Abstract
The life cycles of apicomplexan parasites such as Plasmodium spp. and Toxoplasma gondii are complex, consisting of proliferative and latent stages within multiple hosts. Dramatic transformations take place during the cycles, and they demand precise control of gene expression at all levels, including translation. This review focuses on the mechanisms that regulate translational control in Plasmodium and Toxoplasma, with a particular emphasis on the phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2α). Phosphorylation of eIF2α (eIF2α∼P) is a conserved mechanism that eukaryotic cells use to repress global protein synthesis while enhancing gene-specific translation of a subset of mRNAs. Elevated levels of eIF2α∼P have been observed during latent stages in both Toxoplasma and Plasmodium, indicating that translational control plays a role in maintaining dormancy. Parasite-specific eIF2α kinases and phosphatases are also required for proper developmental transitions and adaptation to cellular stresses encountered during the life cycle. Identification of small-molecule inhibitors of apicomplexan eIF2α kinases may selectively interfere with parasite translational control and lead to the development of new therapies to treat malaria and toxoplasmosis.
INTRODUCTION
Malaria is a devastating disease, with mortality in adults being higher than previously estimated. Malaria killed 1.24 million people in 2010 alone (1). Human malaria, transmitted by Anopheles mosquitoes, is caused by four species of Plasmodium, Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale. Infection with the monkey malaria parasite Plasmodium knowlesi has been reported but remains rare. Malaria parasites undergo complex intracellular and extracellular stages in invertebrate vectors and vertebrate hosts (Fig. 1). The transition from one stage of the life cycle to the next is tightly regulated by gene expression at the level of epigenetics, transcription, translation, and posttranslational modification (2–4). These multiple levels of gene expression control help to ensure that parasite proliferation and differentiation take place appropriately within the diverse environments encountered by Plasmodium.
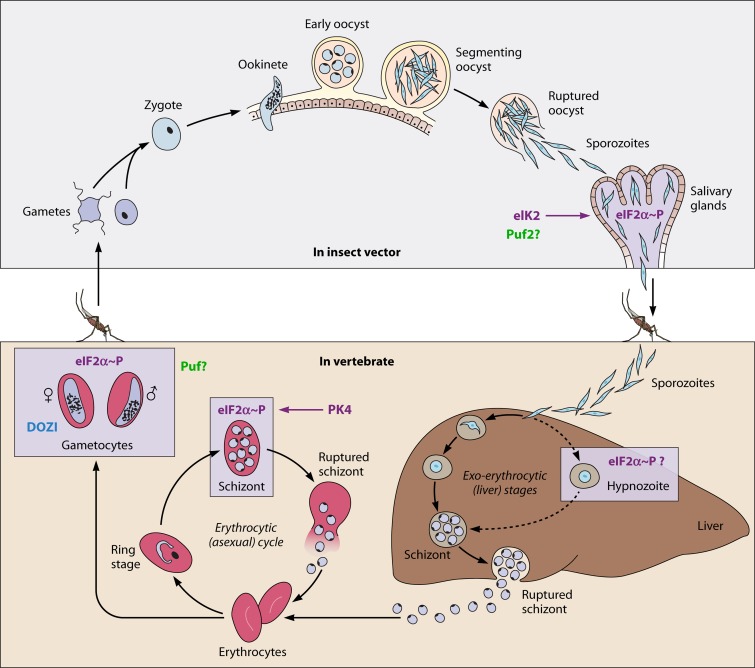
Fig 1.
Translational repression in the life cycle of Plasmodium. P. falciparum gametocytes maintain their infectivity for periods as long as 22 days (72). DOZI, a DDX6-class RNA helicase, binds to the ribonucleoprotein complex in female gametocytes and represses translation. These untranslated mRNAs are stored for translation after fertilization. RNA-binding proteins (Puf proteins) that repress translation by binding to the mRNA 3′ untranslated region are specifically upregulated in gametocytes (Puf1 and Puf2) (27, 28) and sporozoites (Puf2) (46, 47). The phosphorylation of eIF2α in salivary gland sporozoites, mature schizonts, and gametocytes induces translational repression and storage of untranslated mRNAs as stress granules (42, 52). Plasmodium sporozoites maintain infectivity for days in the salivary glands (69, 70). EIF2α kinase eIK2 controls the latency of sporozoites (42). Another eIF2α kinase, PK4, leads to the arrest of global protein synthesis in schizonts and is essential for the development of the erythrocytic cycle (52). In P. vivax and P. ovale, hypnozoites can persist in the liver for years and cause relapses (71). The mechanism behind the development into dormancy and the activation of hypnozoites is not known.
Toxoplasma gondii switches between proliferative (tachyzoite) and latent (bradyzoite) stages within its human host, giving rise to acute and chronic infection, respectively (5). Toxoplasma can chronically infect virtually any warm-blooded vertebrate, and the bradyzoite tissue cysts bestow a unique ability to transmit to new hosts through predation. In addition, infectious oocysts can be excreted by the definitive feline host (Fig. 2). Chronic infections with Toxoplasma are widespread among humans, placing individuals who become immunocompromised at great risk for recrudescence of acute infections. During acute infection, the parasite is also capable of crossing the placental barrier, a process which can result in spontaneous abortion or birth defects that include hydrocephalus, encephalitis, mental retardation, and ocular disease (6).
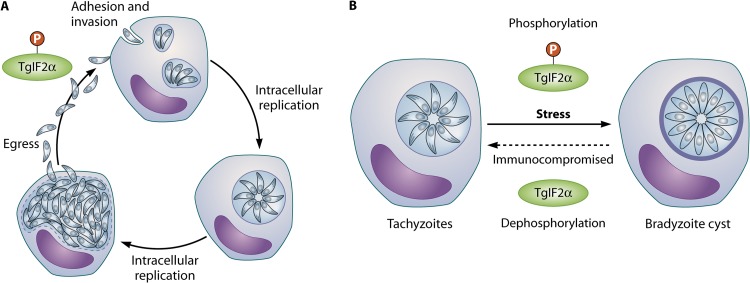
Fig 2.
Proliferation and development of Toxoplasma. (A) The Toxoplasma tachyzoite lytic cycle consists of the following three basic steps: attachment and invasion of a host cell, intracellular replication, and egress into the extracellular environment. Following egress, the parasites will repeat the process by invading a neighboring host cell and continuing to proliferate in the infected tissues. (B) During a primary infection, Toxoplasma tachyzoites quickly develop into latent bradyzoites, which are encased in a thick cyst wall inside host cells. Conversion from tachyzoite to bradyzoite can be mimicked in vitro through the application of cellular stress (e.g., heat shock, oxidative). In vivo, cyst generation and maintenance are reliant on the host immune response. Formation of bradyzoite cysts causes a chronic, incurable infection, as they can persist within the host indefinitely. If the host becomes immunocompromised, bradyzoites can reconvert to replicating tachyzoites, resulting in tissue destruction in cyst-prone areas of the body, such as the brain and heart. Dark-purple oval designates host cell nucleus.
TRANSLATIONAL CONTROL AND THE CELLULAR STRESS RESPONSE
During translation initiation, Met-tRNA interference (tRNAi), together with GTP, eukaryotic initiation factors (eIF), and the small ribosomal subunit (40S), forms a complex with the mRNA. eIF2 is composed of α (eIF2α), β, and γ subunits. An inactive eIF2-GDP leaves the 40S ribosomal subunit after translation initiation. eIF2-GDP is exchanged to eIF2-GTP by a guanine nucleotide exchange factor (eIF2B), at which point a new round of translation is able to commence. In response to cellular stress, phosphorylation of a regulatory serine residue on eIF2α (Ser51 in yeast and mammals) increases the binding affinity of eIF2 to eIF2B, favoring the formation of an inactive eIF2-eIF2B complex. eIF2B is present in limited amounts compared to eIF2; therefore, a small increase in phosphorylation of eIF2α (eIF2α∼P) blocks the recycling of eIF2-GTP and downregulates global protein synthesis (7). However, in mammalian cells, a subset of mRNAs, including those that code for “master regulator” activating transcription factor 4 (ATF4) or general control nonderepressible 4 (GCN4) in metazoans or yeast, respectively, can be preferentially translated when eIF2α is phosphorylated. The preferential translation of ATF4 and GCN4 allows cells to alleviate stress damage by reprogramming gene expression (8–10). In mammalian cells, there are four signature eIF2α kinases activated upon exposure to different kinds of cellular stresses: GCN2 (general control nonderepressible 2) responds to amino acid deprivation, PERK (protein kinase PKR-like endoplasmic reticulum kinase) responds to endoplasmic reticulum (ER) stress, PKR (protein kinase R) responds to viral double-stranded RNA (dsRNA), and HRI (heme-regulated inhibitory kinase) responds to heme deficiency (10).
Translationally inactive mRNAs accumulate in stress granules, composed of stalled preinitiation complexes, or processing bodies (P bodies) associated with mRNA decay. P bodies and stress granules are morphologically indistinguishable nonmembranous cytoplasmic structures ranging in size from 0.1 to 2.0 μm. Both granules are in dynamic equilibrium with the translational pool, allowing rapid shifts between translation, storage, and decay (11). Stress granules are characterized by the presence of 40S ribosomal subunits, poly(A)-binding protein (PABP), and a subset of translation initiation factors (eIF2, eIF3, eIF4E, and eIF4G). In contrast, P bodies contain mRNAs associated with the decapping machinery (12, 13).
TRANSLATION IN THE PARASITE MITOCHONDRIA AND APICOPLAST
In Plasmodium and Toxoplasma, RNA translation takes place in three subcellular compartments: the parasite cytosol, the single tubular mitochondrion, and a relict plastid called the apicoplast (14). While thousands of genes encoded in Apicomplexa genomes are translated in the cytoplasm, fewer than 50 are translated in the apicoplast and only 3 are translated in the mitochondrion. Toxoplasma and Plasmodium are susceptible to prokaryotic translation inhibitors, including clindamycin, macrolides, and tetracyclines, as these drugs interfere with translation in the bacterium-derived apicoplast (15, 16). Mutagenesis experiments have confirmed that clindamycin-resistant parasites result from a mutation within apicoplast rRNA that prevents clindamycin binding (17). Interestingly, the mitochondrial genome lacks aminoacyl-tRNA genes, and charged tRNAs must be imported into the parasite mitochondria (18, 19). Nevertheless, translation in the mitochondrion is active and essential for parasite survival (20, 21). While translational regulation in the mitochondrion and apicoplast is essential for parasite viability and an intriguing area of investigation, the remainder of this review will focus on cytoplasmic control of protein synthesis and its role in regulating stress response and developmental transitions in Plasmodium and Toxoplasma.
TRANSLATIONAL CONTROL IN PLASMODIUM SEXUAL STAGES
In contrast to other eukaryotes, the structure of rRNA differs among the various stages of the Plasmodium life cycle. The A type of rRNA is associated with the asexual blood stages and with liver stages, whereas the O and S types of rRNA predominate in mosquito stages (22). These striking structural changes suggest that rRNAs employed during the insect and mammalian stages may have varied functional properties, but confirmation of this idea awaits further investigation. It has been shown that the life cycles in mosquitoes and rodents of Plasmodium berghei parasites lacking the S-type genes are indistinguishable from those of the wild type, so it remains unclear why this unique organization of rRNA genes has been maintained in Plasmodium evolution (23).
Comparisons of Plasmodium transcriptomes and proteomes show that gene silencing via translational repression of mRNA is a frequent event during the life cycle. This was first described during development of the parasite's sexual stages. Male and female gametocytes circulate in the blood of the mammalian host. After being ingested by Anopheles mosquitoes, they differentiate into gametes and fertilization takes place in the lumen of the insect midgut. Soon the zygotes transform into the motile ookinetes that leave the hostile proteolytic environment of the lumen and take refuge in the wall of the mosquito stomach. Repression of mRNA translation encoding a surface protein of P. berghei (Pb21) was first documented during sexual development of gametocytes (24–26). Although the Pb21 mRNA is expressed in female gametocytes circulating in the vertebrate host, the synthesis of Pb21 protein starts only in the insect vector. Posttranscriptional silencing of several additional messages was revealed by comparing transcriptomes and proteomes of Plasmodium, suggesting that translational repression is an important regulatory mechanism (27). Notably, the 3′ untranslated regions of several repressed mRNAs contain a nucleotide motif known to bind to Pumilio (Puf) proteins, which repress translation and regulate RNA stability (27–29). The nontranslated mRNAs accumulate in cytoplasmic granules that contain an RNA-binding helicase named DOZI. The central role of DOZI in parasite development was revealed in a DOZI knockout. The mutants progressed normally through the blood cycle, but the complete absence in the female gametocytes led to an arrest of parasite development in mosquitoes (3). Analysis of the cytoplasmic granules in female gametocytes showed that they contain nontranslated mRNAs, eIF4E, and PABP but lack the RNA degradation elements that are characteristic of P bodies in higher eukaryotes (30).
TRANSLATIONAL REGULATION OF PLASMODIUM SPOROZOITES
Thirty or more genes are upregulated in salivary gland sporozoites (UIS genes) compared to midgut sporozoites (31, 32). Among them, UIS3 and UIS4 are highly transcribed in the salivary glands, but the corresponding proteins are barely detectable (33). Both genes are expressed instead in early liver stages (34, 35). UIS3 is localized to the parasitophorous vacuolar membrane (PVM) surrounding the liver stage parasites. UIS3 binds to liver fatty acids and must participate in the acquisition of lipids that are essential for the growing parasite (36). UIS4 encodes a small transmembrane localized in the PVM. In its absence, the late liver stages do not generate efficient blood stage infections (37).
Several other transcripts that accumulate in salivary gland sporozoites are translated only later in the life cycle. For example, liver stage-associated proteins 1 and 2 (LSAP-1/PFL0065w and LSAP-2/PFB0105c, respectively) are among the most abundant transcripts in the salivary gland transcriptome (33) but have not been detected in proteomic surveys of sporozoites (38, 39). Rather, these genes are translated only in liver stages (40). In contrast, the translation of the abundant proteins CSP and TRAP from the salivary gland sporozoites starts in oocyst sporozoites (41). In fact, a better correlation exists between levels of transcripts in oocyst sporozoites and protein abundance in salivary gland sporozoites than between transcripts and proteins from the salivary gland (42).
Three eIF2α serine/threonine kinases, eIK1, eIK2, and PK4, have been identified in Plasmodium (43). They are transcriptionally controlled. eIK2 is predominantly transcribed in salivary gland sporozoites. eIK1 and PK4 are mostly transcribed in the asexual blood stages (42). eIK2, also called UIS1, is the most upregulated gene in salivary sporozoites relative to sporozoites from the midgut, in which its transcription is undetectable (31). eIK2 phosphorylates parasite eIF2α and inhibits global translation in the salivary sporozoites. Many stalled mRNAs encode proteins that are required during development of early-liver-stage forms. The nontranslated messages accumulate in the cytoplasm of the parasites as “stress” or RNA granules.
In the P. berghei eIK2 knockout (PbeIK2−) sporozoites, eIF2α is not phosphorylated and translational repression is alleviated (42). The RNA granules disappear, the stalled liver stage messages are translated, and the salivary sporozoites prematurely transform into early liver stages. The sporozoite's inner membrane complex disassembles, the organelles redistribute in the cytoplasm, and a bulb-like structure appears in the center of the mutant parasite. The radical remodeling is proteasome dependent (42). The PbeIK2− sporozoites do not glide, and they lose infectivity. Comparison of transcribed genes between PbeIK2− sporozoites and the wild type showed that many genes found predominantly in liver stages, including some in the ubiquitin pathway, are upregulated in the mutant. This transformation of sporozoites into liver stages can start by incubation of the parasites at 37°C in the presence of serum (44).
Under physiological conditions, the latency of salivary sporozoites is disrupted only after their introduction into the mammalian host, when a phosphatase must be activated to dephosphorylate eIF2α∼P. The parasite phosphatase has not been identified, but its activity can be inhibited by salubrinal, as first described for the eIF2α∼P phosphatases in mammals (42, 45). Treatment of wild-type salivary sporozoites with salubrinal increases the phosphorylation of parasite eIF2α, implying that the phosphatase is present in salivary sporozoites. However, the kinase activity is predominant and leads to the latent state. It has been suggested that the phosphatase in the salivary glands may be repressed by the RNA-binding protein Puf-2. Indeed, when Puf2 is knocked out in P. berghei, the sporozoites start transforming into liver stages while inside the mosquito salivary glands (46, 47).
Activation of Plasmodium sp. IK2 begins when midgut sporozoites enter the salivary sporozoites. This life cycle transition does not involve an obvious stress. However, the shift from a nutrient-rich midgut environment to the salivary glands may deprive the parasite of essential metabolic factors.
TRANSLATIONAL REPRESSION IN PLASMODIUM ASEXUAL ERYTHROCYTIC STAGES
The few sporozoites that are injected into the host by mosquitoes develop into the clinically silent liver (or exoerythrocytic) stages. After a few days, a large number of membrane-bound sacs, named merosomes, containing infective merozoites, are released into the blood. The merozoites rapidly invade erythrocytes and begin the asexual intraerythrocytic life cycle that gives rise to malarial symptoms (48). The metabolism of the blood stages is largely dependent on the degradation of host cell hemoglobin, which produces a toxic monomeric α-hematin by-product. The hematin is a pro-oxidant and catalyzes the production of reactive oxygen species. Oxidative stress is believed to be generated during the conversion of heme to hematin. Free hematin can also bind and disrupt cell membranes, damaging cell structures and causing the lysis of the host erythrocyte (49). The malaria parasite detoxifies the hematin by biocrystallization, which converts it into insoluble and chemically inert β-hematin crystals (called hemozoin) (50, 51). Consequently, pigment appears as the parasite grows: it is absent in the ring stage and becomes detectable in the late trophozoite and the schizont.
Protein synthesis is more active in the P. berghei ring stage and young trophozoite, while translation is repressed in mature schizont. This is in accordance with the heavy phosphorylation of eIF2α in P. berghei schizonts and the accumulation of stalled mRNAs in stress granules (52). A schizont is the intraerythrocytic parasite that is undergoing or has undergone repetitive nuclear division. Nevertheless, the synthesis of some of the molecules needed for parasite multiplication, including DNA, starts in the trophozoite stage (53, 54).
The translational repression in schizonts is mediated by the eIF2α kinase PK4 (52, 55). Independent research groups have reported that PK4 is essential for the erythrocytic cycle in P. berghei and P. falciparum (52, 56). This conclusion is strongly supported by mutagenesis studies of the regulatory phosphorylation site Ser59 of eIF2α in Plasmodium, which corresponds to Ser51 of yeast/human eIF2α. Blood stage parasites bearing eIF2α Ser59Ala (nonphosphorylatable) or Ser59Asp (mimics a phosphorylated serine) mutations are not viable. However, Ser59Thr mutant parasites complete their life cycles normally (52). The implication of these studies is that drugs that inhibit PK4 are likely to alleviate malaria disease and inhibit transmission.
IMPORTANCE OF TRANSLATION CONTROL IN TOXOPLASMA
Proteomic surveys have demonstrated that mRNA levels are not necessarily an accurate predictor of protein levels in Toxoplasma tachyzoites (57, 58). A large number of genes that are not detectable by microarray analysis and/or are in expressed sequence tag (EST) libraries can be detected at a protein level. Likewise, many abundant transcripts are not detected by current proteomic methodologies, suggesting that posttranscriptional regulation, including translational control, is an important factor regulating the proteome. Furthermore, proteomic analysis revealed that an attenuated parasite strain had markedly reduced levels of an eIF4A isoform relative to levels detected in the mouse-virulent strain (59). eIF4A is a DEAD box RNA helicase that facilitates translation initiation by unwinding the secondary structure within the 5′ untranslated region of the transcript to allow for ribosomal scanning. Reduced eIF4A in attenuated parasites suggests that eIF4A may be required for the translation of virulence factors.
TRANSLATIONAL CONTROL DURING THE TOXOPLASMA LYTIC CYCLE
Cellular stress causes the phosphorylation of the Toxoplasma eIF2α homologue (TgIF2α) on a regulatory serine residue, Ser71, which is analogous to Ser59 in Plasmodium as well as Ser51 in yeast and humans (10, 52, 60). In contrast to Plasmodium, Toxoplasma tachyzoites harboring a point substitution at Ser71 to alanine (S71A) are viable (52, 61). However, parasites deficient in stress-induced TgIF2α phosphorylation (S71A-TgIF2α) showed a pronounced reduction in viability and slowed progression through the lytic cycle in the absence of exogenous cellular stress (61). Toxoplasma tachyzoites rapidly progress through a virus-like lytic cycle in any nucleated cell, which includes adherence and invasion of a host cell, multiple rounds of intracellular replication, and egress from the host cell (Fig. 2). Upon release into the extracellular milieu, the parasites search for a nearby host cell to repeat the process. Studies have shown that S71A-TgIF2α mutant parasites are ill-equipped to cope with the extracellular environment following egress from the host cell. Consequently, S71A-TgIF2α parasites suffer reduced viability in the extracellular environment and slowed replication following infection of a new host cell (61). Furthermore, parasites lacking the GCN2-like eIF2α kinase called TgIF2K-D fail to recover from extracellular stress as efficiently as the wild type, a defect that was also observed for the S71A-TgIF2α mutant (62). Parasites lacking TgIF2K-D fail to phosphorylate TgIF2α during extracellular stress yet can still phosphorylate TgIF2α in response to ionophore treatment (62). It is well established that GCN2 eIF2α kinases are activated by nutrient deprivation, making it likely that nutrient starvation is the primary stress experienced by parasites outside their host cells. These results suggest that stress-induced TgIF2α phosphorylation and translational control are critical for Toxoplasma to conserve resources and to reprogram gene expression during conditions of nutrient stress.
Cytoplasmic ribonucleoprotein (RNP) granules containing the hallmark stress granule marker PABP were observed in parasites soon after host cell egress (63). The primary cause for the formation of these RNP granules is the change in the ionic conditions that occur when the parasites egress from the parasitophorous vacuole (63). Parasites released from the host cell in a high K+ buffer (which mimics the ionic conditions within the host cytosol) triggered the formation of stress granules at a higher rate and frequency than parasites released in a high Na+ buffer (which mimics the ionic conditions in the extracellular medium). Interestingly, numerous reports demonstrated that high K+ causes an increase in global protein synthesis, suggesting that during “externally triggered egress,” a dynamic shift in translation results in the repression of a subset of mRNAs (which are targeted to stress granules) while allowing for the enhanced translation of mRNAs that are likely to be important for parasite survival upon exiting the host cell (64, 65). This is supported by the fact that granule-forming parasites demonstrated higher viability and an increased invasion and growth rate compared to non-granule-forming parasites (63). Exogenous cellular stress (sodium arsenite) and pharmacological agents that repress translation (salubrinal) also increase the size and number of these granules in extracellular parasites.
P bodies have not been well characterized in Toxoplasma, but a homologue of Argonaute (TgAGO) was localized to punctuate regions within the parasite cytosol that copurified with conventional P body markers, including numerous DEAD box helicases and a KH-type splicing regulatory protein (KSRP) that facilitates RNA decay (66). TgAGO did not appear to colocalize with the RNP granules observed in extracellular parasites, suggesting that stress granules and P bodies represent distinct sites of translation suppression and mRNA decay (63). Given the role of Puf proteins in Plasmodium RNP granules, it is likely that similar RNA-binding proteins regulate the repression and decay of mRNAs in Toxoplasma. Toxoplasma possesses two predicted Puf-containing proteins in its genome, but they have not been characterized to date.
Toxoplasma tachyzoites possess a PERK/PEK-like eIF2α kinase called TgIF2K-A that localizes to the parasite ER and is regulated through an association with the ER-resident chaperone BiP/GRP78 (67). Tunicamycin is a potent inducer of ER stress through its inhibition of N-linked protein glycosylation in the ER, and it induces robust phosphorylation of TgIF2α and bradyzoite formation in vitro (67). TgIF2K-B is another eIF2α kinase in Toxoplasma, and it appears to be unique to this parasite, devoid of any signature activation domain found on eIF2α kinases in other species. TgIF2K-C is a second GCN2-like eIF2α kinase, but its role has yet to be elucidated. It appears that TgIF2K-C is not entirely redundant to TgIF2K-D, as the TgIF2K-D knockout is defective in its response to extracellular stress (62). The expanded number of eIF2α kinases in these apicomplexan parasites is notable and may be reflective of their complex life cycles, which involve exposure to a multitude of different environments and potential stresses.
TRANSLATIONAL CONTROL AND BRADYZOITE DEVELOPMENT IN TOXOPLASMA
Central to pathogenesis and transmission is the ability of Toxoplasma to convert from proliferative tachyzoites to latent bradyzoite cysts. The development and maintenance of bradyzoites during the primary infection may occur in response to various cellular stresses encountered in the new host, such as heat shock (fever), oxidative stress (reactive oxygen species produced from immune effector cells), and nutrient deprivation caused by gamma interferon (IFN-γ) stimulation of host macrophage (5). It was established that stresses, including those that trigger bradyzoite development, induce the phosphorylation of TgIF2α (60). Additionally, mature bradyzoite cysts induced in vitro have significantly elevated levels of TgIF2α∼P compared to those of tachyzoites (67). These data indicate that translational control mediated by TgIF2α∼P may be important for the development and maintenance of bradyzoite cyst forms. Consistent with this idea, we found that salubrinal, a pharmacological inhibitor of TgIF2α dephosphorylation, leads to increased levels of phosphorylated TgIF2α, activation of bradyzoite gene expression, and cyst wall formation (67). These findings implicate translational control as an important factor involved in the establishment of chronic toxoplasmosis. While the specific kinase(s) required for the development of bradyzoite cysts remains unclear, TgIF2K-B is an attractive candidate, as it shares the highest degree of sequence homology with IK2, which is required for regulating developmental transitions of Plasmodium.
It is of great interest to determine the mRNAs that are preferentially translated during the developmental transition to form bradyzoites. This may be achieved through the purification of polyribosomal fractions from parasites exposed to various bradyzoite induction triggers over a time course. The mRNAs may be identified from polysomes via hybridization to microarrays or through ribosome profiling techniques (68). Similar approaches may be employed to identify preferentially translated mRNAs during key transitions during the Plasmodium life cycle.
CONCLUSIONS AND OUTLOOK
Plasmodium and Toxoplasma belong to Apicomplexa, a phylum of diverse obligate intracellular parasites that includes a number of medically and agriculturally significant pathogens. Translational repression mediated by phosphorylation of eIF2α plays important roles in Plasmodium and Toxoplasma life cycles. Elevated levels of eIF2α∼P have been observed in vitro when Toxoplasma tachyzoites are subjected to stress and transform into bradyzoites. These are latent tissue cyst forms and can reconvert back into the rapidly replicating tachyzoites. Bradyzoites also appear spontaneously during culture of tachyzoites. The mechanisms that lead to bradyzoite formation in vivo are not clear, but they are maintained in a latent stage by the immune system. Plasmodium eIF2α is highly phosphorylated in sporozoites, which are quiescent and persist in mosquito salivary glands for the long term without significantly changing their transcriptional program while maintaining their infectivity (69, 70). We do not know the nature of the physiological signal that triggers the stress response in salivary gland sporozoites. Perhaps there are insufficient nutrients in the gland or the parasites are subjected to oxidative stress. Elevated levels of eIF2α∼P have also been observed in Plasmodium gametocytes and mature schizonts. In these stages, the host erythrocyte hemoglobin is likely expended, leading to a nutritional stress.
The hypnozoites are dormant hepatic forms present in P. vivax and P. ovale. Some hypnozoites can remain dormant in the liver for months or even years before reactivation. There is no morphological marker for hypnozoites, but it is assumed that they are small round forms. After reactivation, they transform into normal liver stages and release merozoites that infect the patient's red blood cells, causing relapse, clinically indistinguishable from the primary attacks (71). Arrest of translation is a conserved mechanism used by Apicomplexa (Plasmodium and Toxoplasma) to achieve latency. Therefore, it is not farfetched to speculate that the phosphorylation of eIF2α is involved in the generation of the still-enigmatic dormant stages of the malaria parasite of humans, P. vivax.
ACKNOWLEDGMENTS
Support for this research was made possible through funding from the National Institutes of Health (AI077502 and AI084031 to W.J.S. and AI093271 to B.R.J.) and the American Heart Association (0920034G to B.R.J.).
Footnotes
Published ahead of print 14 December 2012
REFERENCES
- 1. Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. 2012. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431 [DOI] [PubMed] [Google Scholar]
- 2. Hakimi MA, Deitsch KW. 2007. Epigenetics in Apicomplexa: control of gene expression during cell cycle progression, differentiation and antigenic variation. Curr. Opin. Microbiol. 10:357–362 [DOI] [PubMed] [Google Scholar]
- 3. Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, Khan SM, Dimopoulos G, Janse CJ, Waters AP. 2006. Regulation of sexual development of Plasmodium by translational repression. Science 313:667–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Painter HJ, Campbell TL, Llinas M. 2011. The Apicomplexan AP2 family: integral factors regulating Plasmodium development. Mol. Biochem. Parasitol. 176:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sullivan WJ, Jr, Jeffers V. 2012. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol. Rev. 36:717–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976 [DOI] [PubMed] [Google Scholar]
- 7. Van Der Kelen K, Beyaert R, Inze D, De Veylder L. 2009. Translational control of eukaryotic gene expression. Crit. Rev. Biochem. Mol. Biol. 44:143–168 [DOI] [PubMed] [Google Scholar]
- 8. Mueller PP, Hinnebusch AG. 1986. Multiple upstream AUG codons mediate translational control of GCN4. Cell 45:201–207 [DOI] [PubMed] [Google Scholar]
- 9. Vattem KM, Wek RC. 2004. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 101:11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wek RC, Jiang HY, Anthony TG. 2006. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34:7–11 [DOI] [PubMed] [Google Scholar]
- 11. Anderson P, Kedersha N. 2009. Stress granules. Curr. Biol. 19:R397–R398 [DOI] [PubMed] [Google Scholar]
- 12. Balagopal V, Parker R. 2009. Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr. Opin. Cell Biol. 21:403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kedersha N, Anderson P. 2007. Mammalian stress granules and processing bodies. Methods Enzymol. 431:61–81 [DOI] [PubMed] [Google Scholar]
- 14. Jackson KE, Habib S, Frugier M, Hoen R, Khan S, Pham JS, Ribas de Pouplana L, Royo M, Santos MA, Sharma A, Ralph SA. 2011. Protein translation in Plasmodium parasites. Trends Parasitol. 27:467–476 [DOI] [PubMed] [Google Scholar]
- 15. Dahl EL, Rosenthal PJ. 2008. Apicoplast translation, transcription and genome replication: targets for antimalarial antibiotics. Trends. Parasitol. 24:279–284 [DOI] [PubMed] [Google Scholar]
- 16. Fichera ME, Roos DS. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature 390:407–409 [DOI] [PubMed] [Google Scholar]
- 17. Camps M, Arrizabalaga G, Boothroyd J. 2002. An rRNA mutation identifies the apicoplast as the target for clindamycin in Toxoplasma gondii. Mol. Microbiol. 43:1309–1318 [DOI] [PubMed] [Google Scholar]
- 18. Esseiva AC, Naguleswaran A, Hemphill A, Schneider A. 2004. Mitochondrial tRNA import in Toxoplasma gondii. J. Biol. Chem. 279:42363–42368 [DOI] [PubMed] [Google Scholar]
- 19. Pino P, Aeby E, Foth BJ, Sheiner L, Soldati T, Schneider A, Soldati-Favre D. 2010. Mitochondrial translation in absence of local tRNA aminoacylation and methionyl tRNA Met formylation in Apicomplexa. Mol. Microbiol. 76:706–718 [DOI] [PubMed] [Google Scholar]
- 20. Afonso A, Neto Z, Castro H, Lopes D, Alves AC, Tomás AM, Rosário VD. 2010. Plasmodium chabaudi chabaudi malaria parasites can develop stable resistance to atovaquone with a mutation in the cytochrome b gene. Malar. J. 9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Painter HJ, Morrisey JM, Mather MW, Vaidya AB. 2007. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 446:88–91 [DOI] [PubMed] [Google Scholar]
- 22. Waters AP, Syin C, McCutchan TF. 1989. Developmental regulation of stage-specific ribosome populations in Plasmodium. Nature 342:438–440 [DOI] [PubMed] [Google Scholar]
- 23. van Spaendonk RM, Ramesar J, van Wigcheren A, Eling W, Beetsma AL, van Gemert GJ, Hooghof J, Janse CJ, Waters AP. 2001. Functional equivalence of structurally distinct ribosomes in the malaria parasite, Plasmodium berghei. J. Biol. Chem. 276:22638–22647 [DOI] [PubMed] [Google Scholar]
- 24. Paton MG, Barker GC, Matsuoka H, Ramesar J, Janse CJ, Waters AP, Sinden RE. 1993. Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol. Biochem. Parasitol. 59:263–275 [DOI] [PubMed] [Google Scholar]
- 25. Thompson J, Sinden RE. 1994. In situ detection of Pbs21 mRNA during sexual development of Plasmodium berghei. Mol. Biochem. Parasitol. 68:189–196 [DOI] [PubMed] [Google Scholar]
- 26. Vervenne RA, Dirks RW, Ramesar J, Waters AP, Janse CJ. 1994. Differential expression in blood stages of the gene coding for the 21-kilodalton surface protein of ookinetes of Plasmodium berghei as detected by RNA in situ hybridisation. Mol. Biochem. Parasitol. 68:259–266 [DOI] [PubMed] [Google Scholar]
- 27. Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, James K, Rutherford K, Harris B, Harris D, Churcher C, Quail MA, Ormond D, Doggett J, Trueman HE, Mendoza J, Bidwell SL, Rajandream MA, Carucci DJ, Yates JR, III, Kafatos FC, Janse CJ, Barrell B, Turner CM, Waters AP, Sinden RE. 2005. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307:82–86 [DOI] [PubMed] [Google Scholar]
- 28. Cui L, Fan Q, Li J. 2002. The malaria parasite Plasmodium falciparum encodes members of the Puf RNA-binding protein family with conserved RNA binding activity. Nucleic Acids Res. 30:4607–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braks JA, Mair GR, Franke-Fayard B, Janse CJ, Waters AP. 2008. A conserved U-rich RNA region implicated in regulation of translation in Plasmodium female gametocytes. Nucleic Acids Res. 36:1176–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mair GR, Lasonder E, Garver LS, Franke-Fayard BM, Carret CK, Wiegant JC, Dirks RW, Dimopoulos G, Janse CJ, Waters AP. 2010. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 6:e1000767 doi:10.1371/journal.ppat.1000767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matuschewski K, Ross J, Brown SM, Kaiser K, Nussenzweig V, Kappe SH. 2002. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J. Biol. Chem. 277:41948–41953 [DOI] [PubMed] [Google Scholar]
- 32. Mikolajczak SA, Silva-Rivera H, Peng X, Tarun AS, Camargo N, Jacobs-Lorena V, Daly TM, Bergman LW, de la Vega P, Williams J, Aly AS, Kappe SH. 2008. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol. Cell. Biol. 28:6196–6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Roch KG, Johnson JR, Florens L, Zhou Y, Santrosyan A, Grainger M, Yan SF, Williamson KC, Holder AA, Carucci DJ, Yates JR, III, Winzeler EA. 2004. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 14:2308–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SH. 2005. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl. Acad. Sci. U. S. A. 102:3022–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mueller AK, Labaied M, Kappe SH, Matuschewski K. 2005. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433:164–167 [DOI] [PubMed] [Google Scholar]
- 36. Sharma A, Yogavel M, Akhouri RR, Gill J. 2008. Crystal structure of soluble domain of malaria sporozoite protein UIS3 in complex with lipid. J. Biol. Chem. 283:24077–24088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mackellar DC, O'Neill MT, Aly AS, Sacci JB, Jr, Cowman AF, Kappe SH. 2010. Plasmodium falciparum PF10_0164 (ETRAMP10.3) is an essential parasitophorous vacuole and exported protein in blood stages. Eukaryot. Cell 9:784–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holder AA, Sinden RE, Yates JR, Carucci DJ. 2002. A proteomic view of the Plasmodium falciparum life cycle. Nature 419:520–526 [DOI] [PubMed] [Google Scholar]
- 39. Lasonder E, Janse CJ, van Gemert GJ, Mair GR, Vermunt AM, Douradinha BG, van Noort V, Huynen MA, Luty AJ, Kroeze H, Khan SM, Sauerwein RW, Waters AP, Mann M, Stunnenberg HG. 2008. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog. 4:e1000195 doi:10.1371/journal.ppat.1000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siau A, Silvie O, Franetich JF, Yalaoui S, Marinach C, Hannoun L, van Gemert GJ, Luty AJ, Bischoff E, David PH, Snounou G, Vaquero C, Froissard P, Mazier D. 2008. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte infection. PLoS Pathog. 4:e1000121 doi:10.1371/journal.ppat.1000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou Y, Ramachandran V, Kumar KA, Westenberger S, Refour P, Zhou B, Li F, Young JA, Chen K, Plouffe D, Henson K, Nussenzweig V, Carlton J, Vinetz JM, Duraisingh MT, Winzeler EA. 2008. Evidence-based annotation of the malaria parasite's genome using comparative expression profiling. PLoS One 3:e1570 doi:10.1371/journal.pone.0001570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang M, Fennell C, Ranford-Cartwright L, Sakthivel R, Gueirard P, Meister S, Caspi A, Doerig C, Nussenzweig RS, Tuteja R, Sullivan WJ, Jr, Roos DS, Fontoura BM, Ménard R, Winzeler EA, Nussenzweig V. 2010. The Plasmodium eukaryotic initiation factor-2α kinase IK2 controls the latency of sporozoites in the mosquito salivary glands. J. Exp. Med. 207:1465–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ward P, Equinet L, Packer J, Doerig C. 2004. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics 5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaiser K, Camargo N, Kappe SH. 2003. Transformation of sporozoites into early exoerythrocytic malaria parasites does not require host cells. J. Exp. Med. 97:1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. 2005. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307:935–939 [DOI] [PubMed] [Google Scholar]
- 46. Gomes-Santos CS, Braks J, Prudêncio M, Carret C, Gomes AR, Pain A, Feltwell T, Khan S, Waters A, Janse C, Mair GR, Mota MM. 2011. Transition of Plasmodium sporozoites into liver stage-like forms is regulated by the RNA binding protein Pumilio. PLoS Pathog. 7:e1002046 doi:10.1371/journal.ppat.1002046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Müller K, Matuschewski K, Silvie O. 2011. The Puf-family RNA-binding protein Puf2 controls sporozoite conversion to liver stages in the malaria parasite. PLoS One 6:e19860 doi:10.1371/journal.pone.0019860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cowman AF, Berry D, Baum J. 2012. The cell biology of disease: the cellular and molecular basis for malaria parasite invasion of the human red blood cell. J. Cell Biol. 198:961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Esposito A, Tiffert T, Mauritz JM, Schlachter S, Bannister LH, Kaminski CF, Lew VL. 2008. FRET imaging of hemoglobin concentration in Plasmodium falciparum-infected red cells. PLoS One 3:e3780 doi:10.1371/journal.pone.0003780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fitch CD, Kanjananggulpan P. 1987. The state of ferriprotoporphyrin IX in malaria pigment. J. Biol. Chem. 262:15552–15555 [PubMed] [Google Scholar]
- 51. Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. 2000. The structure of malaria pigment beta-haematin. Nature 404:307–310 [DOI] [PubMed] [Google Scholar]
- 52. Zhang M, Mishra S, Sakthivel R, Rojas M, Ranjan R, Sullivan WJ, Jr, Fontoura BM, Ménard R, Dever TE, Nussenzweig V. 2012. PK4, a eukaryotic initiation factor 2α(eIF2α) kinase, is essential for the development of the erythrocytic cycle of Plasmodium. Proc. Natl. Acad. Sci. U. S. A. 109:3956–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH. 2000. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol. Today 16:427–433 [DOI] [PubMed] [Google Scholar]
- 54. White JH, Kilbey BJ. 1996. DNA replication in the malaria parasite. Parasitol. Today 12:151–155 [DOI] [PubMed] [Google Scholar]
- 55. Mohrle JJ, Zhao Y, Wernli B, Franklin RM, Kappes B. 1997. Molecular cloning, characterization and localization of PfPK4, an eIF-2α kinase-related enzyme from the malarial parasite Plasmodium falciparum. Biochem. J. 328:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Solyakov L, Halbert J, Alam MM, Semblat JP, Dorin-Semblat D, Reininger L, Bottrill AR, Mistry S, Abdi A, Fennell C, Holland Z, Demarta C, Bouza Y, Sicard A, Nivez MP, Eschenlauer S, Lama T, Thomas DC, Sharma P, Agarwal S, Kern S, Pradel G, Graciotti M, Tobin AB, Doerig C. 2011. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat. Commun. 2:565. [DOI] [PubMed] [Google Scholar]
- 57. Wastling JM, Xia D, Sohal A, Chaussepied M, Pain A, Langsley G. 2009. Proteomes and transcriptomes of the Apicomplexa—where's the message? Int. J. Parasitol. 39:135–143 [DOI] [PubMed] [Google Scholar]
- 58. Xia D, Sanderson SJ, Jones AR, Prieto JH, Yates JR, Bromley E, Tomley FM, Lal K, Sinden RE, Brunk BP, Roos DS, Wastling JM. 2008. The proteome of Toxoplasma gondii: integration with the genome provides novel insights into gene expression and annotation. Genome Biol. 9:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gastens MH, Fischer HG. 2002. Toxoplasma gondii eukaryotic translation initiation factor 4A associated with tachyzoite virulence is down-regulated in the bradyzoite stage. Int. J. Parasitol. 32:1225–1234 [DOI] [PubMed] [Google Scholar]
- 60. Sullivan WJ, Jr, Narasimhan J, Bhatti MM, Wek RC. 2004. Parasite-specific eukaryotic initiation factor-2 (eIF2) kinase required for stress-induced translation control. Biochem. J. 380:523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Joyce BR, Queener SF, Wek RC, Sullivan WJ., Jr 2010. Phosphorylation of eukaryotic initiation factor-2α promotes the extracellular survival of obligate intracellular parasite Toxoplasma gondii. Proc. Natl. Acad. Sci. U. S. A. 107:17200–17205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Konrad C, Wek RC, Sullivan WJ., Jr 2011. A GCN2-like eukaryotic initiation factor-2 kinase increases the viability of extracellular Toxoplasma gondii parasites. Eukaryot. Cell 10:1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lirussi D, Matrajt M. 2011. RNA granules present only in extracellular Toxoplasma gondii increase parasite viability. Int. J. Biol. Sci. 7:960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beckers CJ, Roos DS, Donald RG, Luft BJ, Schwab JC, Cao Y, Joiner KA. 1995. Inhibition of cytoplasmic and organellar protein synthesis in Toxoplasma gondii. Implications for the target of macrolide antibiotics. J. Clin. Invest. 95:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takeuchi T, Fujiwara T, Akao S. 1980. Na+, K+-dependent ATPase activity and effect of K+ on in vitro protein synthesis and NAD pyrophosphorylase in Toxoplasma gondii. J. Parasitol. 66:591–595 [PubMed] [Google Scholar]
- 66. Braun L, Cannella D, Ortet P, Barakat M, Sautel CF, Kieffer S, Garin J, Bastien O, Voinnet O, Hakimi MA. 2010. A complex small RNA repertoire is generated by a plant/fungal-like machinery and effected by a metazoan-like Argonaute in the single-cell human parasite Toxoplasma gondii. PLoS Pathog. 6:e1000920 doi:10.1371/journal.ppat.1000920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Narasimhan J, et al. 2008. Translation regulation by eukaryotic initiation factor-2 kinases in the development of latent cysts in Toxoplasma gondii. J. Biol. Chem. 283:16591–16601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. 2009. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324:218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Porter RJ, Laird RL, Dusseau EM. 1954. Studies on malarial sporozoites. II. Effect of age and dosage of sporozoites on their infectiousness. Exp. Parasitol. 3:267–274 [DOI] [PubMed] [Google Scholar]
- 70. Beier JC, Vanderberg JP. 1998. Sporogonic development in the mosquito, p 49–61 In Sherman IW. (ed), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, DC [Google Scholar]
- 71. Markus MB. 2011. The hypnozoite concept, with particular reference to malaria. Parasitol. Res. 108:247–252 [DOI] [PubMed] [Google Scholar]
- 72. Smalley ME, Sinden RE. 1977. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology 74:1–8 [DOI] [PubMed] [Google Scholar]