Abstract
Mannheimia haemolytica, a major causative agent in bovine respiratory disease, inflicts extensive losses each year on cattle producers. Commercially available vaccines are only partially efficacious. Immunity to M. haemolytica requires antibodies to secreted toxins and outer membrane proteins (OMPs) of the bacterium. Gram-negative bacteria produce membrane blebs or vesicles, the membrane components of which are primarily derived from OMPs. Accordingly, vesicles have been used as immunogens with various degrees of success. This study characterized components of M. haemolytica vesicles and determined their immunogenicity in mice and cattle. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of vesicles from this bacterium identified 226 proteins, of which 58 (25.6%) were OMPs and periplasmic and one (0.44%) was extracellular. Vesicles were used to vaccinate dairy calves and BALB/c mice. Analyses of sera from calves and mice by enzyme-linked immunosorbent assay (ELISA) showed that circulating antibodies against M. haemolytica whole cells and leukotoxin were significantly higher on days 21 and 28 (P < 0.05) than on day 0. For control calves and mice, there were no significant differences in serum anti-whole-cell and leukotoxin antibody levels from days 0 and 21 or 28, respectively. Lesion scores of lungs from vaccinated calves (15.95%) were significantly (P < 0.05) lower than those from nonvaccinated calves (42.65%). Sera from mice on day 28 and calves on day 21 showed 100% serum bactericidal activity. Sera from vesicle-vaccinated mice neutralized leukotoxin.
INTRODUCTION
Mannheimia haemolytica is the major causative agent of severe, often fatal, respiratory disease in cattle (1). Vaccination of cattle with commercial M. haemolytica vaccines is only partially efficacious, and antimicrobial treatment of M. haemolytica cases is costly and impractical (2).
Immunity to M. haemolytica is based on the immune response to leukotoxin (LKT) and outer membrane proteins (OMPs) (3). An immunoproteomic study of M. haemolytica OMPs conducted in our laboratory identified 57 OMPs that may have the potential to be developed into vaccines (4). The immunogenicity of recombinant forms of several M. haemolytica OMPs, including PlpE, OmpA, PlpF, OmpP2, serotype 1-specific antigen (SSA-1), and OmpD15, has been studied (5–10). Vaccination of calves with recombinant PlpE partially protects cattle against challenge with virulent M. haemolytica and significantly enhances the efficacy of commercial vaccines (7–9). Chimeric vaccines comprising one or more copies of the immunodominant epitope of PlpE and the neutralizing epitope of LKT (11) stimulated antibodies with potent complement-mediated cell killing and LKT-neutralizing activities, whereas cattle vaccinated with chimeric vaccines in combination with M. haemolytica bacterins had 71% fewer lung lesions than did control cattle (7).
Commercial animal health companies market culture supernatants or bacterin-toxoid combination vaccines (12). Supplementing commercial vaccines with recombinant OMPs substantially enhanced their efficacy (8, 9). However, with the low profit margin on bovine vaccines, commercialization of recombinant-protein-based or recombinant-protein-augmented vaccines has not come to fruition.
Inexpensive, efficacious, and alternative approaches to bovine bacterial vaccines are needed as substitutes for traditional bacterins and recombinant proteins. One such approach from other bacterial studies is bacterial vesicle vaccines. Growing, Gram-negative bacteria produce closed outer membrane blebs that detach as vesicles, which contain OMPs, lipopolysaccharide (LPS), periplasmic proteins, peptidoglycans, and secretory components such as toxins (13, 14). Because they contain a full complement of surface antigens, secretory proteins, and toxins, use of membrane vesicles as a nonliving, acellular vaccine has been studied with several bacteria (15–18). In addition, vesicles can serve as their own adjuvants, which can further decrease production costs (19). To our knowledge, outer membrane vesicles have not previously been demonstrated in M. haemolytica . We, therefore, undertook to identify proteins in M. haemolytica vesicles (MHVs) and to determine the immunogenicity of MHVs.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
Mannheimia haemolytica serotype S1, strain 89010807N, originally isolated from a case of calf pneumonia, was used for this study (20). Growth conditions of the bacterium have been described previously (4).
Preparation of M. haemolytica vesicles.
Membrane vesicles were extracted and purified as previously described with slight modifications (21, 22). An overnight starter culture was used to seed larger volumes of brain heart infusion (BHI) broth in 1- to 2-liter Erlenmeyer flasks. The culture was incubated in a 37°C shaker incubator until the optical density at 600 nm (OD600) was 1.0. The cells were removed by centrifugation at 10,000 × g, and the supernatant was filtered through an 0.2-μm filter. The supernatant was concentrated by a centrifugal filter device with a molecular mass cutoff of 100,000 Da (EMD Millipore, Billerica, MA). Debris was removed by centrifugation at 20,000 × g, 4°C, and pellets containing vesicles were collected by centrifugation at 150,000 × g, 4°C, for 3 h. Pellets were resuspended in Dulbecco's phosphate-buffered saline (DPBS), and the concentration of vesicles was determined by the bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL).
The shapes and appearances of negatively stained MHV preparations were determined with a JEOL JEM-2100 scanning transmission electron microscope (JEOL, Tokyo, Japan) in scanning electron microscope mode.
Identification of proteins with LC-MS/MS.
Proteins from MHVs were cleaned using a two-dimensional (2-D) cleanup kit (Bio-Rad, Hercules, CA), dissolved in Tris-buffered urea, reduced and alkylated using Tris(2-carboxyethyl)phosphine and iodoacetamide (11), and then diluted 4-fold and digested with trypsin. Peptides were separated by nano-liquid chromatography (nano-LC) on C18 columns and eluted directly into an LTQ-OrbitrapXL mass spectrometer for one full-range Fourier transform mass spectroscopy (FT-MS) scan and six concurrent data-dependent tandem mass spectrometry (MS/MS) scans. The LC-MS/MS data were searched via Mascot v2.2.04 (Matrix Science) and X! Tandem v2007.01.01.1 (http://www.thegpm.org) (11). Searches used a parent ion mass tolerance of 15 ppm, a fragment ion tolerance of 0.8 Da, and the variable peptide modifications pyroglutamate cyclization of N-terminal Gln, oxidation of Met, iodoacetamide adducts of Cys, and/or formylation or acetylation of the protein N terminus. The search database consisted of 16,722 M. haemolytica sequences from Mhdatabase111111, which also contains 120,904 human protein sequences downloaded from Uniprot on 16 December 2008. Peptide and protein identifications were validated using Scaffold ver_3.4.9. (Proteome Software) and the PeptideProphet algorithm (49). Probability thresholds were greater than 99% probability for protein identifications, based upon at least 2 peptides identified with 95% certainty.
Immunization of mice and calves with M. haemolytica vesicles.
All studies were done with the approval of the Oklahoma State University Institutional Animal Care and Use Committee (protocol VM1045). A total of 170 female BALB/c mice (Charles River Laboratories, Wilmington, MA) were used in this study. Details of vaccine formulations, doses, adjuvants, vaccination, and bleed times are given in Table 1. Ten mice from group 4 were sacrificed and bled on days 0, 14, and 28. Similarly, 10 mice from each vaccinated group were sacrificed and bled on days 14 and 28. Sera were harvested and stored at −80°C.
Table 1.
Vaccine formulations for immunization of micea
| Group | Antigen dose | FIA | No. of mice |
|---|---|---|---|
| 1 | 10 μg | Yes | 20 |
| 25 μg | 20 | ||
| 50 μg | 20 | ||
| 2 | 10 μg | No | 20 |
| 25 μg | 20 | ||
| 50 μg | 20 | ||
| 3 (Pulmo-Guard PHM-1) | 1/10 cattle dose | No | 20 |
| 4 (Control) | 0 | Yes | 30 |
Vaccination was performed on days 0 and 14. Bleeding was performed on days 14 and 28 (10 mice).
Eight 4-week-old Holstein calves were divided between two groups of four calves each and immunized, challenged, and bled according to the protocol given in Table 2. Calves were subcutaneously vaccinated with a 2-ml vaccine. Each calf was challenged intrabronchially with 20 ml of 109 CFU/ml of logarithmic-phase M. haemolytica S1, strain 89010807N (24). Following challenge, calves were evaluated with a 3-point clinical scoring every day as previously described (25). Five days later, calves were humanely sacrificed, and the lungs were evaluated for percentage of lung with pneumonia. Lungs were cultured for M. haemolytica , and the number of colonies was subjectively scored from 0 to 3 (0 = none and 3 = too numerous to count). Clinical and necropsy evaluations were done without knowledge of whether the calf was from the principal or control group.
Table 2.
Protocol for experiment with calves
| Group | Antigen dose | FIA | No. of calves | Vaccination days | Bleed times |
|---|---|---|---|---|---|
| Vesicle 1 | 150 μg | Yes | 4 | 0 and 14 | Days 0, 7, 14, 21, and 28 |
| Control | PBS | Yes | 4 | 0 and 14 | Days 0, 7, 14, 21, and 28 |
| Total | 8 |
ELISA.
Antibody endpoint titers to formalin-killed M. haemolytica and partially purified LKT were determined by enzyme-linked immunosorbent assay (ELISA) using 2-fold serial dilutions of murine sera ranging from 1:400 to 1:819,200, whereas bovine serum antibodies were determined by a single-dilution ELISA (5, 9, 26). Sera collected from vaccinated or control mice and calves were used as primary antibodies. Affinity-purified, horseradish peroxidase-conjugated goat anti-mouse or goat anti-bovine IgG(H+L) (Kirkegaard and Perry Labs, Gaithersburg, MD) was used as secondary antibody, and o-phenylenediamine (Amresco, Solon, OH) was used as the substrate as described previously (3). Plates were read at 490 nm on a Vmax kinetic microtiter plate reader (Molecular Devices, Sunnyvale, CA). Statistically defined endpoint titers were calculated by the method of Frey et al. (27).
Complement-mediated, serum bactericidal assay.
Complement-mediated killing assays were performed as previously described (11). Briefly, decapsulated M. haemolytica cells were incubated with heat-inactivated sera in the presence or absence of complement source for 30 min at 37°C. Aliquots were plated on BHI blood agar plates at the beginning (T0) and after 30 min (T30) of incubation. All plates were incubated at 37°C overnight. Percent killing was calculated as [(T0 growth − T30 growth)/T0 growth] × 100. Bovine hyperimmune serum from a calf vaccinated with rPlpE (26) and naïve bovine serum were used as positive and negative controls, respectively (8).The assay was repeated at least three times.
Leukotoxin neutralization assay.
A colorimetric microtitration assay for quantifying LKT cytotoxicity to the BL3.1 cell line was done as previously described (11). The viability of control and LKT-treated target cells with and without sera was determined using the methylthiazole tetrazolium (MTT) assay (Sigma, St. Louis, MO). Mouse sera from three mice each from the two 50-μg MHV, Pulmo-Guard PHM-1, and control groups were used in the assay. Percent neutralization was calculated using the formula 1 − [(OD of untreated control cells − OD of test sample wells containing toxin)/(OD of untreated control cells − OD of complete-toxicity wells)] × 100.
Statistical analyses.
Antibody responses, cumulative clinical and M. haemolytica reisolation scores, and percent lung lesions between vesicle-vaccinated and control calves were compared by the Student t test (23). Antibody responses among mouse groups were compared by analysis of variance (ANOVA).
RESULTS
Electron microscopy.
Electron microscopic examination of M. haemolytica demonstrated numerous membrane vesicles that were oval to round and 10 to 20 nm in diameter (Fig. 1)
Fig 1.
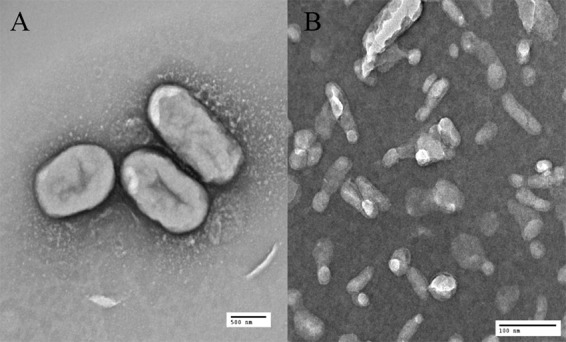
Electron micrographs of M. haemolytica cells (A) magnified ×6,000 and vesicles (B) with a magnification of ×50,000.
Proteomic analysis of M. haemolytica vesicles.
A total of 226 proteins (see Table S1 in the supplemental material) were identified in vesicle preparations of M. haemolytica grown in BHI broth to an A600 of 1.0. The subcellular locales of identified proteins were predicted by PSORTb (Fig. 2). A total of 104 (46%) proteins were characterized as being of cytoplasmic origin, 58 (25.6%) were from either the periplasmic or the outer membrane, one (0.4%) was extracellular, 5 (2.2%) were from the cytoplasmic membrane, and 58 (25.6%) were of unknown locale. Analysis of 58 unknown proteins by LipoP, an algorithm that predicts whether the proteins form signal peptides or lipoproteins with signal peptides, showed that of the 58 proteins, 16 (27.5%) were predicted to be cytoplasmic, 22 (38%) were lipoproteins with signal peptides, and 20 (34.5%) were proteins with signal peptides. Overall, 100 (44.2%) of the proteins identified in vesicle preparations of this bacterium are proteins that are identified in other bacterial vesicle preparations.
Fig 2.
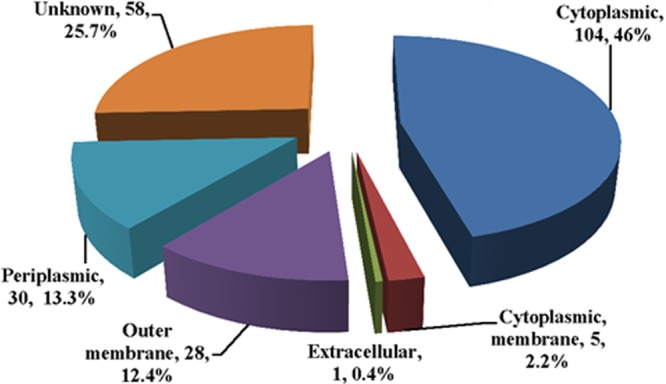
Chart showing subcellular locations of proteins identified in M. haemolytica vesicles as determined by PSORTb. Of particular interest are the 58 (25.66%) proteins that constitute periplasmic and outer membrane proteins and the single extracellular protein, which happens to be leukotoxin.
Functional analysis of proteins of MHVs shows that 31.8% (72 proteins) of the MHV proteins are involved in transport, cell structure, virulence, etc., and therefore may contribute to immunity against M. haemolytica .
Serologic responses of mice.
Anti-whole-cell and LKT antibodies in sera from mice vaccinated with MHV or Pulmo-Guard PHM-1 were determined. Statistical analyses showed that there was a significant increase (P < 0.05) in antibodies to both ligands between days 0 and 28 in sera from mice vaccinated with MHV and Pulmo-Guard PHM-1 (Fig. 3A). Antibody responses to both ligands in sera from nonvaccinated mice remained low. Analysis of the data demonstrated a significant increase of anti-LKT and anti-whole-cell antibodies with increasing MHV doses (P < 0.05). A more consistent increase in antibody responses occurred with increase in dose in the absence of Freund's incomplete adjuvant (FIA). Antibody responses of Pulmo-Guard PHM-1-vaccinated mice to both ligands were significantly higher (P < 0.05) than those of MHV vaccinates. Ten-fold-diluted sera from MHV-plus-FIA, MHV alone, or Pulmo-Guard PHM-1 groups exhibited significant LKT neutralization (P < 0.05) (Table 3).
Fig 3.
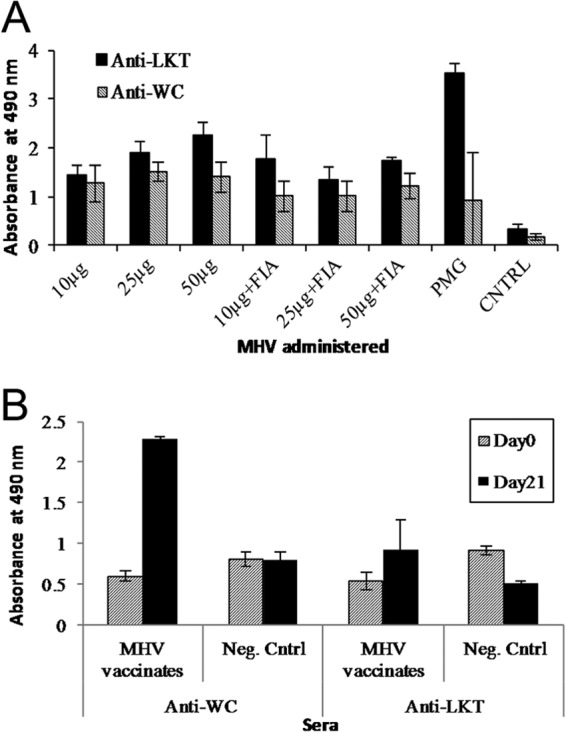
(A) Mean ELISA showing anti-whole-cell (Anti-WC) and antileukotoxin (Anti-LKT) antibody levels in day 28 sera collected from 8 groups of mice (n = 10/group) vaccinated with MHV or Pulmo-Guard PHM-1 (PMG) and nonvaccinates (CNTRL). (B) Circulating anti-whole-cell and anti-LKT antibodies in day 0 and 21 sera collected from 4 calves vaccinated with MHV and 4 nonvaccinates (Neg. Cntrl).
Table 3.
Leukotoxin neutralization by 1:10 dilution of sera from mice vaccinated with 50 μg of MHV with or without FIA, a 1/10 cattle dose of Pulmo-Guard PHM-1, or PBS with FIA
| Target with or without LKT | Sera from vaccinates | Mean OD690 ± SDa | % neutralizationb |
|---|---|---|---|
| BL3 cells only | None | 1.2 ± 0.28 | |
| BL3 cells + LKT | None | 0.24 ± 0.08 | 0 |
| BL3 cells + LKT | MHV + FIA | 0.87 ± 0.17 A | 65.6 |
| BL3 cells + LKT | MHV alone | 0.67 ± 0.10 B | 44.8 |
| BL3 cells + LKT | Pulmo-Guard PHM-1 | 0.71 ± 0.11 B | 49.0 |
| BL3 cells + LKT | PBS + FIA | 0.27 ± 0.05 C | 3.1 |
OD values followed by different capital letters were significantly different (P > 0.05).
% neutralization = 1 − [(OD of untreated control cells − OD of test sample wells containing toxin)/(OD of untreated control cells − OD of complete-toxicity wells)] × 100.
Calf immunization and challenge.
Calves vaccinated with MHV showed a significant increase in anti-whole-cell and anti-LKT antibody titers between days 0 and 21 (P < 0.05) and a significantly higher antibody response than that of controls (P < 0.05) (Fig. 3B). Following challenge, calves from each group developed mild to moderate clinical signs of respiratory disease, including dyspnea, fever, and depression. The mean cumulative clinical score for control calves (9.5 ± 2.1) was significantly higher (P < 0.05) than that for the vaccinated calves (5.3 ± 2.8) (Table 4). Lung lesions were 62.9% smaller for MHV-vaccinated calves (15.9% ± 9.8%) than for control calves (42.7% ± 18.0%) (P < 0.05).
Table 4.
Clinical, microbiologic, and pathological data from M. haemolytica vesicle-vaccinated and control calves after challenge with live M. haemolytica
| Group | Adjuvant | Vaccination days | Challenge day | Mean cumulative clinical score ± SD (% reduction compared to control value) | Mean cumulative M. haemolytica isolation score ± SD (% reduction compared to control value) | Mean % pneumonia ± SD (% reduction compared to control value) |
|---|---|---|---|---|---|---|
| Vesicle vaccinated | FIA | 0, 14 | 21 | 5.3 ± 2.8a (44.2) | 1.4 ± 0.9b (60) | 15.9 ± 9.8a (62.8) |
| Control | FIA | 0, 14 | 21 | 9.5 ± 2.1 | 3.5 ± 1.7 | 42.7 ± 18.0 |
P < 0.05 compared to control values.
P = 0.0515 compared to control values.
Serum bactericidal assay.
Sera from nonvaccinated mice on days 0 and 28 or sera from MHV-vaccinated mice on day 0 had no bactericidal activity in the presence or absence of complement (Fig. 4 and 5). Complement-mediated killing by day 28 sera from mice vaccinated with MHV was 100% in the presence of complement and almost nonexistent in the absence of complement. Even though Pulmo-Guard PHM-1 induced the strongest immune response as measured by ELISA, sera from those mice did not exhibit any bactericidal activity.
Fig 4.
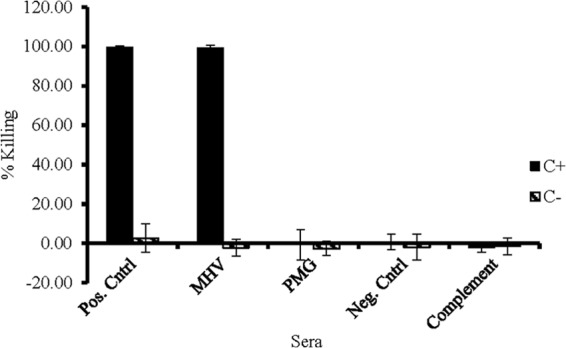
Mean complement-mediated bacterial killing of sera from mice vaccinated with M. haemolytica MHV and Pulmo-Guard PHM-1 (PMG) and nonvaccinated control mice (Neg. Cntrl). Serum from a calf vaccinated with rPlpE, a highly immunogenic outer membrane protein from M. haemolytica , was used as a positive control. C+ and C− designate the presence and absence, respectively, of complement in the assays.
Fig 5.
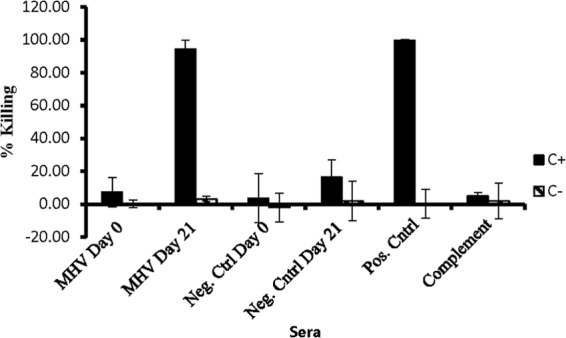
Mean bactericidal activity of sera from Holstein calves that were vaccinated with M. haemolytica MHV and nonvaccinated control calves (Neg. Cntrl). C+ and C− designate the presence and absence, respectively, of complement in each assay. The positive control is serum from a calf vaccinated with the recombinant form of the highly immunogenic M. haemolytica outer membrane protein PlpE. Note that there is no killing activity in the absence of complement in all cases.
Due to natural seroconversion, the bactericidal activity of sera from calves was more complicated. Undiluted sera from calves that were vaccinated and nonvaccinated with MHV exhibited 100% killing activity in the presence of a source of complement. However, when both sets of calf sera were diluted 100-fold, bactericidal activity of nonvaccinates on days 0 and 21 was reduced to background level, whereas MHV-vaccinated calves remained at or close to 100% killing in the presence of complement.
DISCUSSION
Comparison of proteins identified in our earlier immunoproteomic analysis of M. haemolytica OMPs (4) to the current findings shows that generally more proteins (226) were identified in MHV preparations than in the former study, in which 132 proteins were characterized. Prediction with PSORTb of subcellular locales of both sets of proteins followed the same trend. Accordingly, the MHV and immunoproteomic OMP study identified 28 and 16 proteins as OMPs, 30 and 8 as periplasmic proteins, 104 and 55 as cytoplasmic proteins, 5 and 7 as cytoplasmic membrane proteins, 1 and 1 as extracellular proteins, and 58 and 45 as unknown proteins, respectively. The apparent difference is because the immunoproteomic study identified only proteins that reacted with immune sera obtained from cattle either naturally exposed to M. haemolytica or vaccinated with components of the bacterium, as opposed to all proteins in the MHV study. Closer scrutiny of OMPs identified in both studies shows that only 11 of the 16 OMPs identified in the immunoproteomic analysis were found in vesicles of M. haemolytica , even though 28 OMPs were identified in MHV. Possible explanations for the differences could be due to methods of extraction of MHVs and OMPs and databases used in the two studies. The database used in our earlier studies was an incomplete M. haemolytica genome sequence, whereas the MHV findings were compared to current, more complete genomic data.
MHVs or blebs are constantly shed by Gram-negative bacteria and are believed to be a mechanism for secretion and transfer of macromolecules to animals (28, 29). Moreover, bacterial vesicles have been successfully used as vaccines or components of vaccines in pathogenic bacteria, including Vibrio cholerae (30), the oral pathogen Porphyromonas gingivalis (31), serogroup B strains of Neisseria meningitidis (32), enterotoxigenic Escherichia coli (33), and pulmonary Burkholderia pseudomallei infection (15). The current study is the first study that demonstrates that membrane vesicles from M. haemolytica induce robust immune responses both in mice and in calves. Antibody-mediated, complement-mediated killing and LKT neutralization indicated that MHV-induced antibodies are functional against M. haemolytica . Those findings are substantiated in vivo in that lesion scores of lungs from MHV-vaccinated calves were reduced by 62.8% compared to nonvaccinated calves.
The finding in mice that MHV vaccination without adjuvant was as good as, and perhaps superior to, vaccination with adjuvant was an interesting, though not unexpected, finding. Adjuvant properties have been demonstrated using meningococcal and E. coli bacterial vesicles (19, 34). Assets of the vesicles responsible for adjuvant property include lipopolysaccharide, a strong B-cell stimulant, and other OMPs, such as OmpA, that can function as pathogen-associated molecular patterns for pattern recognition by the innate and adaptive arms of the immune system (16, 35). Several studies have proposed that bacterial vesicles can be used as substitutes for chemical adjuvants with viral vaccines, conjugated to other bacterial immunogens, or for intranasal delivery (36, 37). Bacterial vesicle vaccines, however, are not uniformly efficacious in that meningococcal vesicles conjugated with capsular polysaccharide are weak immunogens in infants and neonatal mice (38).
In this study, we evaluated MHV vaccine efficacy using an M. haemolytica challenge model in young dairy calves. M. haemolytica vaccines have traditionally been tested using one to two doses of vaccine with intratracheal, intrabronchial, or transthoracic challenge approximately 14 days after the last vaccination (12, 39). Clinical signs are usually evaluated and cattle are euthanized and necropsied 4 to 6 days after challenge, with lung lesions quantified and compared to lesions in nonvaccinates. The assessment of clinical signs and lesions 4 to 6 days after challenge has been commonly used in assessment of M. haemolytica vaccine (7, 39–42). As early as 1977, it was reported that clinical signs peaked at 9 to 18 h after challenge, and lesions of acute pneumonia were readily accessible at day 3 with evidence of early healing by day 7 (43, 44). In the current study, clinical scores peaked on day 24 after challenge and remained steady in control calves (data not shown). Therefore, given the literature, our previous experience with M. haemolytica challenge, and the clinical findings in this experiment, day 5 was selected for necropsy and evaluation. In our cattle vaccine experiment, we were restricted by the number of cattle available, and because of their young ages, we opted to include adjuvant in our vaccine preparations. Future studies are needed to determine if adjuvant is required for MHV vaccine in cattle and, if so, to examine numerous adjuvants for their efficacy in augmenting MHV vaccine-induced immunity.
In the current study, we used a commercial M. haemolytica bacterin-toxoid as a positive control for antibody production in mice. The 1/10 cattle dose is the USDA-recommended dose for testing a cattle vaccine in mice (3). A previous cattle study using that vaccine as part of a bovine respiratory disease control program demonstrated efficacy in a feedlot (45). Mice vaccinated with the bacterin-toxoid developed high anti-whole-cell and LKT antibodies. Surprisingly, serum from those mice failed to stimulate complement-mediated killing; however, it did have an LKT-neutralizing function. The cause of the lack of functional bactericidal antibodies is not known. Others have shown differences between immune responses and vaccine efficacy among different mouse strains (46). In other Gram-negative bacteria, development of blocking antibodies against OMPs and of serum resistance has been demonstrated (47). Blocking antibodies directed against an N. meningitidis lipoprotein reduced meningococcal killing (48).
In conclusion, MHV vaccines stimulated a protective immune response in calves. Interestingly, some of the proteins identified by the proteomic analysis of MHV are OMPs such as PlpE (8, 9, 26); PlpF (6); and OmpD15, OmpP2, SSA-1 (6), etc., that we have shown to be highly immunogenic and, in the case of PlpE, protective. Further characterization of MHV as an alternative M. haemolytica vaccine is warranted.
Supplementary Material
ACKNOWLEDGMENT
This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2009-01626 from the USDA National Institute of Food and Agriculture.
Footnotes
Published ahead of print 12 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00622-12.
REFERENCES
- 1. Panciera RJ, Confer AW. 2010. Pathogenesis and pathology of bovine pneumonia. Vet. Clin. North Am. Food Anim. Pract. 26:191–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perino LJ, Hunsaker BD. 1997. A review of bovine respiratory disease vaccine field efficacy. Bovine Practitioner 31:59–66 [Google Scholar]
- 3. Confer AW. 1993. Immunogens of Pasteurella. Vet. Microbiol. 37:353–368 [DOI] [PubMed] [Google Scholar]
- 4. Ayalew S, Confer AW, Hartson SD, Shrestha B. 2010. Immunoproteomic analyses of outer membrane proteins of Mannheimia haemolytica and identification of potential vaccine candidates. Proteomics 10:2151–2164 [DOI] [PubMed] [Google Scholar]
- 5. Ayalew S, Shrestha B, Montelongo M, Wilson A, Confer AW. 27 August 2011. Identification and immunogenicity of Mannheimia haemolytica S1 outer membrane lipoprotein PlpF. Vaccine [Epub ahead of print.] doi:10.1016/j.vaccine.2011.08.074 [DOI] [PubMed] [Google Scholar]
- 6. Ayalew S, Shrestha B, Montelongo M, Wilson AE, Confer AW. 2011. Immunogenicity of Mannheimia haemolytica recombinant outer membrane proteins serotype 1-specific antigen, OmpA, OmpP2, and OmpD15. Clin. Vaccine Immunol. 18:2067–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Confer AW, Ayalew S, Montelongo M, Step DL, Wray JH, Hansen RD, Panciera RJ. 2009. Immunity of cattle following vaccination with a Mannheimia haemolytica chimeric PlpE-LKT (SAC89) protein. Vaccine 27:1771–1776 [DOI] [PubMed] [Google Scholar]
- 8. Confer AW, Ayalew S, Panciera RJ, Montelongo M, Whitworth LC, Hammer JD. 2003. Immunogenicity of recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE and augmentation of a commercial vaccine. Vaccine 21:2821–2829 [DOI] [PubMed] [Google Scholar]
- 9. Confer AW, Ayalew S, Panciera RJ, Montelongo M, Wray JH. 2006. Recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE enhances commercial M. haemolytica vaccine-induced resistance against serotype 6 challenge. Vaccine 24:2248–2255 [DOI] [PubMed] [Google Scholar]
- 10. Pandher K, Confer AW, Murphy GL. 1998. Genetic and immunologic analyses of PlpE, a lipoprotein important in complement-mediated killing of Pasteurella haemolytica serotype 1. Infect. Immun. 66:5613–5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayalew S, Confer AW, Payton ME, Garrels KD, Shrestha B, Ingram KR, Montelongo MA, Taylor JD. 2008. Mannheimia haemolytica chimeric protein vaccine composed of the major surface-exposed epitope of outer membrane lipoprotein PlpE and the neutralizing epitope of leukotoxin. Vaccine 26:4955–4961 [DOI] [PubMed] [Google Scholar]
- 12. Shewen PE, Wilkie BN. 1988. Vaccination of calves with leukotoxic culture supernatant from Pasteurella haemolytica. Can. J. Vet. Res. 52:30–36 [PMC free article] [PubMed] [Google Scholar]
- 13. Kato S, Kowashi Y, Demuth DR. 2002. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 32:1–13 [DOI] [PubMed] [Google Scholar]
- 14. Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23:4538–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, McLachlan JB, Roy CJ, Morici LA. 2011. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine 29:8381–8389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuehn MJ, Kesty NC. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655 [DOI] [PubMed] [Google Scholar]
- 17. Park SB, Jang HB, Nho SW, Cha IS, Hikima J, Ohtani M, Aoki T, Jung TS. 2011. Outer membrane vesicles as a candidate vaccine against edwardsiellosis. PLoS One 6:e17629 doi:10.1371/journal.pone.0017629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, Granoff DM. 2011. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 29:4728–4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders H, Feavers IM. 2011. Adjuvant properties of meningococcal outer membrane vesicles and the use of adjuvants in Neisseria meningitidis protein vaccines. Expert Rev. Vaccines 10:323–334 [DOI] [PubMed] [Google Scholar]
- 20. Murphy GL, Whitworth LC, Clinkenbeard KD, Clinkenbeard PA. 1995. Hemolytic activity of the Pasteurella haemolytica leukotoxin. Infect. Immun. 63:3209–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwon SO, Gho YS, Lee JC, Kim SI. 2009. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol. Lett. 297:150–156 [DOI] [PubMed] [Google Scholar]
- 22. Horstman AL, Kuehn MJ. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 275:12489–12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrie A, Watson P. 1999. Statistics for veterinary and animal science. Blackwell Press, London, United Kingdom [Google Scholar]
- 24. Burciaga-Robles LO, Krehbiel CR, Step DL, Holland BP, Richards CJ, Montelongo MA, Confer AW, Fulton RW. 2010. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and Mannheimia haemolytica challenge on animal performance, nitrogen balance, and visceral organ mass in beef steers. J. Anim. Sci. 88:2179–2188 [DOI] [PubMed] [Google Scholar]
- 25. Ayalew S, Step DL, Montelongo M, Confer AW. 2009. Intranasal vaccination of calves with Mannheimia haemolytica chimeric protein containing the major surface epitope of outer membrane lipoprotein PlpE, the neutralizing epitope of leukotoxin, and cholera toxin subunit B. Vet. Immunol. Immunopathol. 132:295–302 [DOI] [PubMed] [Google Scholar]
- 26. Ayalew S, Confer AW, Blackwood ER. 2004. Characterization of immunodominant and potentially protective epitopes of Mannheimia haemolytica serotype 1 outer membrane lipoprotein PlpE. Infect. Immun. 72:7265–7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frey A, Di Canzio J, Zurakowski D. 1998. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 221:35–41 [DOI] [PubMed] [Google Scholar]
- 28. Beveridge TJ. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller SI, Bader M, Guina T. 2003. Bacterial vesicle formation as a mechanism of protein transfer to animals. Cell 115:2–3 [DOI] [PubMed] [Google Scholar]
- 30. Bishop AL, Tarique AA, Patimalla B, Calderwood SB, Qadri F, Camilli A. 2012. Immunization of mice with vibrio cholerae outer-membrane vesicles protects against hyperinfectious challenge and blocks transmission. J. Infect. Dis. 205:412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakao R, Hasegawa H, Ochiai K, Takashiba S, Ainai A, Ohnishi M, Watanabe H, Senpuku H. 2011. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS One 6:e26163 doi:10.1371/journal.pone.0026163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toneatto D, Ismaili S, Ypma E, Vienken K, Oster P, Dull P. 2011. The first use of an investigational multicomponent meningococcal serogroup B vaccine (4CMenB) in humans. Hum. Vaccines 7:646–653 [DOI] [PubMed] [Google Scholar]
- 33. Roy K, Hamilton DJ, Munson GP, Fleckenstein JM. 2011. Outer membrane vesicles induce immune responses to virulence proteins and protect against colonization by enterotoxigenic Escherichia coli. Clin. Vaccine Immunol. 18:1803–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP, Putnam D. 2010. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:3099–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeannin P, Magistrelli G, Goetsch L, Haeuw JF, Thieblemont N, Bonnefoy JY, Delneste Y. 2002. Outer membrane protein A (OmpA): a new pathogen-associated molecular pattern that interacts with antigen presenting cells-impact on vaccine strategies. Vaccine 20(Suppl. 4):A23–A27 [DOI] [PubMed] [Google Scholar]
- 36. Aghasadeghi MR, Salmani AS, Sadat SM, Javadi F, Memarnejadian A, Vahabpour R, Zabihollahi R, Moshiri A, Siadat SD. 2011. Application of outer membrane vesicle of Neisseria meningitidis serogroup B as a new adjuvant to induce strongly Th1-oriented responses against HIV-1. Curr. HIV Res. 9:630–635 [DOI] [PubMed] [Google Scholar]
- 37. Haneberg B, Dalseg R, Oftung F, Wedege E, Hoiby EA, Haugen IL, Holst J, Andersen SR, Aase A, Meyer Naess L, Michaelsen TE, Namork E, Haaheim LR. 1998. Towards a nasal vaccine against meningococcal disease, and prospects for its use as a mucosal adjuvant. Dev. Biol. Stand. 92:127–133 [PubMed] [Google Scholar]
- 38. Fukasawa LO, Dias WO, Schenkman RP, Raw I, Tanizaki MM. 2004. Adjuvant can improve protection induced by OMV vaccine against Neisseria meningitidis serogroups B/C in neonatal mice. FEMS Immunol. Med. Microbiol. 41:205–210 [DOI] [PubMed] [Google Scholar]
- 39. Shewen PE, Lee CW, Perets A, Hodgins DC, Baldwin K, Lo RY. 2003. Efficacy of recombinant sialoglycoprotease in protection of cattle against pneumonic challenge with Mannheimia (Pasteurella) haemolytica A1. Vaccine 21:1901–1906 [DOI] [PubMed] [Google Scholar]
- 40. Briggs RE, Tabatabai LB, Tatum FM. 2012. Mucosal and parenteral vaccination against pneumonic pasteurellosis in cattle with a modified-live in-frame lktA deletion mutant of Mannheimia haemolytica. Microb. Pathog. 52:302–309 [DOI] [PubMed] [Google Scholar]
- 41. Crouch CF, LaFleur R, Ramage C, Reddick D, Murray J, Donachie W, Francis MJ. 2012. Cross protection of a Mannheimia haemolytica A1 Lkt-/Pasteurella multocida DeltahyaE bovine respiratory disease vaccine against experimental challenge with Mannheimia haemolytica A6 in calves. Vaccine 30:2320–2328 [DOI] [PubMed] [Google Scholar]
- 42. Panciera RJ, Corstvet RE. 1984. Bovine pneumonic pasteurellosis: model for Pasteurella haemolytica- and Pasteurella multocida-induced pneumonia in cattle. Am. J. Vet. Res. 45:2532–2537 [PubMed] [Google Scholar]
- 43. Friend SC, Wilkie BN, Thomson RG, Barnum DA. 1977. Bovine pneumonic pasteurellosis: experimental induction in vaccinated and nonvaccinated calves. Can. J. Comp. Med. 41:77–83 [PMC free article] [PubMed] [Google Scholar]
- 44. Friend SC, Thomson RG, Wilkie BN. 1977. Pulmonary lesions induced by Pasteurella hemolytica in cattle. Can. J. Comp. Med. 41:219–223 [PMC free article] [PubMed] [Google Scholar]
- 45. Wildman BK, Perrett T, Abutarbush SM, Guichon PT, Pittman TJ, Booker CW, Schunicht OC, Fenton RK, Jim GK. 2008. A comparison of 2 vaccination programs in feedlot calves at ultra-high risk of developing undifferentiated fever/bovine respiratory disease. Can. Vet. J. 49:463–472 [PMC free article] [PubMed] [Google Scholar]
- 46. Twine S, Shen H, Harris G, Chen W, Sjostedt A, Ryden P, Conlan W. 2012. BALB/c mice, but not C57BL/6 mice immunized with a DeltaclpB mutant of Francisella tularensis subspecies tularensis are protected against respiratory challenge with wild-type bacteria: association of protection with post-vaccination and post-challenge immune responses. Vaccine 30:3634–3645 [DOI] [PubMed] [Google Scholar]
- 47. Rice PA, McQuillen DP, Gulati S, Jani DB, Wetzler LM, Blake MS, Gotschlich EC. 1994. Serum resistance of Neisseria gonorrhoeae. Does it thwart the inflammatory response and facilitate the transmission of infection? Ann. N. Y. Acad. Sci. 730:7–14 [DOI] [PubMed] [Google Scholar]
- 48. Ray TD, Lewis LA, Gulati S, Rice PA, Ram S. 2011. Novel blocking human IgG directed against the pentapeptide repeat motifs of Neisseria meningitidis Lip/H. 8 and Laz lipoproteins. J. Immunol. 186:4881–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keller A, Purvine S, Nesvizhskii AI, Stolyar S, Goodlett DR, Kolker E. 2002. Experimental protein mixture for validating tandem mass spectral analysis. OMICS 6:207–212 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


