]
- 17. Kron M, Wang C, Vodanovic-Jankovic S, Howard O, Kuhn LA. 2012. Interleukin-8-like activity in a filarial asparaginyl-tRNA synthetase. Mol. Biochem. Parasit. 185:66–69 [DOI] [PubMed] [Google Scholar]
- 18. Baumgart DC, Thomas S, Przesdzing I, Metzke D, Bielecki C, Lehmann SM, Lenhardt S, Dorffel Y, Sturm A, Scheffold A, Schmitz J, Radbruch A. 2009. Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin. Exp. Immunol. 157:423–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boismenu R, Chen Y. 2000. Insights from mouse models of colitis. J. Leukoc. Biol. 67:267–278 [DOI] [PubMed] [Google Scholar]
- 20. Howard OM, Dong HF, Yang D, Raben N, Nagaraju K, Rosen A, Casciola-Rosen L, Hartlein M, Kron M, Yang D, Yiadom K, Dwivedi S, Plotz PH, Oppenheim JJ. 2002. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J. Exp. Med. 196:781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang DW, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4:44–57 [DOI] [PubMed] [Google Scholar]
- 22. O'Hayre M, Salanga CL, Handel TM, Hamel DJ. 2010. Emerging concepts and approaches for chemokine-receptor drug discovery. Expert Opin. Drug Discov. 5:1109–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Proudfoot AE, Power CA, Schwarz MK. 2010. Anti-chemokine small molecule drugs: a promising future? Exp. Opin. Invest. Drugs 19:345–355 [DOI] [PubMed] [Google Scholar]
- 24. Metwali A, Setiawan T, Blum AM, Urban J, Elliott DE, Hang L, Weinstock JV. 2006. Induction of CD8 regulatory T cells in the intestine by Helicosomoides polygyrus infection. Am. J. Physiol. Gastrointest. Liver Physiol. 291:G253–G259 [DOI] [PubMed] [Google Scholar]
- 25. Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, Liew FY, Harnett W, Harnett MM. 2005. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J. Immunol. 174:284–293 [DOI] [PubMed] [Google Scholar]
- 26. Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, Doenhoff MJ, van der Bosch J, Mohrs K, Haas H, Mohrs M, Yazdanbakhsh M, Schramm G. 2009. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 8:1673–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hubner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, Gondorf F, Hoerauf A, Killoran KE, Stocker JT, Davies SJ, Tarbell KV, Mitre E. 2012. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-Beta. J. Immunol. 188:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, Soumaoro L, Coulibaly ME, Sanogo D, Doumbia SS, Traore SF, Mahanty S, Klion A, Nutman TB. 2010. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 184:5375–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Endharti AT, Okuno Y, Misawa N, Toyokuni S, Ito M, Isobe K, Suzuki H. 2011. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J. Immunol. 186:41–52 [DOI] [PubMed] [Google Scholar]
- 30. Rizzo A, Waldner MJ, Stolfi C, Sarra M, Fina D, Becker C, Neurath MF, Macdonald TT, Pallone F, Monteleone G, Fantini MC. 2011. Smad7 expression in T cells prevents colitis-associated cancer. Cancer Res. 71:7423–7432 [DOI] [PubMed] [Google Scholar]
- 31. Pender MJ. 2012. CD8+ T-cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to autoimmunity: a unifying hypothesis. Autoimmune Dis. 2012:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, Aandahl EM. 2008. Generation of highly suppressive adaptive CD8+CD25+FOXP3+ regulatory T cells by continuous antigen stimulation. Eur. J. Immunol. 38:640–646 [DOI] [PubMed] [Google Scholar]
- 33. Lee JC, Lyons PA, McKinney EF, Sowerby JM, Carr EJ, Bredin F, Rickman HM, Ratlamwala H, Hatton A, Rayner TF, Parkes M, Smith KG. 2011. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn's disease and ulcerative colitis. J. Clin. Invest. 121:4170–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Semnani RT, Mahapatra L, Moore V, Sanprasert V, Nutman TB. 2011. Functional and phenotypic characteristics of alternative activation induced in human monocytes by interleukin-4 or the parasitic nematode Brugia malayi. Infect. Immun. 79:3957–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tzima E, Schimmel P. 2006. Inhibition of tumor angiogenesis by a natural fragment of a tRNA synthetase. Trends Biochem. Sci. 31:7–10 [DOI] [PubMed] [Google Scholar]
- 36. Liu F, Cui SJ, Hu W, Feng Z, Wang ZQ, Han ZG. 2009. Excretory secretory proteome of the adult development stage of the human blood fluke, Schistosoma japonicum. Mol. Cell. Proteomics 8:1236–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo M, Yang X-L, Schimmel P. 2010. New functions of tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 11:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


