Abstract
It is unclear whether a mother who is negative for hepatitis B virus surface antigen (HBsAg) but positive for hepatitis B virus (HBV) is at potential risk for mother-to-child transmission of HBV. This study, using a paired mother-teenager population, aimed to assess whether maternal HBsAg-negative HBV infection (hnHBI) is a significant source of child HBV infection (HBI). A follow-up study with blood collection has been conducted on the 93 mother-teenager pairs from the initial 135 pregnant woman-newborn pairs 13 years after neonatal HBV vaccination. Serological and viral markers of HBV have been tested, and phylogenetic analysis of HBV isolates has been done. The HBI prevalence was 1.9% (1 hnHBI/53) for teenage children of non-HBI mothers, compared with 16.7% (1 hnHBI/6) for those of hnHBI mothers and 2.9% (1 HBsAg-positive HBV infection [hpHBI]/34) for those of hpHBI mothers. Similar viral sequences have been found in one pair of whom both the mother and teenager have had hnHBI. In comparison with the hpHBI cases, those with hnHBI had a lower level of HBV load and a higher proportion of genotype-C strains, which were accompanied by differentiated mutations (Q129R, K141E, and Y161N) of the “a” determinant of the HBV surface gene. Our findings suggest that mother-to-teenager transmission of hnHBI can occur among those in the neonatal HBV vaccination program.
INTRODUCTION
Infection with the hepatitis B virus (HBV) accounts for a significant portion of morbidity and mortality worldwide (1). With the introduction of a safe and effective HBV vaccination for neonates, the prevalence of chronic carriers who are identified as being hepatitis B virus surface antigen (HBsAg) positive has markedly dropped to 1% to ∼2% among the vaccinees (2, 3).
The HBV vaccination protocols for neonates vary according to their mothers' HBV statuses (3). Since it has been established that the combined three 10-μg-dose HBV vaccines plus hepatitis B immune globulin (HBIG) would provide better protection than the three 5-μg- or 10-μg-dose HBV vaccines alone (4), the Chinese government introduced a compulsory neonatal HBV vaccination program in 1992 (2): for babies born to HBsAg-positive mothers, three 10-μg-dose HBV vaccines plus a dose of 200 IU HBIG are to be provided, whereas for those born to HBsAg-negative mothers, only three 5-μg-dose vaccines are to be used.
Determining HBsAg status has been routinely undertaken for the mothers during a prenatal visit or before delivery through serological methods, which target the major “a” determinant of HBsAg. However, current available commercial assays could not recognize the following scenarios: the early-window period of acute HBV infection (HBI), occult hepatitis B virus infection (OBI) (defined as the presence of HBV DNA in the liver [with or without detectable HBV DNA in the serum] combined with a negative HBsAg result) with an HBV load below 200 IU/ml (5, 6), and a false OBI test result due to the presence of a modified HBsAg (caused by the “a” determinant mutations) (7–10). In current practice, differentiation among the scenarios noted above is unlikely unless follow-up studies are performed. Therefore, nearly all serology-based studies have treated such HBsAg-negative HBI (hnHBI) cases as OBIs (11). The substantial impact of hnHBI, including the reactivation or transmission of HBV, the progression of liver diseases, the development of hepatocellular carcinoma, etc., occurs in a variety of clinical settings (12–21).
Mother-to-child transmission of HBsAg-positive HBI (hpHBI) but not hnHBI has been well documented (4, 22). Scientific evidence suggests that HBV DNA, rather than HBsAg, is the determinant of this transmission (23). However, the inability to identify hnHBI routinely has meant that an hnHBI pregnant woman would be treated as a non-HBI case and that her newborn baby would be vaccinated with only the three 5-μg-dose HBV vaccines. Contrasted with hpHBI, the prevalence of hnHBI was much higher among the vaccinees or even those with high-level antibodies against HBsAg (anti-HBs) (24–26). Recent publications reported that the prevalence of hnHBI was 10.9% for vaccinees aged 1 to 13 years in Taiwan, China (25), 20.0% for those under 15 years of age in Singapore (24), and 3.25% for those aged 19 to 20 years in Qidong, China (26). One study reported a 28% prevalence of hnHBI among children born to hpHBI mothers despite prophylaxis with HBV vaccines and HBIG (27). Among teenagers who had a history of hpHBI but who no longer tested positive for HBsAg, only 24% responded to HBV vaccines marked by positive anti-HBs (28). Therefore, it would be hypothesized that hnHBI in the vaccinees may have originated mainly from their mothers.
In this study, we used a paired mother-teenager population to ascertain whether maternal hnHBI is a significant source of hnHBI for the child by analyzing the occurrence of hnHBI, determining the phylogenetic relationship between concurrent isolates, and assessing the risk of child hnHBI attributable to maternal hnHBI.
MATERIALS AND METHODS
Participants.
From 7 October 1996 to 17 May 1997, 135 pregnant woman-newborn pairs were enrolled in a follow-up vaccination program in Deqing County, Zhejiang Province, China (29). Of the 135 pregnant women, 100 were categorized as non-HBI and 35 had hpHBI; further, 16 of the 35 with hpHBI were also HBV e antigen positive. At 0, 1, and 6 months after birth, the newborns received HBV vaccinations, with each administered a 5-μg dose of yeast-derived recombinant hepatitis B vaccine (Shenzhen Kangtai Biological Products Co., Ltd., Shenzhen, China). The infants' anti-HBs levels were quantified at the ages of both 7 and 12 months. All HBV indexes were determined by using radioimmunoassay-based commercial kits (Shanghai Kehua Bio-engineering Co., Ltd., Shanghai, China) (29).
Follow-up and data collection.
From July to August 2010, a repeat study was conducted on the 135 initial pregnant woman-newborn pairs, who were now mother-teenager pairs. Informed consent was obtained from the teenagers' parents or participating mothers prior to specimen collection. Demographic data on the teenagers and mothers were obtained by using a structured questionnaire, and 5-ml blood samples were collected. Data on the administration of HBV booster vaccines for the teenagers were obtained from their vaccination records at the Center for Disease Prevention and Control, Deqing County, Zhejiang Province, China.
Serological and virological testing.
All serum specimens were divided into aliquots in two separate sterile tubes. The first tube of serum was used for the alanine aminotransferase (ALT) assay by using commercial kits based on the method of analysis of lactate dehydrogenase by UV radiation (Shanghai Kehua Bio-engineering Co., Ltd., Shanghai, China); the second tube was used for the detection of HBsAg, anti-HBs, and antibodies against hepatitis B virus core antigen (anti-HBc) by using commercial kits for an electrochemiluminescence immunoassay (Elecsys; Roche Diagnostics, Inc.). To avoid potential false-negative results caused by a single test, the HBsAg was further tested by enzyme-linked immunosorbent assay (ELISA)-based HBsAg kits (Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., Beijing, China).
Viral DNA extractions from 100 μl of serum were performed in parallel for both the first and second tube. The levels of HBV DNA load were measured by using Premix Ex Taq (Perfect Real Time) kits from TaKaRa Biotechnology (Dalian) Co. Ltd., Dalian, China, and primer sets, described elsewhere (21). Positive results in both tubes indicated a positive HBV load. All tests were performed strictly according to the manufacturer's instructions.
Nested PCR for HBV surface gene, sequencing, and phylogenetic analysis.
The second tube of serum (200 μl) was also used for performing a nested PCR with the HBV surface gene (21). The DNA extraction was performed using QIAamp DNA blood minikits (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Briefly, a 544-nucleotide (nt) segment of the HBV surface gene, nt 223 to 766 relative to the HBV prototype (GenBank accession no. AB033557), was amplified using primers S1 (5′-CCTGCTGGTGGCTCCAGTTC-3′) and S2 (5′-ATACCCAAAGACAAAAGAAAA-3′) for the first round of PCR and primers S3 (5′-GCGGGGTTTTTCTTGTTGAC-3′) and S4 (5′-GGGACTCAAGATGTTGTACAG-3′) for the second round. The PCR cycling conditions for both rounds consisted of denaturing for 40 s at 94°C, annealing for 40 s at 55°C, and extension for 40 s at 72°C, with 35 and 25 cycles used for the first and second rounds, respectively.
The PCR products for the HBV surface gene were purified and further sequenced on an ABI Prism 3130X automatic genetic analyzer (Applied Biosystems Life Technologies Corporation). The viral sequences were aligned using Lasergene (version 7.10; DNASTAR Inc.). Genetic distances between pairs of virus isolates were calculated using the Tamura-Nei method. A phylogenetic tree was constructed using the maximum-likelihood method and evaluated using the bootstrap test with 500 replications in MEGA software (version 5.05; available at http://www.megasoftware.net/). Prototype HBV strains of all genotypes (A to I) were used as references in the analysis; their GenBank accession numbers are as follows: genotype A, AY373432 and DQ315784; genotype B, BX97850, AY800391, AY206390, and AY206391; genotype C, AB112063 and AB033557; genotype D, EU939680 and X65259; genotype E, AB032431 and AB091256; genotype F, AB036905 and AB116654; genotype G, AB056515 and AB064313; genotype H, AB059661 and AB375161; and genotype I, AF241408 and AF241409. The serotypes of all identified HBV strains were determined by their amino acid sequences as previously reported (30).
A positive HBV DNA result was defined as being positive for either HBV load or the HBV surface gene for the HBsAg-positive participant or being positive for both HBV load and the HBV surface gene for the HBsAg-negative participant. Current HBI (in 2010) was defined as being either hpHBI or hnHBI. Prenatal HBI (in 1996 or 1997) was defined as being HBsAg positive only.
For teenagers without current HBI, a protective immunity to HBV was defined as an anti-HBs level ≥ 10.0 IU/liter (22). Initial vaccine response was defined as an anti-HBs level ≥ 10.0 IU/liter for the infants at 7 or 12 months.
The genotype-specific surface gene mutants of HBV in this study were compared with archived HBV strains (95 genotype B and 48 genotype C) that had been isolated from hpHBI from the general population in the same county (31).
Statistical analysis.
The means, medians, or proportions are presented as descriptive statistics. In the bivariate analyses, the Student's t test, the Mann-Whitney test, and Pearson χ2 test or Fisher's exact test were used to compare the means, medians, and proportions, respectively. All P values were 2 sided. The results were considered statistically significant when P < 0.05. All analyses were performed using SAS 8.02 for Windows (SAS Institute, Cary, NC).
Ethical approval was granted by the Human Subjects Committee Review Board of the School of Public Health, Fudan University.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences of the 31 strains determined in this work are KC117267 to KC117297.
RESULTS
Characterization of the follow-up and HBI cases identified in the mother-teenager pairs.
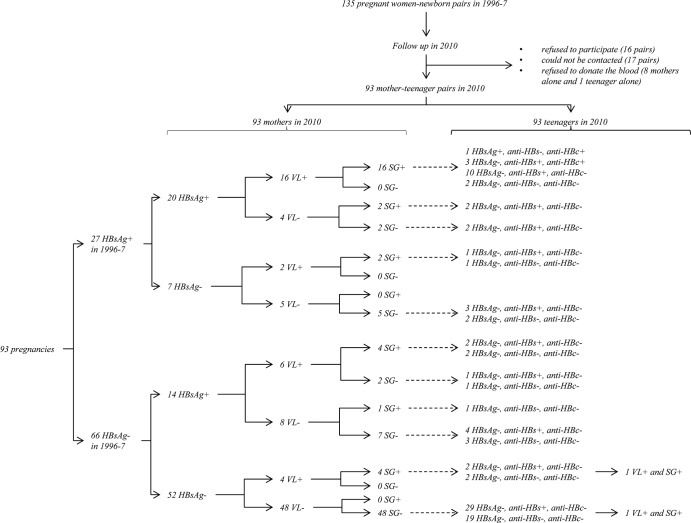
There were 16 mother-teenager pairs who refused to participate, 17 pairs who could not be contacted, and 8 mothers and 1 teenager alone who refused to provide blood samples. The remaining 93 pairs (68.9%) who had completed the questionnaire data and provided blood samples were included for further analysis (Fig. 1). The follow-up rate, 77.1%, for the 35 HBsAg-positive pregnant women diagnosed in 1996 or 1997 was not significantly different from that of 66.0% (66/100) for HBsAg-negative pregnant women (χ2 = 1.05, P = 0.22) (Fig. 1).
Fig 1.
Flow chart for study design and results of hepatitis B virus-related markers. VL, viral load; SG, hepatitis B virus surface gene.
The HBI status of the 93 pairs described above was determined again in 2010 with the more sensitive assays, i.e., PCR, sequencing methods, and new HBsAg assays. Forty HBI mothers (34 hpHBI and 6 hnHBI) and 3 HBI teenagers (1 hpHBI and 2 hnHBI) were identified (Fig. 1). For the mothers, their HBI status exhibited a 75.3% concordance between the data determined in 1996 or 1997 and in 2010 (McNemar χ2 = 7.3, P = 0.01); all the six hnHBI mothers classified in 2010 were also HBsAg negative in 1996 or 1997 (Fig. 1).
Feature comparison of the teenagers and the mothers by mothers' HBI statuses.
In comparison with those of the non-HBI mothers, the ages of HBI mothers were slightly lower and the proportion of mothers who were anti-HBs positive was lower; however, the other features of the mothers (education level, occupation, annual family income, abnormal ALT level, and anti-HBc status) and of the teenagers (age, sex ratio, delivery and feeding methods, infant growth and development, history of diseases, dental treatment, injuries, surgical operation, transfusion, toothbrush sharing, passive smoking, initial response to and boost with HBV vaccine, and abnormal ALT level) were similar (Table 1).
Table 1.
Features of the teenagers and the mothers by the mothers' HBI statusesa
| Feature | HBI mothers (n = 40) |
Non-HBI mothers (n = 53) |
P valueb | ||
|---|---|---|---|---|---|
| No. | Prop (%) | No. | Prop (%) | ||
| Mothersc | |||||
| Education: junior middle school or above | 31 | 77.5 | 39 | 73.5 | 0.66 |
| Occupation: peasant | 22 | 55.0 | 28 | 52.8 | 0.50 |
| Annual family income ≥ 50,000 yuan | 26 | 65.0 | 32 | 60.4 | 0.65 |
| ALT ≥ 40 U/liter | 2 | 5.0 | 6 | 11.3 | 0.46* |
| anti-HBc positive | 32 | 80.0 | 41 | 77.4 | 0.76 |
| anti-HBs positive | 8 | 20.0 | 30 | 56.6 | <0.01 |
| Teenagersd | |||||
| Sex (male) | 22 | 55.0 | 31 | 58.5 | 0.74 |
| Vaginal delivery: yes | 34 | 85.0 | 40 | 75.5 | 0.26 |
| Full-term delivery: yes | 40 | 100.0 | 51 | 96.2 | 0.50* |
| Only breastfeeding up to 6 months: yes | 32 | 80.0 | 43 | 81.1 | 0.89 |
| Normal infantile growth and development: yes | 33 | 82.5 | 42 | 79.2 | 0.69 |
| Passive smoking: yes | 22 | 55.0 | 34 | 64.2 | 0.33 |
| Dental treatment: yes | 18 | 45.0 | 23 | 43.4 | 0.88 |
| History of diseases: yes | 4 | 10.0 | 2 | 3.8 | 0.40* |
| History of injury: yes | 36 | 90.0 | 49 | 92.5 | 0.72* |
| Sharing of toothbrush: yes | 3 | 7.5 | 1 | 1.9 | 0.31* |
| Transfusion history: yes | 2 | 5.0 | 0 | 0.0 | 0.18* |
| History of surgical operation: yes | 4 | 10.0 | 1 | 1.9 | 0.16* |
| Initial HBV vaccine response: yes | 34 | 85.0 | 46 | 86.8 | 0.81 |
| History of booster: yes | 34 | 85.0 | 41 | 77.4 | 0.36 |
| ALT ≥ 40 U/liter | 1 | 2.5 | 4 | 7.5 | 0.46 |
HBI, hepatitis B virus infection; Prop, proportion; ALT, alanine aminotransferase; anti-HBs, antibody to hepatitis B virus surface antigen; anti-HBc, antibody to hepatitis B virus core antigen.
*, Fisher's exact test.
Age in years (mean ± standard deviation), 38.5 ± 2.4 (HBI mothers) or 39.9 ± 3.9 (non-HBI mothers) (P = 0.04).
Age in years (mean ± standard deviation), 13.7 ± 0.1 (HBI mothers) or 13.7 ± 0.1 (non-HBI mothers) (P = 0.72); birth weight in kilograms (mean ± standard deviation), 3.26 ± 0.40 (HBI mothers) or 3.35 ± 0.37 (non-HBI mothers) (P = 0.26).
Relationship of HBI status among the 93 mother-teenager pairs.
For all teenagers, the prevalences of HBI (1 hpHBI plus 2 hnHBI), of being anti-HBs positive, and of being anti-HBc positive were 3.2%, 60.2%, and 4.3%, respectively (Table 2).
Table 2.
Relationship of HBI statuses among the 93 mother-teenager pairsa
| Group | Mother's HBI status (total no.) | No. (%) of teenagers with indicated HBI status |
No. (%) of anti-HBc+ teenagers | ||
|---|---|---|---|---|---|
| HBIb | Non-HBI |
||||
| Anti-HBs+ | Anti-HBs− | ||||
| ① | HBI (40) | 2 (5.0) | 26 (65.0) | 12 (30.0) | 4 (10.0) |
| ② | hnHBI, HBV DNA+ (6) | 1* (16.7) | 2 (33.3) | 3 (50.0) | 0 (0.00) |
| ③ | hpHBI, HBV DNA+ (25) | 1# (4.0) | 18 (72.0) | 6 (24.0) | 4 (16.0) |
| ④ | hpHBI, HBV DNA− (9) | 0 (0.0) | 6 (66.7) | 3 (33.3) | 0 (0.00) |
| ⑤ | Non-HBI (53) | 1* (1.9) | 30 (56.6) | 22 (41.5) | 0 (0.00) |
| Total | 3 (3.2) | 56 (60.2) | 34 (36.6) | 4 (4.3) | |
HBI, hepatitis B virus infection; HBV, hepatitis B virus; anti-HBs, antibody to hepatitis B virus surface antigen; anti-HBc, antibody to hepatitis B virus core antigen. Statistical significance was determined by Fisher's exact test. For teenagers' HBI statuses, overall P = 0.46 for ① and ⑤, P = 0.14 for ②, ③ plus ④, and ⑤, P = 0.28 for ②, ③, ④, and ⑤, P = 0.63 for ② plus ③, ④, and ⑤, and P = 1.00 for ② plus ⑤ and ③ plus ④. For teenagers' anti-HBc statuses, overall P = 0.04 for ① and ⑤, P = 0.05 for ②, ③ plus ④, and ⑤, P = 0.02 for ②, ③, ④, and ⑤, P = 0.02 for ② plus ③, ④, and ⑤, and P = 0.02 for ② plus ⑤ and ③ plus ④.
*, hnHBI; #, hpHBI.
Four anti-HBc-positive cases were identified exclusively among teenagers whose mothers were hpHBI in both 1996 or 1997 and 2010, together with a detectable HBV DNA in 2010 (Fig. 1), but only one of the teenagers developed hpHBI. And the difference in the proportions of being anti-HBc positive was statistically significant in teenage children of mothers with different HBI status based on the results determined in 1996 or 1997 or in 2010 (Table 2, Fig. 1).
The proportions of HBI were 16.7% (1/6), 2.9% (1/34), and 1.9% (1/53), respectively, in teenage children of the mothers with hnHBI, hpHBI, and non-HBI. However, the difference in the proportions of HBI was not statistically significant in teenage children of mothers with different HBI statuses based on the results determined in 1996 or 1997 or in 2010 (Table 2, Fig. 1).
Viral and phylogenetic analysis of HBsAg-negative and HBsAg-positive HBV strains.
Of the 93 mother-teenager pairs, 29 mothers and 2 teenagers were identified as being HBV DNA positive, with a median HBV DNA load of 3.47 × 102 IU/ml. The hpHBI cases exhibited a slightly higher but statistically nonsignificant median HBV DNA level, 3.92 × 102 IU/ml (23 cases; range, <5.0 IU/ml to ∼1.91 × 109 IU/ml), compared with the level seen with hnHBI cases, 2.47 × 102 IU/ml (8 cases; range, 4.33 × 101 IU/ml to ∼6.25 × 105 IU/ml).
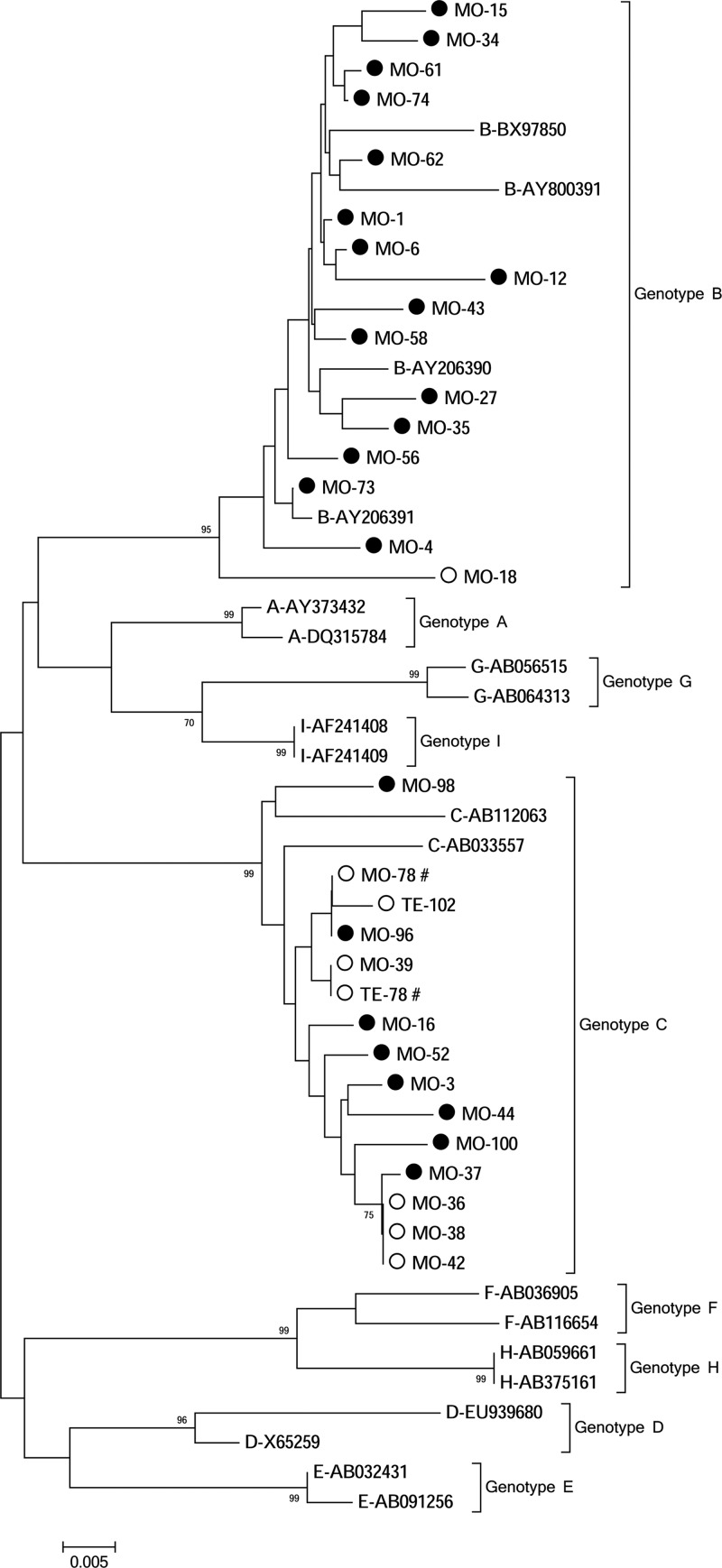
A total of 31 HBV strains, 29 from mothers (6 from hnHBI and 23 from hpHBI) and 2 from teenagers (both from cases of hnHBI), were identified, and the overall ratio of genotype B to genotype C was 16/15 (Fig. 2). The proportion of genotype C was significantly higher for cases with hnHBI (7/8) than for those with hpHBI (8/23, P = 0.02, Fisher's exact test). All genotype-B and genotype-C HBV strains identified in this study had the serotypes of adw and adr, respectively.
Fig 2.
Phylogenetic tree of the 31 hepatitis B virus (HBV) strains isolated from a paired mother-teenager population. Each hepatitis B virus (HBV) strain is presented with a name connected by a hyphen. Those beginning with the letters from A to I denote genotypes A to I of the reference HBV and are further connected by hyphens with their corresponding GenBank accession numbers. Those beginning with the letters “MO” and “TE” denote our HBV strains isolated from the mothers and teenagers in this study. And those letters are further connected by the hyphens with one to three digits that represent the paired mother-teenager numbers. The dotted and hollow circles denote HBsAg-positive and HBsAg-negative HBV strains, respectively. The # symbol at the right of our strain names indicates that the strains were isolated from a mother-teenager pair. The GenBank accession numbers are KC117267 to KC117297 for our 31 strains.
In comparison with the archived HBV strains that were isolated from individuals with hpHBI in the same region, the overall mutation rate per strain was consistent between the hnHBI cases in this study and archived hpHBI cases (7.04 per 1,000 and 7.19 per 1,000, respectively). In the hpHBI cases from this study, the observed mutations of the 100th, 120th, 126th, 156th, 158th, and 164th amino acid positions for genotype-B strains and those of the 120th and 141st amino acid positions for genotype-C strains were exactly the same as those archived. Neither the specific mutations listed above nor those with high frequencies, such as those at the 133rd and 161st positions, from the hpHBI cases were identified in the hnHBI cases in this study. The two alleles identified in the hnHBI cases in this study (Y161N and K141E) showed mutation profiles different from those archived (Y161F/S and K141G). In addition, the Q129R allele identified in two hnHBI cases (one teenager and one mother) was not observed in those archived.
There was one mother-teenager pair (MO-78 and TE-78) both with hnHBI and infected with HBV strains (Fig. 2). Further sequence analysis showed that the viruses from this mother and her child are closely related, with the only difference at the site of Q129R (Table 3).
Table 3.
Population characteristics of the eight mother-teenager pairs with either HBI teenagers or HBsAg-negative HBI mothersa
| M-T pairs | Sex (T) | Age in yr (M/T) | ALT, U/liter (M/T) | Case (M/T) | HBsAg/HBeAgb (M) | HBsAg/anti-HBs/anti-HBc (M) | HBsAg/anti-HBs/anti-HBc (T) | Booster at age in yr (T) | Anti-HBs, IU/literb (7 mo/12 mo/13 yr, T) | HBV DNA, IU/ml (M/T) | HBV-SG (M/T) | Genotype/serotype/mutation (M, T) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 73 | G | 38.8/13.7 | 21/13 | hpHBI/hpHBI | +/+ | +/−/+ | +/−/+ | No | ND/ND/7.8 | 1.03 × 104/<5 | s/ND | B/adw/wt, ND |
| 78 | B | 41.0/13.8 | 14/15 | hnHBI/hnHBI | −/− | −/+/+ | −/+/− | 5.75 | 1003.5/58.8/372.0 | 1.42 × 104/7.83 × 101 | s/s | C/adr/wt, C/adr/Q129R |
| 102 | G | 37.8/13.9 | 45/11 | non-HBI/hnHBI | −/− | −/−/− | −/+/− | 5.92 | 59.7/109.4/2.1 | <5/5.58 × 102 | ND/s | ND, C/adr/K141E |
| 18 | B | 39.2 13.6 | 6 | hnHBI/non-HBI | −/− | −/+/+ | −/+/− | 6.17 | ND/31.1/32.3 | 3.02 × 102/<5 | s/ND | B/adw/F161N, ND |
| 36 | G | 37.9/13.7 | 7 | hnHBI/non-HBI | −/− | −/−/+ | −/−/− | 10.92 | ND/ND/6.2 | 6.25 × 105/<5 | s/ND | C/adr/wt, ND |
| 38 | B | 37.7/13.8 | 20 | hnHBI/non-HBI | −/− | −/+/+ | −/−/− | No | 26.5/45.49/<2.0 | 1.28 × 102/<5 | s/ND | C/adr/wt, ND |
| 39 | B | 38.2/13.8 | 8 | hnHBI/non-HBI | −/− | −/−/+ | −/−/− | 4.92 | 688.9/5136.9/2.1 | 1.08 × 102/<5 | s/ND | C/adr/Q129R, ND |
| 42 | B | 38.4/13.6 | 11 | hnHBI/non-HBI | −/− | −/+/+ | −/+/− | 4.75 | ND/920.0/328 | 1.92 × 102/<5 | s/ND | C/adr/wt, ND |
HBI, hepatitis B virus infection; HBV, hepatitis B virus; anti-HBs, antibody to hepatitis B virus surface antigen; anti-HBc, antibody to hepatitis B virus core antigen; M, mother; T, teenager; G, girl; B, boy; ND, not detectable; wt, wild type; ALT, alanine aminotransferase; HBV-SG, HBV surface gene.
The results were from 1996 to 1997.
DISCUSSION
Our results show that the mother and the teenager in one pair were both infected with hnHBI and that the virus sequences are highly similar, which suggests that mother-teenager transmission of hnHBI has occurred among 13-year-old teenagers who have received neonatal HBV vaccination.
In this study, 3.2% of the 93 teenagers had been infected with either hnHBI or hpHBI even though all of them had received HBV vaccination since birth and 81% of them had received at least one booster dose before the age of 13 years. Further, this prevalence among teenage children of hnHBI mothers, 16.7% (1/6), was markedly higher than the prevalences among those of hpHBI and non-HBI mothers, 2.9% (1/34) and 1.9% (1/53), respectively; but the difference was not statistically significant. Thus, it remains inconclusive whether maternal hnHBI could play a major role in its transmission to her child.
The mechanism of transmission of hpHBI has been well understood. Early-life HBV infection from mother to child, which is mainly determined by the mother's HBV DNA level, can occur during the prenatal (through the placenta), perinatal, or postnatal stages of life (32–34). Furthermore, natural and chronic-carrier infection of HBV can still occur over time, even for those who have received neonatal HBV vaccinations (22) or have been living in areas of high endemicity (35–37). Though hnHBI has been established in a variety of populations, including those with neonatal HBV vaccination (24–26), the source of this infection remains largely unknown. In this study, we did observe the transmission of a one mother's hnHBI to her child (one case), which would imply a role of maternal hnHBI that is possibly similar to that of maternal hpHBI. But other sources (father, siblings, friends, etc.) rather than the mother remain possible, as indicated by the ME-102 and TE-102 pair in this study.
In agreement with previous studies (21, 26), we found more genotype-C HBV strains and a lower viral load in hnHBI cases than in hpHBI cases. The site-specific amino acid analysis identified three mutations (Q129R, K141E, and Y161N) that were unique to hnHBI cases. The Q129R or K141E mutant, which has been suggested as a diagnostic-escape strain (9), had established its infection among the vaccinees. Interestingly, both hnHBI teenagers identified had received an extra booster dose of HBV vaccine in the past 13 years and had a detectable level of anti-HBs (Table 3).
Our findings raise the issue of whether maternal hnHBI can play a main role in its transmission to the child in the current HBV vaccination program. In comparison with the prevalence of hpHBI among the pregnant women (approximately 3% to 4% [L.-N. Tao, personal communication]) and in the general population (7.2%) (2), the prevalence of hnHBI among the pregnant women is not statistically insignificant or is even higher (38), as indicated also by our results showing that the hnHBI prevalence was 10.2% (6/59) among the tested mothers. And the inability to routinely identify hnHBI in prenatal women means that they would be treated as non-HBV cases and that their newborns would receive the less effective HBV vaccination protocol (4). Thus, mother-to-child transmission of hnHBI needs further investigation.
In conclusion, mother-to-teenager transmission of hnHBI occurs among those in a neonatal HBV vaccination program. As a consequence of the limitations of this small study, the exact role of maternal hnHBI remains unknown. A study of a large cohort of pregnant women with a longer follow-up period for both mothers and children would be ideal for investigating mother-to-child transmission of hnHBI and the effectiveness of current HBV vaccinations against both hpHBI and hnHBI.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Science and Technology of the People's Republic of China (2008ZX10002-012), the Shanghai Leading Academic Discipline Project (B118), and Zhejiang Medicines & Health Science and Technology Project (2009A203).
We thank Shan Wei for her English editing.
Footnotes
Published ahead of print 19 December 2012
REFERENCES
- 1. Lavanchy D. 2004. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J. Viral Hepat. 11:97–107 [DOI] [PubMed] [Google Scholar]
- 2. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Wang F, Zheng H, Guo J, Jia Z, Ma J, Wang H, Luo H, Li L, Jin S, Hadler SC, Wang Y. 2009. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine 27:6550–6557 [DOI] [PubMed] [Google Scholar]
- 3. Zhou YH, Wu C, Zhuang H. 2009. Vaccination against hepatitis B: the Chinese experience. Chin. Med. J. (Engl). 122:98–102 [PubMed] [Google Scholar]
- 4. Lee C, Gong Y, Brok J, Boxall EH, Gluud C. 2006. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ 332:328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, Craxi A, Donato F, Ferrari C, Gaeta GB, Gerlich WH, Levrero M, Locarnini S, Michalak T, Mondelli MU, Pawlotsky JM, Pollicino T, Prati D, Puoti M, Samuel D, Shouval D, Smedile A, Squadrito G, Trepo C, Villa E, Will H, Zanetti AR, Zoulim F. 2008. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J. Hepatol. 49:652–657 [DOI] [PubMed] [Google Scholar]
- 6. Raimondo G, Pollicino T, Romano L, Zanetti AR. 2010. A 2010 update on occult hepatitis B infection. Pathol. Biol. (Paris) 58:254–257 [DOI] [PubMed] [Google Scholar]
- 7. El Chaar M, Candotti D, Crowther RA, Allain JP. 2010. Impact of hepatitis B virus surface protein mutations on the diagnosis of occult hepatitis B virus infection. Hepatology 52:1600–1610 [DOI] [PubMed] [Google Scholar]
- 8. Gerlich WH, Bremer C, Saniewski M, Schuttler CG, Wend UC, Willems WR, Glebe D. 2010. Occult hepatitis B virus infection: detection and significance. Dig. Dis. 28:116–125 [DOI] [PubMed] [Google Scholar]
- 9. Huang CH, Yuan Q, Chen PJ, Zhang YL, Chen CR, Zheng QB, Yeh SH, Yu H, Xue Y, Chen YX, Liu PG, Ge SX, Zhang J, Xia NS. 2012. Influence of mutations in hepatitis B virus surface protein on viral antigenicity and phenotype in occult HBV strains from blood donors. J. Hepatol. 57:720–729 [DOI] [PubMed] [Google Scholar]
- 10. Zuckerman JN, Zuckerman AJ. 2003. Mutations of the surface protein of hepatitis B virus. Antiviral Res. 60:75–78 [DOI] [PubMed] [Google Scholar]
- 11. Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Brechot P. 2001. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology 34:194–203 [DOI] [PubMed] [Google Scholar]
- 12. Allain JP. 2004. Occult hepatitis B virus infection: implications in transfusion. Vox Sang. 86:83–91 [DOI] [PubMed] [Google Scholar]
- 13. Chan HL, Tsang SW, Leung NW, Tse CH, Hui Y, Tam JS, Chan FK, Sung JJ. 2002. Occult HBV infection in cryptogenic liver cirrhosis in an area with high prevalence of HBV infection. Am. J. Gastroenterol. 97:1211–1215 [DOI] [PubMed] [Google Scholar]
- 14. Di Stefano M, Volpe A, Stallone G, Tartaglia L, Prato R, Martinelli D, Pastore G, Gesualdo L, Fiore JR. 2009. Occult HBV infection in hemodialysis setting is marked by presence of isolated antibodies to HBcAg and HCV. J. Nephrol. 22:381–386 [PubMed] [Google Scholar]
- 15. Ghisetti V, Marzano A, Zamboni F, Barbui A, Franchello A, Gaia S, Marchiaro G, Salizzoni M, Rizzetto M. 2004. Occult hepatitis B virus infection in HBsAg negative patients undergoing liver transplantation: clinical significance. Liver Transpl. 10:356–362 [DOI] [PubMed] [Google Scholar]
- 16. Gupta S, Singh S. 2010. Occult hepatitis B virus infection in ART-naive HIV-infected patients seen at a tertiary care centre in north India. BMC Infect. Dis. 10:53 doi:10.1186/1471-2334-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ozaslan E, Demirezer A, Yavuz B. 2009. Occult hepatitis B virus infection in Turkish healthy individuals. Eur. J. Gastroenterol. Hepatol. 21:1436–1437 [DOI] [PubMed] [Google Scholar]
- 18. Raimondo G, Navarra G, Mondello S, Costantino L, Colloredo G, Cucinotta E, Di Vita G, Scisca C, Squadrito G, Pollicino T. 2008. Occult hepatitis B virus in liver tissue of individuals without hepatic disease. J. Hepatol. 48:743–746 [DOI] [PubMed] [Google Scholar]
- 19. Samal J, Kandpal M, Vivekanandan P. 2012. Molecular mechanisms underlying occult hepatitis B virus infection. Clin. Microbiol. Rev. 25:142–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torbenson M, Thomas DL. 2002. Occult hepatitis B. Lancet Infect. Dis. ii:479–486 [DOI] [PubMed] [Google Scholar]
- 21. Yuan Q, Ou SH, Chen CR, Ge SX, Pei B, Chen QR, Yan Q, Lin YC, Ni HY, Huang CH, Yeo AE, Shih JW, Zhang J, Xia NS. 2010. Molecular characteristics of occult hepatitis B virus from blood donors in southeast China. J. Clin. Microbiol. 48:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. 2009. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J. Infect. Dis. 200:1390–1396 [DOI] [PubMed] [Google Scholar]
- 23. Giles ML, Visvanathan K, Lewin SR, Sasadeusz J. 2012. Chronic hepatitis B infection and pregnancy. Obstet. Gynecol. Surv. 67:37–44 [DOI] [PubMed] [Google Scholar]
- 24. Chen WN, Oon CJ. 2000. Hepatitis B virus surface antigen (HBsAg) mutants in Singapore adults and vaccinated children with high anti-hepatitis B virus antibody levels but negative for HBsAg. J. Clin. Microbiol. 38:2793–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mu SC, Lin YM, Jow GM, Chen BF. 2009. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J. Hepatol. 50:264–272 [DOI] [PubMed] [Google Scholar]
- 26. Xu L, Wei Y, Chen T, Lu J, Zhu CL, Ni Z, Huang F, Du J, Sun Z, Qu C. 2010. Occult HBV infection in anti-HBs-positive young adults after neonatal HB vaccination. Vaccine 28:5986–5992 [DOI] [PubMed] [Google Scholar]
- 27. Shahmoradi S, Yahyapour Y, Mahmoodi M, Alavian SM, Fazeli Z, Jazayeri SM. 2012. High prevalence of occult hepatitis B virus infection in children born to HBsAg-positive mothers despite prophylaxis with hepatitis B vaccination and HBIG. J. Hepatol. 57:515–521 [DOI] [PubMed] [Google Scholar]
- 28. Taheri H, Hasanjani Roushan MR, Soleimani Amiri MJ, Pouralijan M, Bijani A. 2011. Efficacy of hepatitis B vaccine in those who lost hepatitis B surface antigen during follow-up: efficacy of HBV vaccine in those who lost HBsAg. Hepat. Mon. 11:119–122 [PMC free article] [PubMed] [Google Scholar]
- 29. Wang FD, Huang BD, Liu XH, Wu QW, Wang XL, Wang XC. 1999. The efficacy of recombinant hepatitis B vaccine on the blocking of mother-to-infant transmission. Zhejiang Prev. Med. 11:19–20 [Google Scholar]
- 30. Magnius LO, Norder H. 1995. Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology 38:24–34 [DOI] [PubMed] [Google Scholar]
- 31. Zheng Y, Ge S, Zhang J, Guo Q, Ng MH, Wang F, Xia N, Jiang Q. 2006. Swine as a principal reservoir of hepatitis E virus that infects humans in eastern China. J. Infect. Dis. 193:1643–1649 [DOI] [PubMed] [Google Scholar]
- 32. Chang MH. 2006. Impact of hepatitis B vaccination on hepatitis B disease and nucleic acid testing in high-prevalence populations. J. Clin. Virol. 36(Suppl 1):S45–S50 [DOI] [PubMed] [Google Scholar]
- 33. Ranger-Rogez S, Denis F. 2004. Hepatitis B mother-to-child transmission. Expert Rev. Anti Infect. Ther. 2:133–145 [DOI] [PubMed] [Google Scholar]
- 34. Tang JR, Hsu HY, Lin HH, Ni YH, Chang MH. 1998. Hepatitis B surface antigenemia at birth: a long-term follow-up study. J. Pediatr. 133:374–377 [DOI] [PubMed] [Google Scholar]
- 35. Hadler SC, Francis DP, Maynard JE, Thompson SE, Judson FN, Echenberg DF, Ostrow DG, O'Malley PM, Penley KA, Altman NL, Braff E, Shipman GF, Coleman PJ, Mandel EJ. 1986. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N. Engl. J. Med. 315:209–214 [DOI] [PubMed] [Google Scholar]
- 36. Liao SS, Li RC, Li H, Yang JY, Zeng XJ, Gong J, Wang SS, Li YP, Zhang KL. 1999. Long-term efficacy of plasma-derived hepatitis B vaccine: a 15-year follow-up study among Chinese children. Vaccine 17:2661–2666 [DOI] [PubMed] [Google Scholar]
- 37. Poorolajal J, Mahmoodi M, Majdzadeh R, Nasseri-Moghaddam S, Haghdoost A, Fotouhi A. 2010. Long-term protection provided by hepatitis B vaccine and need for booster dose: a meta-analysis. Vaccine 28:623–631 [DOI] [PubMed] [Google Scholar]
- 38. Kwon CI, Hwang SG, Shin SJ, Chang SW, Kim SY, Ko KH, Hong SP, Park PW, Rim KS, Kang MS, Chung HJ. 2008. Occult hepatitis B virus infection in pregnant woman and its clinical implication. Liver Int. 28:667–674 [DOI] [PubMed] [Google Scholar]