Abstract
The Mycobacterium avium-M. intracellulare complex (MAC) causes a pulmonary disease (PD) similar to tuberculosis (TB). Diagnosis of MAC-PD is complicated and time-consuming. In this study, the serodiagnostic potential of the newly identified MAV2054 and MAV5183 proteins was evaluated in subjects with MAC-PD, pulmonary TB, or latent TB and in noninfected healthy controls (HC), together with HspX and the 38-kDa antigen, well-known serodiagnostic M. tuberculosis antigens. All four antigens evoked significantly higher IgG responses in MAC-PD and active TB than in latent TB and HC subjects. Among the antigens, MAV2054 elicited the highest antibody responses in pulmonary TB and MAC-PD patients. IgG titers against MAV2054 and MAV5183 were significantly higher in MAC-PD than in pulmonary TB subjects. In addition, the levels of IgG against all antigens in the M. intracellulare and fibrocavitary forms were higher than those in the M. avium and nodular bronchiectatic forms, respectively. Based on sensitivity and receiver operator characteristic curve analysis, the best candidates for detection of MAC-PD and pulmonary TB were MAV2054 and the 38-kDa antigen, respectively. In total, 76.0% of MAC-PD and 65.0% of active TB patients were reactive to at least two antigens. In contrast, only 2.8% of HC subjects were reactive with two or more antigens. Our findings suggest that an enzyme-linked immunosorbent assay (ELISA) using the four antigens would be valuable for screening for mycobacterial lung disease, including MAC-PD and pulmonary TB, although it does not provide good discrimination of the disease-causing pathogens.
INTRODUCTION
More than 100 species are recognized in the genus Mycobacterium, and other than the two well-known mycobacterial species Mycobacterium tuberculosis and M. leprae, most are ubiquitous in the environment in soil, dust, plants, natural waters, food, and biofilms (1–3). These mycobacteria, previously known as atypical mycobacteria, are now more commonly called nontuberculous mycobacteria (NTM). The pathogens most commonly associated with NTM disease are the M. avium complex (MAC), M. abscessus, and M. kansasii (4). NTM can cause chronic pulmonary disease (PD) in humans similar to slowly progressive pulmonary tuberculosis (TB), but unlike tuberculosis, infection by NTM is not transmitted from person to person. Currently, the diagnosis of NTM-PD remains a challenge due to the complex diagnostic criteria, and the management of NTM-PD is difficult due to the prolonged treatment duration and resistance to antimycobacterial drugs (5).
MAC is the most common cause of NTM lung diseases and is an opportunistic infectious agent encountered frequently in patients who are immunocompromised, such as individuals with human immunodeficiency virus (HIV) infection (6). Furthermore, the prevalence of MAC-PD in immunocompetent patients has increased recently (5). The diagnosis of MAC-PD is complicated and time-consuming, because MAC may colonize the respiratory tract, and the isolation of MAC from sputum specimens often has no clinical or microbiologic significance due to its ubiquity in nature. In addition, discrimination of MAC-PD from pulmonary TB is difficult. Although the American Thoracic Society (ATS) has outlined guidelines for the diagnosis of NTM, the diagnosis of MAC-PD requires combined clinical, radiographic, and microbiologic evidence (5). Therefore, there have been many efforts to overcome these difficulties in diagnosis of MAC-PD.
There have been few attempts to develop a serologic test for diagnosis of MAC-PD and to distinguish it from other lung diseases, such as TB (7). Serodiagnostic tests using multiple mycobacterial antigens (Ag) are attractive for diagnosis of the disease due to their simplicity and economics. Use of glycopeptidolipids (GPLs) as markers for differentiation of MAC-PD from pulmonary TB and other mycobacterial infections has been reported (8–11). However, mycobacterial glycolipids, such as GPL and cord factor, are difficult to purify in quantity, and their preparation requires great labor and is associated with a high cost. M. avium KatG is the only protein reported as a novel diagnostic marker of M. avium infection (12). Therefore, identification of seroreactive proteins from MAC culture filtrate and evaluation of their usefulness to distinguish MAC-PD from pulmonary TB or healthy controls (HC) still need to be investigated more extensively.
We previously reported the serodiagnostic potential of a mycobacterial antigen cocktail for detection of TB using an enzyme-linked immunosorbent assay (ELISA) (13). In the present study, to apply the combined ELISA for serologic diagnosis of MAC-PD, we identified two candidates, MAV2054 and MAV5183, by screening using multidimensional fractionation of M. avium culture filtrate proteins (CFP) and probing with sera from patients with MAC-PD. In addition, HspX and 38-kDa protein (PstS1), well-known serodiagnostic M. tuberculosis antigens, were included in the antibody detection assay. The serodiagnostic potential of all four antigens was evaluated in subjects with MAC-PD, pulmonary TB, or latent TB and in HC. MAV2054 and MAV5183 proteins elicited significantly higher antibody responses in MAC-PD than in pulmonary TB and HC subjects.
MATERIALS AND METHODS
Subjects.
Sera were obtained from 175 patients with MAC-PD (median age = 60 years; age range = 27 to 85 years; percentage of males, 38%), 123 with active pulmonary TB (median age = 52 years; age range = 18 to 91 years; percentage of males, 63%), 151 with latent TB infection (LTBI) as defined by positive tuberculin skin test (TST) results (median age = 22 years; age range = 14 to 63 years; percentage of males, 54%), and 141 HC as defined by negative TST results (median age = 23 years; age range = 8 to 69 years; percentage of males, 62%). A total of 175 MAC patients who satisfied the diagnostic criteria of the American Thoracic Society (ATS) (5) were prospectively enrolled at the Asan Medical Center (Seoul, South Korea) or Samsung Medical Center (Seoul, South Korea). All serum samples obtained from MAC-PD patients from the two hospitals were accompanied by information about the isolated disease-causing bacteria (M. avium or M. intracellulare), while some included more-detailed information regarding TST results or diagnosis of one of two distinct subtypes, the nodular bronchiectatic form or fibrocavitary form, on the basis of radiographic features as identified by chest computed tomography.
Sera were obtained from patients with active TB or MAC-PD either before or during the first 2 weeks of medication. A basic diagnosis of TB was determined by acid-fast bacillus (AFB) staining and culture results as well as clinical evaluation, such as chest X-ray. Of 123 pulmonary TB sera, 97 were smear or culture positive and 26 were smear and culture negative. Colonies were identified as M. tuberculosis or M. avium complex using the AccuProbe test (Gen-Probe Inc., San Diego, CA) or a duplex PCR kit (M&D, Wonju, South Korea). MAC species were identified using a PCR-restriction fragment length polymorphism method, based on the rpoB gene (14).
Serum samples from LTBI patients were selected from the healthy participants from the Asan Medical Center based on TST results, including or not including a history of previous TB disease or exposure to risk factors for TB. TSTs were performed using 2 tuberculin units (TU) of PPD RT23 (Statens Serum Institute, Copenhagen, Demark) and the Mantoux method (15), and induration size was measured after 48 to 72 h using the ballpoint method. An induration diameter of more than 10 mm was regarded as a positive result, and consequently, sera from a total of 151 individuals with positive TST results and 141 HC with negative TST results and no history of clinical TB were collected. In addition, an gamma interferon release assay (IGRA) in some subjects with MAC-PD or LTBI and in HC was performed using QuantiFERON-TB Gold (QFT-G; Cellestis Ltd., Carnegie, Victoria, Australia) (16). All serum samples were collected after written informed consent had been provided by participants, and the study protocol was approved by the Institutional Review Board (IRB) of the Asan Medical and Samsung Medical Centers.
Multidimensional fractionation of CFP.
The M. avium 104 strain was grown for 6 weeks at 37°C as surface pellicles on Sauton's medium as described previously (17, 18). Culture filtrate proteins (CFP) were filter sterilized and concentrated by ultrafiltration (Amicon Ultra centrifugal filter unit with 3-kDa molecular mass cutoff; Millipore, Bedford, MA). Multidimensional fractionation was performed as described previously (19). In brief, the CFP was precipitated with ammonium sulfate (50% and 80% saturation). The resulting precipitates were dissolved in 50 mM potassium phosphate buffer (PB) containing 1 M ammonium sulfate, and then were separated by hydrophobic interaction chromatography (HIC) using phenyl Sepharose (GE Healthcare Biosciences, Piscataway, NJ) with a linear decreasing gradient of 1 to 0 M ammonium sulfate–50 mM PB and further eluted with 1 mM PB. Each eluate fraction was divided into three subfractions according to its major band pattern upon resolution by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Each of the primary fractions was concentrated separately, dialyzed against 1 mM PB, and then was further fractionated by hydroxyapatite chromatography with elution using a gradient of 1 to 300 mM PB. The eluate was pooled into individual subfractions according to its protein band pattern and concentrated. A third fractionation step was performed using diethylaminoethanol (DEAE) ion-exchange chromatography and a linear salt gradient of 0 to 300 mM NaCl–20 mM Tris-HCl (pH 8.0). All pooled fractions were concentrated and stored at −70°C until use.
Protein identification.
The major bands of the different fractions that strongly reacted with sera from patients with MAC-PD were selected and subjected to liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) at the Yonsei Proteomics Research Center (Yonsei University, Seoul, South Korea) as described previously (13, 17).
Preparation of recombinant proteins.
For this study, four recombinant antigens, the newly identified MAC proteins, MAV2054 and MAV5183, and the well-characterized M. tuberculosis proteins HspX and 38-kDa, were prepared. The HspX and 38-kDa proteins were produced in Escherichia coli and purified as described previously (13).
To produce MAV2054 and MAV5183, the corresponding genes were amplified by PCR using M. avium 104 genomic DNA as the template and the following primers: MAV2054-forward, 5′-CATATGACGTCGGCTCAAAATGAGTCT-3′; MAV2054-reverse, 5′-AAGCTTCTTGTACTCATGGAACTGATC-3′; MAV5183-forward, 5′-CATATGTCCAAGCCGGGGCTTCCGGTG-3′; and MAV5183-reverse, 5′-AAGCTTGGTGGCGGGCTGGGCGGGTTG-3′. Using the Signal P 4.0 server (http://www.cbs.dtu.dk/services/SignalP), which predicts the presence and location of N-terminal signal peptide cleavage sites within amino acid sequences, MAV5183 was determined to be a secretory protein with a putative signal sequence. Therefore, MAV5183 was amplified by PCR without the forward primer, which covered the sequence for secretion. Each PCR product was cut with NdeI and HindIII and then inserted into the pET22b(+) vector (Novagen, Madison, WI). The recombinant proteins were overexpressed in E. coli BL21 cells carrying bacteriophage DE3 and were purified by nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography in accordance with the manufacturer's instructions (Qiagen, Chatsworth, CA). The purified protein was pooled, concentrated, and dialyzed against phosphate-buffered saline (PBS; pH 7.4). Lastly, purified recombinant proteins were filter sterilized and stored at −70°C. Protein concentration was estimated using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL) with bovine serum albumin (BSA) as the standard.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was performed as described previously (20). Briefly, the recombinant antigens (1 μg/ml) were diluted in a coating buffer (KPL, Inc., Gaithersburg, MD), and 100 μl was added to each well in 96-well microtiter plates (Maxisorp; Nalge Nunc International, Rochester, NY). After overnight incubation at 4°C, the plates were washed three times with 1× washing buffer (KPL, Inc.), and then the wells were blocked with 5% BSA–PBS at room temperature for 3 h. After three more washes, 100 μl/well human serum (1:200 dilution) was added to each plate, and the plates were incubated at room temperature for 1 h. After washing, 100 μl of peroxidase-conjugated goat anti-human IgG (Sigma-Aldrich, St. Louis, MO) at a 1:6,000 dilution was added to each well. The plates were then incubated for an additional 30 min at room temperature. After seven more washes, the reactions were developed with a 5-mg o-phenylenediamine (OPD) tablet (Sigma) in 12 ml of substrate buffer and 0.05 M phosphate-citrate buffer followed by 12 μl of 30% H2O2. The reaction was stopped with 2 N sulfuric acid after 20 min of incubation in the dark. The optical density (OD) was measured at 490 nm (OD490) with an ELISA microplate reader (Molecular Devices, Sunnyvale, CA).
Evaluation of tests and statistical analysis.
In this study, there were more than 100 individuals in each subject group; therefore, we considered that the statistical data followed a normal distribution, regardless of the results of the normality test. The mean plus standard deviation (SD) of each group was used as the parameter, and values are expressed as the mean OD ± SD. We used the means plus 2 SD of control subjects as cutoff values for distinguishing between a positive and negative result. For assessment of the significance of differences among the four groups, mean differences were evaluated by a receiver operator characteristic (ROC) curve analysis and Tukey's multiple-comparison one-way analysis of variance (ANOVA). Differences in the median ODs of sera in the MAC-PD and LTBI subgroups were evaluated by the nonparametric Mann-Whitney test. Most statistical analyses were performed using Prism software, version 4.03 (GraphPad Software, San Diego, CA).
RESULTS
Identification of the MAV2054 and MAV5183 proteins.
It is important to identify and test serologically active mycobacterial antigens to determine the most appropriate mixture of antigens for serodiagnosis. Therefore, we conducted broad-scale fractionation of CFP of M. avium and determined seroreactivity by ELISA. The CFP was fractionated through a sequential multistep chromatographic process that included hydrophobic interaction, hydroxyapatite, and ion exchange. IgG titers against all fractions in the sera of some patients with MAC-PD or active pulmonary TB and of HC were determined by ELISA. IgG titers against fraction 15 (F15) and F30 in MAC-PD patients were significantly higher than in HC or active TB patients. Furthermore, the sensitivities of F15 and F30 for diagnosis of MAC-PD were 50% and 62.5%, respectively, which were higher than those of the other fractions. The major protein bands contained in F15 and F30 were selected for identification, and the two proteins indicated by rectangular boxes in Fig. 1 were subjected to LC-tandem MS (LC-MS/MS) analysis. The proteins with the highest scoring match to the query and the highest amino acid sequence homology were MAV2054 and MAV5183, respectively.
Fig 1.
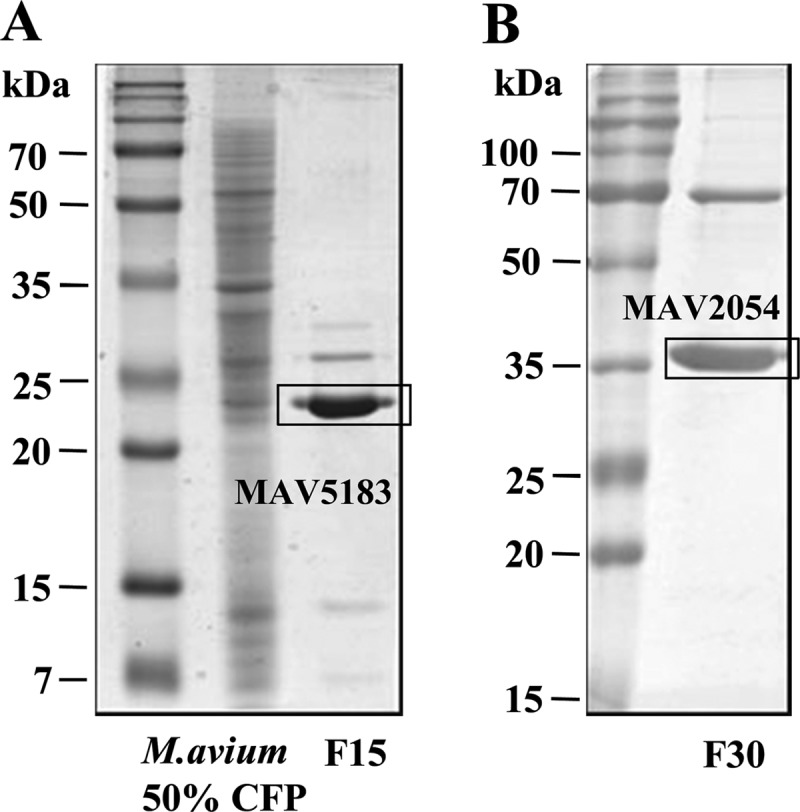
Identification of strong MAV2054 and MAV5183 seroreactivity in MAC-PD patients. An ammonium sulfate precipitate of M. avium CFP was fractionated by hydrophobic interaction chromatography using phenyl Sepharose. Each of the primary fractions was further fractionated by hydroxyapaptite chromatography. The third fractionation was conducted by DEAE ion-exchange chromatography. IgG titers in all fractions of sera from MAC-PD, pulmonary TB, and HC subjects were determined by ELISA. Two fractions, F15 (A) and F30 (B), were selected, subjected to SDS-PAGE, and stained with Coomassie brilliant blue.
Purification of the recombinant proteins.
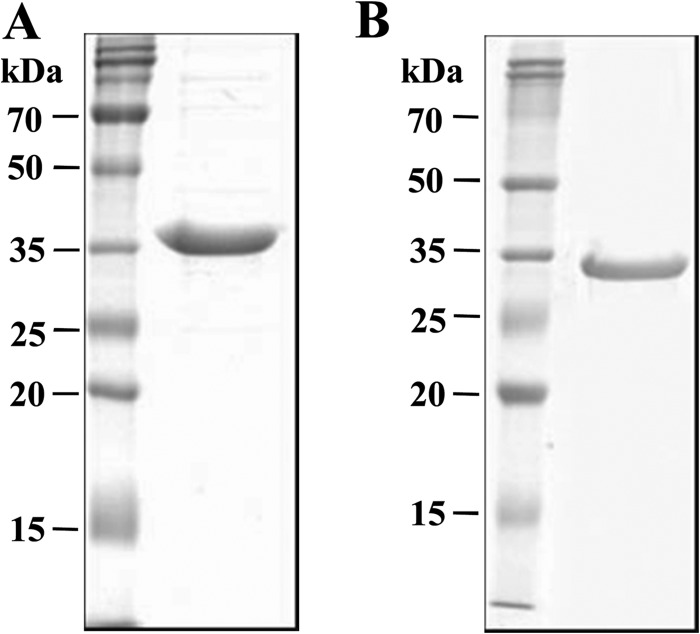
The newly identified MAV2054 and MAV5183 proteins were expressed as 6×His-tagged recombinant proteins in E. coli strain BL21 and then purified by Ni-NTA affinity chromatography. Due to the presence of a signal sequence at the N terminus of MAV5183, recombinant MAV5183 without the signal peptide (47 amino acids) was produced. The purity of both recombinant proteins was confirmed by SDS-PAGE (Fig. 2).
Fig 2.
SDS-PAGE analysis of purified recombinant MAV2054 (A) and MAV5183 (B) proteins. The proteins were overexpressed in E. coli, purified by Ni-NTA affinity chromatography, and analyzed by SDS-PAGE with Coomassie brilliant blue staining. Each lane was loaded with 20 μg protein. Bars indicate molecular mass.
Overall seroreactive patterns of the four proteins.
To investigate the serodiagnostic potential of the newly identified MAC proteins, serum IgG levels against each protein were measured in patients with MAC-PD, pulmonary TB, or LTBI and in HC. The HspX and 38-kDa antigen, well-known TB antigens that were prepared and described previously (13), were also included. As shown in Table 1 and in Fig. S1 in the supplemental material, all four antigens evoked significantly higher IgG responses in the MAC-PD and pulmonary TB groups than in the LTBI and HC groups. Among the antigens, MAV2054 elicited the highest antibody responses in pulmonary TB as well as MAC-PD patients. IgG titers against MAV2054 and MAV5183, but not HspX or 38-kDa antigen, were significantly different between the MAC-PD and pulmonary TB groups. However, MAC-PD and pulmonary TB patients reacted with both the TB and MAC antigens to considerable levels. In addition, antibody titers against the other three antigens were not significantly different between LTBI and HC subjects. There were no significant differences between smear- or culture-positive subjects and smear- and culture-negative subjects (data not shown).
Table 1.
Overall comparison of IgG responses to mycobacterial antigens in subjects with M. avium complex pulmonary disease, pulmonary TB, or latent TB infection and in healthy controlsa
| Antigen | Group (no. of subjects) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MAC-PD (175) |
Pulmonary TB (123) |
LTBI (151) |
HC (141) |
||||||||
| Mean OD ± SD | % sensitivity (no. of positive samples) | AUC (μg · h/ml) (95% confidence interval) | Mean OD ± SD | % sensitivity (no. of positive samples) | AUC (μg · h/ml) (95% confidence interval) | Mean OD ± SD | % sensitivity (no. of positive samples) | AUC (μg · h/ml) (95% confidence interval) | Mean OD ± SD | % specificity (no. of positive samples) | |
| MAV2054 | 0.521 ± 0.306a | 52 (91) | 0.814 (0.768–0.861) | 0.386 ± 0.275b | 36 (44) | 0.701 (0.636–0.766) | 0.306 ± 0.138c | 13 (20) | 0.699 (0.638–0.759) | 0.215 ± 0.115d | 97 (5) |
| MAV5183 | 0.378 ± 0.311a | 44 (77) | 0.786 (0.736–0.836) | 0.273 ± 0.200b | 24 (30) | 0.697 (0.632–0.761) | 0.206 ± 0.083c | 5 (8) | 0.655 (0.592–0.718) | 0.165 ± 0.086c | 96 (6) |
| HspX | 0.291 ± 0.190a | 35 (62) | 0.757 (0.705–0.810) | 0.249 ± 0.213a | 24 (30) | 0.649 (0.581–0.717) | 0.202 ± 0.086b | 5 (8) | 0.647 (0.584–0.711) | 0.163 ± 0.073b | 94 (8) |
| 38-kDa | 0.232 ± 0.336a | 45 (78) | 0.787 (0.736–0.837) | 0.190 ± 0.243a | 36 (44) | 0.726 (0.663–0.788) | 0.099 ± 0.050b | 11 (16) | 0.669 (0.607–0.731) | 0.080 ± 0.032b | 95 (7) |
MAC-PD, M. avium complex pulmonary disease; LTBI, latent TB infection; HC, healthy controls; AUC, area under the concentration-time curve. Mean OD ± SD values are expressed as means of OD490 ± SD. The cutoff value for each antigen was calculated as the mean OD obtained with sera from 141 healthy controls plus 2 SD. The superscript roman letters a, b, c, and d indicate mean antibody titers that were significantly different (P < 0.05) among the four groups.
Considering the mean plus 2 SD of HC as a cutoff value and a specificity of 94 to 97% for all antigens, MAV2054 showed the highest sensitivity for diagnosis of MAC-PD. Sensitivity of MAV2054 and the 38-kDa antigen for pulmonary TB was higher than that of the other two antigens. In general, the sensitivity of all antigens for diagnosis of MAC-PD was higher than that for pulmonary TB. MAV2054 for MAC-PD and 38-kDa antigen for pulmonary TB showed the highest areas under the concentration-time curve, respectively, compared to the other antigens. Taken together, these results suggest that the best candidates for the diagnosis of MAC-PD and active TB are MAV2054 and the 38-kDa antigen, respectively.
Antibody responses according to TST or IGRA results.
There were no significant differences between HspX, the 38-kDa antigen, and MAV5183 in the LTBI (tuberculin reactor) and HC (tuberculin nonreactor) group results. However, it is possible that MAC-PD patients recognized the TB antigens due to coinfection with M. tuberculosis. To determine this, TST and IGRA were performed in suspect MAC-PD cases. A subset of the MAC-PD patients was classified into two groups on the basis of their TST or IGRA positivity. As shown in Table 2, no significant differences were observed in the IgG responses to any antigen between the TST- or IGRA-positive and TST- or IGRA-negative groups. These results suggest that cross-reactivity to the four antigens between MAC-PD and TB patients may be due to the similarity of seroreactive epitopes among the antigens, rather than coinfection.
Table 2.
Comparison of IgG responses to mycobacterial antigens among MAC-PD subjects according to TST and IGRA resultsa
| Antigen | ELISA result for MAC-PD (n = 71) |
|||
|---|---|---|---|---|
| TST or IGRA positive (n = 34) |
TST and IGRA negative (n = 24) |
|||
| Median titer (IQR) | % sensitivity (no. of positive samples) | Median titer (IQR) | % sensitivity (no. of positive samples) | |
| MAV2054 | 0.540 (0.30–0.70) | 61.8 (21) | 0.480 (0.28–0.69) | 54.2 (13) |
| MAV5183 | 0.308 (0.23–0.45) | 50.0 (17) | 0.392 (0.29–0.50) | 58.3 (14) |
| HspX | 0.309 (0.20–0.43) | 50.0 (17) | 0.316 (0.27–0.41) | 50.0 (12) |
| 38-kDa | 0.142 (0.11–0.21) | 44.1 (15) | 0.131 (0.10–0.21) | 62.5 (15) |
IQR, interquartile range between 25th and 75th percentile. There were no differences between the TST- or IGRA-positive group and TST- and IGRA-negative group by a Mann-Whitney test that compared two unpaired groups (P < 0.05).
In this study, discrimination of the LTBI and HC groups was based on TST results. In addition, IGRA was performed in some subjects with LTBI; these were divided into IGRA-positive and -negative groups for comparison of their antibody responses. Interestingly, the median antibody level of the IGRA-positive group was higher than that of the IGRA-negative group (see Table S1 in the supplemental material). In particular, significant differences in IgG titers against MAV2054 and HspX between the IGRA-positive and -negative groups were observed. Additionally, the sensitivities of all antigens in the IGRA-positive group were significantly higher than in the IGRA-negative group.
Antibody responses among MAC-PD subgroups.
The 175 MAC-PD patients were divided into two subgroups according to the presence of disease-causing bacteria, M. avium (48%; n = 84) and M. intracellulare (52%; n = 91). As shown in Table 3, levels of IgG for all antigens in the M. intracellulare group were higher than those in the M. avium group. In particular, significant differences in IgG titers against MAV5183 and the 38-kDa antigen between the groups were observed. Furthermore, the sensitivity of MAV5183 and the 38-kDa antigen for the M. intracellulare group was higher than that for the M. avium group.
Table 3.
Comparison of IgG responses to mycobacterial antigens according MAC-PD typea
| Antigen | Pathogen of MAC-PDb (n = 175) |
Radiographic findings of MAC-PDc (n = 89) |
||||||
|---|---|---|---|---|---|---|---|---|
|
M. avium (n = 84) |
M. intracellulare (n = 91) |
Nodular bronchiectasis (n = 69) |
Fibrocavitary (n = 20) |
|||||
| Median titer (IQR) | % sensitivity (no. of positive samples) | Median titer (IQR) | % sensitivity (no. of positive samples) | Median titer (IQR) | % sensitivity (no. of positive samples) | Median titer (IQR) | % sensitivity (no. of positive samples) | |
| MAV2054 | 0.459 (0.20–0.65)c | 51.2 (43) | 0.471 (0.31–0.74)c | 52.7 (48) | 0.389 (0.22–0.54)e | 40.6 (28) | 0.489 (0.28–0.68)f | 60.0 (12) |
| MAV5183 | 0.251 (0.15–0.44)c | 36.9 (31) | 0.337 (0.23–0.51)d | 50.5 (46) | 0.203 (0.12–0.39)e | 27.5 (19) | 0.379 (0.22–0.85)f | 65.0 (13) |
| HspX | 0.233 (0.14–0.37)c | 31.0 (26) | 0.266 (0.20–0.35)c | 39.6 (36) | 0.208 (0.14–0.30)e | 23.2 (16) | 0.216 (0.18–0.27)e | 15.0 (03) |
| 38-kDa | 0.100 (0.06–0.15)c | 26.2 (22) | 0.160 (0.11–0.30)d | 61.5 (56) | 0.107 (0.07–0.17)e | 34.8 (24) | 0.196 (0.11–0.45)f | 60.0 (12) |
Values indicated by superscript roman letters (“c” and “d” for comparisons between M. avium and M. intracellulare data and “e” and “f” for comparisons between nodular bronchiectasis and fibrocavitary data) represent significant differences according to a Mann-Whitney test that compared two unpaired groups (P < 0.05).
Classification by the disease-causing mycobacteria.
Classification by the characteristic radiologic finding.
The 89 MAC-PD patients for whom detailed radiographic information was available were classified into two subgroups: those with the nodular bronchiectatic form of the disease and those with the fibrocavitary form. Interestingly, the median antibody levels against MAV2054, MAV5183, and the 38-kDa antigen of the fibrocavitary group were significantly higher than those of the nodular bronchiectatic group, and the sensitivity of these three antigens for the fibrocavitary group was markedly higher than for the nodular bronchiectatic group (Table 3).
Effect of antigen combination on sensitivity.
To investigate the multiple-antigen reactivity of a subject, we analyzed the frequency of reactivity against the four antigens in individual subjects (see Table S2 in the supplemental material). Of the 175 MAC-PD patients, 91 (52.0%) showed reactivity to two or more antigens. Of the 123 serum samples from active TB patients, 40 (32.5%) were reactive to two or more antigens. In contrast, only 8/151 (5.3%) LTBI and 4/141 (2.8%) HC subjects were reactive to two or more antigens.
Next, we compared reactivity to a single antigen or to combined antigens in each group (Table 4). As expected, combinations of two or more antigens had higher diagnostic sensitivity for each patient group than use of a single antigen alone. Using a combination of two antigens, the sensitivity was the highest with MAV2054 plus the 38-kDa antigen. The combination of MAV2054, the 38-kDa antigen, and MAV5183 resulted in the best diagnostic performance in terms of combinations of three antigens. When four antigens were combined, sensitivities of 65.0% for active TB, 76.0% for MAC-PD, and 26.5% for LTBI resulted, with a specificity of 83%.
Table 4.
Effects of antigen combinations on sensitivity
| Antigen(s) | % sensitivity (no. of positive sera) |
% specificity (no. of positive sera) for HC (n = 141) | ||
|---|---|---|---|---|
| MAC-PD (n = 175) | Pulmonary TB (n = 123) | LTBI (n = 151) | ||
| HspX | 35.4 (62) | 24.4 (30) | 5.3 (8) | 94.3 (8) |
| 38-kDa | 44.6 (78) | 35.8 (44) | 10.6 (16) | 95.0 (7) |
| MAV2054 | 52.0 (91) | 35.8 (44) | 13.2 (20) | 96.5 (5) |
| MAV5183 | 44.0 (77) | 24.4 (30) | 5.3 (8) | 95.7 (6) |
| 38-kDa + HspX | 57.1 (100) | 48.8 (60) | 15.2 (23) | 89.4 (15) |
| 38-kDa + MAV5183 | 61.7 (108) | 45.5 (56) | 15.2 (23) | 90.8 (13) |
| 38-kDa + MAV2054 | 67.4 (118) | 56.9 (70) | 21.9 (33) | 91.5 (12) |
| MAV2054 + MAV5183 | 62.9 (110) | 46.3 (57) | 15.2 (23) | 92.2 (11) |
| NAV2054 + MAV5183 + HspX | 66.3 (116) | 51.2 (63) | 17.9 (27) | 87.9 (17) |
| 38-kDa + HspX + MAV5183 | 68.6 (120) | 56.1 (69) | 18.5 (28) | 85.1 (21) |
| 38-kDa + HspX + MAV2054 | 71.4 (125) | 59.3 (73) | 24.5 (37) | 87.2 (18) |
| 38-kDa + MAV2054 + MAV5183 | 74.3 (130) | 61.8 (76) | 23.8 (36) | 87.2 (18) |
| 38-kDa + HspX + MAV2054 + MAV5183 | 76.0 (133) | 65.0 (80) | 26.5 (40) | 83.0 (24) |
DISCUSSION
The rates of recovery of NTM from AFB smear-positive clinical specimens have increased worldwide, and NTM is responsible for an increasing proportion of mycobacterial disease in many developed countries (21, 22). When NTM are cultured and identified in respiratory specimens, a diagnosis of NTM lung disease requires differentiation between contamination and colonization (23). Thus, serological reactivity facilitates the diagnosis of such diseases. Differentiation of NTM lung disease from pulmonary TB is important to the clinician. MAC is the most common cause of NTM lung disease in most regions of the world. Although extensive studies to identify protein antigens suitable for serodiagnosis of TB have been performed, little effort has focused on identification and evaluation of protein antigens for diagnosis of NTM lung disease. Here we evaluated serologic responses to newly identified M. avium proteins (MAV2054 and MAV5183) and well-known M. tuberculosis proteins (HspX and the 38-kDa protein) in patients with MAC-PD or pulmonary TB and in HC. Interestingly, the newly identified MAV2054 elicited the highest antibody responses in both TB and MAC-PD patients.
The HspX and 38-kDa proteins reacted with sera from MAC-PD to levels similar to their reactivities with sera from active TB patients. One possible explanation for this apparent cross-reactivity is M. tuberculosis coinfection in MAC-PD patients. However, there was no significant difference in antibody responses to any antigen tested in TST-positive and -negative MAC-PD patients. MAC contains phosphate-binding protein, which is similar to the 38-kDa protein (PstS1) of M. tuberculosis, and alpha crystallin family protein, which is similar to HspX (Rv2031c, alpha crystallin). Our results suggest that epitopes contained in both proteins may also be present in MAC proteins. Therefore, when TB is diagnosed serologically using M. tuberculosis proteins, it is possible that the presence of MAC-PD may lead to false-positive results.
The newly identified MAV2054 and MAV5183 proteins elicited significantly higher antibody responses in the MAC-PD group than in those with pulmonary TB. However, considerable reactivity against both antigens in active TB was also observed. On an amino acid level, MAV2054 shares 100% homology with 35-kDa major membrane protein 1 (MMP-1) of M. avium subsp. paratuberculosis (MAP) and shows 92% identity and 97% positivity with respect to the MMP of M. leprae. MMP-1, which was originally discovered in M. leprae as a 35-kDa immunodominant protein (24), is a serodiagnostic antigen that elicits strong humoral immune responses in tuberculoid leprosy (25–27). In addition, the gene that encodes this protein is present in M. abscessus and M. massiliense; however, it is absent from other mycobacteria such as M. tuberculosis and M. bovis bacillus Calmette-Guérin (BCG) (27, 28). The MAV5183 gene encodes antigen 85C (Ag85C), which consists of the antigen 85 complex of M. tuberculosis (29). Ag85C was reported as a seroreactive protein in TB (30, 31) and is highly conserved among most mycobacteria except several species of NTM such as M. fortuitum or M. chelonae. In addition, MAV5183 had 85% amino acid sequence identity and 93% homology with M. tuberculosis. Therefore, seroreactivity against MAV2054 and MAV5183 in TB patients may due to the presence of similar epitopes in the various M. tuberculosis proteins or due to frequent exposure to MAC, which is ubiquitous in nature.
GPL is used extensively as an antigen for serodiagnosis of MAC-PD and differential diagnosis from pulmonary TB (7–9). GPLs are major components of the outer layer of many NTM cell envelopes and are serotype specific. In addition, GPLs are not present in the M. tuberculosis complex (32). Therefore, a combination multiple-lipid antigen ELISA that includes GPL (7) and an enzyme immunoassay (EIA) kit detecting serum IgA antibody specific for GPL core (8) were useful for diagnosis of MAC-PD and for differentiating MAC-PD from pulmonary TB. However, 24.8% of TB patients and only 4.2% of HC subjects were anti-GPL IgG positive (7). Those data and our results suggest that cross-reactivity might not be fully explicable by exposure or MAC coinfection in TB patients.
MAC lung disease has been classified as two distinct subtypes, the fibrocavitary and the nodular bronchiectatic forms. The fibrocavitary form is usually seen in middle-aged or elderly male alcoholics and/or smokers with coexistent chronic obstructive pulmonary disease (33). Chest radiography frequently demonstrates apical cavity changes, similar to those in reactivated TB. This form is generally progressive, and if left untreated can lead to extensive lung destruction and/or death. The nodular bronchiectatic form occurs predominantly in nonsmoking middle-aged or elderly females without previous or underlying lung disease (4, 34). In the present study, of 175 MAC-PD patients, 69 were classified as having the nodular bronchiectatic form and 20 the fibrocavitary form according to radiographic findings. Interestingly, fibrocavitary-form patients showed significantly higher antibody responses to three antigens than did those with the nodular bronchiectatic form, and these antigens showed a greater sensitivity for patients with the fibrocavitary form. The cavitary form is generally progressive and has a higher burden of bacteria than the nodular bronchiectatic form (33). Therefore, cavitary disease with more-severe manifestations may elicit strong antibody responses to antigen. However, Kitada et al. (8) reported significantly higher antibody levels to GPL in nodular bronchiectatic-form than in fibrocavitary-form patients. To explain this discrepancy between antibody responses in the two forms of MAC-PD, further multivariate analysis using more clinical cases is necessary.
The two MAC species, M. avium and M. intracellulare, are the most frequently encountered causes of NTM pulmonary disease. In fact, they are isolated equally frequently from MAC lung patients (34). Although these species are distinct and can be identified and distinguished using nucleic acid probes, they are phenotypically very similar. Moreover, demographic and radiographic data and responses to therapy are indistinguishable between patients infected with these two species (33). Considering these clinical similarities, the other interesting finding of our study was that the antibody titers to four antigens in the M. intracellulare group were higher than those in the M. avium group. Recently, it was reported that patients with M. intracellulare lung disease exhibited a more severe presentation than patients with M. avium lung disease (35). Therefore, patients with M. intracellulare PD are presumed to have extensive lung lesions, which is consistent with the higher antibody responses in the M. intracellulare group.
It is generally accepted that multiple-antigen ELISAs can overcome the low sensitivity of TB serodiagnosis that results from individual heterogeneity in antibody responses. Rates of positivity for two or more antigens within the same assay were 52%, 32.5%, and 5.3% in MAC-PD, pulmonary TB, and HC subjects, respectively. Rates of positivity for three antigens were 32% and 17.1% in MAC-PD and active TB subjects, respectively. HC subjects did not react in assays that included three antigens. Finally, positivity rates with at least one antigen were 65% and 76% of active TB and MAC-PD subjects, respectively, with a specificity of 83%. These results suggest that subjects who showed positivity for two or more antigens were more likely to have MAC-PD or active TB. Recently, NTM have been responsible for an increasing proportion of mycobacterial lung disease in many developed countries and MAC has been the most common cause of NTM lung disease. Therefore, a cost-effective method for screening of mycobacterial lung disease, including pulmonary TB and MAC-PD, is required. Our findings suggest that an ELISA using the four antigens would be helpful for screening for this disease, although further confirmation is needed to discriminate the disease-causing pathogens. Further investigation on serological reactivity against the two MAV antigens in the patients with other NTM lung disease such as M. kansasii is needed to develop a more reliable screening method.
In conclusion, this is the first study to demonstrate the potential of MAV2054 and MAV5183 proteins as components of a serological test for the diagnosis of TB as well as MAC-PD. Although TB antibody detection is not recommended for the diagnosis of TB by the World Health Organization, it retains clinical value for auxiliary diagnosis and is widely used in countries with a high prevalence of TB. Therefore, multiple-antigen ELISAs that include MAV2054 and MAV5183 may facilitate diagnosis of MAC-PD, in particular for smear- and culture-negative or culture- or PCR-negative cases.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by a fund (2011-E46002-00) by Research of Korea Centers for Disease Control and Prevention and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2012-0005763).
Footnotes
Published ahead of print 26 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00649-12.
REFERENCES
- 1. Herdman AV, Steele JC., Jr 2004. The new mycobacterial species—emerging or newly distinguished pathogens. Clin. Lab. Med. 24:651–690 [DOI] [PubMed] [Google Scholar]
- 2. Falkinham JO, III, Iseman MD, de Haas P, van Soolingen D. 2008. Mycobacterium avium in a shower linked to pulmonary disease. J. Water Health 6:209–213 [DOI] [PubMed] [Google Scholar]
- 3. Huard RC, Lazzarini LC, Butler WR, van Soolingen D, Ho JL. 2003. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J. Clin. Microbiol. 41:1637–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous 1997Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:S1–S25 [DOI] [PubMed] [Google Scholar]
- 5. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K, on behalf of the ATS Mycobacterial Diseases Subcommittee 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 6. Corti M, Palmero D. 2008. Mycobacterium avium complex infection in HIV/AIDS patients. Expert Rev. Anti Infect. Ther. 6:351–363 [DOI] [PubMed] [Google Scholar]
- 7. Fujita Y, Doi T, Maekura R, Ito M, Yano I. 2006. Differences in serological responses to specific glycopeptidolipid-core and common lipid antigens in patients with pulmonary disease due to Mycobacterium tuberculosis and Mycobacterium avium complex. J. Med. Microbiol. 55:189–199 [DOI] [PubMed] [Google Scholar]
- 8. Kitada S, Kobayashi K, Ichiyama S, Takakura S, Sakatani M, Suzuki K, Takashima T, Nagai T, Sakurabayashi I, Ito M, Maekura R. 2008. Serodiagnosis of Mycobacterium avium-complex pulmonary disease using an enzyme immunoassay kit. Am. J. Respir. Crit. Care Med. 177:793–797 [DOI] [PubMed] [Google Scholar]
- 9. Kitada S, Maekura R, Toyoshima N, Naka T, Fujiwara N, Kobayashi M, Yano I, Ito M, Kobayashi K. 2005. Use of glycopeptidolipid core antigen for serodiagnosis of Mycobacterium avium complex pulmonary disease in immunocompetent patients. Clin. Diagn. Lab. Immunol. 12:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitada S, Maekura R, Toyoshima N, Fujiwara N, Yano I, Ogura T, Ito M, Kobayashi K. 2002. Serodiagnosis of pulmonary disease due to Mycobacterium avium complex with an enzyme immunoassay that uses a mixture of glycopeptidolipid antigens. Clin. Infect. Dis. 35:1328–1335 [DOI] [PubMed] [Google Scholar]
- 11. Enomoto K, Oka S, Fujiwara N, Okamoto T, Okuda Y, Maekura R, Kuroki T, Yano I. 1998. Rapid serodiagnosis of Mycobacterium avium-intracellulare complex infection by ELISA with cord factor (trehalose 6, 6′-dimycolate), and serotyping using the glycopeptidolipid antigen. Microbiol. Immunol. 42:689–696 [DOI] [PubMed] [Google Scholar]
- 12. Gupta K, Verma I, Khuller G, Mahajan R. 2010. KatG protein: a novel marker for differential diagnosis of Mycobacterium avium complex infection. Indian J. Med. Microbiol. 28:221–226 [DOI] [PubMed] [Google Scholar]
- 13. Shin AR, Shin SJ, Lee KS, Eom SH, Lee SS, Lee BS, Lee JS, Cho SN, Kim HJ. 2008. Improved sensitivity of diagnosis of tuberculosis in patients in Korea via a cocktail enzyme-linked immunosorbent assay containing the abundantly expressed antigens of the K strain of Mycobacterium tuberculosis. Clin. Vaccine Immunol. 15:1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whang J, Lee BS, Choi GE, Cho SN, Kil PY, Collins MT, Shin SJ. 2011. Polymerase chain reaction-restriction fragment length polymorphism of the rpoB gene for identification of Mycobacterium avium subsp. paratuberculosis and differentiation of Mycobacterium avium subspecies. Diagn. Microbiol. Infect. Dis. 70:65–71 [DOI] [PubMed] [Google Scholar]
- 15.Anonymous 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm. Rep. 49(RR-6):1–51 [PubMed] [Google Scholar]
- 16. Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M, Suzuki K, Inoue Y, Tsuyuguchi K, Sasaki Y, Mazurek GH, Tsuyuguchi I. 2004. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am. J. Respir. Crit. Care Med. 170:59–64 [DOI] [PubMed] [Google Scholar]
- 17. Shin AR, Kim HJ, Cho SN, Collins MT, Manning EJ, Naser SA, Shin SJ. 2010. Identification of seroreactive proteins in the culture filtrate antigen of Mycobacterium avium ssp. paratuberculosis human isolates to sera from Crohn's disease patients. FEMS Immunol. Med. Microbiol. 58:128–137 [DOI] [PubMed] [Google Scholar]
- 18. Shin AR, Lee KS, Lee JS, Kim SY, Song CH, Jung SB, Yang CS, Jo EK, Park JK, Paik TH, Kim HJ. 2006. Mycobacterium tuberculosis HBHA protein reacts strongly with the serum immunoglobulin M of tuberculosis patients. Clin. Vaccine Immunol. 13:869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Byun EH, Kim WS, Shin AR, Kim JS, Whang J, Won CJ, Choi Y, Kim SY, Koh WJ, Kim HJ, Shin SJ. 2012. Rv0315, a novel immunostimulatory antigen of Mycobacterium tuberculosis, activates dendritic cells and drives Th1 immune responses. J. Mol. Med. (Berl) 90:285–298 [DOI] [PubMed] [Google Scholar]
- 20. Kwon YM, Jung KH, Choi GE, Shin AR, Lee BS, Won CJ, Kim WS, Shin SJ, Park JK, Chang CH, Kim HJ. 2009. Identification and diagnostic utility of serologic reactive antigens from Mycobacterium tuberculosis sonic extracts. J. Bacteriol. Virol. 39:329–336 [Google Scholar]
- 21. Alvarez-Uria G. 2010. Lung disease caused by nontuberculous mycobacteria. Curr. Opin. Pulm. Med. 16:251–256 [DOI] [PubMed] [Google Scholar]
- 22. Jeon K, Koh WJ, Kwon OJ, Suh GY, Chung MP, Kim H, Lee NY, Park YK, Bai GH. 2005. Recovery rate of NTM from AFB smear-positive sputum specimens at a medical centre in South Korea. Int. J. Tuberc. Lung Dis. 9:1046–1051 [PubMed] [Google Scholar]
- 23. Yajko DM, Nassos PS, Sanders CA, Madej JJ, Hadley WK. 1994. High predictive value of the acid-fast smear for Mycobacterium tuberculosis despite the high prevalence of Mycobacterium avium complex in respiratory specimens. Clin. Infect. Dis. 19:334–336 [DOI] [PubMed] [Google Scholar]
- 24. Marques MA, Chitale S, Brennan PJ, Pessolani MC. 1998. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae. Infect. Immun. 66:2625–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar A, Girdhar BK, Parkash O. 2010. Immunoproteomic analysis of Mycobacterium leprae derived cell wall membrane antigens. Int. J. Biol. Med. Res. 1:242–247 [Google Scholar]
- 26. Triccas JA, Roche PW, Britton WJ. 1998. Specific serological diagnosis of leprosy with a recombinant Mycobacterium leprae protein purified from a rapidly growing mycobacterial host. J. Clin. Microbiol. 36:2363–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Triccas JA, Roche PW, Winter N, Feng CG, Butlin CR, Britton WJ. 1996. A 35-kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect. Immun. 64:5171–5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ivanyi J, Sinha S, Aston R, Cussell D, Keen M, Sengupta U. 1983. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin. Exp. Immunol. 52:528–536 [PMC free article] [PubMed] [Google Scholar]
- 29. Wiker HG, Harboe M. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim JH, Park JK, Jo EK, Song CH, Min D, Song YJ, Kim HJ. 1999. Purification and immunoreactivity of three components from the 30/32-kilodalton antigen 85 complex in Mycobacterium tuberculosis. Infect. Immun. 67:6187–6190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puech V, Bayan N, Salim K, Leblon G, Daffe M. 2000. Characterization of the in vivo acceptors of the mycoloyl residues transferred by the corynebacterial PS1 and the related mycobacterial antigens 85. Mol. Microbiol. 35:1026–1041 [DOI] [PubMed] [Google Scholar]
- 32. Brennan PJ, Nikaido H. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29–63 [DOI] [PubMed] [Google Scholar]
- 33. Koh WJ, Kwon OJ, Lee KS. 2005. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J. Korean Med. Sci. 20:913–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, Bai GH. 2006. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 129:341–348 [DOI] [PubMed] [Google Scholar]
- 35. Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, Shin SJ, Kwon OJ. 24 May 2012. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M. avium complex lung disease. Chest [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.