Abstract
Haemophilus parasuis and Actinobacillus pleuropneumoniae both belong to the family Pasteurellaceae and are major respiratory pathogens that cause large economic losses in the pig industry worldwide. We previously constructed an attenuated A. pleuropneumoniae serovar 1 live vaccine prototype, SLW05 (ΔapxIC ΔapxIIC ΔapxIV-ORF1), which is able to produce nontoxic but immunogenic ApxIA, ApxIIA, and ApxIVA. This triple-deletion mutant strain was shown to elicit protective immunity against virulent A. pleuropneumoniae. In the present study, we investigated whether immunization with SLW05 could also protect against lethal challenge with virulent H. parasuis SH0165 (serovar 5) or MD0322 (serovar 4). The SLW05 strain was found to elicit a strong humoral antibody response in pigs and to confer significant protection against challenge with a lethal dose of H. parasuis SH0165 or MD0322. IgG subtype analysis revealed that SLW05 induces a bias toward a Th1-type immune response and stimulates interleukin 2 (IL-2) and gamma interferon (IFN-γ) production. Moreover, antisera from SLW05-vaccinated pigs efficiently inhibited both A. pleuropneumoniae and H. parasuis growth in a whole-blood assay. This is the first report that a live attenuated A. pleuropneumoniae vaccine with SLW05 can protect against lethal H. parasuis infection, which provides a novel approach for developing an attenuated H. parasuis vaccine.
INTRODUCTION
Actinobacillus pleuropneumoniae is the etiologic agent of porcine pleuropneumonia and has a serious impact upon animal welfare and economics in the pig rearing industry (1). Several factors have been identified that are involved in the pathogenicity of A. pleuropneumoniae, of which the pore-forming exotoxins are probably the most important (2). The 15 serovars of A. pleuropneumoniae secrete different combinations of four exotoxins (ApxI, ApxII, ApxIII, and ApxIV) belonging to the RTX toxin family (1). ApxI and ApxIII are encoded on classical RTX operons in a CABD manner, and the ApxII operon in all A. pleuropneumoniae serovars is truncated, having only CA genes and missing the secretion genes BD (1). The ApxIV gene is not a classical RTX toxin gene and is expressed only in infected pigs (3). There is a correlation between virulence and the pattern of Apx toxin production, and A. pleuropneumoniae serovars 1, 5, 9, and 11, which produce ApxI and ApxII, are regarded as the most virulent (4).
Live attenuated vaccines hold much promise because protective antigens are produced in a natural context and because live vaccines have a greater ability to stimulate the production of cytokines, including interleukins, tumor necrosis factor, and interferons, which are known to play an important role as immune modulators (5). Previous studies have confirmed the feasibility of engineering mutant strains of A. pleuropneumoniae via the use of selectable antibiotic resistance determinants (6), but such strains are unsuitable as vaccines owing to biosafety concerns (7). We have therefore developed methods for the construction of A. pleuropneumoniae vaccine strains that avoid the use of antibiotic resistance genes. We previously developed a double-deletion ΔapxIC ΔapxIIC mutant strain, SLW03, of A. pleuropneumoniae serovar 1. Upon homologous or heterologous challenge, there was no overt clinical disease or mortality in pigs vaccinated with SLW03. These results, combined with the fact that the strain contains no foreign DNA, emphasize the potential of SLW03 as a live attenuated vaccine (8).
However, previous studies showed that ApxIVA retains weak hemolytic activity in the presence of ORF1, a protein encoded immediately upstream of apxIVA (3), although it remains to be determined whether ORF1 acts in the same way as the ApxC posttranslational activators of other Apx toxins. We therefore constructed a live mutant by introducing an apxIVA-ORF1 deletion into the double-mutant ΔapxIC ΔapxIIC A. pleuropneumoniae strain SLW03 (9). This triple-deletion ΔapxIC ΔapxIIC ΔapxIV-ORF1 mutant strain, named SLW05, was found to have reduced virulence in both mice and pigs compared to that of SLW03 and could elicit protection against A. pleuropneumoniae homologous and heterologous serovar lethal challenge (9).
Haemophilus parasuis is the causative agent of Glässer's disease, and it has become one of the most important bacterial pathogens of livestock worldwide (10). So far, 15 H. parasuis serovars have been described, but up to 25% of isolates in some countries could not be allocated to any known serovar (11, 12). Although vaccination is commonly considered to be the most effective way to control and eradicate infectious disease (13), currently, there is not one vaccine known to induce protection against all pathogenic H. parasuis serovars (14).
It was therefore unexpected that preliminary findings from pig farms employing immunization with A. pleuropneumoniae strain SLW05 suggested that the prevalence of Glässer's disease was markedly diminished in vaccinated herds. Both H. parasuis and A. pleuropneumoniae belong to the family Pasteurellaceae, and this raised the possibility that the A. pleuropneumoniae vaccine strain SLW05 might elicit a degree of cross-protection against H. parasuis. Therefore, the goal of the present study was to evaluate the immune response to and the efficacy of SLW05 as an attenuated vaccine to protect pigs against lethal challenge with H. parasuis serovars 4 and 5.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A. pleuropneumoniae (serovar 1, strain 4074), the live attenuated triple mutant (ΔapxIC ΔapxIIC ΔapxIV-ORF1) strain of A. pleuropneumoniae, SLW05, and H. parasuis (serovar 5, strain SH0165; serovar 4, strain MD0322) (15) were grown in tryptic soy broth (TSB) (Difco) or tryptic soy agar (TSA) (Difco) supplemented with 10 μg/ml NAD (Sigma, St. Louis, MO) and 10% newborn calf serum (Gibco). All bacterial strains were grown at 37°C.
Live-vaccine preparation.
SLW05 was inoculated into 100 ml TSB supplemented with 10% newborn calf serum and NAD (10 μg/ml) and propagated overnight at 37°C. The bacterial suspension was then diluted 10-fold into fresh prewarmed TSB and was cultured for 16 h at 37°C to obtain the log-phase bacteria; then, the log-phase bacteria were washed once in phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 2.8 mM KH2PO4 [pH 7.2]) and mixed with gelatin to yield a concentration of 1.5%, and then the mixture was lyophilized.
For use in pig immunizations, the mixture was diluted in PBS and adjusted with PBS to approximately 5 × 107 CFU/ml.
Protection studies with pigs.
This study was carried out in strict accordance with the recommendations in the China Regulations for the Administration of Affairs Concerning Experimental Animals 1988 and the Hubei Regulations for the Administration of Affairs Concerning Experimental Animals 2005. The protocol was approved by China Hubei Province Science and Technology Department [permit number SYXK(ER) 2010-0029]. The animals were euthanized at the end of the experiments or when they reached a particular clinical score during the experiment.
Forty 30-day-old naturally farrowed early-weaned (NFEW) piglets which were negative for detection of antibody against H. parasuis by INGEZIM Haemophilus 11.HPS.K1 (INGEZIM, Spain), also negative for the isolation of H. parasuis from a nasal cavity swab, and also negative for detection of antibody against A. pleuropneumoniae by the A. pleuropneumoniae ApxI detection kit (Keqian, China) were used. Optical densities (OD) of antibody against A. pleuropneumoniae and H. parasuis were determined (data not shown). The piglets were randomly divided into four experimental groups each of 10 animals. Groups 1 and 3 were vaccinated twice intramuscularly (i.m.) with 1.0 × 108 CFU of SLW05 in 2 ml PBS, whereas groups 2 and 4 (negative-control groups) were injected intramuscularly with 2 ml PBS. A second identical immunization was performed 14 days after the primary vaccination.
On day 14 following the booster immunization, all pigs in groups 1 and 2 were challenged intranasally with 1.5 × 1010 CFU of SH0165 strain; groups 3 and 4 were challenged intranasally with 1.5 × 1010 CFU of MD0322 strain. Pigs were monitored regularly for 14 days following challenge, and clinical signs were scored according to the A. pleuropneumoniae scoring method (16) and H. parasuis scoring method (17). At 14 days postchallenge, all surviving pigs were euthanized and lung lesion scores were recorded as previously described (18).
Antibody measurements.
Sera were isolated from blood samples collected from pigs by anterior vena cava venipuncture on day 14 after the second immunization prior to challenge. Serum antibodies against ApxI of A. pleuropneumoniae or the mixture of 121°C high-pressure H. parasuis serovar 4 and 5 heat-stable antigens for 2 h were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (19, 20), with some modifications. Briefly, 100 μl coating buffer (50 mM sodium carbonate buffer, pH 9.6) containing appropriate ApxI or the mixture of 121°C high-pressure H. parasuis serovar 4 and 5 heat-stable antigens was used at 4°C overnight to coat 96-well ELISA plates. The plates were then washed three times with PBST (PBS supplemented with 0.05% Tween 20) and blocked at 37°C for 1 h with blocking buffer (PBS with 5% bovine serum albumin [BSA]); they were then washed three times with PBST. Serum samples diluted in PBST were added to each well and incubated at 37°C for 1 h. After three washes, horseradish peroxidase (HRP)-conjugated goat anti-porcine IgG diluted 1:5,000 in PBST was added to each well. For determining the IgG isotypes, the sera were added to the coated plates and incubated with mouse anti-pig IgG1-HRP (AbD Serotec, United Kingdom) or IgG2a-HRP (AbD Serotec). After a washing, substrate solution 3,3′,5,5′-tetramethylbenzidine (TMB) and H2O2 were added to each well, the plates were incubated at room temperature for ∼20 min, and 1% SDS was added to stop the reaction. The plates were read at an absorbance of 630 nm, and serum antibody IgG titer was calculated as the reciprocal of the serum dilution which gave an OD at 630 nm of 0.3 above that of the preimmune sera.
Determination of cytokines by ELISA.
Sera isolated from blood samples as described above were analyzed for interleukin 2 (IL-2), IL-4, and gamma interferon (IFN-γ) using ELISA kits (R&D systems) according to the manufacturer's instructions. Briefly, sera were added to the coated plates, incubated for 3 h, washed with PBST, and blocked with blocking buffer (PBS with 5% BSA). Cytokine concentrations were determined using an ELISA reader.
Blood bactericidal assay.
The bactericidal assay was performed as described previously (21), with some modifications. Briefly, live bacteria of H. parasuis strain SH0165 or MD0322, or A. pleuropneumoniae strain 4074, were washed and diluted in sterile physiological saline. Samples of diluted bacteria (20 μl; 106 cells) were mixed with 180 μl inactivated test sera (the test sera were inactivated in a 56°C water bath for half an hour) diluted 2-fold with physiological saline, or diluted bacteria were mixed with saline alone. After incubation at 30 min at 37°C and then 30 min on ice, nonimmune whole heparinized (10 U/ml) pig blood (100 μl) was added, and the mixture was incubated at 37°C with shaking for 1 h. Bacteria were then plated on TSA containing 10% newborn calf serum and 1 mM/ml NAD (Sigma). Bacterial colony counts (CFU) were recorded after 24 h. Results were expressed as percent killing according to the following formula: (CFU after 1 h of growth with control sera − CFU after 1 h of growth with immune sera)/CFU after 1 h of growth with control sera × 100. Data presented are the means of three independent assays.
Histopathological analysis.
Lung and brain tissues were fixed by immersion in 10% neutral buffered formalin and embedded in paraffin; 4-μm tissue sections were then cut and stained with hematoxylin and eosin according to a standard protocol and examined under light microscopy.
Statistical analysis.
The experimental data were expressed as means ± standard deviations (SD). The difference between two groups was analyzed using the two-tailed Student t test, and survival analysis was done with the log rank test. A P value of <0.05 was considered to indicate a statistically significant result.
RESULTS
Immune response.
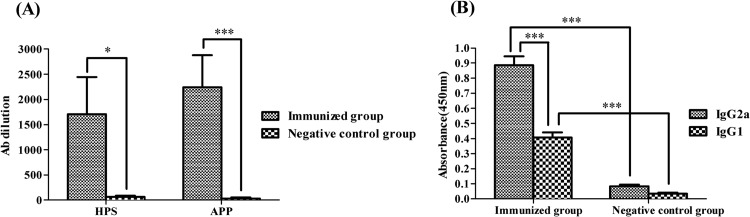
Two weeks after booster immunization with the triple-mutant strain SLW05, blood samples from all pigs were collected via anterior vena cava venipuncture. The results showed that all pigs immunized with SLW05 had significant antibody titers, whereas no antibody was detected in the negative-control groups (H. parasuis serovar 4 and 5 heat-stable antigens, P < 0.05; ApxI, P < 0.001) (Fig. 1A). There were no significant differences in the antibody titers between the different animals immunized with SLW05 (P > 0.05). We then performed isotyping to determine the specific antibody types against the mixture of H. parasuis serovar 4 and 5 heat-stable antigens of H. parasuis induced by vaccination. Levels of isotypes IgG1 and IgG2a were significantly higher in the immunized group than in the negative-control group (P < 0.001) (Fig. 1B). IgG2a titers predominated over IgG1 titers, suggesting a bias toward a Th1-type immune response (P < 0.001) (Fig. 1B).
Fig 1.
(A) Antibody titers in pigs immunized with SLW05 against the mixture of H. parasuis serovar 4 and 5 heat-stable antigens (HPS) or ApxI (APP). Groups of pigs were immunized with SLW05 and boosted after 14 days. Negative controls received PBS. Blood samples were collected 2 weeks after the booster injection, and the antibody response was determined by ELISA; optical density readings of ≥0.3 were scored as positive. *, significance at a P value of <0.05; ***, significance at a P value of <0.001. (B) IgG1 and IgG2a levels in pigs immunized with SLW05 of the antibodies against the H. parasuis serovar 4 and 5 heat-stable antigens (ELISA). Results are expressed as means ± SD; ***, significance at a P value of <0.001.
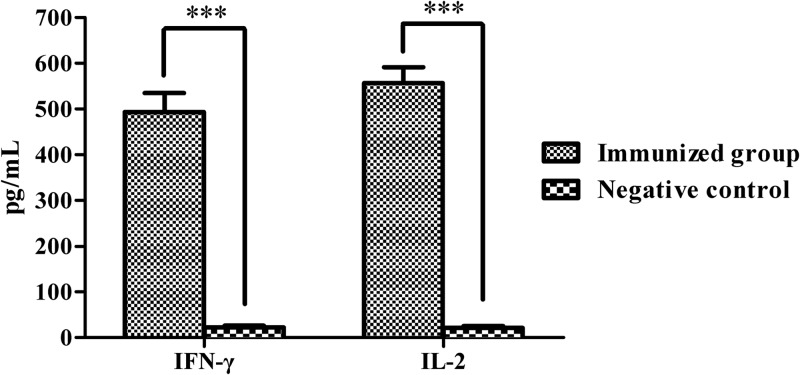
We also determined levels of cytokines induced by immunization. Blood serum samples from day 14 after the last immunization were analyzed by ELISA. Levels of IFN-γ were significantly higher in sera from immunized animals than in sera from negative controls (P < 0.001) (Fig. 2). Levels of IL-2 production were also significantly higher in immunized animals (P < 0.001) (Fig. 2). However, there were no significant increases in IL-4 levels in vaccinated animals versus negative controls (data not shown).
Fig 2.
Serum levels of IFN-γ and IL-2 production in serum of pigs immunized with SLW05. Results are means ± SD; ***, significance at a P value of <0.001.
Protective efficacy against lethal challenge with H. parasuis serovars 4 and 5.
The protective efficacy of SLW05 against lethal challenge with H. parasuis serovar 4 or serovar 5 was evaluated in terms of survival and clinical signs in pigs, including appetite, dyspnea, lethargy, lameness, and neurological signs. Separate groups of pigs were challenged intranasally with a lethal dose of 1.5 × 1010 CFU of H. parasuis strain SH0165 or strain MD0322 2 weeks after the booster immunization; animals were monitored after challenge, and survival, clinical scores, and lung lesion scores were recorded (Table 1). All pigs in the nonimmunized control groups developed clinical disease, with an elevated average rectal temperature (41.5°C), and were inappetant and depressed (Table 1). However, pigs in the immunized groups displayed little or no depression, anorexia, dyspnea, lameness, or neurological signs, and lung lesions assessed by tissue analysis following necropsy 14 days postchallenge were significantly diminished (P < 0.05) (Table 1). In addition, there was no pathological change at the injection site. Overall survival rates were markedly and significantly (P < 0.05) increased in animals immunized with SLW05 compared to those in control groups (Fig. 3). The majority of immunized animals survived lethal challenge with either SH0165 (90% survival) or MD0322 (80% survival), although the difference in the survival rates between the two challenge groups was not statistically significant (P > 0.05) (Fig. 3). These results demonstrated that SLW05 could provide significant immunoprotection against H. parasuis infection.
Table 1.
Protective effect of the live mutant against H. parasuis SH0165 and MD0322 challenge in pigs
| H. parasuis strain used for challenge | Group | Appetitea | Dyspneaa | Lethargya | Lamenessa | Neurological signsa | Lung lesion scoreb |
|---|---|---|---|---|---|---|---|
| SH0165 | Immunized group | 0.2 ± 0.4 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.3 | 0.1 ± 0.3 | 1.9 ± 2.4 |
| PBS (negative control)c | 2.6 ± 0.5 | 2.7 ± 0.5 | 2.8 ± 0.4 | 1.0 ± 1.0 | 0.6 ± 1.0 | 18.7 ± 4.8 | |
| MD0322 | Immunized group | 0.3 ± 0.5 | 0.4 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.3 | 0 | 2.6 ± 3.1 |
| PBS (negative control)c | 2.7 ± 0.5 | 2.6 ± 0.5 | 2.6 ± 0.5 | 0.9 ± 1.0 | 0.5 ± 1.0 | 21.1 ± 6.1 |
Clinical signs were scored as described by Jolie et al. (16) and Blanco et al. (17). Appetite was scored as follows: 0, did eat, and 1, did not eat. Total score = number of 12-h periods of not eating over the 36-h observation period. Dyspnea was scored as follows: 0, normal; 1, slight; 2, moderate; and 3, severe. Lethargy was scored as follows: 0, normal; 1, slight inactivity; 2, moderate; and 3, severe. Lameness was scored as follows: 0, normal; 1, lameness in 1 limb; 2, lameness in 2 limbs; and 3, severe lameness. Neurological signs were scored as follows: 0, normal; 1, muscular rigidity or tremors; 2, convulsions; and 3, paralysis.
The lung lesion score was determined as described by Hannan et al. (18).
The clinical signs of negative control groups were recorded for the animals prior to death. The final scores were obtained by the average of all the data within the observation time and are expressed as arithmetic means ± SD.
Fig 3.
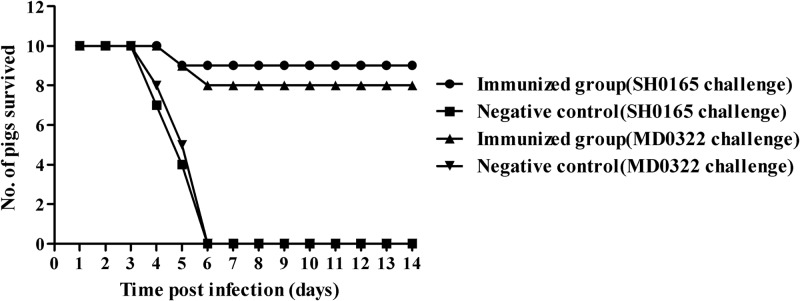
Survival of pigs immunized with A. pleuropneumoniae SLW05 and negative-control pigs following intranasal challenge with 1.5 × 1010 CFU of H. parasuis strain SH0165 or MD0322.
Blood bactericidal assay.
We then evaluated whether immunization with SLW05 induces the production of antibody that is bactericidal and/or opsonic against live H. parasuis and A. pleuropneumoniae in vitro. The results showed that sera from nonimmunized animals failed to inhibit the growth of either H. parasuis or A. pleuropneumoniae. In contrast, sera from immunized animals caused a marked reduction in the viable titers of both H. parasuis SH0165 and MD0322, as well as A. pleuropneumoniae 4074 (P < 0.001) (Fig. 4). There was a 55% reduction the viability of SH0165 following incubation with immune sera and a 51% reduction in MD0322 viability, demonstrating that immunization with SLW05 elicits the production of bactericidal antibodies against H. parasuis. In addition, there was a 61% reduction the viability of A. pleuropneumoniae 4074 following incubation with immune sera.
Fig 4.
Blood bactericidal activity of SLW05-vaccinated and negative-control animals. Results are expressed as the percent reduction of viable bacteria in the presence of immune blood serum. Results are determined from three independent assays. A >50% reduction in the viable titer in at least one test was considered to be a positive result. ***, significance at a P value of <0.001. APP, A. pleuropneumoniae.
Histopathological analysis.
To evaluate the protective efficacy of SLW05 against the development of disease induced by challenge with H. parasuis strains SH0165 and MD0322, brain and lung samples of the surviving animals from the vaccine-immunized groups 14 days postchallenge and the animals from the negative-control groups postchallenge were euthanized when the clinical disease became severe and then were examined for tissue pathology. In the negative-control groups challenged with either SH0165 or MD0322, all animals developed severe disease (Fig. 5A, C, E, and G). As shown in Fig. 5A and C, lung tissue showed extensive edema, with massive proliferation of fibroblasts and the formation of connective tissue. Bronchioles were filled with cellular exudate containing large numbers of neutrophils. Portions of the parenchyma were completely necrotic or collapsed. Congestion of brain blood vessels was prominently observed in histological specimens from negative-control groups (Fig. 5E and G). In contrast, only minor signs of clinical disease were observed in tissues of pigs immunized with SLW05 vaccine (Fig. 5B, D, F, and H). These data demonstrate that immunization with SLW05 can prevent pathological changes following challenge infection with H. parasuis and confirm that the triple-deletion mutant strain of A. pleuropneumoniae can induce robust immunoprotection against H. parasuis infection in pigs.
Fig 5.
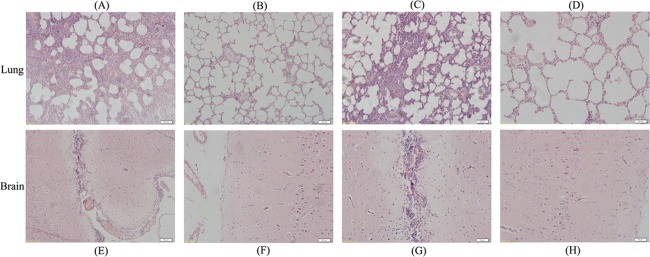
Representative lung and brain sections of pigs from different treatment groups. (A and E) Negative-control group challenged with strain H. parasuis SH0165; (B and F) A. pleuropneumoniae SLW05-immunized group challenged with H. parasuis strain SH0165; (C and G) negative-control group challenged with H. parasuis strain MD0322; (D and H) A. pleuropneumoniae SLW05-immunized group challenged with H. parasuis strain MD0322.
DISCUSSION
Haemophilus parasuis and Actinobacillus pleuropneumoniae are two of the most serious porcine respiratory diseases and cause large economic losses in the pig industry worldwide (22, 23). A live vaccine is considered to offer a better prospect of obtaining cross-serovar protection against A. pleuropneumoniae serovars or H. parasuis, and live attenuated deletion mutants have been shown to be effective in controlling infection by A. pleuropneumoniae (24, 25). Based on the observation of cross-protection of SLW03 against heterologous A. pleuropneumoniae infection in recovery pigs, we constructed SLW05. It was shown to induce broad-range protective immunity against A. pleuropneumoniae infection in vaccinated pigs (9).
At present, commerical vaccines against H. parasuis are predominantly inactivated vaccines based on well-characterized strains, and there are commercially produced autogenous bacterins that are used some places. These vaccines usually induce strong immunoprotection against homologous serovar challenge (14, 26, 27), but some inconsistent results have been obtained for cross-protection, depending on serovars and strains of H. parasuis (26, 28). Although recombinant vaccines based on H. parasuis outer membrane proteins have been constructed, they only provide limited protective efficacy and fail to confer cross-protection (29). Attention has therefore focused on inoculation of animals with live attenuated vaccines because these present a wide spectrum of immunogenic antigens in a native form (25), so a live attenuated vaccine is considered to offer a better prospect of obtaining cross-serovar protection against H. parasuis. However, the development of attenuated vaccines for commercial use has been hindered by the lack of methods for modifying the H. parasuis genome without the concomitant insertion of an antibiotic resistance marker.
H. parasuis and A. pleuropneumoniae both belong to the family Pasteurellaceae, and it is therefore possible that they share antigenic sites on the cell wall and might afford a measure of cross-protection. Our results showed that immunization with SLW05 elicits the production of bactericidal antibodies against H. parasuis, and this is the first report that the antibodies against A. pleuropneumoniae have the characteristic of bactericidal activity. At present, some common antigens are being identified between A. pleuropneumoniae and H. parasuis serovars 4 and 5 by a proteomics approach in our laboratory.
The route of immunization may also have an effect on raising the level of cross-immunoprotection. A. pleuropneumoniae is a respiratory pathogen, and the endotracheal or intranasal administration route is thought to be a better way to simulate the natural routes of infection (30). However, compared with other immunization routes such as endotracheal administration, the i.m. administration route is more convenient and used widely in practice. Furthermore, our laboratory and other researchers also proved that i.m. immunization could also elicit protection equal to that of the intranasal or tracheal route against challenge with homologous or heterologous serovars of a virulent A. pleuropneumoniae strain (31, 32), so in this study we used the i.m. administration route.
The predominant immunological effector response against extracellular bacteria is generally thought to operate via circulating antibodies that lead to inactivation of the pathogen either via complement activation or by opsonization and phagocytosis (33). Examination of sera from pigs inoculated with the A. pleuropneumoniae vaccine confirmed that SLW05 could induce a significant humoral immune response. It has been reported that the specific IgG subtype and the Th type are crucial determinants of protective immunity against a particular disease (34). Enhanced production of IgG1 indicates the induction of a Th2 immune response, whereas IgG2a is representative of a Th1 response. Although antibody subtype analysis of SLW05-immunized animals indicates that the vaccine induces the production of both IgG1 and IgG2a, the higher levels of IgG2a suggest a bias toward a Th1-type immune response; this was further confirmed by cytokine assay. But one study showed that anti-HLY (Apx) antibodies of A. pleuropneumoniae were consistently associated with protection and that IgG1 may be more efficacious than IgG2 at low-dose challenge (30); specific mechanisms need further study. Th1 cells mainly secrete IL-2 and IFN-γ, and Th2 cells mainly secrete IL-4. In this study, SLW05 induced higher levels of IL-2 and IFN-γ production, which may be related to the stimulation of the bacteria. Sera from immunized animals demonstrated pronounced bactericidal activity against both H. parasuis and A. pleuropneumoniae; we surmise that this is mediated by antibody binding followed by complement activation, but further experiments will be required to determine the mechanism of cell killing in vitro.
The majority of studies addressing immunoprotection against H. parasuis have employed intraperitoneal challenge of mice; however, this does not mimic the natural route of infection (35). The present study therefore used intranasal challenge with virulent strains of H. parasuis. Ninety percent of immunized pigs survived lethal challenge with SH0165, whereas 80% of immunized pigs survived challenge with MD0322. This is the first report that the SLW05 strain can confer a high level of immunoprotection against heterogenetic attack by H. parasuis of either serovar 5 or 4, so our next goal is the development of SLW05 as a commerical vaccine by exploring the more accurate immunization doses and routes.
In summary, the present study demonstrates that immunization of pigs with SLW05 induces a significant increase of antibody titers against H. parasuis compared with negative-control groups. The response was predominantly of the Th1 type, and sera from immunized animals showed in vitro bactericidal activity against live H. parasuis. Importantly, immunization with the triple-mutant SLW05 conferred marked resistance against lethal challenge with virulent heterologous H. parasuis. We conclude that the triple-deletion mutant SLW05 has significant potential in the development of an attenuated vaccine to prevent and control H. parasuis infection. In addition to being a potential vaccine against both A. pleuropneumoniae and H. parasuis, the mutant strain also holds promise as a delivery vehicle for protective antigens of other pathogens and has great potential in the development of new multivalent vaccines. The work is ongoing in our laboratory to standardize production of the SLW05 vaccine, and field trials are under way.
ACKNOWLEDGMENTS
This research is supported by grants from the National Nature Science Foundation of China (no. 30970109 and no. 31172352), the National Programs for High Technology Research and Development of China (no. 2011AA10A210), the Hubei Province Technology Program (no. 2011BBB082), and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (no. 31121004).
Footnotes
Published ahead of print 5 December 2012
REFERENCES
- 1. Frey J. 2003. Detection, identification, and subtyping of Actinobacillus pleuropneumoniae. Methods Mol. Biol. 216:87–95 [DOI] [PubMed] [Google Scholar]
- 2. Frey J. 1995. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 3:257–261 [DOI] [PubMed] [Google Scholar]
- 3. Schaller A, Kuhn R, Kuhnert P, Nicolet J, Anderson TJ, MacInnes JI, Segers RP, Frey J. 1999. Characterization of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae. Microbiology 145:2105–2116 [DOI] [PubMed] [Google Scholar]
- 4. Bandara AB, Lawrence ML, Veit HP, Inzana TJ. 2003. Association of Actinobacillus pleuropneumoniae capsular polysaccharide with virulence in pigs. Infect. Immun. 71:3320–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mekalanos JJ. 1994. Live attenuated vaccine vectors. Int. J. Technol. Assess. Health Care 10:131–142 [DOI] [PubMed] [Google Scholar]
- 6. Inzana TJ, Glindemann G, Fenwick B, Longstreth J, Ward D. 2004. Risk assessment of transmission of capsule-deficient, recombinant Actinobacillus pleuropneumoniae. Vet. Microbiol. 104:63–71 [DOI] [PubMed] [Google Scholar]
- 7. Dröge M, Pühler A, Selbitschka W. 1998. Horizontal gene transfer as a biosafety issue: a natural phenomenon of public concern. J. Biotechnol. 64:75–90 [DOI] [PubMed] [Google Scholar]
- 8. Lin L, Bei W, Sha Y, Liu J, Guo Y, Liu W, Tu S, He Q, Chen H. 2007. Construction and immunogenicity of a ΔapxIC/ΔapxIIC double mutant of Actinobacillus pleuropneumoniae serovar 1. FEMS Microbiol. Lett. 274:55–62 [DOI] [PubMed] [Google Scholar]
- 9. Yuan F, Liu J, Guo Y, Tan C, Fu S, Zhao J, Chen H, Bei W. 2011. Influences of ORF1 on the virulence and immunogenicity of Actinobacillus pleuropneumoniae. Curr. Microbiol. 63:574–580 [DOI] [PubMed] [Google Scholar]
- 10. Rapp-Gabrielson VJ, Kocur GJ, Clark JT, Muir SK. 1997. Haemophilus parasuis: immunity in swine after vaccination. Vet. Med. 9:83–90 [Google Scholar]
- 11. Tadjine M, Mittal KR, Bourdon S, Gottschalk M. 2004. Development of a new serological test for serotyping Haemophilus parasuis isolates and determination of their prevalence in North America. J. Clin. Microbiol. 42:839–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turni C, Blackall PJ. 2005. Comparison of the indirect haemagglutination and gel diffusion test for serotyping Haemophilus parasuis. Vet. Microbiol. 106:145–151 [DOI] [PubMed] [Google Scholar]
- 13. Rappuoli R, Miller HI, Falkows S. 2002. Medicine: the intangible value of vaccination. Science 297:937–939 [DOI] [PubMed] [Google Scholar]
- 14. Takahashi K, Naga S, Yagihashi T, Ikehata T, Nakano Y, Senna K, Maruyama T, Murofushi J. 2001. A cross-protection experiment in pigs vaccinated with Haemophilus parasuis serovars 2 and 5 bacterins, and evaluation of a bivalent vaccine under laboratory and field conditions. J. Vet. Med. Sci. 63:487–491 [DOI] [PubMed] [Google Scholar]
- 15. Cai X, Chen H, Blackall PJ, Yin Z, Wang L, Liu Z, Jin M. 2005. Serological characterization of Haemophilus parasuis isolates from China. Vet. Microbiol. 111:231–236 [DOI] [PubMed] [Google Scholar]
- 16. Jolie RA, Mulks MH, Thacker BJ. 1995. Cross-protection experiments in pigs vaccinated with Actinobacillus pleuropneumoniae subtypes 1A and 1B. Vet. Microbiol. 45:383–391 [DOI] [PubMed] [Google Scholar]
- 17. Blanco I, Canals A, Evans G, Mellencamp MA, Cia C, Deeb N, Wang L, Galina-Pantoja L. 2008. Differences in susceptibility to Haemophilus parasuis infection in pigs. Can. J. Vet. Res. 72:228–235 [PMC free article] [PubMed] [Google Scholar]
- 18. Hannan PC, Bhogal BS, Fish JP. 1982. Tylosin tartrate and tiamutilin effects on experimental piglet pneumonia induced with pneumonic pig lung homogenate containing mycoplasmas, bacteria and viruses. Res. Vet. Sci. 33:76–88 [PubMed] [Google Scholar]
- 19. Shin MK, Kang ML, Cha SB, Lee WJ, Sung JH, Yoo HS. 2011. An immunosorbent assay based on the recombinant ApxIa, ApxIIa, and ApxIIIa toxins of Actinobacillus pleuropneumoniae and its application to field sera. J. Vet. Diagn. Invest. 23:736–742 [DOI] [PubMed] [Google Scholar]
- 20. Wasiński B, Pejsak Z. 2012. Reactivity of heat-stable Leptospira antigenic preparation used in enzyme-linked immunosorbent assay for detection of antibodies in swine serum. Pol. J. Vet. Sci. 15:31–36 [DOI] [PubMed] [Google Scholar]
- 21. Furano K, Campagnari AA. 2003. Inactivation of the Moraxella catarrhalis 7169 ferric uptake regulator increases susceptibility to the bactericidal activity of normal human sera. Infect. Immun. 71:1843–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gottschalk M, Taylor DJ. 2006. Actinobacillus pleuropneumoniae, p 563–576 In Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ. (ed), Diseases of swine. Blackwell Publishing Professional, Ames, IA [Google Scholar]
- 23. Oliveira S, Pijoan C. 2004. Haemophilus parasuis: new trends on diagnosis, epidemiology and control. Vet. Microbiol. 99:1–12 [DOI] [PubMed] [Google Scholar]
- 24. Bei W, He Q, Yan L, Fang L, Tan Y, Xiao S, Zhou R, Jin M, Guo A, Lv J, Huang H, Chen H. 2005. Construction and characterisation of a live, attenuated apxIICA inactivation mutant of Actinobacillus pleuropneumoniae lacking a drug resistance marker. FEMS Microbiol. Lett. 243:21–27 [DOI] [PubMed] [Google Scholar]
- 25. Liu J, Chen X, Lin L, Tan C, Chen Y, Guo Y, Jin M, Guo A, Bei W, Chen H. 2007. Potential use an Actinobacillus pleuropneumoniae double mutant strain ΔapxIICΔapxIVA as live vaccine that allows serological differentiation between vaccinated and infected animals. Vaccine 25:7696–7705 [DOI] [PubMed] [Google Scholar]
- 26. Bak H, Riising HJ. 2002. Protection of vaccinated pigs against experimental infections with homologous and heterologous Haemophilus parasuis. Vet. Rec. 151:502–505 [DOI] [PubMed] [Google Scholar]
- 27. Hoffmann CR, Bilkei G. 2002. The effect of a homologous bacterin given to sows prefarrowing on the development of Glässer's disease in postweaning pigs after i.v. challenge with Haemophilus parasuis serotype 5. Dtsch. Tierarztl. Wochenschr. 109:271–276 [PubMed] [Google Scholar]
- 28. Palzer A, Ritzmann M, Heinritzi K. 2007. A field trial for early vaccination against Gässer's disease using Porcilis Glässer. Schweiz. Arch. Tierheilkd. 149:389–394 [DOI] [PubMed] [Google Scholar]
- 29. Martín de la Fuente AJ, Rodríguez-Ferri EF, Frandoloso R, Martínez S, Tejerina F, Gutiérrez-Martín CB. 2009. Systemic antibody response in colostrum-deprived pigs experimentally infected with Haemophilus parasuis. Res. Vet. Sci. 86:248–253 [DOI] [PubMed] [Google Scholar]
- 30. Furesz SE, Mallard BA, Bossé JT, Rosendal S, Wilkie BN, MacInnes JI. 1997. Antibody- and cell-mediated immune responses of Actinobacillus pleuropneumoniae-infected and bacterin-vaccinated pigs. Infect. Immun. 65:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bei W, He Q, Zhou R, Yan L, Huang H, Chen H. 2007. Evaluation of immunogenicity and protective efficacy of Actinobacillus pleuropneumoniae HB04C− mutant lacking a drug resistance marker in the pigs. Vet. Microbiol. 125:120–127 [DOI] [PubMed] [Google Scholar]
- 32. Fuller TE, Thacker BJ, Duran CO, Mulks MH. 2000. A genetically-defined riboflavin auxotroph of Actinobacillus pleuropneumoniae as a live attenuated vaccine. Vaccine 18:2867–2877 [DOI] [PubMed] [Google Scholar]
- 33. Chang YF, Chen CS, Palaniappan RU, He H, McDonough SP, Barr SC, Yan W, Faisal SM, Pan MJ, Chang CF. 2007. Immunogenicity of the recombinant leptospiral putative outer membrane proteins as vaccine candidates. Vaccine 25:8190–8197 [DOI] [PubMed] [Google Scholar]
- 34. Chiang CH, Huang WF, Huang LP, Lin SF, Yang WJ. 2009. Immunogenicity and protective efficacy of ApxIA and ApxIIA DNA vaccine against Actinobacillus pleuropneumoniae lethal challenge in murine model. Vaccine 27:4565–4570 [DOI] [PubMed] [Google Scholar]
- 35. Zhou M, Guo Y, Zhao J, Hu Q, Hu Y, Zhang A, Chen H, Jin M. 2009. Identification and characterization of nove immunogenic outer membrane proteins of Haemophilus parasuis serovar 5. Vaccine 27:5271–5277 [DOI] [PubMed] [Google Scholar]