Abstract
Respiratory syncytial virus (RSV) infects elderly (≥65 years) adults, causing medically attended illness and hospitalizations. While RSV neutralizing antibody levels correlate inversely with RSV-associated hospitalization in the elderly, the role of RSV-specific T cells in preventing disease in the elderly remains unclear. We examined RSV-specific humoral, mucosal, and cellular immune profiles in healthy elderly (65 to 85 years) and young (20 to 30 years) adults. RSV neutralization antibody titers in the elderly (10.5 ± 2.2 log2) and young (10.5 ± 2.1 log2) were similar. In contrast, levels of RSV F protein-specific gamma interferon (IFN-γ)-producing T cells were lower in elderly (180 ± 80 spot-forming cells [SFC]/106 peripheral blood mononuclear cells [PBMC]) than in young adults (1,250 ± 420 SFC/106 PBMC). Higher levels of interleukin-13 (IL-13; 3,000 ± 1,000 pg/ml) in cultured PBMC supernatants and lower frequency of RSV F-specific CD107a+ CD8+ T cells (3.0% ± 1.6% versus 5.0% ± 1.6%) were measured in PBMC from elderly than young adults. These results suggest that deficient RSV F-specific T cell responses contribute to susceptibility to severe RSV disease in elderly adults.
INTRODUCTION
Respiratory syncytial virus (RSV) causes annual outbreaks of respiratory disease. In North America and western Europe, these outbreaks are seasonal, occurring in winter and lasting for about 4 months. While the high global disease burden of RSV in young children and infants is well documented (1–5), the epidemiology of RSV illness in elderly adults is less well defined. Data from a variety of studies (6–14) suggest that in U.S. adults over 65 years of age, the overall annual incidence of RSV illness is ∼3 to 4%, with an estimated annual RSV-associated hospitalization rate of ∼0.1 to 0.4% and an estimated 10,000 RSV-associated deaths per year (Table 1).
Table 1.
RSV epidemiology in U.S. elderly (≥65 years)
| Parameter | Incidence rate (%) | Total no. of casesa |
|---|---|---|
| RSV illnesses/year | ∼30/1,000 (∼3) | ∼1,200,000 |
| RSV medically attended illnesses/year | ∼10/1,000 (∼1) | ∼420,000 |
| RSV-associated hospitalizations/year | ∼1.5/1,000 (∼0.15) | ∼45,000–50,000 |
| RSV-associated deaths/year | ∼0.25/1,000 (∼0.025) | ∼10,000 |
Total cases based on a U.S. population of ∼40,000,000 elderly, i.e., ≥65 years of age.
The immune correlates associated with increased susceptibility to severe RSV illness in the elderly are not well understood. Serum anti-RSV neutralizing antibody titers have been reported to inversely correlate with an increased risk of RSV-associated hospitalizations in the elderly (15). Other studies have found that the RSV-specific memory CD8+ T cells are reduced in the peripheral blood of healthy elderly adults (16, 17), and that a switch from a CD4+ Th1 to a Th2 functional phenotype occurs with age (17). One report suggested that aging is associated with a defect in T cell responses to RSV, and this defect in cellular immunity is related to RSV disease susceptibility in older adults (18). These studies suggest that either waning RSV-specific neutralizing antibodies or declining cell-mediated immunity, or a combination of both, contribute to the greater severity of RSV disease in elderly compared to young adults.
Our immune profiling studies revealed that plasma from healthy young and elderly adults had comparably high RSV neutralizing antibody titers. However, RSV F protein-specific memory CD4+ and CD8+ T cell responses were significantly lower in the elderly than young donors, suggesting that deficient RSV F-specific T cell responses contribute to susceptibility to severe RSV disease in this population. Further characterization of RSV-specific immune deficits in the elderly may help elucidate the underlying mechanisms mediating protection against severe RSV disease, thereby facilitating the design and development of RSV vaccines for the elderly.
MATERIALS AND METHODS
Study cohort.
Thirty young adults who were 20 to 30 years old (median age, 26 years) and 30 elderly individuals who were 65 to 85 years old (median age, 74 years) were enrolled. All subjects were healthy and free of respiratory illness and had no hospitalization episodes for a 2-month period prior to sample collection by SeraCare Life Sciences, Inc. (Milford, MA), and Bioreclamation (Hicksville, NY). Informed consents given by all subjects were approved by Bioreclamation's Independent Institutional Review Board. Since the amount of available peripheral blood mononuclear cells (PBMC) was insufficient to perform every assay on every donor sample, we used the indicated number of donor samples in each assay to enable reasonable comparisons between the age cohorts. The subjects' demographic characteristics and the number and type of samples assessed in each immunological assay are shown in Table 2.
Table 2.
Demographic characteristics of the study cohort and assays performed
| Demographic or assay parameter | Demographic or assay result by age |
P value | |
|---|---|---|---|
| 20–30 yr old (young) | 65–85 yr old (elderly) | ||
| Demographics | |||
| Age (yr; median ± SD) | 26 ± 3 | 74 ± 6 | <0.0001 |
| Age (yr; range) | 20–30 | 65–85 | |
| Gender (no. [%]) | |||
| Male | 14 (46.7) | 12 (40) | 0.6021 |
| Female | 16 (53.3) | 18 (60) | 0.6020 |
| Race (no. [%]) | |||
| Caucasian | 8 (26.7) | 7 (23.3) | 0.3903 |
| Hispanic | 11 (36.7) | 7 (23.3) | 0.4196 |
| African-American | 11 (36.7) | 16 (53.3) | 0.2776 |
| Assay parameters [mean ± SD (no. of samples/assay)] | |||
| Sample | |||
| Plasma | |||
| RSV neutralization (log2 titer) | 10.5 ± 2.2 (30) | 10.5 ± 2.1 (30) | 0.7758 |
| RSV F IgG ELISA (×103 titer) | 0.90 ± 0.31 (20) | 0.93 ± 0.36 (20) | 0.8821 |
| Nasal wash | |||
| RSV F IgA ELISA (RLU) | 1.4 × 104 ± 1.2 × 104 (10) | 1.0 × 104 ± 1.1 × 104 (20) | 0.7694 |
| PBMC | |||
| Memory B cell ELISPOT (% F-specific ASC/total IgG ASC) | 0.22 ± 0.25 (10) | 0.18 ± 0.5 (20) | 0.2413 |
| Memory T cell ELISPOT (SFC/106 PBMC) | |||
| RSV A2 virus | 360 ± 130 (12) | 93 ± 41 (20) | 0.0262 |
| RSV F protein | 1,250 ± 420 (20) | 180 ± 80 (20) | <0.0001 |
| CD4+ T cell peptides | 450 ± 180 (12) | 100 ± 60 (12) | <0.0001 |
| CD8+ T cell peptides | 400 ± 210 (12) | 90 ± 55 (12) | <0.0001 |
| CD107a flow cytometry (% CD107a+ CD8+ T cells) | |||
| RSV F protein | 5.0 ± 1.6 (14) | 3.0 ± 1.7 (14) | 0.0173 |
| CD8+ T cell peptides | 4.7 ± 1.9 (14) | 2.3 ± 1.8 (14) | 0.0045 |
| Th1-Th2 cytokines (pg/ml) | 7,500 ± 3,100 (10) | 3,000 ± 800 (10) | 0.0004 |
| RSV F IFN-γ | 475 ± 450 (10) | 5,000 ± 4,000 (10) | 0.0017 |
| RSV F IL-13 | 1,000 ± 560 (10) | 3,000 ± 1,000 (10) | 0.0033 |
Specimen collection and processing.
All specimens (whole blood, plasma, and nasal washes) were collected between the months of May and July and transported at ambient temperature to the processing site within 2 h of sample draw. PBMC were isolated from fresh whole blood using serum-free medium conditions, and frozen PBMC were placed at −80°C overnight before being transferred to liquid nitrogen for storage. Samples were shipped overnight on dry ice to MedImmune within 2 weeks of freezing and were thawed for enzyme-linked immunosorbent spot (ELISPOT) assay evaluation within 3 days of arrival. Plasma and nasal wash samples were aliquoted into 1-ml cryovials (Thermo Scientific, Rochester, NY) and frozen at −80°C at the processing site within 2 h of collection. Samples were shipped overnight on dry ice to MedImmune within 2 weeks of freezing and were thawed for evaluation within 1 week of arrival.
RSV antigens.
The soluble fusion F protein of RSV A2 was expressed in Chinese hamster ovary (CHO) cells and immunoaffinity purified with an anti-RSV F monoclonal antibody (palivizumab; MedImmune, Gaithersburg, MD) to >95% purity. The sequence of the soluble RSV F protein lacking the transmembrane and cytoplasmic tail has been previously described (19). Wild-type (wt) RSV A2 and recombinant green fluorescent protein (GFP)-tagged RSV A2 were produced in Vero cells. RSV F-specific CD4+ and CD8+ T cell immunodominant peptides described elsewhere (20–23) were custom designed and ordered at >90% purity from ProImmune (Oxford, United Kingdom). Although the major histocompatibility complex (MHC) restriction of some of the peptides in the pools was unknown, we utilized the information available in the published reports (20–23) to optimize our RSV F-specific CD4+ and CD8+ T cell peptide pools for use in ELISPOT and cytokine multiplex assays.
RSV microneutralization assay.
Donor plasma samples were heat inactivated at 56°C and serially diluted 2-fold in growth medium. Equal volumes of the diluted plasma were mixed with GFP-tagged RSV A2 virus at a concentration of 500 PFU/well. Samples were preincubated at 33°C for 1 h to facilitate antibody neutralization of the GFP-tagged RSV A2 virus. Samples were used to infect confluent monolayers of Vero cells (ATCC, Manassas, VA), and the cell plates were incubated at 33°C for 22 h. Fluorescent viral foci were enumerated using an IsoCyte Reader (Blueshift Biotechnologies, Sunnyvale, CA), and a 50% reduction in fluorescent viral foci was calculated using a 4-parameter curve-fit algorithm. Data were expressed as log2 neutralization titers.
RSV F-specific IgG by ELISA.
RSV F-specific IgG antibody titers in plasma were measured by enzyme-linked immunosorbent assay (ELISA). The plasma samples were serially diluted 2-fold and incubated on RSV F-coated plates for 1 h at 37°C. Horseradish peroxidase (HRP)-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch, West Grove, PA) was diluted 1:20,000 and incubated with the samples for 1 h at 37°C. Titers were reported as endpoint titers.
RSV F-specific nasal wash IgA.
Nasal wash samples were prediluted 1:4 in matrix inhibition buffer (MIB), which contains bovine serum albumin (BSA), polysorbate 20, EDTA, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, and NaCl in phosphate-buffered saline (PBS), followed by a 1:3 serial dilution in MIB into duplicate wells of a 96-well plate coated with 0.4 μg/ml RSV F or 0.2 μg/ml purified goat anti-human IgA+IgG+IgM (H+L) (KPL, Gaithersburg, MD). A pooled human reference plasma standard (AllCells, LLC, Emeryville, CA) was included on each plate for comparison, and all plates were incubated for 1 h. A SulfoTag-labeled goat anti-human IgA antibody (Jackson ImmunoResearch, West Grove, PA) was added to the plates at a 1:2,000 dilution in MIB, and plates were incubated at 37°C for 1 h. After a final plate wash, the MSD read buffer (Meso Scale Discovery, Gaithersburg, MD) was added, and the plates were imaged using the MSD Sector Imager 6000. The RSV F-specific and total IgA endpoint titers were calculated using SoftMax Pro. The total protein concentration in the nasal wash samples was determined using the Pierce BCA (bicinchoninic acid) assay kit (Thermo Scientific, Rockford, IL).
Memory B cell expansion and RSV F-specific ELISPOT assay.
Peripheral blood mononuclear cells (PBMC) were stimulated using a polyclonal stimulation cocktail containing pokeweed mitogen (Sigma-Aldrich, St. Louis, MO), S. aureus strain Cowan protein A (Sigma-Aldrich), and CpG oligonucleotide (ODN 2006; InvivoGen, San Diego, CA) for 5 days at 37°C as described previously (24). Unstimulated PBMC served as the negative control. Expanded memory B cells were incubated in ELISPOT plates (Millipore, Billerica, MA) coated with 10 μg/ml RSV F or goat anti-human IgA+IgG+IgM antibody (KPL, Gaithersburg, MD) to detect antigen-specific antibody-secreting cells (ASC) or total IgG/IgA-producing ASC, respectively. Spots were enumerated using an ImmunoSpot analyzer (Cellular Technologies Ltd., Cleveland, OH).
RSV-F-specific IFN-γ ELISPOT assay.
Human gamma interferon (IFN-γ) ELISPOT kits containing anti-IFN-γ-precoated plates and anti-IFN-γ detection antibodies were purchased from Mabtech, Inc. (Mariemont, OH), and the assay was carried out per the manufacturer's instructions. PBMC were incubated for 24 h at 37°C on the ELISPOT plates together with wt RSV A2 virus (1 PFU/10 cells) or 5 μg/ml of RSV F or 2 μg/ml of RSV F-specific CD4+/CD8+ T cell peptide pools (ProImmune, Oxford, United Kingdom). The peptide pools were custom designed as described in the review by Olson and Varga to represent 12 CD8+ T cell epitopes and 21 CD4+ T cell epitopes of RSV F, respectively (21). Cells incubated with CTL-test medium (Cellular Technology Ltd., Cleveland, OH) or phorbol 12-myristate 13-acetate (PMA) and ionomycin (Sigma, St. Louis, MO) served as the negative and positive controls, respectively. Data were expressed as spot-forming cells (SFC)/106 PBMC after background subtraction of medium wells to indicate a positive response. Valid samples were defined as those with viabilities of ≥70%, medium background of ≤50 SFC/106 PBMC, and PMA/ionomycin response of ≥500 SFC/106 PBMC.
Th1/Th2 cytokine profiling by Luminex.
PBMC were incubated with 5 μg/ml of RSV F for 72 h. Medium alone or PMA with ionomycin stimulation served as the negative and positive control for each sample, respectively. Human cytokine multiplex kits were custom designed to include IFN-γ, interleukin-2 (IL-2), IL-4, IL-5, IL-10, IL-12(p70), IL-13, IL-17, IP-10, and tumor necrosis factor alpha (TNF-α; Millipore, Billerica, MA). The assay was performed per the manufacturer's instructions. The culture supernatants were analyzed on a Bio-Rad BioPlex 2200 (Bio-Rad, Hercules, CA), and cytokine concentrations are expressed in pg/ml.
CD107a expression on CD8+ T cells.
Donor samples that were tested in the IFN-γ ELISPOT assay were also stained for CD107a expression following RSV F-specific stimulation. Cells were incubated with 5 μg/ml of RSV F or 2 μg/ml of RSV F-specific CD8+ T cell peptide pool for 8 h. Phycoerythrin (PE)-labeled CD107a or isotype control (BD Biosciences, San Jose, CA) antibody was added to the wells. Four hours later, 0.5 μg/ml of the Golgi plug, brefeldin A (BD Biosciences, San Jose, CA), was added for the last 8 h of incubation. Medium alone or PMA with ionomycin-treated cells served as the negative and positive controls, respectively. Cells were surface stained with an antibody cocktail (BD Biosciences) to label CD3, CD8, CD19, and CD56 for 30 min. Cells were fixed, permeabilized, and incubated with PE-labeled CD107a or isotype control antibody for 30 min. All antibody incubations were carried out at 4°C. Samples were analyzed on a FACS Canto (BD Biosciences), and data are expressed as percent CD107a+ CD8+ T cells, fold change relative to the medium, and mean fluorescence intensity (MFI).
Statistical analysis.
The Pearson's correlation coefficient was used to measure the strength of the correlation between the different serological and cellular markers evaluated in the study cohorts. Statistical significance of the difference in the means between the young and elderly IFN-γ SFC/106 PBMC was determined using analysis of variance (ANOVA) and the two-sample t test.
RESULTS
Young and elderly adults have comparable titers of neutralizing antibodies to RSV.
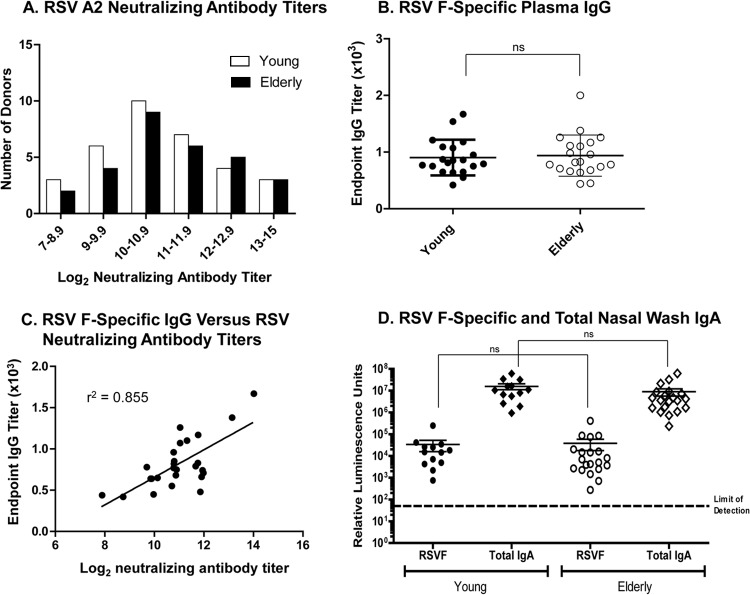
To determine whether the higher incidence of severe RSV disease in the elderly was related to the neutralizing antibody titers against RSV, we first examined RSV neutralizing antibody levels in plasma from 30 healthy young versus 30 elderly adults. Neutralizing antibody titers to RSV were determined using a microneutralization assay to a GFP-RSV A2 virus. The distribution range of the GFP-RSV A2 virus-specific neutralizing antibody titers across donors in the two cohorts demonstrated that the majority of the donors had RSV neutralizing antibody titers between 10 and 12 log2, with the elderly cohort showing slightly higher numbers of donors with titers between 12 and 15 log2 (Fig. 1A). An average neutralizing antibody titer of 10.5 ± 2.2 log2 was observed in both cohorts.
Fig 1.
Equivalent RSV-specific antibody levels in plasma of young and elderly donors. (A) RSV-specific antibody titers in plasma from young (n = 30) and elderly (n = 30) donors were compared by microneutralization of GFP-RSV A2 virus. (B) F-specific IgG ELISA. (C) Correlation analysis between neutralizing antibody titers and F-specific IgG titers. (D) RSV F-specific and total IgA titers in nasal washes of young (n = 10) and elderly (n = 20) adults.
Since neutralizing antibody titers can include antibodies to both RSV F and G proteins, we next examined whether there were measurable differences in the RSV F-specific IgG titers between the young and elderly donors. The young and elderly donors also had equivalent titers of RSV F-specific plasma IgG antibodies (Fig. 1B), and there was a strong correlation (r2 = 0.855) between RSV A2 neutralizing antibody and RSV F-specific IgG titers (Fig. 1C). Presence of RSV F-specific nasal IgA antibodies has been associated with protection from severe RSV disease in the elderly; therefore, we compared RSV F-specific IgA titers in the nasal washes of the young and elderly donors (Fig. 1D). We also measured the total IgA and protein concentration levels (data not shown) in the nasal washes and did not find significant differences between the young and elderly samples. Importantly, the RSV F-specific IgA titers were equivalent between the young and elderly donors (Fig. 1D), suggesting that other deficits in the immune system account for susceptibility to RSV disease in the elderly.
RSV F-specific memory B cell frequencies are comparable in the young and elderly.
We next explored the RSV F-specific memory B cell frequency in PBMC to determine if there were quantitative differences between young and elderly donors. Young adults showed an RSV F-specific IgG ASC frequency ranging from 0.05 to 0.5% of the total IgG ASC, whereas elderly adults showed an RSV F-specific IgG ASC frequency ranging from 0 to 0.68% of the total IgG ASC frequency (Fig. 2). The difference in the frequency of RSV F-specific IgG ASC between the two cohorts as calculated by the unpaired t test was not statistically significant (P = 0.2413). However, an overall trend of reduced RSV F-specific memory B cell frequency in the elderly was noted, except for two donors that possessed relatively high F-specific memory IgG ASC frequency (Fig. 2). Taken together, these data indicated that the elderly were not deficient in neutralizing antibody titers or RSV F-specific memory B cell responses.
Fig 2.
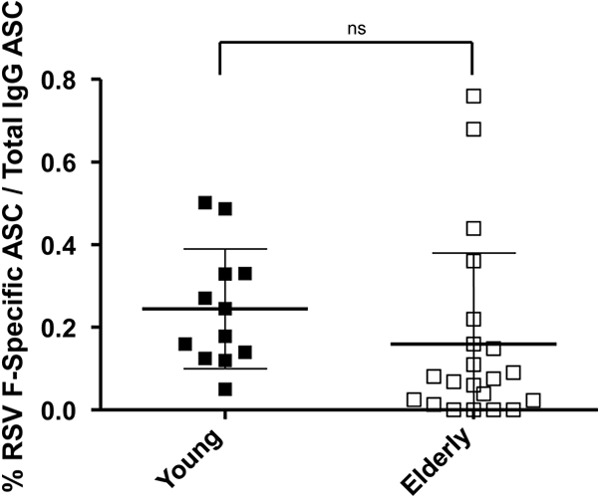
RSV F-specific memory B cells in young and elderly donors. RSV F-specific memory B cells were measured in young (n = 10) and elderly (n = 20) donor PBMC. PBMC cultures were stimulated ex vivo with a mixture of pokeweed mitogen, S. aureus protein A, and CpG DNA. The expanded RSV F-specific antibody-secreting cells (ASC) were measured in a memory B cell ELISPOT assay. Data are expressed as percent RSV F-specific IgG ASC/total IgG ASC.
Elderly adults have lower levels of RSV F-specific IFN-γ-producing cells than young adults.
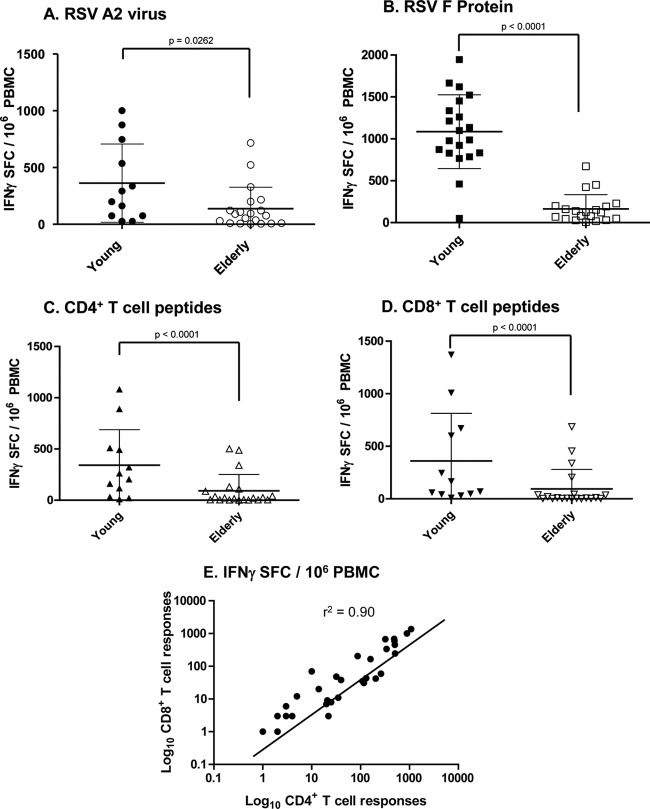
We decided to evaluate whether RSV F-specific memory T cell responses would be significantly different between the young and elderly. Findings on the frequency of RSV F-specific T cell immune responses in PBMC from the young and elderly donors are summarized in Table 2. In contrast to findings for plasma neutralizing antibody titers and memory B cell responses, PBMC from elderly donors had reduced RSV-specific IFN-γ responses to whole virus (Fig. 3A), F antigen (Fig. 3B), and F-specific immunodominant CD4+ and CD8+ T cell peptide pools (Fig. 3C and D) compared to PBMC from young donors. RSV A2 virus-specific IFN-γ-producing memory T cell frequency/106 PBMC was 360 ± 130 SFC in the young and 93 ± 41 SFC in the elderly (P = 0.0262) (Fig. 3A). Total RSV F-specific IFN-γ-producing cell frequency in the young cohort was 1,250 ± 420 SFC, while that observed in the elderly was only 180 ± 80 SFC (P < 0.0001) (Fig. 3B). Further examination of the RSV F-specific memory T cell responses revealed that the IFN-γ-producing CD4+ T cell frequency in the young was 450 ± 180 SFC, while that seen in the elderly was 100 ± 60 SFC (P < 0.0001) (Fig. 3C). The CD8+ T cell frequency in the young was 400 ± 210 SFC and 90 ± 55 SFC in the elderly (P < 0.0001) (Fig. 3D). Finally, there existed a strong correlation (r2 = 0.887) between the RSV F-specific IFN-γ-producing CD4+ and CD8+ T cell numbers in both of these cohorts (Fig. 3E). Thus, the observed deficit in the numbers of functional memory T cells in the elderly existed in both the CD4+ and CD8+ T cell compartments. Taken together, our results suggest that the reduced numbers of RSV F-specific IFN-γ-producing T cells contribute to the lack of complete immunity against RSV, predisposing elderly adults to disease.
Fig 3.
Elderly have significantly lower numbers of RSV F-specific IFN-γ-producing cells. PBMC from young and elderly donors were stimulated ex vivo using (A) wt RSV A2 at 1 PFU/10 cells (n = 12 young and n = 20 elderly), (B) 5 μg/ml of RSV F protein (n = 20 young and n = 20 elderly), (C) 2 μg/ml of RSV F-specific CD4+ T cell peptide pools (n = 12 young and n = 12 elderly), or (D) 2 μg/ml of RSV F-specific CD8+ T cell peptide pools (n = 12 young and n = 12 elderly). The IFN-γ secreted by T cells was measured by ELISPOT assay, and data are expressed as spot-forming cells (SFC)/106 PBMC. (E) Correlation between RSV F-specific CD4+ and CD8+ T cell frequencies.
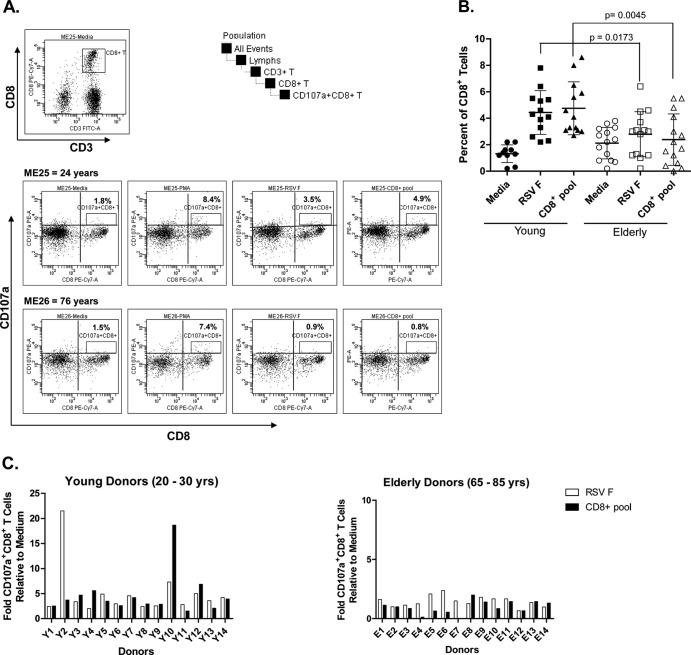
Elderly humans have significantly lower numbers of degranulating RSV F-specific CD107a+ CD8+ T cells.
The comparative cytolytic potential of the RSV F-specific CD8+ T cells in PBMC from the two cohorts was evaluated by quantifying the number of CD107a-expressing degranulating CD8+ T cells following an in vitro stimulation of PBMC with RSV F or a peptide pool containing RSV F-specific immunodominant CD8+ T cell peptides. The CD107a or LAMP1 (for lysosomal associated membrane protein-1) protein is intracellularly expressed in activated CD8+ T cells and natural killer T cells and is a marker of activated cytotoxic T cells (25). The gating strategy utilized in the study and acquisition plots for medium, PMA/ionomycin, RSV F protein, or CD8+ T cell peptide pool stimulation are illustrated for representative young (24 years) and elderly (76 years) donor PBMC (Fig. 4A). Different degrees of background CD107a expression were observed in all samples (Fig. 4B). However, upon incubation with RSV F protein or CD8+ T cell peptide pool, this number increased from a baseline of 1.5 to 5% of the parent CD8+ T cell population in the young cohort compared to an increase from 2.0 to 3.0% of the parent CD8+ T cell population following antigen incubation in PBMC from the elderly (P = 0.005) (Fig. 4B). The fold change relative to medium in the percentage of CD107a+ CD8+ T cells following RSV F or CD8+ peptide pool stimulation was calculated and showed an increase of 2- to 22-fold in young and 0- to 2.5-fold in elderly donor PBMC, respectively (P = 0.005) (Fig. 4C). The mean fluorescence intensity (MFI) of the RSV F-specific CD107a+ CD8+ T cells was equivalent in the elderly and young donor PBMC samples (data not shown), suggesting that the difference between the young and elderly was the frequency of CD8+ T cells expressing CD107a rather than the number of CD107a molecules expressed on each RSV F-specific CD8+ T cell. The lower numbers of RSV F-specific CD107a+ CD8+ T cells in the elderly suggests a potential mechanism for the higher susceptibility to RSV disease in this population.
Fig 4.
Elderly PBMC have lower numbers of RSV F-specific CD107a+ CD8+ T cells. PBMC cultures were incubated in medium alone or were stimulated with RSV F protein or an RSV F-specific CD8+ T cell peptide pool. (A) CD107a gating strategy and acquisition data plots for medium, PMA/ionomycin, RSV F, or CD8+ T cell peptide pool stimulation of representative young (ME25) and elderly (ME26) donor PBMC. (B) The percentages of CD107a+ CD8+ T cells were quantified using multiparameter flow cytometry in young (n = 14) and elderly (n = 14) donor PBMC. (C) Fold CD107a+ CD8+ T cell numbers relative to medium following stimulation of young (n = 14) and elderly (n = 14) donor PBMC samples with RSV F protein or CD8+ T cell peptide pool.
Elderly donor PBMC secrete relatively high basal levels of IL-13 ex vivo.
The functional activity of the RSV F-specific T cells was examined ex vivo by measuring cytokine production in PBMC in response to stimulation with RSV F antigen or a peptide pool containing RSV F-specific immunodominant CD4+ T cell peptides. The cytokines that were undetectable in the cell supernatants of PBMC cultures from either cohort included IL-2, IL-4, IL-5, IL-17, IL-12(p70), and TNF-α. In contrast, cytokines that were present in measurable quantities in the cell supernatants included IFN-γ, IP-10, IL-10, and IL-13. Basal levels of the Th1 cytokine, IFN-γ, were secreted at low levels in both cohorts. However, upon RSV F stimulation, considerably higher levels of IFN-γ (7,500 ± 3,100 pg/ml) were produced by PBMC from young compared to elderly donors (3,000 ± 1,000 pg/ml) (P = 0.0004) (Fig. 5A). This finding was consistent with the results obtained in the IFN-γ T cell ELISPOT assay, where significantly higher numbers of IFN-γ-producing T cells were observed in PBMC from the young cohort (Fig. 3). Interestingly, PBMC from elderly donors secreted relatively high basal levels of the Th2 cytokine, IL-13 (2,500 ± 2,000 pg/ml), compared to the levels produced by PBMC from young donors (800 ± 600 pg/ml) (Fig. 5B). Stimulation of elderly or young PBMC with RSV F slightly increased IL-13 secretion; however, the increase above basal levels was not statistically significant in either cohort (Fig. 5B). The inability of elderly PBMC to secrete high levels of IFN-γ upon RSV F stimulation seemed to reflect the inherently weak Th1 antigenic responses observed in aged individuals. Moreover, the high basal levels of IL-13 are consistent with a Th2 polarization of the CD4+ T cell compartment in this population. Both of these factors may contribute to the increased susceptibility to RSV disease observed in the elderly.
Fig 5.
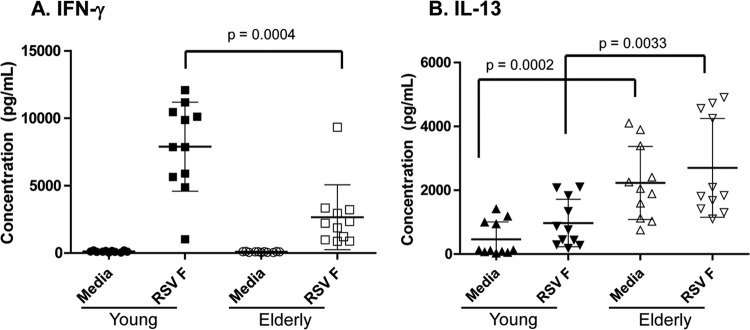
Elderly PBMC secrete relatively low levels of IFN-γ in conjunction with secreting high basal levels of IL-13 ex vivo. PBMC cultures (n = 10 young and n = 10 elderly) were incubated in medium alone or with RSV F protein, and the resulting cytokines secreted into the supernatant were measured using a multiplex Th1-Th2 cytokine bead array panel (Luminex). (A) IFN-γ. (Β) IL-13.
DISCUSSION
In this study, we explored immunological factors that could contribute to the higher susceptibility of elderly adults to severe disease by measuring the RSV F-specific humoral and cellular immune responses in healthy elderly and young adults. Compared to the overall U.S. population (26), the African-American and Hispanic numbers in both our study cohorts appeared relatively overrepresented, while the Caucasian numbers appeared relatively underrepresented (Table 2). However, analysis of the RSV F-specific responses obtained in the different assays by race indicated that the results were not influenced by racial or ethnic origin of the donors (data not shown).
RSV infection in adults elicits a robust serum antibody response (27, 28). The neutralizing antibody titers to GFP-tagged RSV A2 measured in the elderly donors in our study were consistent with previous findings (27–30). RSV F- and G-specific IgA antibodies measured in nasal washes of older adults ≥65 years of age have been shown to correlate with protection from RSV disease (31). We expect a successful RSV vaccine candidate that prevents disease in elderly adults to elicit robust and durable humoral, mucosal, and T cell-mediated immunity (31). It has been shown that RSV-specific IgE antibodies can be detected in the nasopharyngeal secretions of patients with acute RSV bronchiolitis, and interestingly, the levels correlated inversely with the number of circulating RSV-specific CD8+ T cells (32). Evidence indicates that RSV-specific IgE-mediated mast cell degranulation and histamine release in the airway epithelium, along with the induction of Th2-type cytokines in the respiratory tract, potentiates the clinical symptoms of severe RSV disease (33). Our data showed no measurable RSV-specific serologic, mucosal, or memory B cell deficits in elderly adults (Table 2), implying that these immune responses are insufficient to fully protect against RSV disease in this population.
We hypothesized that T cell responses to RSV proteins play an important role in clearing RSV infections, and at least one report has suggested that the persistence of RSV-specific memory CD8+ T cells following primary infection is important in preventing severe RSV disease (16). Moreover, RSV-specific CD8+ T cells have been detected in the peripheral blood of previously infected adults in whom these responses have been associated with decreased clinical symptoms (34, 35). The contribution of the different antigen-specific T cell subsets to recovery from RSV infection has been extensively studied in mouse models, and long-lived RSV-specific cytotoxic T cell function has been demonstrated to be critical for the accelerated clearance of virus from the lungs of infected animals (36–40). Recently, it was demonstrated that RSV-specific CD8+ T cells induced upon vaccination protected mice against RSV infection and pathogenesis, and the authors reported that waning protection correlated with reduced CD8+ T cell cytokine expression (41).
To determine if impaired RSV-specific T cell numbers and/or function could account for the susceptibility to severe RSV illness in the elderly, we compared the RSV F-specific CD4+ and CD8+ T cells in PBMC from elderly and young adults. We utilized previously published RSV F T cell epitope mapping results (20–23) to custom synthesize T cell peptide pools consisting of 12mers or 9mers to evaluate putative CD4+ or CD8+ T cell responses, respectively, realizing that the lack of a response in our ELISPOT assay may also be attributed to HLA mismatch between the stimulating peptide and the donor PBMC. Notably, the young-adult PBMC produced significantly higher IFN-γ responses to the same peptide pools than elderly PBMC. Furthermore, elderly PBMC appeared to have comparable numbers of total CD3+, CD4+, and CD8+ T cells and CD19+ B cells compared to young-adult PBMC based on our flow cytometry studies (data not shown). Future studies will focus on delineating the CD4+ versus CD8+ T cell responses in the ELISPOT assay by performing T cell subset depletions of whole PBMC or using flow cytometry to identify relevant cytokine-producing T cell populations.
Cell surface expression of CD107 has been correlated with the secretion of cytolytic molecules, such as perforin and granzyme B, following cytotoxic T lymphocyte (CTL) activation (42). In vivo, CD107a and CD107b are expressed on the surface of CD8+ T cells following activation with the cognate antigen leading to IFN-γ production and cytolytic activity directed against the target cells (25). Our studies suggest that since the amount of CD107a expressed on CD8+ T cells of elderly PBMC is relatively similar to that seen in young adults, the elderly may benefit from a vaccine that can boost the numbers of RSV F-specific degranulating cytotoxic T cells.
Aging individuals experience deficits in both innate and adaptive immune responses to pathogens, reflecting changes at the molecular, cellular, and tissue levels that accumulate over time (21, 43, 44). As a result, immune responses to vaccination in the elderly are diminished compared to those in young adults, posing serious challenges to the development of effective vaccination strategies.
Taken together, results from the studies presented here demonstrate that elderly individuals possess relatively high titers of RSV neutralizing antibodies that are comparable to those seen in young adults. However, the elderly are deficient in RSV F-specific CD4+ Th1 and/or CD8+ memory T cell responses that may contribute to their higher susceptibility to RSV disease. A longitudinal evaluation in elderly adults involving systematic correlation of RSV F-specific T cell responses with the clinical outcomes of RSV disease should provide significant insight into the observations made in the current study. Further work will be required to elucidate the precise mechanisms governing the distinct RSV F-specific memory T cell responses observed in elderly versus young individuals.
ACKNOWLEDGMENTS
We thank Allen Zeng, Ryan Yamagata, Li Yu, and Stacie Lambert for their contributions to the design of these studies and analysis of the results.
This work was entirely supported by MedImmune, LLC, funds.
All authors report no conflicts of interest.
Footnotes
Published ahead of print 12 December 2012
REFERENCES
- 1. Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus in young children. N. Engl. J. Med. 360:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madhi SA, Levine OS, Hajjeh R, Mansoor OD, Cherian T. 2008. Vaccines to prevent pneumonia and improve child survival. Bull. World Health Organ. 86:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. 2008. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ. 86:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simoes EAF. 1999. Respiratory syncytial virus infection. Lancet 354:847–852 [DOI] [PubMed] [Google Scholar]
- 6. Falsey AR, McCann RM, Hall WJ, Tanner MA, Criddle MM, Formica MA, Irvine CS, Kolassa JE, Barker WH, Treanor JJ. 1995. Acute respiratory tract infection in daycare centers for older persons. J. Am. Geriatr. Soc. 43:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759 [DOI] [PubMed] [Google Scholar]
- 8. Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, Gorse GJ, Edelman R, Hayden FG, McElhaney JE, Neuzil KM, Nichol KL, Simões EA, Wright PF, Sales VM. 2008. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines—nonadjuvanted vaccine or vaccine adjuvanted with alum—given concomitantly with influenza vaccine to high-risk elderly individuals. J. Infect. Dis. 198:1317–1326 [DOI] [PubMed] [Google Scholar]
- 9. Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. 2000. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 283:499–505 [DOI] [PubMed] [Google Scholar]
- 10. Griffin MR, Coffey CS, Neuzil KM, Mitchel EF, Jr, Wright PF, Edwards KM. 2002. Winter viruses: influenza- and respiratory syncytial virus-related morbidity in chronic lung disease. Arch. Intern. Med. 162:1229–1236 [DOI] [PubMed] [Google Scholar]
- 11. Han LL, Alexander JP, Anderson LJ. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 179:25–30 [DOI] [PubMed] [Google Scholar]
- 12. Johnstone J, Majumdar SR, Fox JD, Marrie TJ. 2008. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest 134:1141–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullooly JP, Bridges CB, Thompson WW, Chen J, Weintraub E, Jackson LA, Black S, Shay DK. 2007. Vaccine Safety Datalink Adult Working Group. Influenza- and RSV-associated hospitalizations among adults. Vaccine 25:846–855 [DOI] [PubMed] [Google Scholar]
- 14. Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186 [DOI] [PubMed] [Google Scholar]
- 15. Walsh EE, Peterson DR, Falsey AR. 2004. Risk factors for severe respiratory syncytial virus infection in elderly persons. J. Infect. Dis. 189:233–238 [DOI] [PubMed] [Google Scholar]
- 16. Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P. 2010. Age related changes in T cell mediated immune response and effector memory to respiratory syncytial virus (RSV) in healthy subjects. Immun. Aging 7:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Bree GJ, Heidema J, van Leeuwen EMM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RAW, Out TA. 2005. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J. Infect. Dis. 191:1710–1718 [DOI] [PubMed] [Google Scholar]
- 18. Looney RJ, Falsey AR, Walsh E. 2002. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J. Infect. Dis. 185:682–685 [DOI] [PubMed] [Google Scholar]
- 19. Tang RS, MacPhail M, Schickli JH, Kaur J, Robinson CL, Lawlor HA, Guzzetta JM, Spaete RR, Haller AA. 2004. Parainfluenza virus type 3 expressing the native or soluble fusion (F) protein of respiratory syncytial virus (RSV) confers protection from RSV infection in African green monkeys. J. Virol. 78:11198–11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandenburg AH, de Waal L, Timmerman HH, Hoogerhout P, de Swart RL, Osterhaus AD. 2000. HLA class I-restricted cytotoxic T-cell epitopes of the respiratory syncytial virus fusion protein. J. Virol. 74:10240–10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olson MR, Varga SM. 2008. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev. Vaccines 7:1239–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rock MT, Crowe JE., Jr 2003. Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology 108:474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Bleek GM, Poelen MC, van der Most R, Brugghe HF, Timmermans HA, Boog CJ, Hoogerhout P, Otten HG, van Els CA. 2003. Identification of immunodominant epitopes derived from the respiratory syncytial virus fusion protein that are recognized by human CD4 T cells. J. Virol. 77:980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crotty AR, Glidewell J, Ahmed R. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111–122 [DOI] [PubMed] [Google Scholar]
- 25. Alter G, Malenfant JM, Altfeld M. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15–22 [DOI] [PubMed] [Google Scholar]
- 26. Humes KR, Jones NA, Ramirez RR. 2011. Overview of race and Hispanic origin: 2010, p. 1–23. United States Census Bureau, U.S. Department of Commerce, Washington, DC [Google Scholar]
- 27. Prince GA, Hemming VG, Horswood RL, Baron PA, Chaock RM. 1987. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J. Virol. 61:1851–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siber GR, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, Thompson CM, Walsh EE, Piedra PA, Hemming VG. 1994. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J. Infect. Dis. 169:1368–1373 [DOI] [PubMed] [Google Scholar]
- 29. Falsey AR, Walsh EE. 1998. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J. Infect. Dis. 177:463–466 [DOI] [PubMed] [Google Scholar]
- 30. Falsey AR, Singh HK, Walsh EE. 2006. Serum antibody decay in adults following natural respiratory syncytial virus infection. J. Med. Virol. 78:1493–1497 [DOI] [PubMed] [Google Scholar]
- 31. Walsh EE, Falsey AR. 2004. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J. Infect. Dis. 190:373–378 [DOI] [PubMed] [Google Scholar]
- 32. Welliver RC, Wong DT, Sun M, Middleton E, Vaughn RS, Ogra PL. 1981. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N. Engl. J. Med. 305:841–846 [DOI] [PubMed] [Google Scholar]
- 33. Welliver RC, Kaul TN, Sun M, Ogra PL. 1984. Defective regulation of immune responses in respiratory syncytial virus infection. J. Immunol. 133:1925–1930 [PubMed] [Google Scholar]
- 34. Bangham CRM, Openshaw PJM, Ball LA, King AMQ, Wertz GW, Askonas BA. 1986. Human and murine cytotoxic T-cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J. Immunol. 137:3973–3977 [PubMed] [Google Scholar]
- 35. Isaacs D. 1991. Viral subunit vaccines. Lancet 337:1223–1224 [DOI] [PubMed] [Google Scholar]
- 36. Cannon MJ, Scott EJ, Taylor G, Askonas BA. 1987. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T-cells. Immunology 62:133–138 [PMC free article] [PubMed] [Google Scholar]
- 37. Cannon MJ, Bangham CRM. 1989. Recognition of respiratory syncytial virus fusion protein by mouse cytotoxic T-cell clones and a human cytotoxic T cell line. J. Gen. Virol. 70:79–87 [DOI] [PubMed] [Google Scholar]
- 38. Cherukuri A, Stokes KL, Patton K, Kuo H, Sakamoto K, Lambert S, Stillman E, Moore ML, Lee S. 2012. An adjuvanted respiratory syncytial virus fusion protein induces protection in aged BALB/c mice. Immun. Aging 9:21 doi:10.1186/1742-4933-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Domachowske JB, Rosenberg HF. 1999. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin. Microbiol. Rev. 12:298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muñoz JL, McCarthy CA, Clark ME. 1991. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cell. J. Virol. 65:4494–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee S, Stokes KL, Currier MG, Sakamoto K, Lukacs NW, Celis E, Moore ML. 2012. Vaccine-elicited CD8+ T cells protect against respiratory syncytial virus strain A2-line19F-induced pathogenesis in BALB/c mice. J. Virol. 86:13016–13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup R. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65–78 [DOI] [PubMed] [Google Scholar]
- 43. Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. 2009. Vaccination in the elderly: an immunological perspective. Trends Immunol. 30:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dowling MR, Hodgin PD. 2009. Why does the thymus involute? A selection-based hypothesis. Trends Immunol. 30:295–300 [DOI] [PubMed] [Google Scholar]