Abstract
The antimalarial agent fosmidomycin is a validated inhibitor of the nonmevalonate isoprenoid biosynthesis (methylerythritol 4-phosphate [MEP]) pathway in the malaria parasite, Plasmodium falciparum. Since multiple classes of prenyltransferase inhibitors kill P. falciparum, we hypothesized that protein prenylation was one of the essential functions of this pathway. We found that MEP pathway inhibition with fosmidomycin reduces protein prenylation, confirming that de novo isoprenoid biosynthesis produces the isoprenyl substrates for protein prenylation. One important group of prenylated proteins is small GTPases, such as Rab family members, which mediate cellular vesicular trafficking. We have found that Rab5 proteins dramatically mislocalize upon fosmidomycin treatment, consistent with a loss of protein prenylation. Fosmidomycin treatment caused marked defects in food vacuolar morphology and integrity, consistent with a defect in Rab-mediated vesicular trafficking. These results provide insights to the biological functions of isoprenoids in malaria parasites and may assist the rational selection of secondary agents that will be useful in combination therapy with new isoprenoid biosynthesis inhibitors.
INTRODUCTION
Severe malaria due to infection with the protozoan parasite Plasmodium falciparum has a significant impact on global health (1). Infections with P. falciparum contribute nearly 1 million deaths per year (2). Malaria control efforts are hampered by resistance to existing antimalarial agents, particularly chloroquine (3, 4). Clinical resistance to the more recently introduced artemisinin-based therapies has already been reported, highlighting the ongoing need to identify and exploit new targets for antimalarial drug development (5, 6).
Isoprenoid biosynthesis is a promising antimalarial drug target. Unlike mammalian cells, plasmodia do not use the classically described metabolic route via mevalonate. Instead, the malaria parasite produces isoprenoids through a mevalonate-independent pathway, which proceeds through a different key metabolite, methylerythritol 4-phosphate (MEP) (7, 8). P. falciparum requires isoprenoid biosynthesis through the MEP pathway during intraerythrocytic development, the stage of parasite growth responsible for the clinical symptoms of malaria. The genetic locus for the first dedicated enzyme of this pathway (deoxyxylulose-5-phosphate reductoisomerase [DXR]) is resistant to genetic disruption in P. falciparum and the related apicomplexan Toxoplasma gondii, and chemical inhibition of the MEP pathway by the small molecule fosmidomycin is lethal to malaria parasites (8–10). Fosmidomycin treatment of cells inhibits two enzymes of the MEP pathway (DXR and methylerythritol phosphate cytidyltransferase [IspD]), and growth inhibition by fosmidomycin is rescued by supplementation with downstream isoprenoids, such as isopentenyl pyrophosphate and geranylgeraniol (11, 12). Altogether, these studies validate the MEP pathway as an antimalarial drug target and establish the specificity of fosmidomycin as a chemical probe to address isoprenoid biology in P. falciparum.
Isoprenoids comprise a diverse class of cellular molecules, with over 20,000 natural isoprenoids described (13). The pathogenic stage of P. falciparum occupies a highly unusual ecological niche within human red blood cells and has several peculiar metabolic features that make it unclear which isoprenoids are essential in P. falciparum. For example, although isoprenoids contribute to membrane stability (as cholesterol), the malaria parasite acquires cholesterol from host cells and does not synthesize sterols de novo (14). In contrast, both ubiquinone biosynthesis and protein prenylation appear to be required for P. falciparum development. Ubiquinone, derived from isoprenoids, is an electron carrier and a necessary cofactor for the P. falciparum pyrimidine biosynthesis enzyme dihydroorotate dehydrogenase (DHODH), which is vital for malaria parasite growth (15). Protein prenylation is the posttranslational modification of proteins, such as small GTPases, with either farnesyl (15-carbon) or geranylgeranyl (20-carbon) isoprenyl groups. Isoprenyl moieties are covalently attached to C-terminal cysteines by one of three well-characterized prenyltransferases, which are expressed during the intraerythrocytic cycle (16, 17). Multiple classes of prenyltransferase inhibitors kill the malaria parasite, strongly suggesting that protein prenylation is an essential function of isoprenoid biosynthesis in malaria (18–21).
In our approach, we used the isoprenoid biosynthesis inhibitor fosmidomycin to address the role of protein prenylation as an essential function of isoprenoids in P. falciparum. In these studies, we confirm that ubiquinone is not the sole required isoprenoid in malaria parasites. We demonstrate that de novo isoprenoid biosynthesis via the MEP pathway generates the isoprenyl precursors for protein prenylation and that nonprenylated proteins are mislocalized upon fosmidomycin treatment. Finally, we demonstrate that inhibition of isoprenoid biosynthesis causes a late developmental arrest and vesicular trafficking defect in malaria parasites, consistent with a loss of protein prenylation.
MATERIALS AND METHODS
Materials.
All buffer components, salts, and enzyme substrates were purchased from Sigma, unless otherwise indicated.
Plasmodium falciparum culture and strains.
P. falciparum strains were cultured in vitro in human erythrocytes, as described previously (12), with the following modifications: a 5% O2–5% CO2–90% N2 atmosphere in RPMI 1640 medium supplemented with 27 mM sodium bicarbonate, 11 mM glucose, 5 mM HEPES, 1 mM sodium pyruvate, 0.37 mM hypoxanthine, 0.01 mM thymidine, 0.25 mg/ml gentamicin (Goldbio), and 0.5% Albumax (Invitrogen). The following strains were obtained from the Malaria Research and Reference Reagent Resource Center (MR4): wild-type strain 3D7 (MRA-102), D10 ACP-(leader)-GFP (MRA-568 [22]), and D10 ACP-(signal)-GFP (MRA-570 [22]). The following strains were kindly provided by Akhil Vaidya (Drexel University, Philadelphia, PA) (23): parental clone D10 and transgenic D10+pHHyDHOD-GFP (which heterologously expresses yeast DHODH [yDHODH]). The following strain was kindly provided by Daniel Goldberg (Washington University, St. Louis, MO): 3D7+pPlasmepsin-II-GFP (24).
Flow cytometric analysis.
Cultures were treated twice with a 5% (wt/vol) d-sorbitol solution during ring-stage growth to produce a >90% synchronized culture. Each culture was resynchronized 24 h later by magnetic separation (MS) with MS columns and a MiniMACS separator (Miltenyi Biotech) to remove early-stage parasites, as previously described (25, 26). Giemsa-stained smears were used to monitor growth. Cell cycle analysis was performed using >95% synchronized Plasmodium falciparum 3D7 cultures. Four independent strains were split to 1% parasitemia (percentage of infected erythrocytes), and each was divided into four separate treatment groups (no drug, 5 μM fosmidomycin [Invitrogen], 5 μM fosmidomycin plus 5 μM geranylgeraniol, or 5 μM geranylgeraniol). At each time point, 100 μl of each culture was fixed with an equivalent volume of fixative solution (8% paraformaldehyde–0.015% glutaraldehyde in phosphate-buffered saline [PBS]). DNA content was determined by staining with the fluorophore acridine orange (1.5 μg/ml; Invitrogen), followed by resolution on a BD-FACS flow cytometer, as previously described (27). Data analysis was performed by using FlowJo (TreeStar Inc., Ashland, OR) and Prism 5 (GraphPad Software, La Jolla, CA) software.
Immunoblot analysis.
P. falciparum 3D7 parasites were grown to approximately 10% parasitemia, synchronized by treatment with sorbitol as described above. Newly invaded ring-stage parasites were treated with or without 5 μM fosmidomycin for 24 h. Host erythrocytes were lysed with 0.1% saponin, and parasites were harvested by centrifugation, washed with PBS, and then stored at −80°C until use. Parasite pellets were lysed by sonication in lysis buffer (10% glycerol, 0.1% Triton X-100, 20 mM HEPES [pH 7.5], 150 mM sodium chloride, and protease inhibitors [Complete EDTA-free tablets; Roche]). Immunoblotting was performed as previously described, using rabbit antifarnesyl polyclonal antibody at a 1:500 dilution (Abcam, Cambridge, MA), rabbit anti-Plasmodium falciparum EIF1α antibody (kindly provided by Daniel Goldberg, Washington University [28]) at a 1:3,000 dilution, and horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin polyclonal antibody at a 1:10,000 dilution (Invitrogen).
Microscopic analysis.
P. falciparum parasites were synchronized by treatment with sorbitol as described above. Newly invaded ring-stage parasites were treated with or without 5 μM fosmidomycin, 5 μM fosmidomycin plus 5 μM geranylgeraniol, or 5 μM wortmannin. Live cells were visualized as previously described (24), and images of live cells and Giemsa-stained slides were obtained with an Olympus BH8 microscope. Live cells were stained with Lysotracker Red DND-99 (LR) (Molecular Probes) at 75 nM for 15 min, washed in an equal volume of fresh culture medium, and visualized immediately for no longer than 15 min. For immunofluorescence microscopy, cells were fixed in 4% paraformaldehyde with or without 0.0075% glutaraldehyde and stored at 4°C. Fixed parasites were permeabilized, blocked, and incubated with antibodies, as previously described (29). Rabbit polyclonal antibodies against recombinant P. falciparum Rab5a (Pf-Rab5a) and Pf-Rab5c were kindly provided by Gordon Langsley (Institut Cochin, France) (30) and were used at 1:500 and 1:1,000 dilutions, respectively. The secondary antibody used was Alexa Fluor 488 goat anti-rabbit IgG(H+L) (catalog number A11008; Invitrogen) at a 1:500 dilution, and Hoechst 33258 was used as a nuclear counterstain. Images were obtained with an Olympus Fluoview FV1000 confocal microscope. All images were analyzed by using ImageJ software (31). Minimal adjustments in brightness and contrast were employed and were applied equally to all samples.
For ultrastructural analysis, Plasmodium-infected red blood cells were fixed in 1% glutaraldehyde (Polysciences Inc., Warrington, PA)–1% osmium tetroxide (Polysciences Inc.) in 50 mM phosphate buffer (pH 7.2) for 1 h at 4°C. This low-osmolarity fixation was used to remove dense, soluble cytoplasmic components, allowing unobscured membrane analysis. Cells were washed in phosphate buffer and rinsed extensively in distilled water (dH2O) prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 h. Following several rinses in dH2O, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections of 90 nm were cut, stained with uranyl acetate and lead citrate, and viewed on a Jeol 1200 EX transmission electron microscope (Jeol USA, Peabody, MA) at an accelerating voltage of 80 kV.
RESULTS
Electron transport bypass does not confer resistance to inhibition of isoprenoid biosynthesis.
De novo isoprenoid biosynthesis through the MEP pathway is an essential metabolic pathway in malaria parasites. Because this pathway produces a large number of compounds, we are interested in which of these is required for development of the malaria parasite, P. falciparum. The isoprenoid ubiquinone is an electron acceptor for mitochondrial electron transport and is a necessary cofactor for the essential pyrimidine biosynthesis enzyme dihydroorotate dehydrogenase (DHODH). The cytochrome bc1 complex of the electron transport chain is the target of the antimalarial agent atovaquone (32). Malaria parasites engineered to heterologously express a yeast dihydroorotate dehydrogenase (yDHODH) homolog, which does not require a ubiquinone cofactor, can survive without mitochondrial respiration and are resistant to atovaquone (23). Ubiquinone biosynthesis is therefore expected to be one of the essential functions of isoprenoids in malaria parasites.
In order to determine whether there are other essential functions of isoprenoids, we evaluated whether yDHODH expression also conferred resistance to inhibition of isoprenoid biosynthesis, using the validated MEP pathway inhibitor fosmidomycin. We obtained a strain of P. falciparum that heterologously expresses yDHODH. We independently confirmed that this strain is resistant to atovaquone, as was previously shown (data not shown) (23). We compared the fosmidomycin concentration that inhibited 50% of growth (IC50) at 3 days between the yDHODH-expressing strain and its parental control line. The yDHODH-expressing strain was as sensitive to fosmidomycin as the control parasite line (mean IC50 of 1.2 μM [95% confidence interval {CI}, 0.66 to 2.3], compared to the mean IC50 of the wild-type strain, 0.88 μM [95% CI, 0.67 to 1.14]) (Fig. 1). Since expression of yDHODH does not confer fosmidomycin resistance, ubiquinone production is not likely to be the only essential function of isoprenoid biosynthesis in malaria parasites.
Fig 1.
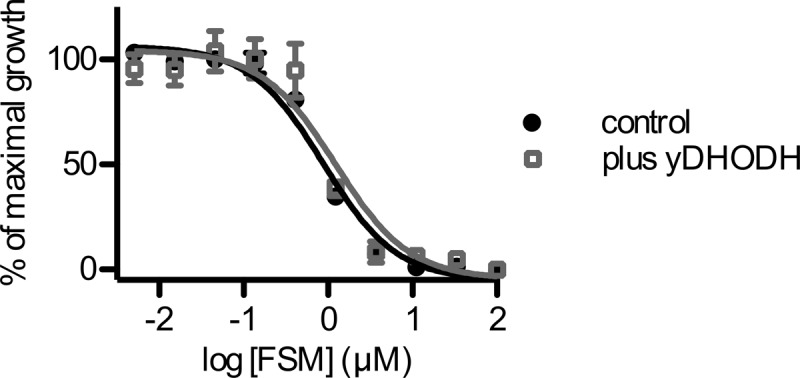
Bypass of electron transport does not confer fosmidomycin resistance. Shown are data for growth inhibition by the isoprenoid biosynthesis inhibitor fosmidomycin (FSM) in P. falciparum parasites (control) compared to parasites that heterologously express yeast dihydroorotate dehydrogenase, which does not require ubiquinone (control + yDHODH). Results are the means and standard deviations from three independent biological replicates.
Inhibition of isoprenoid biosynthesis reduces protein prenylation.
We evaluated whether inhibition of de novo isoprenoid biosynthesis by fosmidomycin, which should reduce the concentration of isoprenyl precursors, would reduce protein prenylation. We analyzed whole protein lysates from parasites grown with and without fosmidomycin (5 μM, approximately five times the IC50) by immunoblotting with a rabbit polyclonal antifarnesyl antibody that recognizes both farnesyl and geranylgeranyl groups. Previous experiments in which P. falciparum was metabolically labeled with [3H]farnesol demonstrated a characteristic protein banding pattern of two dominant prenylated bands, one at 45 kDa and the other at 20 to 25 kDa (16, 33). We found that immunoblotting of parasite lysates with antifarnesyl antibody recapitulated this banding pattern, consistent with the identification of these bands as prenylated proteins (Fig. 2). We used a densitometric evaluation of band intensities to compare prenylation with and without fosmidomycin treatment. The intensity of antifarnesyl staining of the ∼25-kDa lower band was reduced by 97% following fosmidomycin treatment (normalized to the density of control Pf-EF1α immunoblotting of the same blot). The intensity of the upper 45-kDa band, the identity of which is unknown, was reduced by 30% following fosmidomycin treatment. These results indicate that the isoprenyl moieties used for protein prenylation are derived from de novo isoprenoid biosynthesis in P. falciparum and that inhibition of isoprenoid biosynthesis reduces protein prenylation.
Fig 2.
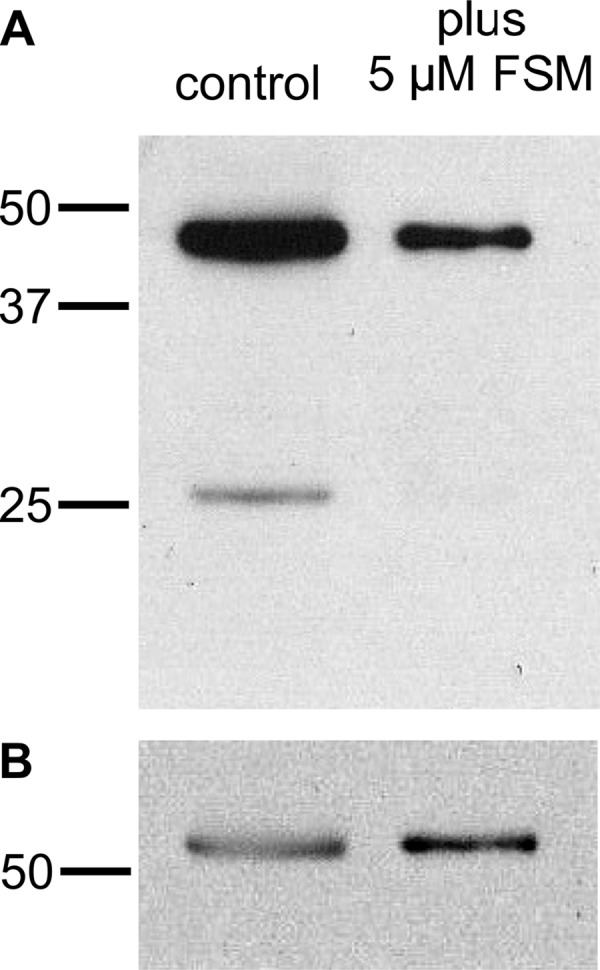
Fosmidomycin treatment inhibits protein prenylation. (A) Antifarnesyl immunoblot of extracts with or without treatment with 5 μM fosmidomycin (FSM) for 24 h. (B) Blot from panel A reprobed with antibodies to Pf-EIF1α to indicate equivalent protein loading. Results are representative of at least three independent biological replicates.
Rab GTPases are mislocalized when isoprenoid biosynthesis is inhibited.
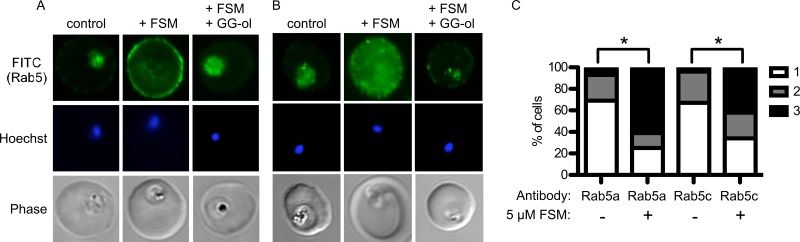
Fosmidomycin inhibited protein prenylation and particularly reduced prenylation of proteins of around 25 kDa. The malaria parasite expresses at least 11 small GTPases with predicted molecular masses of between 23 and 27 kDa, each of which is predicted to be geranylgeranylated (34, 35). These proteins likely comprise the dominant band that labels with [3H]farnesol and are recognized by antifarnesyl antibodies at 25 kDa (16). Since protein prenylation is typically required for the localization and function of small GTPases, such as Rab5 family members, we examined the localization of two candidate geranylgeranyltransferase substrates, Pf-Rab5a and Pf-Rab5c. Rab5 proteins are prototypical markers of early endosomal vesicles in most eukaryotic cells, and Pf-Rab5a was previously localized by immunoelectron microscopy to small hemoglobin-containing vesicles in P. falciparum (36). Pf-Rab5a and Pf-Rab5c were dispersed in punctae throughout malaria parasite cells (Fig. 3). Upon fosmidomycin treatment, a dramatic mislocalization of Rab5a occurred in the majority of treated cells, such that Rab5a was no longer present within the parasite cell but instead was found at the membrane of the host erythrocyte. Similarly, fosmidomycin treatment mislocalized Rab5c, which became diffusely localized throughout both the parasite cell and the host erythrocyte cytoplasm. Previous studies demonstrated that the antimalarial effects of fosmidomycin are rescued by medium supplementation with the downstream isoprenol geranylgeraniol, which we confirmed (see Fig. S1 in the supplemental material) (11, 12). Localizations of both Rab5a and Rab5c were restored by medium supplementation with geranylgeraniol, demonstrating that this effect of fosmidomycin was also due to inhibition of isoprenoid biosynthesis. To quantify these observations, a series of independent representative images (>50 cells under each condition) were scored on a scale of 1 to 3 (where 1 represents a typical intracellular localization and 3 represents primarily erythrocyte membrane staining) by a trained observer who was blinded to the treatment conditions (Fig. 3C). The cellular distributions of Pf-Rab5a and Pf-Rab5c were significantly altered upon fosmidomycin treatment (P < 0.001 for each [t test]).
Fig 3.
Mislocalization of Rab5 proteins by fosmidomycin treatment. (A and B) Confocal immunofluorescence with either anti-PfRab5a (A) or anti-PfRab5c (B) antibody in untreated parasites (control) compared to fosmidomycin-treated (+FSM) and fosmidomycin- and geranylgeraniol-treated (+FSM +GG-ol) parasites. FITC, fluorescein isothiocyanate. (C) Blinded scoring of >50 cells under each condition for severity of mislocalization of Rab5 (1, typical cellular punctae within parasite; 2, partial mislocalization to erythrocyte or erythrocyte membrane; 3, severe mislocalization to erythrocyte or erythrocyte membrane). ∗, P < 0.001 compared to untreated conditions.
Inhibition of isoprenoid biosynthesis causes developmental arrest during schizogony.
Our data indicate that the MEP pathway generates the isoprenyl groups for protein prenylation in P. falciparum and that candidate prenylated proteins are mislocalized upon fosmidomycin treatment. We next evaluated the biological effects of isoprenoid depletion on P. falciparum to assess whether the phenotype of fosmidomycin-treated parasites was consistent with a loss of prenylated protein function. We examined gross effects on parasite development using Giemsa staining and light microscopy of parasites with or without fosmidomycin treatment. Within the erythrocyte host cell, untreated P. falciparum parasites mature from newly invaded parasites with a typical ring morphology into multinucleated schizonts. This is followed by egress as merozoites and reinvasion, in a cycle that typically takes approximately 48 h. We found that fosmidomycin-treated parasites have normal gross development over the first 24 h but arrest in the first cell cycle as schizonts, after the onset of nuclear division but prior to segmentation of daughter merozoites (Fig. 4). To quantify the stage of this arrest, we generated highly synchronized parasite cultures and monitored DNA content throughout the development of each culture using acridine orange staining and flow cytometry. At the initial time point (time zero), each culture was highly enriched for ring-stage parasites (>95% of total cells), which had not yet begun DNA replication (pre-S phase). Control and geranylgeraniol-treated parasites completed multiple rounds of DNA replication and produced daughter parasites (returning to pre-S phase). In contrast, the majority (>80%) of fosmidomycin-treated parasites entered S phase but failed to develop further (Fig. 5). These effects of fosmidomycin were reversed by medium supplemented with a downstream isoprenol, geranylgeraniol (Fig. 5), confirming that they are specific to isoprenoid blockade. Fosmidomycin-treated parasites arrest with an average DNA content of approximately 9.1 ± 1.0 n (where n refers to the haploid DNA content of ring-stage parasites), compared to control parasites, which contain an average maximum DNA content of 15.6 ± 1.3 n (see Fig. S2 in the supplemental material).
Fig 4.
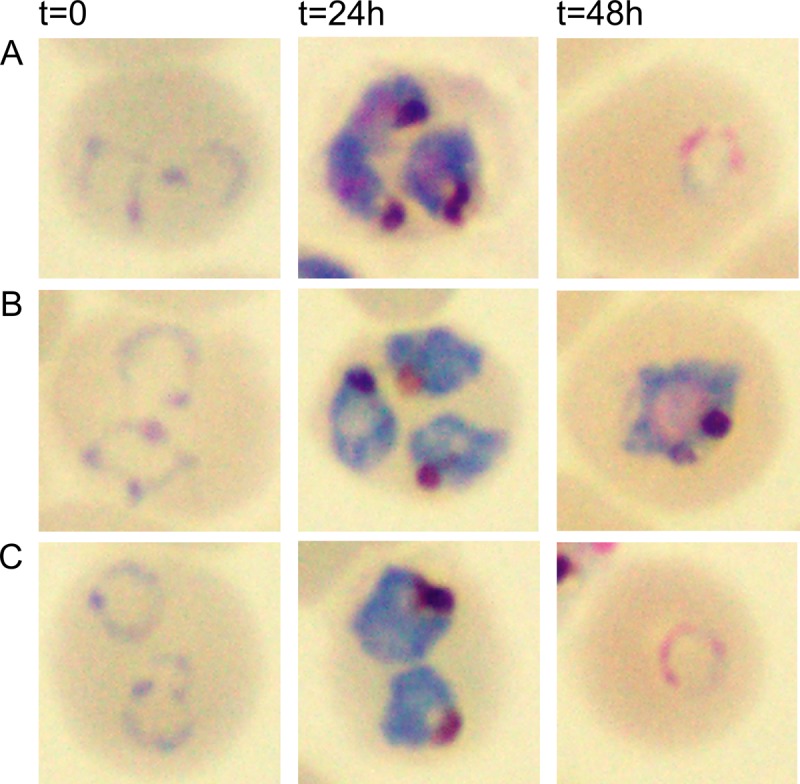
Fosmidomycin treatment causes growth arrest of malaria parasites during schizogony. Shown are Giemsa-stained light micrographs of synchronized ring-stage parasites at the indicated time points of culture for comparison of untreated parasites (A) to those treated with 5 μM fosmidomycin (B) or 5 μM fosmidomycin plus 5 μM the downstream isoprenol geranylgeraniol (C). Images of representative cells are indicative of results from at least five independent biological replicates.
Fig 5.
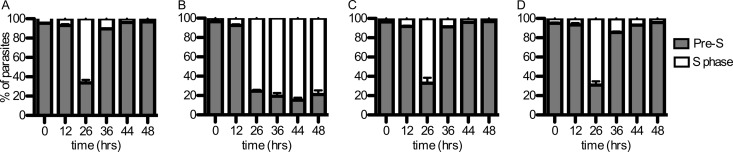
Fosmidomycin-treated parasites arrest during S phase. Shown are proportions of cells with unreplicated DNA (pre-S phase, morphologically early-ring-stage parasites, 12 h after invasion) compared to those in which DNA replication has begun (S phase), as determined by acridine orange staining and flow cytometric evaluation. Untreated parasites (A) are compared to fosmidomycin-treated parasites (B), fosmidomycin- and geranylgeraniol-treated parasites (C), and parasites with geranylgeraniol treatment alone (D). The cell cycle duration under these conditions is approximately 48 h; untreated parasites return to pre-S phase at 36 h.
Inhibition of isoprenoid biosynthesis disrupts food vacuolar morphology.
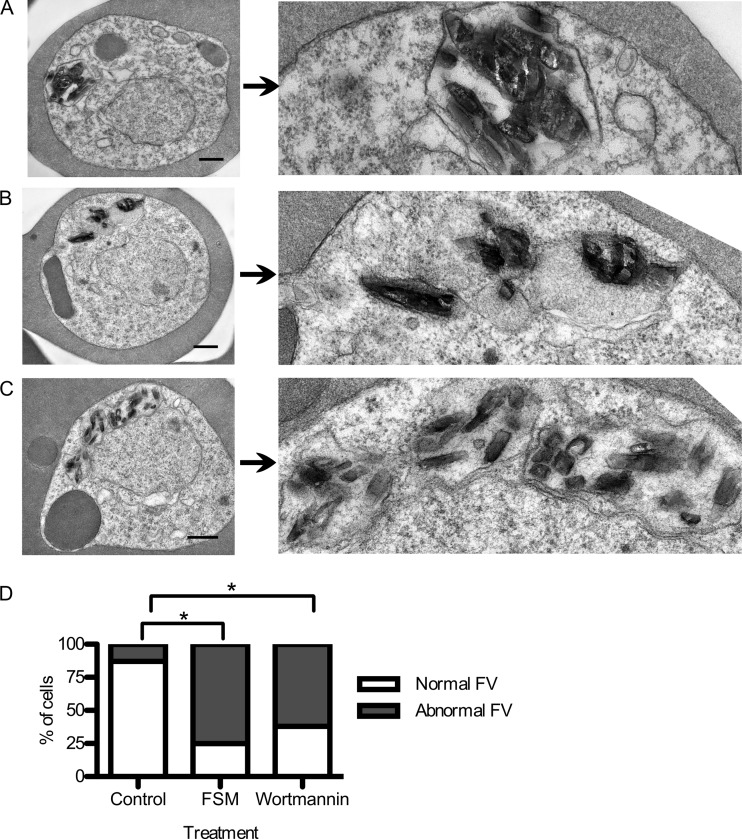
Because Rab GTPases are required for vesicular trafficking in most eukaryotic cells, we further examined the ultrastructural effects of isoprenoid biosynthesis inhibition on P. falciparum parasites. Highly synchronized cultures (enriched for ring-stage parasites) were cultured with or without fosmidomycin and then fixed at the indicated time points. Typical cellular features of P. falciparum include nuclei, mitochondria, apicoplast organelles, as well as the food vacuole (FV), where hemoglobin digestion occurs. We found that fosmidomycin-treated parasites were ultrastructurally indistinguishable from control parasites at early stages of development through early trophozoite stages (through 16 h of drug treatment [data not shown]), including normal formation and development of the digestive FV. By 24 h of treatment, however, fosmidomycin-treated parasites began to demonstrate morphological abnormalities of the FV (Fig. 6). A typical malarial FV contains a single focus of hemozoin pigment crystals (the final product of heme detoxification from hemoglobin digestion) within a distinct food vacuolar membrane. In contrast, fosmidomycin-treated parasites demonstrated a variety of abnormal FV morphologies. In less severely affected parasites, these FVs appeared as multiple discontiguous vacuoles. In more severely affected parasites, the vacuolar membrane was absent, and hemozoin crystals were free within the cellular cytoplasm.
Fig 6.
Food vacuolar defect in fosmidomycin- and wortmannin-treated parasites. (A to C) Transmission electron microscopic evaluation of control parasites (A) compared to parasites treated for 24 h with either the isoprenoid inhibitor fosmidomycin (B) or the PI3-K inhibitor wortmannin (C). On the right is a magnified view of a hemozoin-containing FV. (D) Scoring of electron micrographs of control versus fosmidomycin (FSM)- and wortmannin-treated cells. Abnormal FVs were defined as FVs that either lacked an FV membrane or contained more than one discontiguous membrane-bound collection of hemozoin (n > 25 under each condition). ∗, P < 0.001 compared to untreated conditions (Fisher's 2-tailed test).
Inhibition of phosphatidylinositol 3-kinase function also disrupts food vacuolar morphology.
The morphological effects of fosmidomycin-treated P. falciparum suggest a defect in hemoglobin trafficking in these cells. The phosphatidylinositol 3-kinase (PI3-K) inhibitor wortmannin was previously demonstrated to inhibit P. falciparum PI3-K in vitro and inhibit hemoglobin uptake in malaria parasites (37). Since Rab GTPases often function through the activation of PI3-Ks, we hypothesized that the ultrastructural abnormalities of fosmidomycin-treated parasites would be similar to those of wortmannin-treated parasites. We found that wortmannin caused a disruption in food vacuolar morphology similar to that caused by fosmidomycin. In wortmannin-treated parasites, the FV appeared to be fragmented into multiple discontiguous vacuoles (Fig. 6C). While a number of abnormal traits characterized the FV of both fosmidomycin- and wortmannin-treated cells, the most quantifiable change was the loss of FV membrane or apparent fragmentation of a single FV into multiple FVs. A series of representative electron micrographs (>25 cells under each condition) were scored for FV morphology. Abnormal FVs were defined by an absence of FV membrane or the presence of more than one focus of hemozoin. Using these criteria, FV morphology was significantly altered upon fosmidomycin and wortmannin treatments (P < 0.001 for each [t test]). While the ultrastructural effects that we observed differed slightly from what was previously reported for wortmannin-treated P. falciparum, this may reflect the superior membrane preservation achieved by the low-osmolality fixation technique used here (37).
Inhibition of isoprenoid biosynthesis or PI3-K function disrupts food vacuolar integrity.
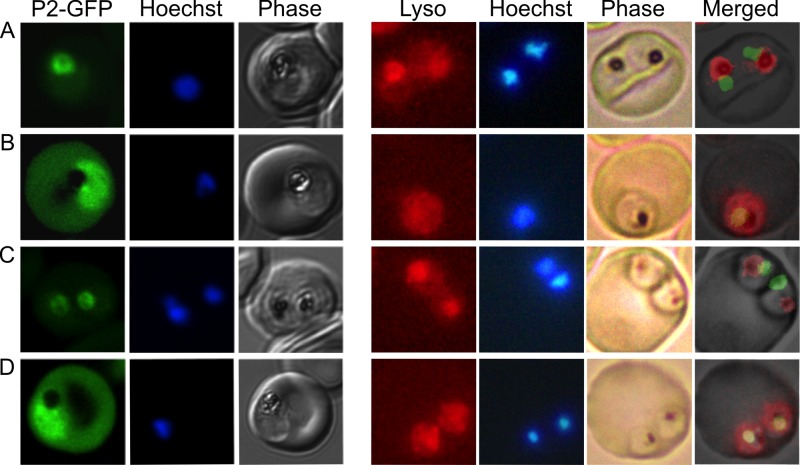
Both fosmidomycin and wortmannin treatment of P. falciparum resulted in abnormal FV morphologies, as visualized by electron microscopy. To independently assess the integrity of the FV in fosmidomycin- and wortmannin-treated parasites, we utilized a malaria parasite strain expressing the hemoglobin-digesting enzyme plasmepsin II tagged with green fluorescent protein (PMII-GFP). In malaria parasites, PMII-GFP is localized to the FV (24). Using confocal immunofluorescence, we confirmed that PMII-GFP was localized to the FV, which is distinguished microscopically in phase images by the presence of dark hemozoin pigment (Fig. 7, left). Following fosmidomycin treatment, PMII-GFP was no longer discretely localized within the FV. Instead, PMII-GFP was dispersed throughout the parasite cytoplasm, consistent with the physical disruption of the FV that was visualized by electron microscopy (Fig. 7). PMII-GFP localization was restored by medium supplementation with the isoprenoid geranylgeraniol, confirming that this effect of fosmidomycin is caused by an inhibition of isoprenoid biosynthesis. PMII-GFP was diffusely present throughout the cytoplasm of wortmannin-treated parasites, confirming that FVs are also disrupted upon PI3-K inhibition. Similar effects of fosmidomycin and wortmannin on FV integrity were confirmed in live parasite cells treated with Lysotracker Red (LR), a fluorophore that indicates acidic organelles. LR accumulated within the FV of control parasites but was dispersed throughout the cytoplasm of fosmidomycin- and wortmannin-treated parasites (Fig. 7, right). FV localization was restored in fosmidomycin-treated parasites that were rescued with medium supplementation with geranylgeraniol.
Fig 7.
Loss of food vacuolar integrity upon fosmidomycin and wortmannin treatment. Shown is the confocal fluorescence microscopic localization of a plasmepsin II-GFP (P2-GFP) construct (left) or live fluorescence imaging of Lysotracker Red-stained malaria parasites (right). Control parasites (A) are compared to parasites treated for 24 h with either fosmidomycin (B), fosmidomycin plus geranylgeraniol (C), or wortmannin (D). Images are representative of at least three independent biological experiments. Visualization of PMII-GFP in FSM- and wortmannin-treated parasites required higher detector gain levels, resulting in increased observed erythrocyte autofluorescence.
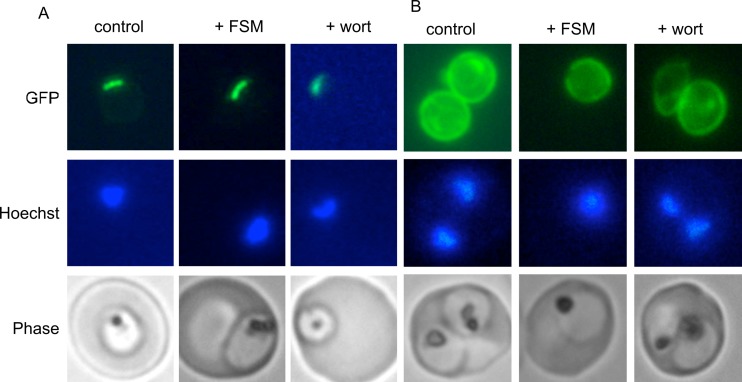
To examine the possibility that the mislocalization of PMII-GFP was due to general defects in protein traffic or membrane permeability, we evaluated the ability of drug-treated cells to appropriately localize two additional GFP fusion proteins with well-characterized localizations: the apicoplast localization sequence from acyl carrier protein (ACP), ACPL-GFP (which traffics to the apicoplast), and the signal sequence from ACP, ACPs-GFP (which is exported to the parasitophorous vacuole space) (22). Both ACPs- and ACPL-GFP were appropriately localized in both fosmidomycin- and wortmannin-treated cells (Fig. 8). ACPL-GFP fluorescence in parasites with and without drug treatments demonstrated a typical apicoplast appearance, with a long, thin, single structure early in intraerythrocytic development (38). With and without drug treatment, ACPs-GFP fluorescence was present within the parasitophorous vacuole and was frequently seen in the tubovesicular network. These results indicate that general protein trafficking mechanisms are still in place in both fosmidomycin- and wortmannin-treated cells and support that the FV phenotype and PMII-GFP localization observed in these cells are not an indirect effect of acute cellular injury.
Fig 8.
Apicoplast and parasitophorous vacuolar targeting in fosmidomycin- and wortmannin-treated parasites. Shown is live-cell fluorescence of malaria parasites that express either the leader sequence (ACPL-GFP) (A) or signal sequence (ACPs-GFP) (B) from P. falciparum acyl carrier protein, fused to GFP, which traffic to the apicoplast or parasitophorous vacuole, respectively (27). Untreated parasites (control) are compared to fosmidomycin (+FSM)- and wortmannin (+wort)-treated parasites. Images are representative of at least three independent biological experiments.
DISCUSSION
Isoprenoid biosynthesis via the nonmevalonate (MEP) pathway is required for intraerythrocytic development of P. falciparum malaria parasites. Fosmidomycin-treated parasites arrest in the first cell cycle following drug treatment, in contrast to the “delayed death” that occurs in the second cell cycle of parasites treated with other apicoplast-targeting antimalarial agents (such as clindamycin and doxycycline) (34). This is not unexpected, given that isoprenoids are expected to have multiple cellular functions outside apicoplast maintenance. We find that fosmidomycin-treated parasites successfully generate digestive food vacuoles (FVs), initiate hemoglobin digestion, and begin DNA replication prior to developmental arrest. De novo isoprenoid biosynthesis therefore does not appear to be required for these complex and energy-intensive cellular tasks, and this finding indicates a narrower role for isoprenoids in malaria cellular functions than had been suspected.
Our results suggest that the biological effects of isoprenoid inhibition by fosmidomycin in P. falciparum are in part due to an inhibition of protein prenylation. While the mitochondrial electron carrier ubiquinone is also likely essential, we find that malaria parasites that have been engineered to bypass the need for electron transport remain highly sensitive to fosmidomycin. Our results implicate at least one further essential function of isoprenoid biosynthesis in malaria parasites and are consistent with findings reported previously by Yeh and DeRisi (unpublished data described in reference11). We present the first evidence that the isoprenyl metabolites used in protein prenylation are derived from de novo isoprenoid biosynthesis, since protein prenylation is reduced by isoprenoid biosynthesis inhibition. Protein prenylation is necessary for proper protein localization and function of prenylated proteins, and indeed, we find that inhibition of isoprenoid biosynthesis causes a dramatic mislocalization of two candidate prenyltransferase substrates (Rab5a and Rab5c). Finally, inhibition of isoprenoid biosynthesis causes food vacuolar morphological defects that are consistent with a disruption of protein prenylation. Inhibition of protein prenylation directly with prenyltransferase inhibitors causes a more rapid cell cycle arrest than we observed (16); however, this is consistent with our previously reported findings that the levels of isoprenoid precursors do not change for 6 h following fosmidomycin treatment (12).
The intraerythrocytic stage of P. falciparum depends on digestion of host cell hemoglobin as its source for most amino acids (27). Hemoglobin is transported from the host cell to a specialized acidic digestive structure, the FV, where proteolysis and heme detoxification occur. Hemoglobin trafficking to the FV is a complicated, actin-dependent process, the molecular details of which have not been fully elucidated (36, 39, 40). The Rab family of small GTPases is likely to be important for this process. There are at least 11 Rab family homologs in the P. falciparum genome (41). Of these, Rab5 homologs in particular are important for early endocytosis in other eukaryotes, including other protozoans, such as the related apicomplexan Toxoplasma gondii as well as Leishmania donovani and Entamoeba histolytica (42–44). In P. falciparum, Rab5a, a prototypical early endosomal marker, has been localized to small hemoglobin-containing vesicles by immunoelectron microscopy, suggesting that these are endocytic vesicles en route to the FV (36).
Activated GTP-bound Rab5s typically function through activation of phosphatidylinositol-3 kinases (PI3-Ks) to increase local levels of phosphatidylinositol 3-phosphate [PI(3)P]. Although casein kinase physically associates with Rab5b, the downstream effectors of Rab5s in malaria are not known (30). A single essential, wortmannin-sensitive PI3-K has been described for P. falciparum, which can synthesize PI(3)P, PI(3,4)P2, and PI(3,4,5)P3 (35, 37). PI(3)P is the dominant phosphorylated phosphatidylinositol in malaria-infected erythrocytes and appears to have a large number of functional roles in malaria parasites (35). Using specific PI(3)P-binding proteins, PI(3)P has been localized to the food vacuole, the apicoplast, and the endoplasmic reticulum in malaria parasites (35, 45, 46). Surprisingly, PI(3)P binding by export signals appears to be required for malaria protein export to the host cell, and PI3-K itself is exported to the host erythrocyte (37, 45).
The growth arrest and morphological changes of fosmidomycin-treated malaria parasites may reflect multiple cellular insults. Decreased protein prenylation upon fosmidomycin treatment is likely to result in mislocalization of multiple prenyltransferase substrates, including all Rab family members. For example, a loss of Rab5 prenylation and defective trafficking to the FV could explain the ultrastructural defects observed for fosmidomycin-treated parasites (graphically represented in Fig. 9). Expression of a constitutively active Rab5a (Q102L) allele produces a bloated, enlarged FV, the opposite effect of what we observed upon fosmidomycin treatment and Rab5 mislocalization (36). In addition to the effects on food vacuolar morphology that we observed, fosmidomycin was also previously reported to interrupt apicoplast development in P. falciparum (9). The lipid PI(3)P has been localized to both the apicoplast and food vacuole, and PI3-K inhibition with wortmannin disrupts apicoplast development (35, 47) and alters food vacuolar morphology (this study). While PI(3)P signaling and isoprenoid biology are both complex, these phenotypic similarities suggest that the two pathways could converge and are consistent with the hypothesis that PI3-K may be a Rab5 effector in P. falciparum.
Fig 9.
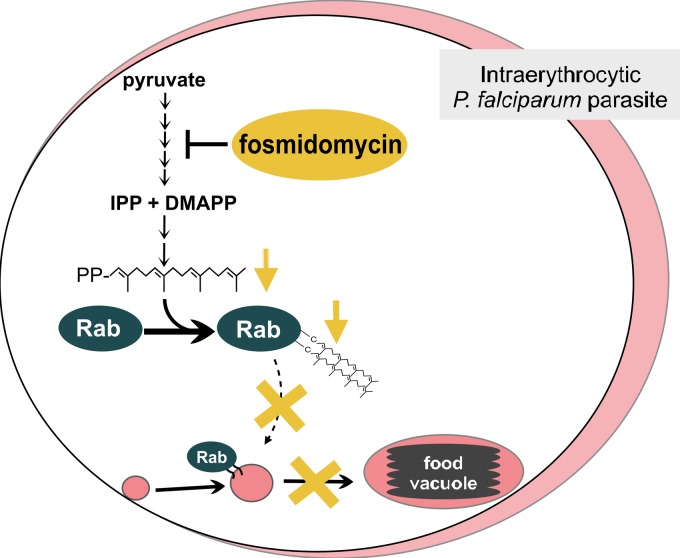
Model of fosmidomycin effects on malaria parasites. Fosmidomycin blocks isoprenoid biosynthesis and causes a defect in growth and vesicular trafficking to the food vacuole (FV). These effects are rescued by geranylgeraniol (GG-ol), indicating that the essential isoprenoids in malaria are metabolically derived from geranylgeranyl pyrophosphate (GG-PP). GG-PP is the substrate for geranylgeranyltransferase (GGTase), which modifies the endocytosis regulator Rab5 (a small GTPase) in most eukaryotes. Blocking of isoprenoid biosynthesis (with fosmidomycin) decreases protein prenylation, causes Rab5 mislocalization, and alters FV morphology. IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate.
Upon fosmidomycin treatment, both Rab5a and Rab5c are found within the host erythrocyte. This mislocalization does not appear to be due to general membrane permeability, since other protein constructs are retained within the parasite plasma membrane or parasitophorous vacuole (PMII-GFP [Fig. 7] and ACPL-GFP and ACPs-GFP [Fig. 8]). Since neither Rab5a nor Rab5c is predicted to contain a host-targeting (HT) or Plasmodium export element (PEXEL) motif, the localization of these proteins to the host cell suggests an alternate mechanism of export that is unmasked when the proteins are not tethered by prenylation. Further experimentation is required to determine whether there is a population of Rabs in P. falciparum that have biological functions within the host erythrocyte under normal growth conditions.
As novel inhibitors are developed to target nonmevalonate isoprenoid biosynthesis, an understanding of the biological consequences of isoprenoid biosynthesis inhibition in P. falciparum may inform the rational selection of secondary agents that will be useful in combination therapy. For example, since fosmidomycin-treated parasites arrest prior to completion of DNA synthesis, small molecules that act on parasite egress, such as PfSUB1 protease inhibitors (48), may be less useful alongside inhibition of isoprenoid biosynthesis. Our results suggest that parasite-specific prenyltransferase inhibitors may be particularly useful in combination antimalarial therapy with isoprenoid biosynthesis inhibitors.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID grant K08 AI079010, a Doris Duke Charitable Foundation clinical scientist development award, and the Children's Discovery Institute. Audrey R. Odom was a scholar of the Child Health Research Center of Excellence at Washington University.
We thank Wandy Beatty for technical assistance with electron microscopy. We are also grateful to Akhil Vaidya for P. falciparum strains, Gordon Langsley for antibodies, and Daniel Goldberg for P. falciparum strains and antibodies. We are grateful to Daniel Goldberg, David Hunstad, and Sebastian Lourido for critical reading of the manuscript.
Footnotes
Published ahead of print 7 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00073-12.
REFERENCES
- 1. Sachs J, Malaney P. 2002. The economic and social burden of malaria. Nature 415:680–685 [DOI] [PubMed] [Google Scholar]
- 2. Aregawi M, Cibulskis R, Kita Y, Otten H, Williams R, World Health Organization. 2010. World malaria report 2010. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3. Baird JK. 2005. Effectiveness of antimalarial drugs. N. Engl. J. Med. 352:1565–1577 [DOI] [PubMed] [Google Scholar]
- 4. Olliaro P. 2005. Drug resistance hampers our capacity to roll back malaria. Clin. Infect. Dis. 41(Suppl 4):S247–S257 doi:10.1086/430785 [DOI] [PubMed] [Google Scholar]
- 5. Dondorp A, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muller O, Sie A, Meissner P, Schirmer R. 2009. Artemisinin resistance on the Thai-Cambodian border. Lancet 374:1419 doi:10.1016/S0140-6736(09)61857-2 [DOI] [PubMed] [Google Scholar]
- 7. Cassera MB, Gozzo FC, D'Alexandri FL, Merino EF, del Portillo HA, Peres VJ, Almeida IC, Eberlin MN, Wunderlich G, Wiesner J, Jomaa H, Kimura EA, Katzin AM. 2004. The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum. J. Biol. Chem. 279:51749–51759 [DOI] [PubMed] [Google Scholar]
- 8. Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573–1576 [DOI] [PubMed] [Google Scholar]
- 9. Nair SC, Brooks CF, Goodman CD, Strurm A, McFadden GI, Sundriyal S, Anglin JL, Song Y, Moreno SNJ, Striepen B. 2011. Apicoplast isoprenoid precursor synthesis and the molecular basis of fosmidomycin resistance in Toxoplasma gondii. J. Exp. Med. 208:1547–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Odom AR, Van Voorhis WC. 2010. Functional genetic analysis of the Plasmodium falciparum deoxyxylulose 5-phosphate reductoisomerase gene. Mol. Biochem. Parasitol. 170:108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeh E, DeRisi JL. 2011. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 9:e1001138 doi:10.1371/journal.pbio.1001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang B, Watts KM, Hodge D, Kemp LM, Hunstad DA, Hicks LM, Odom AR. 2011. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 50:3570–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gershenzon J, Dudareva N. 2007. The function of terpene natural products in the natural world. Nat. Chem. Biol. 3:408–414 [DOI] [PubMed] [Google Scholar]
- 14. Labaied M, Jayabalasingham B, Bano N, Cha SJ, Sandoval J, Guan G, Coppens I. 2011. Plasmodium salvages cholesterol internalized by LDL and synthesized de novo in the liver. Cell. Microbiol. 13:569–586 [DOI] [PubMed] [Google Scholar]
- 15. Painter HJ, Morrisey JM, Vaidya AB. 2010. Mitochondrial electron transport inhibition and viability of intraerythrocytic Plasmodium falciparum. Antimicrob. Agents Chemother. 54:5281–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chakrabarti D. 2002. Protein farnesyltransferase and protein prenylation in Plasmodium falciparum. J. Biol. Chem. 277:42066–42073 [DOI] [PubMed] [Google Scholar]
- 17. Chakrabarti D, Azam T, DelVecchio C, Qiu L, Park YI, Allen CM. 1998. Protein prenyl transferase activities of Plasmodium falciparum. Mol. Biochem. Parasitol. 94:175–184 [DOI] [PubMed] [Google Scholar]
- 18. Buckner FS, Eastman RT, Yokoyama K, Gelb MH, Van Voorhis WC. 2005. Protein farnesyl transferase inhibitors for the treatment of malaria and African trypanosomiasis. Curr. Opin. Investig. Drugs 6:791–797 [PubMed] [Google Scholar]
- 19. Glenn MP, Chang SY, Horney C, Rivas K, Yokoyama K, Pusateri EE, Fletcher S, Cummings CG, Buckner FS, Pendyala PR, Chakrabarti D, Sebti SM, Gelb M, Van Voorhis WC, Hamilton AD. 2006. Structurally simple, potent, Plasmodium selective farnesyltransferase inhibitors that arrest the growth of malaria parasites. J. Med. Chem. 49:5710–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glenn MP, Chang SY, Hucke O, Verlinde CL, Rivas K, Horney C, Yokoyama K, Buckner FS, Pendyala PR, Chakrabarti D, Gelb M, Van Voorhis WC, Sebti SM, Hamilton AD. 2005. Structurally simple farnesyltransferase inhibitors arrest the growth of malaria parasites. Angew. Chem. Int. Ed. Engl. 44:4903–4906 [DOI] [PubMed] [Google Scholar]
- 21. Nallan L, Bauer KD, Bendale P, Rivas K, Yokoyama K, Hornéy CP, Pendyala PR, Floyd D, Lombardo LJ, Williams DK, Hamilton A, Sebti S, Windsor WT, Weber PC, Buckner FS, Chakrabarti D, Gelb MH, Van Voorhis WC. 2005. Protein farnesyltransferase inhibitors exhibit potent antimalarial activity. J. Med. Chem. 48:3704–3713 [DOI] [PubMed] [Google Scholar]
- 22. Waller RF, Reed MB, Cowman AF, McFadden GI. 2000. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19:1794–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Painter HJ, Morrisey JM, Mather MW, Vaidya AB. 2007. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 446:88–91 [DOI] [PubMed] [Google Scholar]
- 24. Klemba M, Beatty W, Gluzman I, Goldberg DE. 2004. Trafficking of plasmepsin II to the food vacuole of the malaria parasite Plasmodium falciparum. J. Cell Biol. 164:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahn SY, Shin MY, Kim YA, Yoo JA, Kwak DH, Jung YJ, Jun G, Ryu SH, Yeom JS, Ahn JY, Chai JY, Park JW. 2008. Magnetic separation: a highly effective method for synchronization of cultured erythrocytic Plasmodium falciparum. Parasitol. Res. 102:1195–1200 [DOI] [PubMed] [Google Scholar]
- 26. Ribaut C, Berry A, Chevalley S, Reybier K, Morlais I, Parzy D, Nepveu F, Benoit-Vical F, Valentin A. 2008. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 7:45 doi:0.1186/1475-2875-7-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. 2006. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc. Natl. Acad. Sci. U. S. A. 103:8840–8845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mamoun CB, Goldberg DE. 2001. Plasmodium protein phosphatase 2C dephosphorylates translation elongation factor 1beta and inhibits its PKC-mediated nucleotide exchange activity in vitro. Mol. Microbiol. 39:973–981 [DOI] [PubMed] [Google Scholar]
- 29. Ponpuak M, Klemba M, Park M, Gluzman IY, Lamppa GK, Goldberg DE. 2007. A role for falcilysin in transit peptide degradation in the Plasmodium falciparum apicoplast. Mol. Microbiol. 63:314–334 [DOI] [PubMed] [Google Scholar]
- 30. Rached FB, Ndjembo-Ezougou C, Chandran S, Talabani H, Yera H, Dandavate V, Bourdoncle P, Meissner M, Tatu U, Langsley G. 2012. Construction of a Plasmodium falciparum Rab-interactome identifies CK1 and PKA as Rab-effector kinases in malaria parasites. Biol. Cell 104:34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rasband WS. 1997-2012, posting date ImageJ. US National Institutes of Health, Bethesda, MD: http://imagej.nih.gov/ij/ [Google Scholar]
- 32. Fry M, Pudney M. 1992. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 43:1545–1553 [DOI] [PubMed] [Google Scholar]
- 33. Moura IC, Wunderlich G, Uhrig ML, Couto AS, Peres VJ, Katzin AM, Kimura EA. 2001. Limonene arrests parasite development and inhibits isoprenylation of proteins in Plasmodium falciparum. Antimicrob. Agents Chemother. 45:2553–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dahl EL, Rosenthal PJ. 2007. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob. Agents Chemother. 51:3485–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tawk L, Chicanne G, Dubremetz J-F, Richard V, Payrastre B, Vial HJ, Roy C, Wengelnik K. 2010. Phosphatidylinositol 3-phosphate, an essential lipid in Plasmodium, localizes to the food vacuole membrane and the apicoplast. Eukaryot. Cell 9:1519–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elliott DA, McIntosh MT, Hosgood HD, Chen S, Zhang G, Baevova P, Joiner KA. 2008. Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 105:2463–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vaid A, Ranjan R, Smythe WA, Hoppe HC, Sharma P. 2010. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood 115:2500–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Foth BJ, Ralph SA, Tonkin CJ, Struck NS, Fraunholz M, Roos DS, Cowman AF, McFadden GI. 2003. Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum. Science 299:705–708 [DOI] [PubMed] [Google Scholar]
- 39. Lazarus MD, Schneider TG, Taraschi TF. 2008. A new model for hemoglobin ingestion and transport by the human malaria parasite Plasmodium falciparum. J. Cell Sci. 121:1937–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smythe WA, Joiner KA, Hoppe HC. 2008. Actin is required for endocytic trafficking in the malaria parasite Plasmodium falciparum. Cell. Microbiol. 10:452–464 [DOI] [PubMed] [Google Scholar]
- 41. Ward GE, Tilney LG, Langsley G. 1997. Rab GTPases and the unusual secretory pathway of Plasmodium. Parasitol. Today 13:57–62 [DOI] [PubMed] [Google Scholar]
- 42. Robibaro B, Stedman TT, Coppens I, Ngo HM, Pypaert M, Bivona T, Nam HW, Joiner KA. 2002. Toxoplasma gondii Rab5 enhances cholesterol acquisition from host cells. Cell. Microbiol. 4:139–152 [DOI] [PubMed] [Google Scholar]
- 43. Saito-Nakano Y, Yasuda T, Nakada-Tsukui K, Leippe M, Nozaki T. 2004. Rab5-associated vacuoles play a unique role in phagocytosis of the enteric protozoan parasite Entamoeba histolytica. J. Biol. Chem. 279:49497–49507 [DOI] [PubMed] [Google Scholar]
- 44. Singh SB, Tandon R, Krishnamurthy G, Vikram R, Sharma N, Basu SK, Mukhopadhyay A. 2003. Rab5-mediated endosome-endosome fusion regulates hemoglobin endocytosis in Leishmania donovani. EMBO J. 22:5712–5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhattacharjee S, Stahelin RV, Speicher KD, Speicher DW, Haldar K. 2012. Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell. Cell 148:201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McIntosh MT, Vaid A, Hosgood HD, Vijay J, Bhattacharya A, Sahani MH, Baevova P, Joiner KA, Sharma P. 2007. Traffic to the malaria parasite food vacuole: a novel pathway involving a phosphatidylinositol 3-phosphate-binding protein. J. Biol. Chem. 282:11499–11508 [DOI] [PubMed] [Google Scholar]
- 47. Tawk L, Dubremetz J-F, Montcourrier P, Chicanne G, Merezegue F, Richard V, Payrastre B, Meissner M, Vial HJ, Roy C, Wengelnik K, Lebrun M. 2011. Phosphatidylinositol 3-monophosphate is involved in Toxoplasma apicoplast biogenesis. PLoS Pathog. 7:e1001286 doi:10.1371/journal.ppat.1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gemma S, Giovani S, Brindisi M, Tripaldi P, Brogi S, Savini L, Fiorini I, Novellino E, Butini S, Campiani G, Penzo M, Blackman MJ. 2012. Quinolylhydrazones as novel inhibitors of Plasmodium falciparum serine protease PfSUB1. Bioorg. Med. Chem. Lett. 22:5317–5321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.