Abstract
Paenibacillus larvae is the causative agent of American foulbrood (AFB), a disease affecting honey bee larvae. First- and second-instar larvae become infected when they ingest food contaminated with P. larvae spores. The spores then germinate into vegetative cells that proliferate in the midgut of the honey bee. Although AFB affects honey bees only in the larval stage, P. larvae spores can be distributed throughout the hive. Because spore germination is critical for AFB establishment, we analyzed the requirements for P. larvae spore germination in vitro. We found that P. larvae spores germinated only in response to l-tyrosine plus uric acid under physiologic pH and temperature conditions. This suggests that the simultaneous presence of these signals is necessary for spore germination in vivo. Furthermore, the germination profiles of environmentally derived spores were identical to those of spores from a biochemically typed strain. Because l-tyrosine and uric acid are the only required germinants in vitro, we screened amino acid and purine analogs for their ability to act as antagonists of P. larvae spore germination. Indole and phenol, the side chains of tyrosine and tryptophan, strongly inhibited P. larvae spore germination. Methylation of the N-1 (but not the C-3) position of indole eliminated its ability to inhibit germination. Identification of the activators and inhibitors of P. larvae spore germination provides a basis for developing new tools to control AFB.
INTRODUCTION
American foulbrood (AFB) is a bacterial disease of honey bees that kills the developing larvae (1, 2). Paenibacillus larvae spores are the infectious agents for AFB, but it is the vegetative cells that cause disease (3, 4). In 2005, a survey of almond-pollinating bee colonies indicated that 4% of colonies had a significant AFB load (5). Once a beekeeping operation is contaminated, the bacterial spores are not easily removed (6). Although autoclaving and high concentrations of chemical disinfectants effectively kill the spores, these treatments are not viable for the beekeeping industry (7). Traditionally, Terramycin and other antibiotics have been used for the treatment and prevention of AFB. However, antibiotic treatment is ineffective in the spore stage of P. larvae, and overuse of the antibiotics leads to resistant strains (8, 9). Presently, the only accepted practice for controlling the spread of AFB is burning both the infected colonies and beekeeping equipment (3, 6).
AFB occurs when first- or second-instar larvae (within 48 h after the egg hatches) ingest food that is contaminated with the P. larvae spores (10). Twelve hours after ingestion, P. larvae spores germinate and the new vegetative cells start to proliferate inside the larval gut (11). Several days postinfection, extreme bacteremia causes the death of the honey bee larvae (12–14). After the nutrient levels of the honey bees are depleted, P. larvae cells stop dividing and then sporulate. As a result, billions of spores are found in the dead remains of each bee larva (15, 16). Within the colony, spores are transmitted by adult bees that eat larval remains (17, 18). P. larvae spores are transmitted between colonies through bees that rob honey from neighboring infected colonies and through the use of contaminated beekeeping equipment (19).
Because P. larvae spore germination is the first step of infection, controlling spore germination might lead to new approaches to prevent AFB (20). However, little is known about the environmental cues required to trigger P. larvae spore germination. In Clostridia and Bacilli species, spores require sugars, nucleosides, amino acids, and/or inorganic salts to stimulate germination (21). The complexity of germination signals varies and commonly requires several types of germinants (22). We, and others, have also identified molecules that can inhibit spore germination (23–28).
In this work, we tested the ability of metabolites to promote P. larvae spore germination. We found that P. larvae spores exclusively recognize l-tyrosine and uric acid as cogerminants. We determined the germination 50% effective concentrations (EC50s) of l-tyrosine and uric acid. Because l-tyrosine and uric acid are strong germinants in vitro, we screened chemical analogs for their ability to inhibit spore germination. Indole and phenol, the side chains of tryptophan and tyrosine, were thus identified as germination inhibitors. Methylation of the N-1 position of indole inactivates its inhibitory activity. In contrast, methylation of the C-3, C-5, or C-7 position has no effect on the inhibitory potential of indole.
MATERIALS AND METHODS
Materials.
Chemicals were purchased from the Sigma-Aldrich Corporation (St. Louis, MO). The dehydrated culture medium was purchased from BD Difco (Franklin Lakes, NJ). Paenibacillus larvae subsp. pulvifaciens strain ATCC 49843 was purchased from the American Tissue Culture Collection (ATCC). Environmental American foulbrood scales (the remains of infected larvae collected from infected hives) were kindly donated by Jay D. Evans at the USDA Bee Research Facility (Beltsville, MD). The environmental strain was identified as a strain of Paenibacillus larvae subsp. larvae based on its phenotypic characteristics and 16S rRNA analysis (29).
P. larvae spore preparation.
P. larvae strains were grown on BD tryptic soy agar plates for 7 days in a 5% CO2 incubator at 37°C. The resulting bacterial lawns were collected by flooding with ice-cold deionized water. Spores were pelleted by centrifugation and resuspended in fresh deionized water. After three washing steps, the spores were separated from their vegetative and partially sporulated forms by centrifugation through a 20%-to-50% HistoDenz gradient. The spore pellet was washed five times with water and stored at 4°C (23). Spore preparations were >90% pure as determined by microscopic observation of Schaeffer-Fulton-stained samples (30).
Preparation of germinant solution.
Sixteen complex media (Mueller-Hinton broth, yeast extract, potassium phosphate, glucose, and pyruvate [MYPGP]; tryptic soy broth [TSB]; brain heart infusion [BHI]; Nutrient; LB; Tris-maleate buffer, yeast extract, glucose, and sodium pyruvate [TMYGP]; NZ amine; NZCYM [NZ amine, NaCl, Bacto yeast extract, Casamino Acids, MgSO4·7H2O, adjusted to pH 7.0 with NaOH]; Lactobacillus; SOC [Bacto tryptone, Bacto yeast extract, 5 M NaCl, 1 M KCl, 1 M MgCl2, 1 M MgSO4, and 1 M glucose]; Bailey; Clostridium; Michael; Terrific; MD [potassium phosphate (pH 7.5), trisodium citrate, 2% (wt/vol) glucose, ferric ammonium citrate, 0.25% (wt/vol) potassium aspartate, magnesium sulfate, l-tryptophan, and l-phenylalanine]; and Jansen [J] broths) were prepared (31–33). A defined medium was prepared as described previously (34). An artificial worker jelly (AWJ) medium was prepared based on modifications to the published composition of worker jelly (35). For AWJ, the following stock solutions were prepared: 100 mM inosine in 220 mM NaOH, 400 mM for each sugar (fructose, glucose, and arabinose) in water, 30 mM for each of the 20 proteinogenic l-amino acids in 0.36 N HCl, 100 mM uric acid in 220 mM NaOH, and 0.2 mg/ml vitamins (thiamine, riboflavin, pyridoxine, β-alanine, para-aminobenzoic acid, nicotinic acid, pantothenic acid, biotin, folic acid, and inositol) in water. To prepare AWJ, inosine, uric acid, sugars, and amino acids were dissolved to a final concentration of 3 mM in 0.1 M sodium phosphate buffer (0.06 mM Na2HPO4 and 0.04 mM NaH2PO4) and adjusted to pH 7.0. This solution was supplemented with vitamins to a final concentration of 1 μg/ml.
Determination of germinants for P. larvae spores.
The decrease in optical density (OD) is inversely proportional to spore germination (36). Changes in light diffraction during spore germination were monitored at 580 nm (optical density at 580 nm [OD580]) on a BioMate 5 (Thermo Electron Corporation, Waltham, MA) or a Tecan Infinite M200 (Tecan Group, Männedorf, Switzerland) spectrophotometer. Experiments were carried out in 96-well plates (200 μl/well). In preparation for germination assays, P. larvae spore suspensions were washed three times with water. Spores were then heat-activated at 70°C for 30 min. The heat-activated spores were allowed to reach room temperature and were transferred to 0.1 M sodium phosphate buffer (pH 7.0) to an approximate OD580 of 0.70. Spores were monitored for autogermination for 30 min. Germination experiments were carried out with spores that did not autogerminate. Putative germinants were added individually or in various combinations to a final concentration of 3 mM. Experiments were performed in triplicate with at least two different spore preparations. After germinant addition, the OD580 of the spore suspension was measured every minute for an hour. Relative OD values were derived by dividing each OD580 reading by the initial OD580. Spore germination rates (v) were calculated from the initial linear decrease in relative OD (23). Germination rates were set to 100% for P. larvae spores that had the highest germination rate in a particular assay. Germination rates for other conditions were divided by the maximum germination rate for that assay and are reported as percent germination (23). Standard deviations were calculated from at least six independent measurements and were typically below 10%. Spore germination was confirmed in selected samples by microscopic observation of Schaeffer-Fulton-stained aliquots (30).
Effect of temperature and pH on P. larvae germination.
For temperature experiments, P. larvae spores were germinated in 3 mM l-tyrosine and 3 mM uric acid. Germination rates were determined as described above, except that the germination temperature varied between 25 and 42°C. The germination rate was set to 100% for the spores that were germinated at 42°C. Germination rates for other conditions were divided by the maximum germination rate at 42°C and are reported as percent germination. Germination rate differences were analyzed using analysis of variance (ANOVA) followed by a Tukey-Kramer procedure (SigmaPlot v.9).
For pH experiments, P. larvae spores were resuspended in 0.1 M sodium phosphate, potassium-sodium phosphate, or citrate phosphate buffer. The pH of the buffers was adjusted between 3.0 and 9.0. Spores were germinated in the presence of 3 mM l-tyrosine and 3 mM uric acid. Germination rates were determined as described above. Germination rate was set to 100% for spores germinated at pH 7.0. Germination rates for other conditions were divided by the maximum germination rate at pH 7 and are reported as percent germination. As discussed above, germination rate differences were analyzed using ANOVA followed by a Tukey-Kramer procedure (SigmaPlot v.9).
Activation of P. larvae spore germination by l-tyrosine and uric acid.
P. larvae spore germination was tested using different combinations of l-tyrosine and uric acid. For l-tyrosine titrations, spores were exposed to various concentrations of l-tyrosine and a constant concentration of 3 mM uric acid. For uric acid titrations, spores were exposed to various concentrations of uric acid and a constant concentration of 3 mM l-tyrosine. Germination rates were determined as described above. The germination rate was set to 100% for P. larvae spores germinated in the presence of 3 mM l-tyrosine–3 mM uric acid. Germination rates for other conditions were divided by the maximum germination rate obtained with 3 mM l-tyrosine–3 mM uric acid and are reported as percent germination. Percent germination was plotted against compound concentrations. The resulting sigmoidal curves were fitted using the four-parameter logistic function of the SigmaPlot v.9 software to calculate EC50s (for enhancers of spore germination). EC50 is defined as the concentration of a germinant required to increase the germination rate to 50% of its maximal value (37, 38).
Agonists of P. larvae spore germination.
To test for possible agonists of P. larvae spore germination, spores were individually supplemented with 3 mM purine analog and 3 mM l-tyrosine. Separately, P. larvae spores were incubated with 3 mM amino acid analog and 3 mM uric acid. Spore germination was monitored as described above. Germination rates for other conditions were divided by the maximum germination rate obtained with 3 mM l-tyrosine–3 mM uric acid and are reported as percent germination.
Antagonists of P. larvae spore germination.
To test for possible antagonists of P. larvae spore germination, spores were individually supplemented with 3 mM purine analog or 3 mM amino acid analog. Spore suspensions were incubated for 15 min at room temperature while monitoring the OD580s. If no germination was detected, l-tyrosine and uric acid were added to final concentrations of 3 mM, and germination was monitored as described above. Germination rates for other conditions were divided by the uninhibited maximum germination rate obtained with 3 mM l-tyrosine–3 mM uric acid and are reported as percent germination.
Inhibition of P. larvae spore germination by indole and phenol.
P. larvae spores were individually incubated with various concentrations of indole, phenol, 1-N-methylindole, 3-methylindole, 5-methylindole, or 7-methylindole. After 15 min incubation, the spores were treated with 3 mM l-tyrosine–3 mM uric acid. Germination rate was set to 100% for P. larvae spores germinated in the absence of inhibitor. Germination rates for other conditions were divided by the uninhibited maximum germination rate obtained with 3 mM l-tyrosine–3 mM uric acid and are reported as percent germination. Percent germination was plotted against the inhibitor concentrations. The resulting sigmoidal curves were fitted using the four-parameter logistic function in SigmaPlot v.9 to calculate the germination 50% inhibitory concentrations (IC50s) (23, 37).
RESULTS
We were unable to detect significant P. larvae spore germination in any of the 16 different complex media tested, even after 24 h incubation. By comparison, spores of Bacillus anthracis and Bacillus cereus germinate within 2 h in rich medium (39, 40). Similarly, P. larvae spores failed to germinate in a defined medium containing metabolites commonly used as germinants by Bacillus and Clostridia species (Fig. 1).
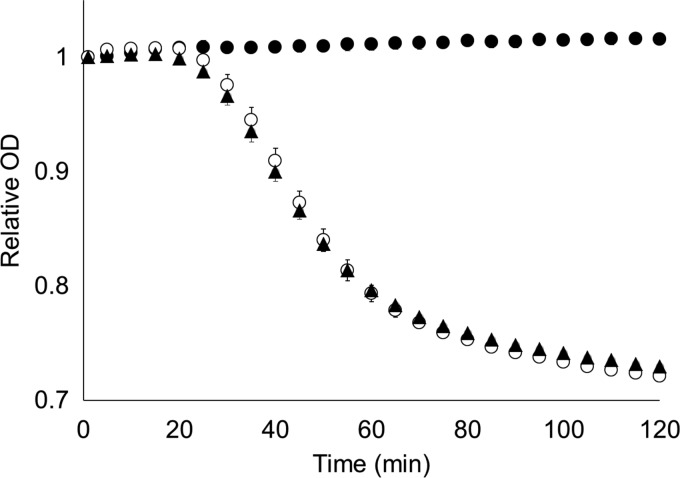
Fig 1.
P. larvae spores germinate in artificial worker jelly. P. larvae strain ATCC 49843 spores were suspended in defined medium (●), artificial worker jelly (○), or uric acid plus l-tyrosine (▲). Data are shown for every 5 min for clarity. Spore germination was followed by decreases of the relative OD over time. Each error bar represents a standard deviation obtained from at least six independent measurements.
Honey bee larvae are fed royal or worker jelly that can be contaminated with P. larvae spores (41). Here, P. larvae spores were resuspended in a chemically defined medium (AWJ) that differs from worker jelly only in its pH value. The optical density of P. larvae spores suspended in AWJ decreased, indicating that the spores were germinating (Fig. 1). Spore germination was confirmed by Schaeffer-Fulton staining (30).
To determine the compounds that are necessary to trigger P. larvae spore germination, groups of compounds were systematically omitted from the AWJ medium. P. larvae spores germinated well in the absence of sugars and vitamins, suggesting that germination onset required uric acid and proteinogenic amino acid(s). Testing of individual amino acids showed that only l-tyrosine was able to synergize with uric acid to produce a sufficiently strong germination response in P. larvae spores (Fig. 1). A mixture of uric acid and the remaining 19 proteinogenic amino acids induced negligible germination responses (data not shown).
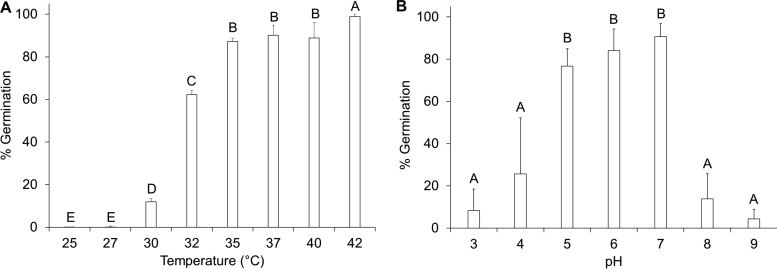
To determine the effects of temperature on P. larvae spore germination, a range of 25 to 42°C was tested (Fig. 2A). At temperatures of <30°C, the germination rate of P. larvae spores was low. The maximal germination rates occurred at temperatures of >35°C. The ability of P. larvae spores to germinate was also tested at different pH values (Fig. 2B). Under acidic or basic conditions, spores failed to germinate. Germination was optimal near neutral pH.
Fig 2.
Effects of temperature and pH on P. larvae spore germination. (A) P. larvae spores were suspended in 3 mM l-tyrosine–3 mM uric acid and exposed individually to temperatures between 25 and 42°C. The maximum germination rate was set to 100% for spores germinated at 42°C. Percent germination for other conditions was calculated relative to 42°C. Each error bar represents a standard deviation obtained from at least six independent measurements. Columns that are labeled with different letters are statistically different (P > 0.05). (B) P. larvae spores were suspended in 3 mM l-tyrosine–3 mM uric acid and were exposed individually to pH values between 3 and 9. The maximum germination rate was set to 100% for spores germinated at pH 7. Percent germination for other conditions was calculated relative to pH 7. Each error bar represents a standard deviation obtained from at least six independent measurements. Columns that are labeled with different letters are statistically different (P > 0.05).
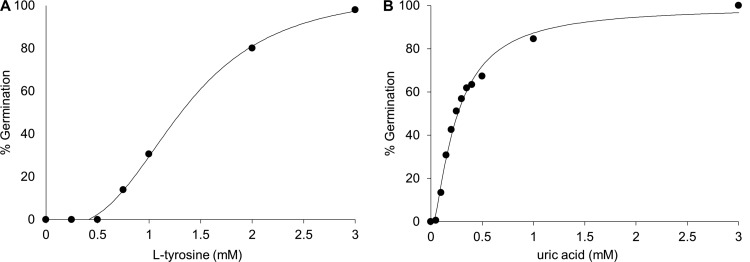
Titration of l-tyrosine at a saturating uric acid concentration yielded an EC50 of 1.2 mM for l-tyrosine activation of P. larvae spore germination (Fig. 3A). Titration of uric acid at a saturating l-tyrosine concentration yielded an EC50 of 0.2 mM for uric acid activation of P. larvae spore germination (Fig. 3B). Both dose-response assays resulted in sigmoidal curves that passed the Durbin-Watson statistical test for autocorrelation.
Fig 3.
Calculation of 50% effective concentration for l-tyrosine and uric acid. (A) Dose-response curve of P. larvae spores germinated at a saturating concentration of uric acid and various concentrations of l-tyrosine. The EC50 for l-tyrosine was determined based on these data. (B) Dose-response curve of P. larvae spores germinated at a saturating concentration of l-tyrosine and various concentrations of uric acid. The EC50 for uric acid was determined based on these data.
Uric acid is a degradation product of purine catabolism. Hence, we tested the ability of purine analogs to act as cogerminants of P. larvae spores. Since l-tyrosine is the only amino acid able to trigger P. larvae spore germination, we also tested its stereoisomer (d-tyrosine) and its side chain (phenol) as cogerminants with uric acid. None of the compounds tested as a cogerminant was able to activate P. larvae spore germination (data not shown).
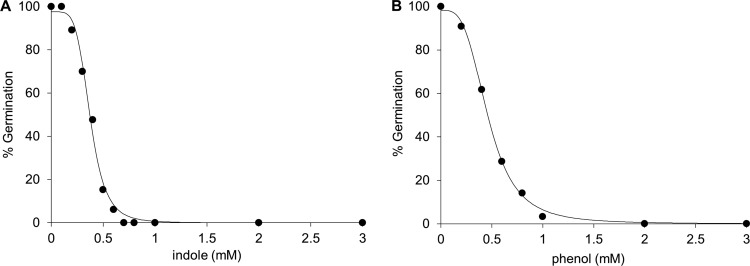
l-Tyrosine and purine analogs were also tested for their ability to inhibit P. larvae germination. None of the purine analogs tested inhibited uric acid–l-tyrosine-induced germination of P. larvae spores (data not shown). Similarly, d-tyrosine did not inhibit P. larvae spore germination. Of the compounds tested, only indole (the side chain of tryptophan) and phenol (the side chain of tyrosine) were able to inhibit P. larvae spore germination. Titrations of indole and phenol yielded IC50s of 0.37 mM (Fig. 4A) and 0.46 mM (Fig. 4B), respectively, for the inhibition of P. larvae spore germination. 3-Methylindole, 5-methylindole, and 7-methylindole retained inhibitory properties, with IC50s of 0.38, 0.37, and 0.28 mM, respectively (Table 1). In contrast, 1-N-methylindole did not activate or inhibit P. larvae spore germination. All dose-response assays resulted in sigmoidal curves that passed the Durbin-Watson statistical test for autocorrelation.
Fig 4.
Calculation of 50% inhibitory concentration. (A) Dose-response curve of P. larvae spores germinated with l-tyrosine–uric acid in the presence of various concentrations of indole. The IC50 for indole was determined based on these data. (B) Dose-response curve of P. larvae spores germinated with l-tyrosine–uric acid in the presence of various concentrations of phenol. The IC50 for phenol was determined based on these data.
Table 1.
Effects of indole methylation on P. larvae spore germinationa
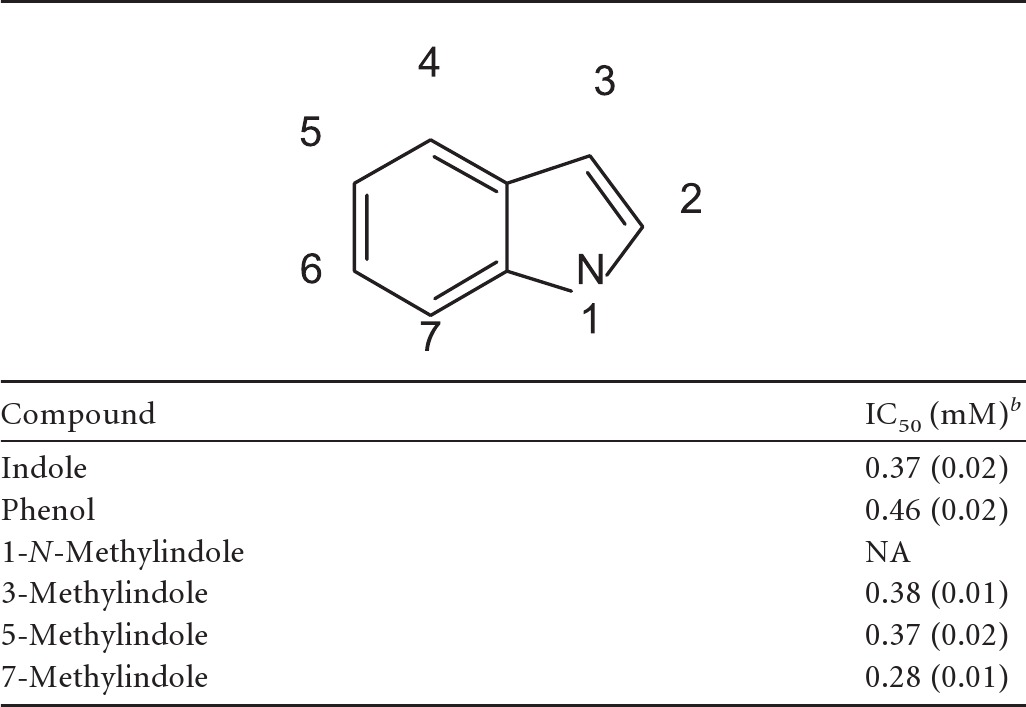
P. larvae spores were incubated with various concentrations of indole analogs for 15 min prior to addition of 3 mM l-tyrosine–3 mM uric acid.
IC50 was calculated by plotting percent germination versus indole analog concentration. Standard deviations are shown in parentheses. NA, no activity under the conditions tested.
To test the generality of P. larvae spore response, we prepared spores from P. larvae subsp. pulvifaciens strain ATCC 49843 and from an environmental AFB sample. Based on phenotypic characteristics, this environmental sample was identified as P. larvae subsp. larvae (13, 14). The spores of both P. larvae subspecies responded identically to l-tyrosine and uric acid. Their germination was similarly inhibited by indole and phenol (data not shown).
DISCUSSION
Bacterial spore germination is a critical step for infection onset in numerous hosts (42, 43). The nature of the spore germination signals has been studied widely in Bacilli and Clostridia (21, 27, 28, 44–50), but the triggers for P. larvae spore germination have not been identified. In order to cultivate P. larvae, specialized media have been produced (1, 31, 51–53). Under the best conditions, <10% of P. larvae spores plated on complex laboratory media germinate (54). In this study, we show that P. larvae spores specifically recognize l-tyrosine and uric acid as germinants.
P. larvae spores germinate at a sluggish pace at room temperature and thus can remain dormant on hive surfaces and beekeeping equipment for long periods of time. Honey bees are endothermic and use heat-producing muscle contractions to maintain colony temperature at a narrow range, around 35°C (41). To develop correctly in the laboratory, reared honey bee larvae are maintained at a constant temperature of 35 to 37°C (55), which is also optimal for P. larvae spore germination.
Maximal germination of P. larvae spores was observed between pH 5 and 7, which matches the intestinal pH levels of both adult bees (pH 5.6 to 6.3) and bee larvae (pH 6.8) (56). Honey, nectar, pollen, and royal jelly are much more acidic (pH 3 to 4). We suggest that the acidity of these products may prevent P. larvae spores from germinating prematurely outside the honey bee gut.
Although the concentration of l-tyrosine in the bee larva gut is not known, honey bee larvae are fed either worker or royal jelly containing free l-tyrosine at a concentration of approximately 0.11 mM (57). Royal jelly also contains proteins that, upon digestion, can increase the concentration of l-tyrosine to 22 mM (57).
Once metabolized, proteinaceous materials are converted to uric acid, which is excreted as a waste product (58). Uric acid in the midgut of honey bee larvae must be at saturating concentrations, because when larvae first defecate, uric acid precipitates as large crystals (58). In fact, the appearance of uric acid crystals has been used as a marker for pupation onset (41) and insect infestation of stored grains (59).
The Bacilli and Clostridia spore germination responses result from complex interacting pathways (26, 60). Indeed, six different strategies have been described that integrate the multiple signals required for spore germination (22). We showed recently that active germination pathways in Bacillus can cooperate or interfere with each other (61). In contrast, the simplicity of signals required for P. larvae spore germination can be described as a single integrator logical gate (22). By narrowing the germination signals to two germinants, P. larvae spores further ensure that germination occurs only in the larval gut, where amino acids and uric acid are abundant.
The midgut and the hindgut of honey bee larvae are disconnected until the final larval molt. Hence, in the AFB-susceptible young larvae, food and waste products will colocalize in the midgut (41). This will ensure that P. larvae spores are exposed simultaneously to high concentrations of uric acid and l-tyrosine, allowing for germination and infection onset. In contrast, in adult honey bees, amino acids are thought to be absorbed in the midgut, and uric acid is present only in the hindgut (58, 62). The spatial and temporal separation between food and waste products in adult honey bees will preclude P. larvae spores from detecting both germination signals, thus preventing germination (41).
Spore-formers can have strain-specific differences in their germination responses. For example, while adenosine is a germinant for B. cereus strain 3711, it inhibits inosine-mediated germination in B. cereus strain 569 (63, 64). P. larvae spores from the two subspecies show identical germination responses. Our results are consistent with polyphasic taxonomic studies that have found few differences between germination responses in P. larvae strains (14).
The spore germination response has been studied in other Paenibacillus species. Spores of Paenibacillus polymyxa germinate in response to the presence of fructose and alanine (48). The difference in germination responses between P. larvae and P. polymyxa was expected since P. polymyxa spores are associated with plant roots, where the concentration of uric acid is low. Other members of the Paenibacillus genus are insect pathogens that specifically target the larval stage. Indeed, Paenibacillus popilliae spores are sold commercially for the control of Japanese beetle infestation (65). It is tempting to speculate that uric acid can serve as a general germination signal for insect larva pathogens. Indeed, while there are no other reports of bacterial spores that germinate in response to the presence of uric acid and l-tyrosine, Bacillus fastidiosus spore germination occurs in a medium containing only uric acid (66), while Clostridium cylindrosporum spores germinate in a medium containing bicarbonate, uric acid, and calcium (49). Both of these bacteria are present in poultry litter and bird droppings, where uric acid is abundant (49, 67). The ability to recognize uric acid and an amino acid cogerminant might allow bacteria to select for specific hosts.
Although d-amino acids are strong spore germination inhibitors of Bacillus species (68–72), d-tyrosine failed to inhibit P. larvae spore germination. Of the analogs tested, only indole (the side chain of tryptophan) and phenol (the side chain of tyrosine) inhibited P. larvae spore germination.
Indole and phenol might inhibit P. larvae spore germination through nonspecific binding to hydrophobic regions of the tyrosine or uric acid binding sites. Methylindole derivatives are more hydrophobic than indole derivatives, but none of these analogs show increased antigermination activity. On the contrary, methylation at the N-1 position eliminates antigermination activity, suggesting that the NH group of indole forms an essential hydrogen bond with the tyrosine and/or uric acid binding sites.
Indole is the last intermediate in the biosynthesis of tryptophan in plants and bacteria (73). Indole also acts as an intracellular and extracellular signal for virulence, biofilm formation, acid resistance, drug resistance, and sporulation in bacteria (74–79). Phenol, on the other hand, is toxic in its free form but is found as a functional group in many secondary metabolites of plants (80). Since honey bees gather pollen and nectar, which are very rich in phenolic compounds, it is possible that collected polyphenols and indole compounds can protect honey bee larvae from infection.
In conclusion, we have found the activators and inhibitors of P. larvae spore germination. We also present evidence to suggest that P. larvae spores have evolved to germinate only in the gut of the larvae and to remain dormant in food, exposed environments, and the adult bee.
ACKNOWLEDGMENTS
We thank Rodney Mehring for beekeeping assistance.
This project is supported by the Agriculture and Food Research Initiative competitive grant 2011-67013-30169 from the USDA National Institute of Food and Agriculture.
Footnotes
Published ahead of print 21 December 2012
REFERENCES
- 1. White GF. 1906. The bacteria of the apiary, with special reference to bee diseases. GPO, Washington, DC [Google Scholar]
- 2. Broodsgaard C, Ritter W, Hansen H. 1998. Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie 29: 569– 578 [Google Scholar]
- 3. Genersch E. 2010. American foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 103: S10– S19 [DOI] [PubMed] [Google Scholar]
- 4. Tarr HLA. 1938. Studies on American foulbrood of bees. III. The resistance of individual larvae to inoculation with the endospores of Bacillus larvae. Ann. Appl. Biol. 25: 807– 814 [Google Scholar]
- 5. Eischen F, Graham H. 2005. American foulbrood survey in honey bees pollinating California almonds. II. A disease equivalent number of spores. Am. Bee J. 145: 390– 391 [Google Scholar]
- 6. Shimanuki H. 1983. Identification and control of honey bee diseases. U.S. Department of Agriculture, Washington, DC [Google Scholar]
- 7. Dobbelaere W, de Graaf DC, Reybroeck W, Desmedt E, Peeters JE, Jacobs FJ. 2001. Disinfection of wooden structures contaminated with Paenibacillus larvae subsp. larvae spores. J. Appl. Microbiol. 91: 212– 216 [DOI] [PubMed] [Google Scholar]
- 8. Alippi AM, López AC, Reynaldi FJ, Grasso DH, Aguilar OM. 2007. Evidence for plasmid-mediated tetracycline resistance in Paenibacillus larvae, the causal agent of American foulbrood (AFB) disease in honeybees. Vet. Microbiol. 125: 290– 303 [DOI] [PubMed] [Google Scholar]
- 9. Lodesani M, Costa M. 2005. Limits of chemotherapy in beekeeping: development of resistance and the problem of residues. Bee World 86: 102– 109 [Google Scholar]
- 10. Crailsheim K, Riessberger-Gallè U. 2001. Honey bee age-dependent resistance against American foulbrood. Apidologie 32: 91– 103 [Google Scholar]
- 11. Yue D, Nordhoff M, Wieler LH, Genersch E. 2008. Fluorescence in situ hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera). Environ. Microbiol. 10: 1612– 1620 [DOI] [PubMed] [Google Scholar]
- 12. Davidson EW. 1973. Ultrastructure of American foulbrood disease pathogenesis in larvae of the worker honey bee, Apis mellifera. J. Invertebr. Pathol. 21: 53– 61 [Google Scholar]
- 13. Genersch E, Ashiralieva A, Fries I. 2005. Strain- and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing American foulbrood disease in honeybees. Appl. Environ. Microbiol. 71: 7551– 7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Genersch E, Forsgren E, Pentikäinen J, Ashiralieva A, Rauch S, Kilwinski J, Fries I. 2006. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int. J. Syst. Evol. Microbiol. 56: 501– 511 [DOI] [PubMed] [Google Scholar]
- 15. Lindstrom A, Korpela S, Fries I. 2008. The distribution of Paenibacillus larvae spores in adult bees and honey and larval mortality, following the addition of American foulbrood diseased brood or spore-contaminated honey in honey bee (Apis mellifera) colonies. J. Invertebr. Pathol. 99: 82. [DOI] [PubMed] [Google Scholar]
- 16. Sturtevant AP. 1932. Relation of commercial honey to the spread of American foulbrood. J. Agric. Res. 45:257 [Google Scholar]
- 17. Fries I, Camazine S. 2001. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 32:199– 214 [Google Scholar]
- 18. Gillard M, Charriere JD, Belloy L. 2008. Distribution of Paenibacillus larvae spores inside honey bee colonies and its relevance for diagnosis. J. Invertebr. Pathol. 99: 92– 95 [DOI] [PubMed] [Google Scholar]
- 19. Fries I, Lindström A, Korpela S. 2006. Vertical transmission of American foulbrood (Paenibacillus larvae) in honey bees (Apis mellifera). Vet. Microbiol. 114: 269– 274 [DOI] [PubMed] [Google Scholar]
- 20. Alvarez Z, Abel-Santos E. 2007. Potential use of inhibitors of bacteria spore germination in the prophylactic treatment of anthrax and Clostridium difficile-associated disease. Expert Rev. Anti Infect. Ther. 5: 783– 792 [DOI] [PubMed] [Google Scholar]
- 21. Paredes-Sabja D, Setlow P, Sarker MR. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19: 85– 94 [DOI] [PubMed] [Google Scholar]
- 22. Ross C, Abel-Santos E. 2010. The Ger receptor family from sporulating bacteria. Curr. Issues Mol. Biol. 12: 147– 158 [PMC free article] [PubMed] [Google Scholar]
- 23. Akoachere M, Squires RC, Nour AM, Angelov L, Brojatsch J, Abel-Santos E. 2007. Identification of an in vivo inhibitor of Bacillus anthracis spore germination. J. Biol. Chem. 282: 12112– 12118 [DOI] [PubMed] [Google Scholar]
- 24. Cortezzo DE, Setlow B, Setlow P. 2004. Analysis of the action of compounds that inhibit the germination of spores of Bacillus species. J. Appl. Microbiol. 96: 725– 741 [DOI] [PubMed] [Google Scholar]
- 25. Yasuda-Yasaki Y, Namiki-Kanie S, Hachisuka Y. 1978. Inhibition of Bacillus subtilis spore germination by various hydrophobic compounds: demonstration of hydrophobic character of the l-alanine receptor site. J. Bacteriol. 136: 484– 490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6: 550– 556 [DOI] [PubMed] [Google Scholar]
- 27. Howerton A, Ramirez N, Abel-Santos E. 2011. Mapping interactions between germinants and Clostridium difficile spores. J. Bacteriol. 193: 274– 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alvarez Z, Lee K, Abel-Santos E. 2010. Testing nucleoside analogues as inhibitors of Bacillus anthracis spore germination in vitro and in macrophage cell culture. Antimicrob. Agents Chemother. 54: 5329– 5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piccini C, D'Alessandro B, Antunez K, Zunino P. 2002. Detection of Paenibacillus larvae subspecies larvae spores in naturally infected bee larvae and artificially contaminated honey by PCR. World J. Microbiol. Biotechnol. 18: 761– 765 [Google Scholar]
- 30. Schaeffer AB, Fulton MD. 1933. A simplified method of staining endospores. Science 77: 194. [DOI] [PubMed] [Google Scholar]
- 31. Bailey L, Lee DC. 1962. Bacillus larvae: its cultivation in vitro and its growth in vivo. J. Gen. Microbiol. 29:711– 717 [DOI] [PubMed] [Google Scholar]
- 32. Dingman DW, Stahly DP. 1983. Medium promoting sporulation of Bacillus larvae and metabolism of medium components. Appl. Environ. Microbiol. 46: 860– 869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zimbro MJ. 2009. Difco & BBL manual: manual of microbiological culture media. Becton, Dickinson, and Company, Sparks, MD [Google Scholar]
- 34. Ramirez N, Abel-Santos E. 2010. Requirements for germination of Clostridium sordellii spores in vitro. J. Bacteriol. 192: 418– 425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rembold H, Dietz A. 1966. Biologically active substances in royal jelly. Vitam. Horm. 23: 359– 382 [DOI] [PubMed] [Google Scholar]
- 36. Powell JF. 1950. Factors affecting the germination of thick suspensions of Bacillus subtilis spores in l-alanine solution. J. Gen. Microbiol. 4: 330– 338 [DOI] [PubMed] [Google Scholar]
- 37. Rodbard D, Lenox RH, Wray HL, Ramseth D. 1976. Statistical characterization of the random errors in the radioimmunoassay dose-response variable. Clin. Chem. 22: 350– 358 [PubMed] [Google Scholar]
- 38. Sebaugh J. 2011. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 10: 128– 134 [DOI] [PubMed] [Google Scholar]
- 39. Johnson K, Nelson C, Busta F. 1983. Influence of temperature on germination and growth of spores of emetic and diarrheal strains of Bacillus cereus in a broth medium and in rice. J. Food Sci. 48: 286– 287 [Google Scholar]
- 40. Sanz P, Teel LD, Alem F, Carvalho HM, Darnell SC, O'Brien AD. 2008. Detection of Bacillus anthracis spore germination in vivo by bioluminescence imaging. Infect. Immun. 76: 1036– 1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winston ML. 1987. The biology of the honey bee. Harvard University Press, Cambridge, MA [Google Scholar]
- 42. Guidi-Rontani C, Pereira Y, Ruffie S, Sirard JC, Weber-Levy M, Mock M. 2002. Identification and characterization of a germination operon on the virulence plasmid pXOl of Bacillus anthracis. Mol. Microbiol. 33: 407– 414 [DOI] [PubMed] [Google Scholar]
- 43. Guidi-Rontani C, Weber-Levy M, Labruyère E, Mock M. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31: 9– 17 [DOI] [PubMed] [Google Scholar]
- 44. Barlass PJ, Houston CW, Clements MO, Moir A. 2002. Germination of Bacillus cereus spores in response to l-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148: 2089– 2095 [DOI] [PubMed] [Google Scholar]
- 45. Broussolle V, Gauillard F, Nguyen-The C, Carlin F. 2008. Diversity of spore germination in response to inosine and l-alanine and its interaction with NaCl and pH in the Bacillus cereus group. J. Appl. Microbiol. 105: 1081– 1090 [DOI] [PubMed] [Google Scholar]
- 46. Dodatko T, Akoachere M, Muehlbauer SM, Helfrich F, Howerton A, Ross C, Wysocki V, Brojatsch J, Abel-Santos E. 2009. Bacillus cereus spores release alanine that synergizes with inosine to promote germination. PLoS One 4: e6398 doi:10.1371/journal.pone.0006398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dodatko T, Akoachere M, Jimenez N, Alvarez Z, Abel-Santos E. 2010. Dissecting interactions between nucleosides and germination receptors in Bacillus cereus 569 spores. Microbiology 156:1244– 1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huo Z, Yang X, Raza W, Huang Q, Xu Y, Shen Q. 2010. Investigation of factors influencing spore germination of Paenibacillus polymyxa ACCC10252 and SQR-21. Appl. Microbiol. Biotechnol. 87: 527– 536 [DOI] [PubMed] [Google Scholar]
- 49. Smith M, Sullivan C. 1989. Germination of Clostridium cylindrosporum spores on medium containing uric acid. Appl. Environ. Microbiol. 55: 1380– 1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Warren SC, Gould GW. 1968. Bacillus cereus spore germination: absolute requirement for an amino acid. BBA-Gen. Subjects 170: 341– 350 [DOI] [PubMed] [Google Scholar]
- 51. Nordstrom S, Fries I. 1995. A comparison of media and cultural conditions for identification of Bacillus larvae in honey. J. Apic. Res. 34: 97– 103 [Google Scholar]
- 52. Allipi AM. 1995. Detection of Bacillus larvae spores in Argentinian honeys by using a semi-selective medium. Microbiologia 11: 343– 350 [PubMed] [Google Scholar]
- 53. Hornitzky M, Nicholls P. 1993. J medium is superior to sheep blood agar and brain heart infusion agar for the isolation of Bacillus larvae from honey samples. J. Apic. Res. 31: 51– 52 [Google Scholar]
- 54. Shimanuki H, Knox D. 2000. Diagnosis of honey bee diseases. U.S. Department of Agriculture; Beltsville, MD [Google Scholar]
- 55. Peng YC, Mussen E, Fong A, Montague MA, Tyler T. 1992. Effects of chlortetracycline of honey bee worker larvae reared in vitro. J. Invertebr. Pathol. 60: 127– 133 [Google Scholar]
- 56. Colibar O, Popovici D, Eugeniu C, Korodi G. 2010. The effect of acidifiant on the development of bee families (Apis mellifica). Med. Vet. 43: 296– 299. [Google Scholar]
- 57. Liming W, Jinhui Z, Xiaofeng X, Yi L, Jing Z. 2009. Fast determination of 26 amino acids and their content changes in royal jelly during storage using ultra-performance liquid chromatography. J. Food Compost. Anal. 22: 242– 249 [Google Scholar]
- 58. Yadav M. 2003. Physiology of insects. Discovery Publishing House Pvt. Limited, New Delhi, India [Google Scholar]
- 59. Jood S, Kapoor AC. 1993. Protein and uric acid contents of cereal grains as affected by insect infestation. Food Chem. 46: 143– 146 [DOI] [PubMed] [Google Scholar]
- 60. Foerster HF, Foster JW. 1966. Response of Bacillus spores to combinations of germinative compounds. J. Bacteriol. 91: 1168– 1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Luu H, Akoachere M, Patra M, Abel-Santos E. 2011. Cooperativity and interference of germination pathways in Bacillus anthracis spores. J. Bacteriol. 193: 4192– 4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Crailsheim K. 1988. Transport of leucine in the alimentary canal of the honeybee (Apis mellifera L.) and its dependence on season. J. Insect Physiol. 34: 1093– 1100 [Google Scholar]
- 63. Abel-Santos E, Dodatko T. 2007. Differential nucleoside recognition during Bacillus cereus 569 (ATCC 10876) spore germination. New J. Chem. 31: 748– 755 [Google Scholar]
- 64. Hornstra LM, de Vries YP, Wells-Bennik MHJ, de Vos WM, Abee T. 2006. Characterization of germination receptors of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 72: 44– 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McSpadden Gardener BB, Driks A. 2004. Overview of the nature and application of biocontrol microbes: Bacillus spp. Phytopathology 94: 1244. [DOI] [PubMed] [Google Scholar]
- 66. Salas JA, Ellar DJ. 1985. Uric acid and allantoin uptake by Bacillus fastidiosus spores. FEBS Lett. 183:256– 259 [Google Scholar]
- 67. Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB. 2009. Bergey's manual of systematic bacteriology, 2nd ed, vol 3: the firmicutes Springer, New York, NY [Google Scholar]
- 68. Romick TL, Tharrington G. 1997. An automated method for quantifying the l-alanine trigger of Bacillus subtilis spore germination and competitive inhibition by d-alanine. J. Rapid Methods Autom. Microbiol. 5: 215– 221 [Google Scholar]
- 69. Woese CR, Morowitz HJ, Hutchison CA., III 1958. Analysis of action of l-alanine analogues in spore germination. J. Bacteriol. 76: 578– 588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O'Connor RJ, Halvorson HO. 1961. l-Alanine dehydrogenase: a mechanism controlling the specificity of amino acid-induced germination of Bacillus cereus spores. J. Bacteriol. 82: 706– 713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fey G, Gould GW, Hitchins AD. 1964. Identification of d-alanine as the auto-inhibitor of germination of Bacillus globigii spores. J. Gen. Microbiol. 35: 229– 236 [DOI] [PubMed] [Google Scholar]
- 72. Hills G. 1949. Chemical factors in the germination of spore-bearing aerobes. The effects of amino-acids on the germination of Bacillus anthracis, with some observations on the relation of optical form to biological activity. Biochem. J. 45: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Radwanski ER, Last RL. 1995. Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell 7:921– 934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hirakawa H, Kodama T, Takumi-Kobayashi A, Honda T, Yamaguchi A. 2009. Secreted indole serves as a signal for expression of type III secretion system translocators in enterohaemorrhagic Escherichia coli O157: H7. Microbiology 155: 541– 550 [DOI] [PubMed] [Google Scholar]
- 75. Lee J, Attila C, Cirillo SL, Cirillo JD, Wood TK. 2009. Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb. Biotechnol. 2: 75– 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee JH, Lee J. 2010. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 34: 426– 444 [DOI] [PubMed] [Google Scholar]
- 77. Lee J, Jayaraman A, Wood TK. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nikaido E, Yamaguchi A, Nishino K. 2008. AcrAB multidrug efflux pump regulation in Salmonella enterica serovar Typhimurium by RamA in response to environmental signals. J. Biol. Chem. 283:24245– 24253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim YG, Lee JH, Cho MH, Lee J. 2011. Indole and 3-indolylacetonitrile inhibit spore maturation in Paenibacillus alvei. BMC Microbiol. 11: 119– 128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Karakaya S, El SN, Tas AA. 2001. Antioxidant activity of some foods containing phenolic compounds. Int. J. Food Sci. Nutr. 52: 501– 508 [PubMed] [Google Scholar]