Abstract
New, more accessible therapies for cryptococcosis represent an unmet clinical need of global importance. We took a repurposing approach to identify previously developed drugs with fungicidal activity toward Cryptococcus neoformans, using a high-throughput screening assay designed to detect drugs that directly kill fungi. From a set of 1,120 off-patent medications and bioactive molecules, we identified 31 drugs/molecules with fungicidal activity, including 15 drugs for which direct antifungal activity had not previously been reported. A significant portion of the drugs are orally bioavailable and cross the blood-brain barrier, features key to the development of a widely applicable anticryptococcal agent. Structural analysis of this set revealed a common chemotype consisting of a hydrophobic moiety linked to a basic amine, features that are common to drugs that cross the blood-brain barrier and access the phagolysosome, two important niches of C. neoformans. Consistent with their fungicidal activity, the set contains eight drugs that are either additive or synergistic in combination with fluconazole. Importantly, we identified two drugs, amiodarone and thioridazine, with activity against intraphagocytic C. neoformans. Finally, the set of drugs is also enriched for molecules that inhibit calmodulin, and we have confirmed that seven drugs directly bind C. neoformans calmodulin, providing a molecular target that may contribute to the mechanism of antifungal activity. Taken together, these studies provide a foundation for the optimization of the antifungal properties of a set of pharmacologically attractive scaffolds for the development of novel anticryptococcal therapies.
INTRODUCTION
Cryptococcus spp. are basidiomycetous yeasts that cause disease in nearly 1 million people each year, leading to more than 600,000 attributable deaths worldwide (1). The majority of cases of cryptococcosis occur in people living with HIV/AIDS, and approximately one-third of all HIV/AIDS-associated deaths are due to cryptococcal disease, surpassing tuberculosis (TB) in this regard. The Cryptococcus species complex that infects humans includes Cryptococcus neoformans and Cryptococcus gattii (2). C. neoformans more commonly causes disease in people with compromised immune function. C. gattii, on the other hand, displays a distinct epidemiology based on molecular types. For example, C. gattii molecular types VGI and VGII have been associated with outbreaks among healthy individuals, while molecular types VGIII and VGIV, like C. neoformans, primarily affect immunocompromised people (3). Although cryptococcosis can affect a variety of organ systems, it most commonly manifests as cryptococcal meningoencephalitis (CEM). In regions with high HIV infection rates, Cryptococcus is the most common cause of meningitis and, accordingly, is one of the most common AIDS-defining opportunistic infections (1).
Currently, the gold standard regimen for the treatment of CEM (4) is a combination of an amphotericin B preparation (AMB) and 5-flucytosine (5FC) and is associated with relatively low mortality rates (10 to 20%). Unfortunately, AMB-5FC is not widely available in resource-limited regions (5) because (i) it is expensive; (ii) it requires intravenous administration; and (iii) it is toxic, and thus requires therapeutic monitoring that is not practical in these regions. In resource-limited regions, fluconazole (FLU) is the most commonly used agent, since it is safe, administered orally, and currently freely available from Pfizer's donation program (5). The mortality with standard-dose FLU is as high as 50%, and thus it is much less effective than AMB-5FC. Recent studies indicate that the difference in efficacy between AMB-5FC (6) and FLU may be due to the fact that AMB-5FC is rapidly fungicidal, while FLU is fungistatic (6). Higher doses of FLU have been studied in a number of clinical trials and appear to provide improved fungicidal activity as well as better outcomes (5); however, further clinical studies are needed before a definitive alternative to AMB-5FC can be established.
From these considerations, it is clear that new drugs are needed for the treatment of cryptococcosis and that these drugs would be of enormous benefit to world health. Ideally, a new anticryptococcal agent would have the following four characteristics: (i) fungicidal activity or ability to combine with a current agent to yield a fungicidal cocktail, (ii) ability to cross the blood-brain barrier, (iii) good oral absorption to allow its use in resource-limited regions, and (iv) activity against C. neoformans within macrophages so as to access all niches occupied by the pathogen. Unfortunately, the pace of new antifungal drug development has been slow. Indeed, the gold standard combination of AMB and 5FC is based on medications that have been used for nearly 50 years (7). The most recent additions to the antifungal pharmacopeia, the echinocandins, are not efficacious against Cryptococcus spp.
As an approach to addressing this unmet clinical need, we initiated a project to identify small molecules that directly kill Cryptococcus by use of a novel high-throughput screening (HTS) assay recently developed in our laboratory (8). The assay is based on the release of the intracellular enzyme adenylate kinase (AK) into the growth medium as a reporter of yeast cell lysis. Molecules that cause cell death lead to compromised cellular integrity and increased levels of AK in the growth medium. We have applied this assay to a variety of organisms (8), and here we report its application to the identification of off-patent drugs with fungilytic activity toward C. neoformans.
We screened the Prestwick library of 1,120 off-patent drugs and molecules with known biological activity (Prestwick Chemical). This type of repurposing approach has several advantages (9). First, in the ideal scenario, a molecule with previously unrecognized but clinically useful activity can be translated rapidly to clinical trials, since it is already approved for another indication. A second, more likely scenario is that molecules with the desired activity will be identified but the potency will require further optimization. In this scenario, the chemical scaffold is already “drug-like,” and therefore medicinal chemistry efforts can focus on optimizing the antifungal activity (in this case) rather than optimizing both activity and pharmacology. Repurposing has emerged as an attractive and successful approach to developing new therapies expediently.
As described below, we identified a set of previously developed drugs with features that make them extremely attractive as potential anticryptococcal scaffolds: (i) oral bioavailability, (ii) ability to cross the blood-brain barrier, (iii) fungicidal activity in the presence of fluconazole, and (iv) ability to target intracellular Cryptococcus.
MATERIALS AND METHODS
Strains, media, and materials.
Cryptococcus neoformans serotype A strain H99 was a gift from Joseph Heitman and was used for all experiments, unless otherwise noted. C. neoformans was cultivated from frozen stocks on yeast extract-peptone-2% dextrose (YPD) agar plates at 30°C and used within 2 weeks for subsequent experiments. Liquid cultures (YPD) were incubated at 30°C unless otherwise noted. YPD medium and plates were prepared using standard recipes. The Prestwick library was obtained from the manufacturer, and a working stock (100 μM in water containing 2% dimethyl sulfoxide [DMSO]) in a 384-well format was prepared. The working stock was used for screening experiments. Individual drugs and chemicals were obtained from Sigma Chemicals (St. Louis, MO) and used as received. Stocks of all drugs and molecules were prepared in DMSO. The final DMSO concentration was 1% for all experiments.
Adenylate kinase assay screen of Prestwick library.
The AK screen was carried out using the protocol described by DiDone et al. (8). Briefly, H99 was grown overnight to mid-logarithmic phase (optical density at 600 nm [OD600] of <0.5). The cells were washed with fresh YPD and suspended to a density of 2 × 106 cells/ml based on counts with a hemocytometer. Using a plate filler, the cell suspension (25 μl) was added to 384-well plates containing library compounds (100 μM) in 25 μl of sterile water (2% DMSO), giving a final volume of 50 μl in each well (50 μM drug and 1% DMSO). The plates were incubated at 37°C for 4 h and then equilibrated to ambient temperature for 1 h. In a separate experiment, the entire 5-h incubation was performed at ambient temperature. Toxa-Light (Lonza) reagent (50 μl) was added to each well and incubated at ambient temperature for 1 h. The luminescence was measured with an Envision plate reader (Beckman). The raw luminescence for each plate was normalized to that for wells of the same plate containing DMSO (1%) only, and compounds inducing a 3-fold or greater increase in relative light units (RLU) were scored as “hits.” Hits were confirmed using the same assay protocol in a 96-well format, using a dilution series of the compound from an independent source.
In vitro antifungal susceptibility assays.
The in vitro antifungal activity of the hits was tested in accordance with CLSI document M27-A2 (12), using RPMI 1640 (with glutamine and phenol red, without bicarbonate) medium buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS), an inoculum of 1.5 × 103 cells/well, and incubation at 37°C. DMSO was used as the solvent for all drugs, and its final concentration was 1%. The MIC was defined as the lowest dilution of the molecule resulting in no visible growth of the organism after 72 h of incubation at 37°C. The fractional inhibitory concentration indexes (FICI) for selected hits in combination with fluconazole were determined using the checkerboard method and formulas as described previously (25). Synergy was defined as an FICI of <0.5. The minimum fungicidal concentration (MFC) was determined by plating dilution series of the well contents from MIC and checkerboard assays on YPD medium. The starting CFU per well was 1.5 × 103, and the MFC was defined as the lowest concentration of compound that reduced the starting inoculum by 2 log10. Propidium iodide (PI) staining was performed as described previously (13). All assays were performed on two or three separate days with independent isolates of H99, and each assay was performed in triplicate. Time-kill assays were performed as described previously (14). C. neoformans cells from overnight cultures were washed twice with Dulbecco's phosphate-buffered saline (DPBS), adjusted to a density of 2 × 107 CFU/ml in YPD (50 ml), and treated with either fluconazole (2 μg/ml), a test compound (2 to 16 μg/ml), or a combination of fluconazole and the test compound at the same concentrations. Cultures were incubated at 37°C, and 10-fold dilutions were plated on YPD at 0 h and 24 h. Plates were incubated at 30°C for 48 h and scored for CFU/ml.
Intracellular replication of C. neoformans in murine phagocytes.
Freshly split, murine J774 phagocyte-like cells were added to a tissue culture-treated 12-well plate at a final density of 1 × 105 cells/well and incubated overnight at 37°C with 5% CO2 in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), Glutamax, and penicillin-streptomycin. Cryptococcus neoformans H99 was grown in YPD overnight at 30°C, harvested, washed twice with DPBS, and opsonized with 18b7 antibody (Ab) (a generous gift of A. Casadevall; final concentration of 2 μg/ml) at 37°C for 1 h, with rotation. The opsonized yeast cells were then added to phagocytes at a final density of 5 × 104 CFU/well (multiplicity of infection [MOI] = 0.5) and incubated at 37°C with 5% CO2 for 80 min. The wells were washed with DPBS to remove nonadherent or extracellular yeast. Fresh growth medium buffered with HEPES (10 mM) containing either drug in DMSO or DMSO alone was added, and the plates were incubated at 37°C with 5% CO2 for 24 h. The supernatants were removed, and the phagocytes were treated with cold Triton X-100 (0.2% solution in water) and incubated at 37°C with 5% CO2 for 10 min to lyse the cells. Lysates were serially diluted, spread onto YPD plates, and incubated at 30°C for 48 h to determine CFU/ml. The potential cytotoxic effects of the drugs on J774 cells were determined by measuring lactate dehydrogenase (LDH) activity in the supernatants of J774 cells treated with drugs in the absence of H99 cells. LDH activity was measured using a commercially available kit (CytoToxONE; Promega) according to the manufacturer's protocol. LDH release triggered in amiodarone (10 μg/ml)-, thioridazine (2 μg/ml)-, and DMSO (1%)-treated cells was not distinguishable from background and was less than 5% of that generated by cells lysed with the manufacturer's lysis reagent.
Expression, purification, and in vitro activity of C. neoformans calmodulin.
C. neoformans var. neoformans CAM1 carried on a pRSET plasmid (a gift from J. Heitman) that also encoded a histidine tag at the C terminus of the protein (Invitrogen) was expressed in Escherichia coli BL21 cells (Invitrogen) by induction of logarithmic-phase cells with IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM) following the manufacturer's protocol. The protein was purified using a Talon affinity purification kit (Clontech) following the manufacturer's instructions and was >90% pure by SDS-PAGE–Coomassie blue analysis.
The function of the purified CnCam1 protein was confirmed by its ability to activate human calcineurin in a commercially available colorimetric calcineurin assay kit (Calbiochem). Briefly, reactions were done at 30°C according to the kit instructions, with each well containing 40 U calcineurin, 0.15 mM RII phosphopeptide substrate, 10 μl distilled water (dH2O), and a final volume of 50 μl. Reactions progressed for 60 min and then were terminated by the addition of 100 μl malachite green and incubation at room temperature for an additional 10 min for the color to fully develop. Absorbance at 620 nm was read on a SPECTROmax 5 M plate reader (Eppendorf). Human calmodulin (hCam) was also prepared according to the kit instructions, with a final concentration of 0.25 μM in reaction wells. In experiments where C. neoformans var. neoformans calmodulin was used, protein stocks of 1 mg/ml in DPBS with calcium chloride were diluted in 2× assay buffer. Concentrations were adjusted to a final concentration of ∼0.25 μM Cam, and CnCam1 was substituted for human calmodulin where indicated.
Thermal shift assay.
Thermal shift assays were performed using the fluorescence resonance energy transfer (FRET) channel of a Bio-Rad CFX96 real-time thermal cycler, using the manufacturer's protocol and software. A DPBS solution (25 μl) of CnCam1 (0.5 mg/ml) containing calcium chloride (1.8 mM), 5× SYPRO orange, and DMSO (4%) was used to generate a thermal denaturation curve in the absence of drug; for curves generated in the presence of drug, the final drug concentration was 400 μg/ml. The thermal cycle was as follows: 40°C for 3 min, 40°C to 100°C in 0.5°C steps (5 s/step), and 100°C for 10 s. The melting temperature (Tm) was identified by analyzing the first derivative of the fluorescence with respect to temperature. The thermal shift data are means for three independent experiments performed in triplicate, with error bars in figures representing standard deviations.
RESULTS
Screen of Prestwick library identifies drug-like molecules with fungilytic activity toward C. neoformans.
The majority of screens for antifungal molecules that have been reported in the literature have used Candida albicans, the most common human fungal pathogen, or Saccharomyces cerevisiae, the most widely studied model yeast. In addition, screening efforts are generally focused on identifying anti-infective agents that have broad spectra of activity. Both C. albicans and S. cerevisiae are ascomycetes, while C. neoformans is a basidiomycete that has diverged considerably since the last common ancestor for these lineages. Since C. neoformans is a pathogen of worldwide importance and has important biological distinctions compared to the ascomycetous fungi, we hypothesized that directly screening a small-molecule library against C. neoformans would identify molecules with anticryptococcal activity that would be missed by screening with ascomycetes. We further focused our screen on molecules that directly kill C. neoformans, because drugs with this type of activity have been shown to be more effective in the treatment of C. neoformans disease than molecules that simply inhibit growth. To identify molecules that directly kill C. neoformans cells, we used an AK release assay recently developed in our laboratory (8, 13). The AK assay detects the release of the intracellular enzyme AK into the growth medium by cells that have lost membrane integrity by coupling its conversion of ADP to ATP with a luciferase reporter reaction. We have previously shown that the assay is robust in the HTS setting and is more sensitive than growth assays (8, 13). Furthermore, it is specific for molecules that directly kill yeast cells; for example, the fungistatic drug fluconazole is completely inactive in this assay (13).
We screened against serotype A C. neoformans var. grubii strain H99, since it is the most common species and serotype isolated from patients. As alluded to above, we screened the Prestwick library of off-patent and biologically active molecules (Prestwick Chemical) at a final drug concentration of 50 μM, using the AK assay as the readout. As previously described (8), positively scoring molecules (hits) were defined as those inducing a 3-fold increase in extracellular AK activity. Each hit was evaluated using a set of three secondary screening assays. First, each was retested to confirm AK release by using a dilution series of the drug to generate a dose-response curve. Second, the MIC for each confirmed hit was then determined using CLSI-approved methods (12). Third, we confirmed the ability of the molecule to directly kill H99 by using a propidium iodide-based viability assay (13).
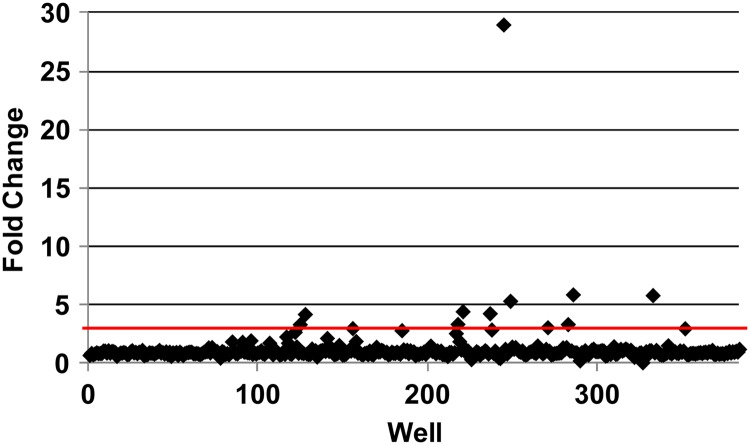
We performed the initial AK screening assay at two temperatures: ambient and 37°C. A scatterplot of the raw AK data from a representative plate is shown in Fig. 1. More drugs (31 versus 9) were active at 37°C, i.e., human body temperature, than at ambient temperature (15). Two molecules were active only at ambient temperature, and the majority of molecules active at low temperature were general antiseptic drugs that directly disrupt plasma membranes (6/11 molecules [55%]). The ability of C. neoformans to grow at 37°C is an important virulence factor, and it is possible that some of the hits directly interfere with this trait (3, 15). Alternatively, the increased physiological stress of high-temperature growth may increase the susceptibility of the organism to the compounds; it is likely that both possibilities are represented within the set of hits.
Fig 1.
Representative primary screening data. The scatterplot shows the fold changes in library drug-treated wells relative to DMSO (1%)-treated wells for a representative plate in the primary AK screen.
We obtained samples of 21/31 compounds (Table 1) from alternative sources/lots and confirmed their activity in dose-response experiments with the AK assay. The dose-response curve for amiodarone is shown in Fig. 2A, as a representative example. All retested molecules were confirmed as hits, indicating a low false-positive rate. We excluded known antifungals and general antiseptics from additional testing. From the remaining set, we determined the MICs against C. neoformans strain H99 and C. albicans SC5314 for 15 of the drugs (Table 1), using standardized CLSI microdilution susceptibility testing (12). We also determined the MFC, which was identical to the MIC for all compounds tested. Interestingly, all molecules identified in the screen were either more active against C. neoformans or equally active against the two organisms. Of the hits tested, all but two drugs had MIC values of 64 μg/ml or lower. The two drugs that did not have MIC values at or below 64 μg/ml were pimozide and fluspirilen (solubility limited the identification of the MIC). Both drugs induced cell lysis as determined by AK release and PI staining, indicating that the AK assay is more sensitive than growth assays and is able to identify molecules with anticryptococcal activity below the MIC. The ability of the AK assay to detect antifungal activity at concentrations well below the MIC is due to the fact that a drug may cause up to 20% cell lysis, but the logarithmic nature of microbial growth over the 24- to 48-h period typical of growth assays masks these effects (8, 13).
Table 1.
Drugs inducing adenylate kinase release at 37°C in C. neoformans var. grubii
| Compound no. | Drug | Therapeutic use | AK releasea | C. neoformans MIC/MFCb | C. albicans MICb | Known antifungalc | Modulation of Camd | Oral administratione | Lysosome tropismf | FICIg |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tomatine | Fungicide | 29 | NT | NT | Y | N | NA | N | NT |
| 2 | Dicyclomine | Anticholinergic | 5 | 64 | >128 | N | N | Y | N | I |
| 3 | Perhexilene | Antiangina | 3 | 8 | 32 | N | N | Y | Y | A |
| 4 | Chlorhexidine | Antiseptic | 3 | NT | NT | Y | N | NA | N | NT |
| 5 | Tamoxifen | Antiestrogen | 4 | 4 | 32 | Y | Y | Y | Y | A |
| 6 | Miconazole | Antifungal | 4 | NT | NT | Y | Y | Y | N | NT |
| 7 | Tolnafate | Antifungal | 6 | NT | NT | Y | N | N | N | NT |
| 8 | Pimozide | Antipsychotic | 6 | >128 | >128 | N | Y | Y | Y | I |
| 9 | Thioridazine | Antipsychotic | 3 | 16 | 64 | N | Y | Y | Y | A |
| 10 | Trifluoperazine | Antipsychotic | 4 | 32 | 128 | Y | Y | Y | Y | A |
| 11 | Amiodarone | Antiarrhythmic | 8 | 8 | >128 | Y | Y | Y | Y | A |
| 12 | Polymyxin E | Antibiotic | 14 | Y | Y | N | N | A | ||
| 13 | Suloctodil | Antihypertensive | 12 | 4 | 16 | Y | N | Y | N | S |
| 14 | Chlorprothixene | Antipsychotic | 4 | 32 | 64 | N | Y | Y | Y | A |
| 15 | Norcyclobenzaprine | Muscle relaxant | 4 | NT | NT | N | N | Y | N | NT |
| 16 | Cyclobenzaprine | Muscle relaxant | 3 | 32 | 64 | N | N | Y | N | I |
| 17 | Bepridil | Antihypertensive | 3 | 32 | >128 | N | Y | Y | Y | A |
| 18 | Methiothepin | Antipsychotic | 5 | 16 | 64 | N | N | Y | N | A |
| 19 | Dimethisoquin | Topical anesthetic | 3 | NT | NT | N | N | Y | N | NT |
| 20 | Prochlorperazine | Antipsychotic | 7 | 64 | 32 | N | Y | Y | Y | A |
| 21 | Hexetidine | Antiseptic | 4 | NT | NT | Y | N | NA | N | NT |
| 22 | Fluspirilen | Antipsychotic | 24 | >128 | >128 | N | Y | Y | Y | S |
| 23 | Thonzonium | Antiseptic | 4 | NT | NT | Y | N | Y | N | NT |
| 24 | Methylbenzethonium chloride | Antiseptic | 6 | NT | NT | Y | N | Y | N | NT |
| 25 | Thiethylperazine | Antipsychotic | 5 | NT | NT | N | Y | Y | NT | |
| 26 | Sertaline | Antidepressant | 5 | 6h | 14–28 | Y | Y | Y | Y | Sh |
| 27 | Meclozine | Antiemetic | 4 | NT | NT | N | N | Y | NT | |
| 28 | Clomiphene | Antiestrogen | 7 | 64 | 64 | Y | Y | Y | Y | A |
| 29 | Pheniramine | Antihistamine | 4 | NT | NT | N | N | Y | N | NT |
| 30 | Alexidene | Antiseptic | 9 | NT | NT | Y | N | NA | N | NT |
| 31 | Benzethonium | Antiseptic | 3 | 4 | 4 | Y | N | NA | N | NT |
Fold change in AK activity relative to that in cells treated with DMSO solvent.
MICs were determined by a standard CLSI protocol. The MFC was the lowest concentration that decreased the number of CFU by 2 log10 units relative to the initial inoculum and was identical to the MIC for all compounds tested. NT, not tested.
Y, reported to have in vitro antifungal activity; N, no previous report of antifungal activity.
Y, drug or drug class has been reported to modulate mammalian calmodulin activity; N, not reported to modulate mammalian calmodulin activity.
Y, drug is administered orally for its primary clinical indication; N, drug is not administered orally; NA, data not available.
Y, reported to traffic to the lysosomes of eukaryotic cells; N, not reported to traffic to the lysosomes of eukaryotic cells.
Determined as described in Materials and Methods. NT, not tested; S, synergy (FICI of <0.5); A, additive (FICI of 0.5 to 1.0); I, indifferent (FICI = 1.0 to 2.0).
See reference 40.
Fig 2.
Representative results of secondary screening assays. (A) Dose-response data for amiodarone (AMD) showing relative light units of released AK activity at the indicated doses relative to those in DMSO (1%) (mock)-treated wells. (B) Percentages of cells staining with propidium iodide relative to DMSO-treated cells, as determined by fluorescence microscopy, for cells treated with amiodarone (AMD), trifluoperazine (TFP), and thioridazine (THZ) at the indicated drug concentrations (n, >100 cells).
One of the drugs identified in our screen, amiodarone, has previously been reported to have activity against S. cerevisiae, C. albicans, and C. neoformans (16, 17). In addition, its activity toward C. albicans and S. cerevisiae has been the subject of a number of mechanistic studies (17–19). The anti-C. albicans activity of amiodarone is pH dependent, and it appears to be active only at pH 5, well below physiologic pH (18). In contrast, we found that amiodarone is equally active toward C. neoformans at pH 5 and pH 7, indicating a significant interspecies difference in activity. Interestingly, dronedarone, a recently approved analog of amiodarone (20), also has similar activity toward C. neoformans but little activity against C. albicans (dronedarone MIC, 8 μg/ml for C. neoformans and >64 μg/ml for C. albicans) at physiologic pH. The distinctions in activity between C. albicans and C. neoformans for this class of molecules provide further support for the notion that the search for new anticryptococcal agents will require the direct screening of C. neoformans in order to maximize the likelihood of finding active molecules.
As a final secondary screening assay, we confirmed that the identified drugs directly killed C. neoformans cells by using the well-established PI stain (13). All compounds tested caused significantly increased levels of PI staining in C. neoformans, either at the MIC or at concentrations of <128 μg/ml if the molecule did not inhibit growth below that concentration; representative examples of PI staining data are shown in Fig. 2B. Based on PI staining, our primary screen did not identify any false-positive hits.
Characteristics of FDA-approved drugs with fungilytic anticryptococcal activity.
With respect to clinical uses, the largest class of molecules with fungilytic anticryptococcal activity was the antipsychotics (8/31 molecules); indeed, 8 of the 17 antipsychotics in the Prestwick library were identified. The next largest class, not surprisingly, was the general membrane-targeted antiseptics (7/31 molecules). Antifungal activity has been described previously for 16/31 hits; consequently, the screen identified 15 new drugs with antifungal activity. For an agent to be effective against cryptococcosis, it must be able to cross the blood-brain barrier and access the central nervous system (CNS). Importantly, nearly half of the drugs identified in our screen are active within the CNS. As discussed in the introduction, new anticryptococcal drugs would ideally be administered orally to increase their applicability in resource-limited settings. Excluding topical antiseptics, 22/24 drugs identified as having anticryptococcal activity are administered orally for their current clinical indications. Taken together, the data from our repurposing screen have identified a set of 17 previously approved drugs that are fungicidal toward C. neoformans, active within the central nervous system, and administered orally. As such, this set represents a potentially rich collection of chemical scaffolds for development as potential anticryptococcal agents.
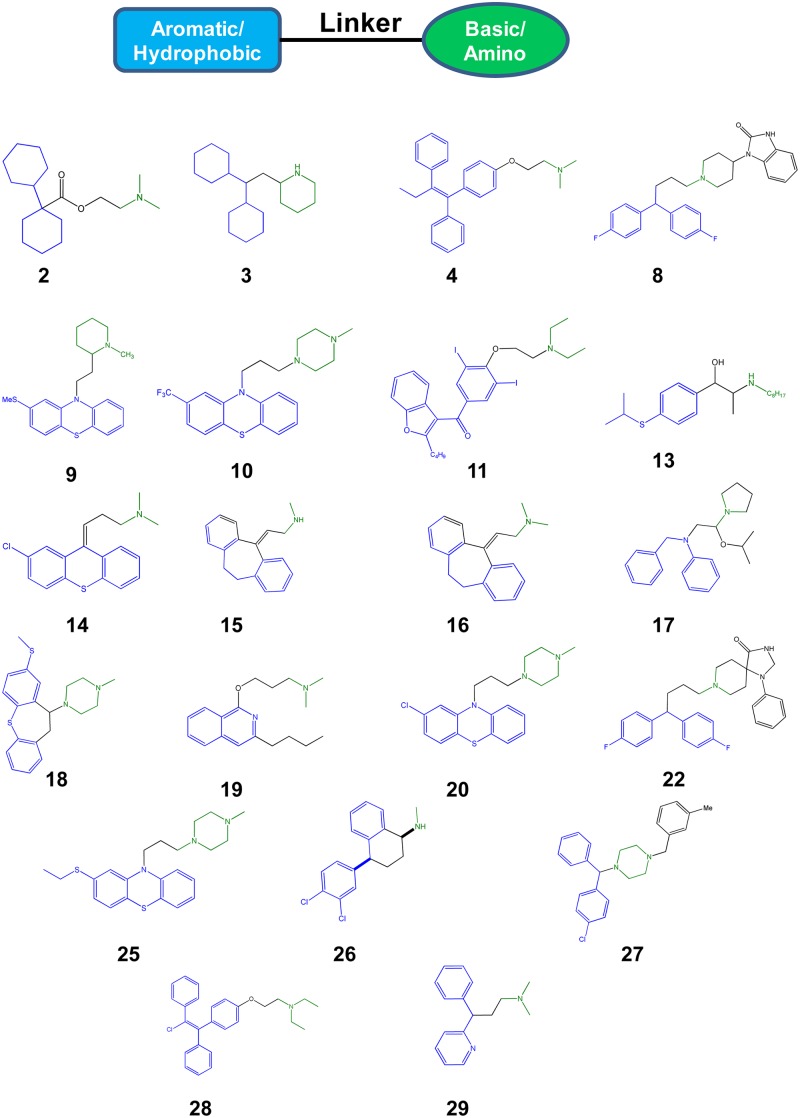
To further analyze the characteristics of our set of hits, we examined the chemical structures for common motifs. As displayed in Fig. 3, a striking pattern was immediately apparent for the molecules that were not general antiseptics: 21/23 structures contained a hydrophobic/aromatic section connected to a basic amine by a 2- to 4-atom linker. This combination of lipophilicity with a basic pKa is characteristic of molecules that access the CNS (10). The same set of physicochemical properties has also been identified as characteristic of molecules with lysosomotropism (11), the ability to target and/or concentrate in host cell phagolysosomes. The lipophilic/hydrophobic portion of the molecule allows it to penetrate cellular membranes, while the basic amine is protonated by the acidic environment, trapping the molecule within the lysosome/phagolysosome. Consistent with this structural analysis, 12/31 hits (39%) (Table 1) from our screen have been identified in other screens of FDA-approved drugs for molecules that target or interact with the phagolysosome (11, 21, 22).
Fig 3.
Common chemotype of hits. Structural analysis of the hits revealed that a common motif for the set was a hydrophobic/aromatic moiety (blue) linked by a flexible chain (black) to a basic amine (green). The chemical structures of the hits that fit this motif are indicated. Structure numbers correlate with Table 1. The structural features are color coded to indicate the regions that match the chemotype.
C. neoformans traffics to the phagolysosome of macrophages, replicates, and then escapes as part of its pathogenic life cycle in humans (23). Unfortunately, the two most widely used anticryptococcal drugs, AMB and FLU, do not appear to have activity toward intraphagocytic C. neoformans (24). Thus, molecules able to target C. neoformans within this important niche could serve as either useful stand-alone agents or, possibly, adjuvants to agents currently in use. Further supporting this principle, Levitz et al. (25) have shown that chloroquine, a canonical lysosomotropic molecule, is active against intraphagocytic C. neoformans. As shown below, two of the hits, amiodarone and thioridazine, also have activity against intracellular C. neoformans. Consequently, the set of molecules identified in our screen not only have fungicidal activity against C. neoformans but also display physicochemical/pharmacological properties that appear to be well suited to targeting the drugs to host niches (phagolysosome and CNS) occupied by the pathogen.
For many of the molecules identified in the screen, the mechanism of antifungal activity may be distinct from the mechanism of its activity in humans and thus will be related to an “off-target” effect. A clue to a possible mechanism of action also emerged from the consensus structural chemotype of our set of hits. Specifically, the lipophilic linker-basic amine motif is also characteristic of molecules that antagonize the essential calcium-binding protein calmodulin (26). Interestingly, over half of the nonantiseptic hits have been reported to inhibit calmodulin or other calcium-related functions in eukaryotic cells (Table 1) (14/23 hits [60%]). Indeed, trifluoperazine, pimozide, and other antipsychotics are well-characterized calmodulin inhibitors (26). Recently, we confirmed that tamoxifen targets calmodulin as part of its antifungal activity against S. cerevisiae (27). As detailed below, we have shown that a number of these molecules directly bind C. neoformans calmodulin, suggesting that this activity may contribute to the mechanism of their antifungal properties.
Combination of fungilytic drugs with fluconazole increases antifungal activity.
In resource-rich countries (31), the treatment of choice for CEM is the combination of AMB with 5FC. This combination has been proven to be more effective clinically than FLU, a fungistatic drug with excellent in vitro activity (5). Recent studies have shown that the difference in clinical efficacy between the two regimens is most likely due to the early fungicidal activity of the AMB-5FC combination, as reflected by the increased rate at which C. neoformans is cleared from the cerebrospinal fluid of patients treated with AMB-5FC compared to those treated with FLU (6). As discussed above, FLU is widely available, nontoxic, and easy to administer in resource-limited regions (5). Therefore, one approach to improving the therapy of CEM in resource-limited regions is to identify an agent that leads to a fungicidal combination when administered with FLU. FLU inhibits the synthesis of ergosterol, a key plasma membrane sterol that is unique to fungi. Previously, we have shown that sets of molecules identified by the AK assay are enriched for those that target the fungal cell wall and plasma membrane, the two most important cellular targets of antifungal drugs (8, 13). Since molecules that target these structures have also been shown to synergize with FLU (28), we hypothesized that the set of molecules identified in our screen may contain molecules that interact with FLU. Supporting this hypothesis, five drugs (trifluoperazine, tamoxifen, suloctodil, polymyxin E, and sertaline) identified in our screen were recently reported to be synergistic with FLU against C. neoformans in checkerboard assays (29).
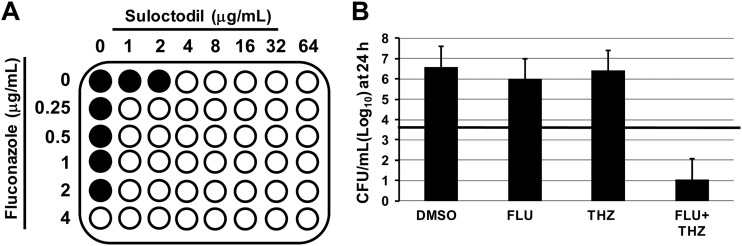
To test our hypothesis, we first carried out checkerboard microdilution assays to characterize the interactions of our set of hit molecules with FLU (28). We determined the FICI (28) for the set of molecules listed, as summarized in Table 1. The data from a representative checkerboard experiment assaying the interaction of suloctodil with fluconazole are depicted in Fig. 4A. Synergistic interactions are defined as those with FICI of <0.5, according to standards in the field (28). Consistent with previous reports (29), we found that the antihypertensive suloctodil, the antibiotic polymyxin E, and the antidepressant sertaline are synergistic with FLU (Table 1). We did not, however, observe the FLU synergy with either tamoxifen or trifluoperazine reported by Spitzer et al. (29). The strain of C. neoformans used by Spitzer et al. (29) had a very high FLU MIC (64 μg/ml). In addition, Spitzer et al. used a modified CLSI protocol for their microdilution susceptibility testing, in which buffered RPMI tissue culture medium was replaced by synthetic complete yeast medium (29). We hypothesize that either or both of these differences in experimental protocols contributed to the variation in FICI data between the two laboratories. Finally, we also observed synergy for the interaction of the atypical antipsychotic fluspirilen with FLU. As noted above, fluspirilen had an MIC of >128 μg/ml when used by itself. The combination of FLU at 1/4 MIC (0.5 μg/ml) and 8 μg/ml of fluspirilen was fungicidal, indicating that the minimum FICI for this combination is 0.31. The observation of this dramatic effect further underscores the utility of the AK assay for identifying antifungal activity at concentrations below the MIC, since this compound would have been missed by traditional growth-based assays.
Fig 4.
Representative interactions of hits with fluconazole. (A) Checkerboard assay showing synergistic interaction of fluconazole with suloctodil. Filled circles indicate growth, and open circles indicate no growth. (B) Subinhibitory concentrations of fluconazole (FLU; 2 μg/ml) and thioridazine (THZ; 16 μg/ml) decreased the initial inoculum (indicated by the horizontal bar) >2 log10 CFU/ml after 24 h.
A second manner in which a drug could interact with fluconazole would be to generate a fungicidal combination at concentrations below which neither drug alone is fungicidal. This type of interaction may not be apparent from checkerboard assays, because fungistatic and fungicidal activities are not distinguishable using such growth assay readouts. To test for this effect, we plated the well contents from checkerboard assays of our hits in combination with a range of FLU concentrations. Fungicidal activity was defined as a 2-log10 decrease in CFU relative to the starting inoculum of 1.5 × 103 CFU/ml (28). From this initial screening, we identified amiodarone, perhexilene, and thioridazine as potential interacting drugs (data not shown). To confirm these screening results, we performed 24-hour time-kill experiments with each drug, using subinhibitory concentrations of the test drug and FLU. The number of viable organisms (CFU/ml) decreased by >2 log10 for each of the three drugs tested in combination with subinhibitory FLU. The time-kill data for thioridazine are shown in Fig. 4B; amiodarone and perhexilene decreased the initial inoculum by 2.5 and 2.1 log10 units, respectively. Thus, these three drugs are able to combine with FLU to yield a fungicidal cocktail.
Amiodarone and thioridazine are active against intracellular C. neoformans.
C. neoformans replicates within the phagolysosome of macrophages as part of its mechanism of pathogenesis, and therefore the phagolysosome is an important niche for this pathogen (23). Consequently, anticryptococcal drugs that are able to penetrate and have activity within the phagolysosome are potentially useful (25). Previous studies have shown that neither AMB nor FLU has significant antifungal activity toward C. neoformans once the organism is within the phagocyte (24). The canonical lysosomotropic drug chloroquine has been shown to have activity against C. neoformans within phagocytes (25). Since lysosomotropic drugs frequently concentrate within the phagolysosome (30, 31), we hypothesized that some of the agents identified in our screen may have activity against intracellular C. neoformans. Of the lysosomotropic agents identified in the screen, thioridazine and amiodarone appeared to be attractive agents in this regard (30, 31). Both are known to accumulate within alveolar macrophages, and thioridazine has emerged as a potential treatment for extremely drug-resistant TB based on this property (32).
To test the hypothesis that these agents may be able to kill intraphagocytic C. neoformans, we infected murine J774 phagocytes, a cell line widely used to study phagocyte-C. neoformans interactions, with opsonized C. neoformans H99 cells, washed the cells to remove extracellular organisms, and treated them with either DMSO or a drug in DMSO. After 24 h, the J774 cells were washed and lysed to determine the amount of intracellular C. neoformans. Thioridazine at a concentration 16-fold below the MIC (2 μg/ml) decreased the intracellular burden of C. neoformans 2.7-fold relative to that in untreated phagocytes (1.8 × 105 ± 3 × 104 CFU/ml in untreated phagocytes versus 6.2 × 104 ± 6 × 103 CFU/ml in thioridazine [2 μg/ml]-treated phagocytes; P < 0.0005 by Student's t test). Importantly, this concentration corresponds to a serum concentration that is achievable by clinical doses of thioridazine (33). Amiodarone was not as active within J774 cells and required a concentration equivalent to its MIC to achieve intracellular antifungal activity (2.5-fold reduction) (9.1 × 104 ± 5 ×103 CFU/ml for DMSO-treated cells versus 4.0 × 104 ± 6 × 103 CFU/ml for amiodarone [10 μg/ml]-treated cells; P < 0.05 by Student's t test). The concentrations of drugs used in these experiments had no detectable effect on J774 cell viability (>95% viable relative to DMSO-treated cells by LDH cytotoxicity assay), adherence, or cellular morphology (data not shown). To our knowledge, the only other lysosomotropic agent that has been shown to have anticryptococcal activity is the previously mentioned drug chloroquine (25); thus, amiodarone and thioridazine represent the first new examples of agents with activity against intracellular C. neoformans since that report.
Anticryptococcal molecules with off-target calmodulin antagonism in mammalian cells bind C. neoformans calmodulin.
As discussed above, 14 of the molecules identified in our screen have been reported to have activity as calmodulin antagonists. This is particularly characteristic of antipsychotics, but other classes of drugs have also shown this activity (Table 1) (26). In addition, the structural motif shared by many of the drugs identified in our screen fits the general chemotype of calmodulin antagonists (26). To determine if calmodulin interactions may contribute to the anticryptococcal activity of the drugs, we examined the ability of a set of our hits to directly bind C. neoformans calmodulin. Although calmodulin molecules are highly conserved, with >95% sequence homology across metazoans, the fungal calmodulins are among the most divergent of all eukaryotic calmodulin molecules (15, 34). Therefore, we sought to directly test the ability of a subset of our hits to bind to C. neoformans calmodulin. To this end, we expressed the cDNA of serotype D C. neoformans var. neoformans CAM1 containing a histidine tag sequence in E. coli and purified it using standard affinity techniques. The resulting protein was functional and activated human calcineurin in a commercially available assay kit (CnCam1-mediated calcineurin activity, 2.2 ± 0.1 arbitrary absorbance units/μM CnCam1/h; and human calmodulin-mediated calcineurin activity, 0.54 ± 0.05 arbitrary absorbance units/μM hCam/h).
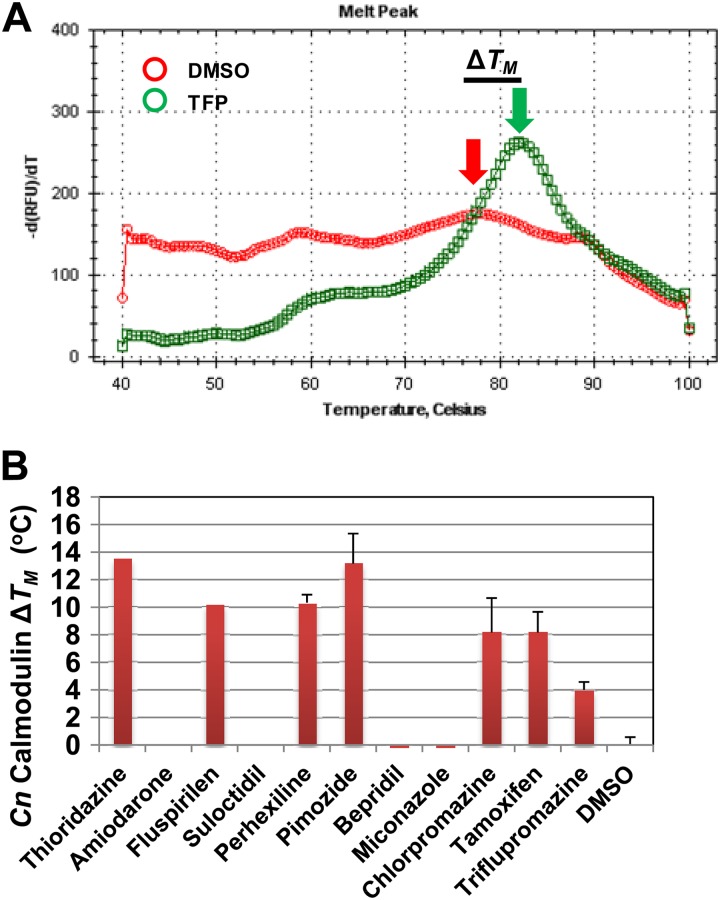
Recently, thermal shift assays have emerged as a powerful and expedient method for determining whether or not a small molecule binds directly to a potential protein target (35). This approach is based on the fact that the ability of a fluorescent dye such as SYPRO orange to bind to protein varies with the folding state of the protein. In the case of calmodulin, the folded protein is highly hydrophobic and binds SYPRO orange, leading to increased fluorescence. As calmodulin denatures, the acidic residues of the protein are exposed, leading to decreased SYPRO binding and, consequently, decreased fluorescence. To measure the Tm of CnCam1 in the presence and absence of drugs, we used differential scanning fluorimetry (DSF). The first derivative of the melting curve for CnCam1 in the presence of DMSO is shown in Fig. 5A, with a Tm of 77°C. In the presence of the canonical calmodulin inhibitor triflupromazine, the Tm of CnCam1 was shifted 4°C and the transition was more distinct, indicating that the drug binds CnCam1. In general, ΔTm values of >2°C are indicative of protein-ligand binding (35). As shown in Fig. 5B, we examined the calmodulin-binding properties of 11 hits by using DSF-based thermal shift assays. Of the nine drugs reported to bind mammalian calmodulin, six (thioridazine, fluspirilen, perhexilene, pimozide, chlorpromazine, and triflupromazine) bound CnCam1, while no binding was observed for amiodarone, bepridil, and miconazole. In addition, suloctodil, a vasodilator with no known calmodulin-binding activity, did not show evidence of binding (Fig. 5B). Although these data do not exclude the possibility that the molecules have multiple targets in C. neoformans, our data are consistent with calmodulin antagonism contributing to the mechanism of their antifungal activity.
Fig 5.
The set of fungicidal anticryptococcal drugs contains a number of calmodulin antagonists. (A) Thermal shift assay for CnCam1 in the presence of DMSO or the well-characterized calmodulin antagonist triflupromazine (TFP; 100 μM). The x axis shows the temperature, and the y axis shows the negative derivative of the fluorescence value. The red arrow indicates the Tm for CnCam1 in the presence of DMSO, and the green arrow indicates that in the presence of TFP. (B) Changes in Tm induced by the indicated drugs relative to that with DMSO (1%). The bars indicate means for three independent experiments performed in triplicate; error bars indicate standard deviations.
DISCUSSION
New treatment approaches for cryptococcosis represent an unmet clinical need with significant importance to global health (1, 3, 5, 36). As an approach to the initial development of new anticryptococcal molecules, we designed a screening strategy that would allow us to identify molecules that satisfied some or all of the characteristics described above. The application of a repurposing approach to the identification of anticryptococcal agents has yielded a number of important lead compounds. For example, calcineurin inhibitors have been under study as potential general antifungal compounds, and they have activity against Cryptococcus (37). Recently, polymyxin and sertaline emerged from screens of off-patent drugs for other fungi and were subsequently found to have activity against Cryptococcus (38, 39). Importantly, our screen identified both polymyxin and sertaline; calcineurin inhibitors such as cyclosporine and FK506 were negative in our screen, consistent with the fact that they are not directly fungicidal by themselves. In addition, many of the drugs we identified were more active toward C. neoformans than against C. albicans, supporting the notion that direct screening with C. neoformans may yield more hits than the more common C. albicans-based screens.
Analysis of the chemical structures of the drugs identified in our screen revealed a striking general structural motif comprised of a hydrophobic/lipophilic moiety linked to a cationic amine substituent. The amphipathic nature of drugs within this general structural class allows them to readily cross biological membranes and thus display two important properties relevant to the treatment of cryptococcosis. First, drugs of this type readily cross the blood-brain barrier and thus can access the most important site of disease caused by Cryptococcus (10). Second, these drugs are typically able to penetrate the lysosome/phagolysosome of host cells and thus can access an important niche for Cryptococcus during pathogenesis (3, 23). Consistent with the general ability of drugs with this chemotype to cross the blood-brain barrier, the largest clinical class of drugs identified by our screen was antipsychotic medications, such as trifluoperazine, fluspirilen, and thioridazine; in addition, the antidepressant sertaline was also identified. The antifungal activities of trifluoperazine (40) and sertaline (38) have been described previously. In addition, a chemogenomic study of drugs with activity against S. cerevisiae indicated that many psychoactive molecules have antifungal activity (41). Our data are consistent with previous reports and extend those findings to pathogenic fungi. In addition, our results indicate that a significant portion of psychoactive drugs with antifungal activity are fungicidal toward C. neoformans.
Within the set of neurotropic drugs identified by our screen, the most common structural class of drug was the phenothiazines (Fig. 4) (see structures in references 9, 10, 14, 25, and 29). The phenothiazine class of molecules is one of the oldest drug scaffolds, and drugs based on these structures have been developed as neuroleptic, antimicrobial, antimalarial, and anticancer agents, to name a few indications (42). As a class, phenothiazines cross the blood-brain barrier and are lysosomotropic. In addition, a wide range of phenothiazine structures have been synthesized and evaluated for a number of biological activities (42). Our results suggest that this class of molecules possesses many characteristics that are ideal for the development of an anticryptococcal agent. However, to our knowledge, no dedicated program has sought to optimize the anticryptococcal activity of this chemical scaffold.
Within our set of fungicidal anticryptococcal drugs, we identified 14 drugs that interacted with fluconazole to yield a more active combination; by growth assay, the majority of these interactions were additive. Recently, a number of studies have reported the interactions of repurposed drugs with FLU (28, 37–39). For example, while our work was in progress, Spitzer et al. reported a screen of the same library looking directly for drugs that had little or no intrinsic antifungal activity but that improved the antifungal activity of FLU, referred to as syncretic combinations (29). They used a growth assay as the readout for this screen. Spitzer et al. identified a number of the drugs that we identified in our screen (29). For example, they also identified a large set of antipsychotics that interact with FLU against C. neoformans. In our studies, we further showed, by time-kill assay, that subinhibitory concentrations of amiodarone, perhexilene, and thioridazine yield fungicidal combinations against C. neoformans. Taking the present data together with the data of Spitzer et al. (29), a number of the scaffolds identified by our two studies have promise as potential adjunctive agents in combination with FLU for the treatment of cryptococcosis.
A second manner in which a drug could function as an adjuvant is by killing C. neoformans in a niche that is inaccessible to fluconazole or amphotericin B. C. neoformans infects and replicates within the phagolysosome of macrophages. Previous work indicates that neither FLU nor AMB has activity against intracellular C. neoformans (24). From our set of lysosomotropic anticryptococcal drugs, we selected two agents known to accumulate within macrophages (amiodarone and thioridazine) and found that both were active against C. neoformans within J774 mouse phagocytes. Of the two drugs, thioridazine appears to be the most clinically promising, since it is active against intracellular C. neoformans at concentrations 8-fold below its MIC. This is most likely due to its previously characterized ability to concentrate within the phagolysosome in murine macrophages (31). Indeed, this property of thioridazine has already been exploited as a repurposing approach to the treatment of multidrug-resistant Mycobacterium tuberculosis infection and has shown promise in both animal studies and small clinical studies (31, 32). The intracellular lifestyle of C. neoformans has been proposed to play a role in latency (29), and thus the addition of an agent that is active within the macrophage to current therapy may decrease the occurrence of relapses. With this possibility in mind, it is important that a serum thioridazine concentration of 2 μg/ml is clinically achievable (33). Of the other drugs, amiodarone, tamoxifen, and perhexilene represent other agents that have either direct activity or activity in combination with FLU that is close to clinically achievable serum drug levels (21, 22). As such, amiodarone, tamoxifen, thioridazine, and perhexilene will be the focus of subsequent animal studies to test the in vivo efficacy of the agents identified in this screen.
Many of the drugs identified in our screen have been analyzed in the setting of Saccharomyces cerevisiae-based chemical-genetic screens as an approach to identifying potential molecular targets for antifungal activity (29, 41). These studies, for example, indicate that a loss of secretory/vesicle transport pathway function or membrane-related genes leads to increased sensitivity to antipsychotics. However, these pathway-based genetic studies have not led to the identification of a specific molecular target. We took an alternative approach to identifying potential molecular targets for the drugs identified in our screen. First, we searched the literature for off-target effects that had previously been identified in other systems. Second, we asked if the structural motif shared by our hits was characteristic of specific inhibitors. Both of these approaches suggested that many of our hits may target calmodulin as part of their mechanism of action. Supporting that hypothesis was the fact that phenothiazines such as trifluoperazine, thioridazine, and prochlorpromazine are well-characterized, canonical calmodulin antagonists (26); similarly, tamoxifen, bepridil, and amiodarone have all been shown to inhibit mammalian calmodulin (27). Curiously, except for our previous studies supporting calmodulin as a target of tamoxifen in S. cerevisiae, the anticalmodulin properties of these drugs have not previously been invoked as a potential mechanism for their antifungal activity (27). We confirmed that a significant number of the drugs identified in our screen bind directly to C. neoformans Cam1p in vitro and thus, for the first time, provide experimental support for a specific fungal protein target for drugs such as trifluoperazine, thioridazine, and fluspirilen.
It is important to note that our data indicate only that calmodulin antagonism is likely to contribute to the mechanism of antifungal activity for some of our hits, so we cannot rule out other potential targets. However, it is equally important that calmodulin regulates processes such as vesicle transport (through Myo2), vacuolar function (through calcineurin), and membrane function (through regulation of both vesicle and calcium homeostasis). Thus, many features of the chemogenomic profiles previously reported for these drugs may be attributable (29, 41), at least in part, to decreased calmodulin function. Since fungal calmodulins as well as parasitic calmodulins have diverged significantly from mammalian calmodulins (34), it may be possible to identify derivatives of the anticryptococcal drugs that are selective for C. neoformans calmodulin. Indeed, calmodulin has also been proposed as a potential target for the development of antiparasitic drugs (34).
In summary, we carried out a repurposing-based screen and identified a collection of off-patent drugs with fungicidal anticryptococcal activity that also possess a number of important pharmacological properties relevant to the treatment of cryptococcosis. We also identified a consensus chemotype common to these scaffolds. In addition, we identified a set of drugs that may be useful adjunctive agents, based on their interactions with FLU and/or ability to target C. neoformans within phagocytes. Finally, we have shown that a number of these drugs and drug classes target calmodulin as part of their mechanism of action. Taken together, these studies provide a foundation for subsequent work aimed at optimizing the antifungal properties of a set of pharmacologically attractive scaffolds for the development of novel anticryptococcal therapies.
ACKNOWLEDGMENTS
We thank Joseph Heitman (Duke University) for helpful discussions and strains, as well as Alan Smrcka and Michael Davenport, High Throughput Screening Core at the University of Rochester Medical Center, for assistance with screening experiments. We thank Arturo Casadevall (Albert Einstein College of Medicine) for providing antibody 18b7.
This work was supported by National Institute of Allergy and Infectious Disease grants R01AI091422 (D.J.K.) and 2T32AI007464-16 (Y.C.-R.).
Footnotes
Published ahead of print 14 December 2012
REFERENCES
- 1. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530 [DOI] [PubMed] [Google Scholar]
- 2. Kwon-Chung KJ, Boekhout T, Wickes BL, Fell J. 2011. Systematics of the genus Cryptococcus and its type species C. neoformans, p 3–17 In Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A. (ed), Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC [Google Scholar]
- 3. Perfect JR. 2012. The triple threat of cryptococcosis: it's the body site, the strain and/or the host. mBio 3:e100165–12 doi:10.1128/mBio.00165-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 50:291–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sloan DJ, Dedicoat MJ, Lalloo DG. 2009. Treatment of cryptococcal meningitis in resource-limited settings. Curr. Opin. Infect. Dis. 22:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, Rebe K, Loyse A, Jarivis J, Bekker LG, Wood R, Limmathurostakul D, Chierakul W, Stepniewska K, White NJ, Jaffar S, Harrison TS. 2009. Independent association of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of combined cohort of 262 patients. Clin. Infect. Dis. 49:702–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butts A, Krysan DJ. 2012. Antifungal drug discovery: something old and something new. PLoS Pathog. 8:e1002870 doi:10.1371/journal.ppat.1002870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiDone L, Scrimale T, Baxter BK, Krysan DJ. 2010. A high-throughput assay of yeast cell lysis for drug discovery and genetic analysis. Nat. Protoc. 5:1107–1114 [DOI] [PubMed] [Google Scholar]
- 9. Bisson WH. 2012. Drug repurposing in chemical genomics: can we learn from the past to improve the future? Curr. Top. Med. Chem. 12:1883–1888 [DOI] [PubMed] [Google Scholar]
- 10. Banks WA. 2009. Characteristics of drugs that cross the blood-brain-barrier. BMC Neurol. 9:53 doi:10.1186/1471-2377-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nadanaciva S, Lu S, Gehard DF, Jessen BA, Pennie WA, Will Y. 2011. A high-content screening assay for identifying lysosomotropic compounds. Toxicol. In Vitro 25:715–723 [DOI] [PubMed] [Google Scholar]
- 12. National Committee for Clinical Laboratory Standards 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 13.Krysan DJ, DiDone L. 2008. A high-throughput screening assay for small molecules that disrupt yeast cell integrity. J. Biomol. Screen. 181:1073–1081 [DOI] [PubMed] [Google Scholar]
- 14. Chabrier-Rosello Y, Gerik K, Koselny K, DiDone LL, Lodge JK, Krysan DJ. 19 October 2012. Cryptococcus neoformans phosphoinositide-dependent kinase 1 (PDK1) ortholog is required for stress tolerance. Eukaryot. Cell doi:10.1128/EC.00235-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kraus PR, Heitman J. 2003. Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem. Biophys. Res. Commun. 311:1151–1157 [DOI] [PubMed] [Google Scholar]
- 16. Gupta SS, Ton UK, Beaudry V, Rulli S, Cunningham K, Rao R. 2003. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J. Biol. Chem. 278:28831–28839 [DOI] [PubMed] [Google Scholar]
- 17. Courchesne WE. 2002. Characterization of broad-based fungicidal activity for the antiarrhythmic drug amiodarone. J. Pharmacol. Exp. Ther. 300:195–199 [DOI] [PubMed] [Google Scholar]
- 18. Gamarra S, Rocha EM, Zhang YQ, Parks S, Rao R, Perlin D. 2010. Mechanism of the synergistic effect of amiodarone and fluconazole in C. albicans. Antimicrob. Agents Chemother. 54:1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moend S, Rao R. 2008. Fungicidal activity of amiodarone is tightly coupled to calcium influx. FEMS Yeast Res. 8:425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pystowosky EN. 2010. Atrial fibrillation: dronedarone and amiodarone—the safety versus efficacy debate. Nat. Rev. Cardiol. 7:5–6 [DOI] [PubMed] [Google Scholar]
- 21. Balgi AD, Fonesca BD, Donahue E, Tsang TCF, Lajoie P, Proud CG, Nabi IR, Roberge M. 2009. Screen for specific modulators of autophagy reveals novel therapeutic inhibitors of TORC1 signaling. PLoS One 4:e7124 doi:10.1371/journal.pone.0007124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W, Zhu H, Yu AD, Xie X, Ma D, Yaun J. 2007. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl. Acad. Sci. U. S. A. 104:19023–19028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voelz K, May RC. 2010. Cryptococcal interactions with the host immune system. Eukaryot. Cell 9:835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herrmann JL, Dubois N, Fourgeaud M, Basset D, Lagrange PH. 1994. Synergic activity of amphotericin-B and gamma interferon against intracellular Cryptococcus neoformans in macrophages. J. Antimicrob. Chemother. 34:1051–1058 [DOI] [PubMed] [Google Scholar]
- 25. Levitz SM, Harrison TS, Tabuni A, Liu X. 1997. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J. Clin. Invest. 100:1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prozialeck WG, Weiss B. 1982. Inhibition of calmodulin by phenothiazines and related drugs: structure-activity relationships. J. Pharmacol. Exp. Ther. 222:509–516 [PubMed] [Google Scholar]
- 27. Dolan K, Montgomery S, Buchheit B, DiDone L, Wellington M, Krysan DJ. 2009. The antifungal activity of tamoxifen: in vitro, in vivo, and mechanistic characterization. Antimicrob. Agents Chemother. 53:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. 2005. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 8:163–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Tyers M, Wright GD. 2011. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 7:499 doi:10.1038/msb.2011.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Futamura Y. 1996. Effect of amiodarone on cytokine release and on enzyme activities of mouse alveolar macrophages, bone marrow macrophages, and blood monocytes. J. Toxicol. Sci. 21:125–134 [DOI] [PubMed] [Google Scholar]
- 31. Ordway D, Viveiros L, Bettencourt R, Aleida J, Martins M, Kristiansen JE, Molnar J, Amaral L. 2003. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 47:917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abbate E, Vescovo M, Natiello M, Cufre M, Garcia A, Gonzalez Montaner P, Ambroggi M, Ritacco V, van Soolingen D. 2012. Successful alternative treatment of extensively drug-resistant tuberculosis in Argentina with a combination of linezolid, moxifloxacin, and thioridazine. J. Antimicrob. Chemother. 67:473–477 [DOI] [PubMed] [Google Scholar]
- 33. Bergling R, Mjondal T, Oreland L, Rapp W, Wold S. 1975. Plasma levels and clinical effects of thioridazine and thiothixene. J. Clin. Pharmacol. 15:178–186 [DOI] [PubMed] [Google Scholar]
- 34. Benain B, Garcia CR. 2011. Targeting calcium homeostasis as a therapy of Chagas' disease and leishmaniasis—a review. Trop. Biomed. 28:471–481 [PubMed] [Google Scholar]
- 35. Senistenra G, Chau I, Vedad M. 2011. Thermal denaturation assays in chemical biology. Assay Drug Dev. Technol. 10:128–136 [DOI] [PubMed] [Google Scholar]
- 36. Schaars CF, Meintjes GA, Mooroni C, Post FA, Maartens G. 2006. Outcome of AIDS-associated cryptococcal meningitis initially treated with 200mg/day and 400mg/day of fluconazole. BMC Infect. Dis. 6:118 doi:10.1186/1471-2334-6-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Del Poeta M, Cruz MC, Cardenas ME, Perfect JR, Heitman J. 2000. Synergistic antifungal activities of bafilomycin A(1), fluconazole, and the pneumocandin MC0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhai B, Wu C, Wang L, Sachs MS, Lin X. 2012. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 56:3758–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhai B, Zho J, Yang L, Zhang J, Jung K, Giam CZ, Xiang XL, Lin X. 2010. Polymixin B, in combination with fluconazole, exerts a potent fungicidal effect. J. Antimicrob. Chemother. 65:931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eilam Y, Polacheck I, Ben-Golgi B, Chernichovosky D. 1987. Activity of phenothiazines against medically important yeasts. Antimicrob. Agents Chemother. 31:834–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ericson E, Bebbia M, Heisler LE, Wildenhain J, Tyers M, Giaver G, Nislow C. 2008. Off-target effects of psychoactive drugs revealed by genome-side assays in yeast. PLoS Genet. 4:e1000151 doi:10.1371/journal.pgen.1000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pluta K, Morak-Moldanska B, Jelen M. 2011. Recent progress in biological activities of synthesized phenothiazines. Eur. J. Med. Chem. 46:3179–3189 [DOI] [PubMed] [Google Scholar]