Abstract
Histopathology and quantitative PCR (qPCR) were used to determine the tissue distribution of Neospora caninum in calves at 80 days postinfection. Our findings revealed that the most appropriate brain areas for researching N. caninum pathogenesis were the amygdala and hippocampus for qPCR and the corpus striatum and diencephalon for histopathology.
TEXT
Neospora caninum is an apicomplexan parasite and a major cause of abortion in cattle (1). Infection with N. caninum causes severe economic losses through fetal death and lower milk production (2, 3); therefore, it is desirable to develop a safe and effective vaccine against bovine neosporosis. The efficacy of vaccine candidates against N. caninum is evaluated by various approaches; these include analysis of antibody production, clinical signs, gamma interferon (IFN-γ) production, mortality, and pathology in animal models (4). Although qualitative or quantitative analysis of parasites in tissues is often performed by PCR (5–8) or quantitative PCR (qPCR) (9–11), little is known about the tissue distribution of N. caninum. The aim of our study was to determine the distribution of N. caninum in experimentally infected cattle and to identify the most suitable organs and tissues for the evaluation N. caninum infection intensity.
Male Holstein calves (n = 8), ages 2 to 4 months, were assessed to investigate tissue distribution of N. caninum. Animals were seronegative for N. caninum antibodies. The systemic distribution of N. caninum was investigated in calves 1 to 4. The distribution of N. caninum in the brain was conducted for calves 5 to 8. Animals were intravenously inoculated with 5 × 107 (calves 1 and 3) or 1 × 107 (calves 2 and 4 to 8) tachyzoites of the N. caninum Nc-1 isolate. Animals were euthanized at 77 days postinoculation (dpi) (calves 1 to 4) or 85 dpi (calves 5 to 8). For qPCR assays, liver, spleen, kidney, heart, lung, adrenal gland, thyroid gland, pancreas, thymus, tongue, parotid gland, mandibular salivary gland, skeletal muscle, brachial and sciatic plexus, sympathetic trunk, cerebrum, cerebellum, spinal cord, eye, optic nerve, pituitary gland, gastrointestinal tract, and lymph nodes were collected. Brain and spinal cord were collected for use in histopathological examination. For further analysis of brain distribution, the prefrontal cortex, caudate putamen, amygdala, hippocampus, hypothalamus, periaqueductal gray, pons, and medulla oblongata were collected from calves 5 to 8 and subjected to qPCR analysis. Samples of liver, kidney, spleen, thymus, skeletal muscle, mesenteric lymph node, cerebrum, cerebellum, and spinal cord from cattle that were seronegative for N. caninum were used as negative controls in the qPCR assays. DNA was extracted from 1 g of tissue using a DNeasy Blood & Tissue kit (Qiagen, Santa Clarita, CA). The DNA concentration was adjusted to 50 ng/μl for each sample, and 50 ng of DNA was used as a template. The qPCR assays specifically targeted parasite DNA (Nc5) and were carried out as previously described (12). Results are expressed as the number of parasites in 50 ng of DNA. The limit of detection was 0.1 parasites in 50 ng of tissue DNA. In some regions, such as the cerebellum, negative controls showed relatively high values. Under our experimental conditions, cell-rich tissue samples, including lymph nodes, showed higher qPCR values despite being negative controls. In the brain, the cell density of the cerebellum is much higher than that of any other region (13). We hypothesize that the increased background in the qPCR result may be due to increased cell density in certain tissue samples. Test samples were considered positive when the parasite number was greater than 0.1 and higher than the values for the negative-control samples.
For histopathological analysis, tissues were fixed in 10% formalin solution, and brains were cut in coronal sections. The frontal lobe, corpus striatum, diencephalon, and mesencephalon included each area that was evaluated by qPCR: the prefrontal cortex, caudate putamen, hippocampus and hypothalamus, and periaqueductal gray. Tissues were embedded in paraffin, cut into sections that were 4 μm thick, and then stained with hematoxylin and eosin (HE). Immunohistochemistry (IHC) for N. caninum was performed with anti-N. caninum polyclonal antiserum (210-70 NC; VMRD, Pullman, WA) as the primary antibody. The secondary antibody was conjugated with horseradish peroxidase-labeled streptavidin biotin (LSAB+ kit, universal; Dako, Burlingame, CA). The chromogen was developed with 3,3′-diaminobenzidine (DAB) (Impact DAB; Vector Laboratories, Burlingame, CA). At least two sections were observed for each area.
Parasite DNA was detected only in the central nervous system (CNS) of calves 1 to 4. For the cerebellum and spinal cord, there was little difference between infected and control tissues (Fig. 1A). Calves 5 to 8 tested positive for the presence of N. caninum in the amygdala and hippocampus. Although the parasite load was relatively low in the hypothalamus, three of four animals were positive (Fig. 1B). Our results suggest that the cerebrum, in particular the amygdala, hippocampus, and hypothalamus, can be used to evaluate the level of N. caninum infection in cattle.
Fig 1.
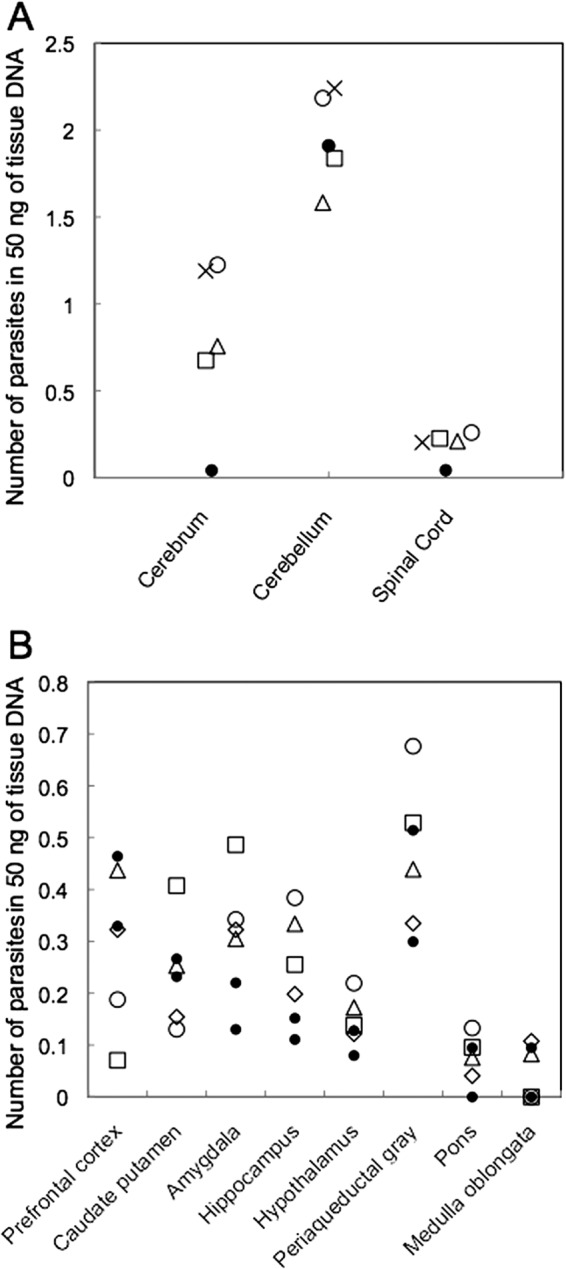
Quantitation of parasites in tissues from cattle infected with N. caninum tachyzoites by qPCR. Samples were considered positive when the number of parasites was greater than 0.1 and was higher than those for negative-control samples. (A) N. caninum distribution in the CNS. Open circle, calf no.1; open triangle, no. 2; open square, no. 3; cross, no. 4; filled circle, control. (B) N. caninum distribution in the brain. Open circle, calf no. 5; open triangle, no. 6; open square, no. 7; open diamond, no. 8; filled circle, control.
Histopathologically, mild lesions, such as focal necrosis, glial activation, and perivascular cuffing, were observed in the cerebrum of infected cattle (Fig. 2A). These changes were frequently found in sections from the corpus striatum and diencephalon (Table 1). Additionally, many lesions were found in the cortex and in the white matter adjacent to the cortex. However, the distribution of lesions was not concentrated in areas that were found to be positive by qPCR. No lesions were seen in the mesencephalon, pons, medulla oblongata, and cerebellum. Our IHC results showed that a small tissue cyst was detected only in calf 4 (Table 1 and Fig. 2B).
Fig 2.
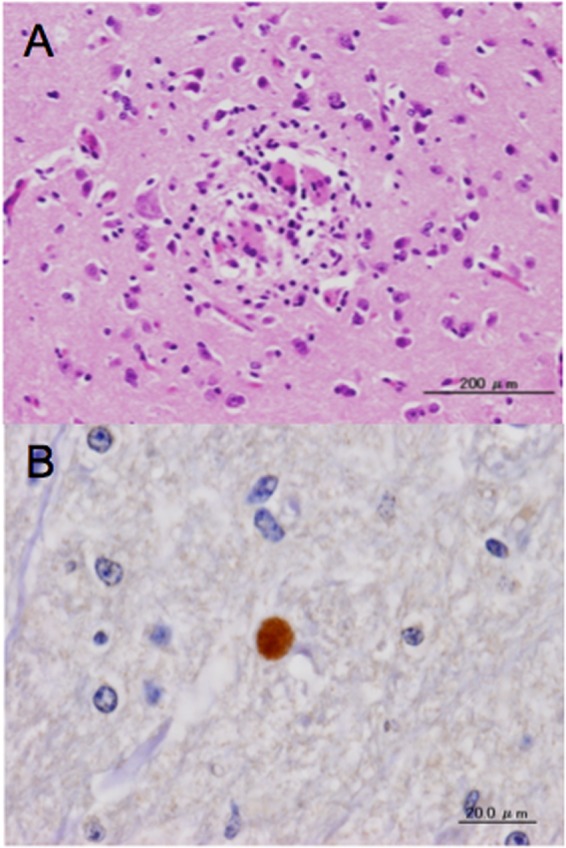
Histological sections of brain from cattle infected with N. caninum tachyzoites. (A) A section of corpus striatum from animal 7. Focal reactive glial cell infiltration as indicated by HE staining (magnification, ×20). (B) A section of mesencephalon from calf 4, showing a cyst as determined by IHC with anti-N. caninum polyclonal antiserum (magnification, ×60).
Table 1.
Histopathological changes in central nervous system
| Area | Finding(s) for calf no.a: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Frontal lobe | M | C | N | |||||
| Corpus striatum | C, G | M, C, GN | C, S | C, G | M | |||
| Amygdala | ∗ | ∗ | ∗ | ∗ | G | |||
| Diencephalon | M, C, G | M | C | C, G | ||||
| Occipital lobe | C, G | C | ||||||
| Mesencephalon | P | |||||||
| Pons | ||||||||
| Medulla oblongata | ||||||||
| Cerebellum | ||||||||
| Spinal cord | S | |||||||
M, mononuclear cell infiltration in meninges; C, perivascular cuffing; G, glial cell activation or aggregation; GN, glial nodule; N, focal necrosis; S, spheroid; P, parasite detected by IHC; ∗, not examined.
In fetuses naturally infected with N. caninum, PCR of brain samples is considered most suitable for diagnosis of bovine neosporosis (14). In experimentally infected calves, Kritzner et al. (15) showed that parasite DNA could be detected in the brain, muscle, and heart but not in the liver, spleen, lung, pancreas, popliteal lymph node, and gastrointestinal tract. In another study, parasite DNA was detected in the brain, spinal cord, heart, lung, diaphragm, and skeletal muscle (16). In the present study, parasite DNA was detected only in the CNS, emphasizing the importance of evaluating the brain in cattle experimentally infected with N. caninum. Additionally, we demonstrated that parasites were detected mainly in the amygdala and hippocampus of the limbic area. In the case of Toxoplasma gondii, closely related to N. caninum, the limbic area is a region where the cysts are known to localize at a high prevalence in murine models (17). Therefore, N. caninum may exhibit similar brain topology.
Brain lesions were also frequently found in the cerebrum, particularly the gray matter in aborted Neospora-infected fetuses (18). The cerebrum showed a significantly higher frequency of lesions than the medulla oblongata and cerebellum (18). Our findings are in accordance with previously published results. Most lesions were found in superficial gray and white matter and to a lesser extent in deeper areas. Lesions indicative of glial and inflammatory cell activation suggest some form of immune reaction against the parasites. It is also possible that the parasites could have already been eradicated, thereby leading to differences in the distribution of lesions and parasite DNA. N. caninum is believed to be disseminated hematogenously, with perivascular cuffing a common tissue response in neosporosis. This was actually observed in seven of the eight experimentally infected animals. Perivascular spaces around the artery in the brain have been shown to play an immunological role. The structure of the perivascular space in basal ganglia, including the amygdala, differs markedly from that seen in the cortex (19). Although it is not clear why few lesions were found in the deeper areas of the brain, the differences in anatomical structure, including the vascular system of each area, possibly affects invasion of the parasite or the host immune reaction.
Using IHC, the parasite was detected in only one experimentally infected animal. In approximate calculation from the qPCR results, the number of parasites per 1 g of tissue sample of amygdala was about 110 parasites on average. Assuming a specific gravity of brain 1, calculated simply, 1 g of tissue sample could provide 2,500 pieces of 1-cm2 tissue sections when sliced 4 μm thick. This means that at least 23 sections are needed to find one parasite even when the parasites are distributed evenly in tissue. In general, the parasites would be sparsely distributed in the tissue as colonies of tachyzoites or cysts. Therefore, detection of the parasite by histopathological and immunohistochemical techniques is difficult with low sensitivity. In contrast, qPCR is highly sensitive and specific, especially in tissues with low levels of parasites.
In the present study, the importance of sampling and analyzing brain tissue was confirmed in cattle experimentally infected with N. caninum. Our results show that the amygdala, hippocampus, and hypothalamus samples are most appropriate for qPCR assays. Additionally, sections from the corpus striatum and diencephalon were most useful in histopathological analysis. Our findings show that these areas are most useful for evaluating the extent of N. caninum infection, investigating the pathogenesis of neosporosis, and evaluating antiparasitic drugs and vaccines against such organisms. Although the Nc-1 isolate has a lower ability for cyst formation, this isolate has been used in many studies of the pathogenesis of neosporosis and can induce fetal death in experimentally infected cattle (20–22). On the other hand, cystogenic isolates, such as Nc-Liv, may show some differences in brain parasite burdens and pathological changes. Thus, in future work, comparative study of different isolates of N. caninum will be important to understand the pathogenesis.
ACKNOWLEDGMENTS
We thank J. P. Dubey (U.S. Department of Agriculture, Agriculture Research Service, Livestock and Poultry Sciences Institute, and Parasite Biology and Epidemiology Laboratory) for the N. caninum Nc-1 isolate. We also thank Susumu Ogawa, Hiromichi Nishida, and Shinya Osada for excellent technical assistance and Kyoritsu Seiyaku Corporation for coordinating experiments using cattle.
This research was supported by the Japan Society for the Promotion of Science through the Funding Program for Next Generation World-Leading Researchers (NEXT Program), initiated by the Council for Science and Technology Policy (2011/LS003).
Footnotes
Published ahead of print 12 December 2012
REFERENCES
- 1. Dubey JP, Schares G, Ortega-Mora LM. 2007. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20:323–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hernandez J, Risco C, Donovan A. 2001. Association between exposure to Neospora caninum and milk production in dairy cows. J. Am. Vet. Med. Assoc. 219:632–635 [DOI] [PubMed] [Google Scholar]
- 3. Thurmond MC, Hietala SK. 1997. Effect of Neospora caninum infection on milk production in first-lactation dairy cows. J. Am. Vet. Med. Assoc. 210:672–674 [PubMed] [Google Scholar]
- 4. Reichel MP, Ellis JT. 2009. Neospora caninum—how close are we to development of an efficacious vaccine that prevents abortion in cattle? Int. J. Parasitol. 39:1173–1187 [DOI] [PubMed] [Google Scholar]
- 5. Nishikawa Y, Inoue N, Xuan X, Nagasawa H, Igarashi I, Fujisaki K, Otsuka H, Mikami T. 2001. Protective efficacy of vaccination by recombinant vaccinia virus against Neospora caninum infection. Vaccine 19:1381–1390 [DOI] [PubMed] [Google Scholar]
- 6. Nishikawa Y, Xuan X, Nagasawa H, Igarashi I, Fujisaki K, Otsuka H, Mikami T. 2001. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine. 19:1710–1716 [DOI] [PubMed] [Google Scholar]
- 7. Nishikawa Y, Zhang H, Ikehara Y, Kojima N, Xuan X, Yokoyama N. 2009. Immunization with oligomannose-coated liposome-entrapped dense granule protein 7 protects dams and offspring from Neospora caninum infection in mice. Clin. Vaccine Immunol. 16:792–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tuo W, Zhao Y, Zhu D, Jenkins MC. 2011. Immunization of female BALB/c mice with Neospora cyclophilin and/or NcSRS2 elicits specific antibody response and prevents against challenge infection by Neospora caninum. Vaccine 29:2392–2399 [DOI] [PubMed] [Google Scholar]
- 9. Alaeddine F, Keller N, Leepin A, Hemphill A. 2005. Reduced infection and protection from clinical signs of cerebral neosporosis in C57BL/6 mice vaccinated with recombinant microneme antigen NcMIC1. J. Parasitol. 91:657–665 [DOI] [PubMed] [Google Scholar]
- 10. Debache K, Alaeddine F, Guionaud C, Monney T, Müller J, Strohbusch M, Leib SL, Grandgirard D, Hemphill A. 2009. Vaccination with recombinant NcROP2 combined with recombinant NcMIC1 and NcMIC3 reduces cerebral infection and vertical transmission in mice experimentally infected with Neospora caninum tachyzoites. Int. J. Parasitol. 39:1373–1384 [DOI] [PubMed] [Google Scholar]
- 11. Debache K, Guionaud C, Alaeddine F, Mevissen M, Hemphill A. 2008. Vaccination of mice with recombinant NcROP2 antigen reduces mortality and cerebral infection in mice infected with Neospora caninum tachyzoites. Int. J. Parasitol. 38:1455–1463 [DOI] [PubMed] [Google Scholar]
- 12. Collantes-Fernández E, Zaballos Á, lvarez-García G, Ortega-Mora LM. 2002. Quantitative detection of Neospora caninum in bovine aborted fetuses and experimentally infected mice by real-time PCR. J. Clin. Microbiol. 40:1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herculano-Houzel S, Lent R. 2005. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J. Neurosci. 25:2518–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubey JP, Schares G. 2006. Diagnosis of bovine neosporosis. Vet. Parasitol. 140:1–34 [DOI] [PubMed] [Google Scholar]
- 15. Kritzner S, Sager H, Blum J, Krebber R, Greif G, Gottstein B. 2002. An explorative study to assess the efficacy of toltrazuril-sulfone (ponazuril) in calves experimentally infected with Neospora caninum. Ann. Clin. Microbiol. Antimicrob. 18:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho MS, Barr BC, Rowe JD, Anderson ML, Sverlow KW, Packham A, Marsh AE, Conrad PA. 1997. Detection of Neospora sp. from infected bovine tissues by PCR and probe hybridization. J. Parasitol. 83:508–514 [PubMed] [Google Scholar]
- 17. Melzer TC, Cranston HJ, Weiss LM, Halonen SK. 2010. Host cell preference of Toxoplasma gondii cysts in murine brain: a confocal study. J. Neuroparasitol. 1:N100505 doi:10.4303/jnp/N100505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helman RG, Stair EL, Lehenbauer TW, Rodgers S, Saliki JT. 1998. Neosporal abortion in Oklahoma cattle with emphasis on the distribution of brain lesions in aborted fetuses. J. Vet. Diagn. Invest. 10:292–295 [DOI] [PubMed] [Google Scholar]
- 19. Pollock H, Hutchings M, Weller RO, Zhang ET. 1997. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J. Anat. 191:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartley PM, Wright SE, Maley SW, Macaldowie CN, Nath M, Hamilton CM, Katzer F, Buxton D, Innes EA. 2012. Maternal and foetal immune responses of cattle following an experimental challenge with Neospora caninum at day 70 of gestation. Vet. Res. 43:38 doi:10.1186/1297-9716-43-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Macaldowie C, Maley SW, Wright S, Bartley P, Esteban-Redondo I, Buxton D, Innes EA. 2004. Placental pathology associated with fetal death in cattle inoculated with Neospora caninum by two different routes in early pregnancy. J. Comp. Pathol. 131:142–156 [DOI] [PubMed] [Google Scholar]
- 22. Maley SW, Buxton D, Rae AG, Wright SE, Schock A, Bartley PM, Esteban-Redondo I, Swales C, Hamilton CM, Sales J, Innes EA. 2003. The pathogenesis of neosporosis in pregnant cattle: inoculation at mid-gestation. J. Comp. Pathol. 129:186–195 [DOI] [PubMed] [Google Scholar]


