Abstract
Streptococcus anginosus is a member of the anginosus group streptococci, which form part of the normal human oral flora. In contrast to the pyogenic group streptococci, our knowledge of the virulence factors of the anginosus group streptococci, including S. anginosus, is not sufficient to allow a clear understanding of the basis of their pathogenicity. Generally, hemolysins are thought to be important virulence factors in streptococcal infections. In the present study, a sag operon homologue was shown to be responsible for beta-hemolysis in S. anginosus strains by random gene knockout. Interestingly, contrary to pyogenic group streptococci, beta-hemolytic S. anginosus was shown to have two tandem sagA homologues, encoding streptolysin S (SLS)-like peptides, in the sag operon homologue. Gene deletion and complementation experiments revealed that both genes were functional, and these SLS-like peptides were essential for beta-hemolysis in beta-hemolytic S. anginosus. Furthermore, the amino acid sequence of these SLS-like peptides differed from that of the typical SLS of S. pyogenes, especially in their propeptide domain, and an amino acid residue indicated to be important for the cytolytic activity of SLS in S. pyogenes was deleted in both S. anginosus homologues. These data suggest that SLS-like peptides encoded by two sagA homologues in beta-hemolytic S. anginosus may be potential virulence factors with a different structure essential for hemolytic activity and/or the maturation process compared to the typical SLS present in pyogenic group streptococci.
INTRODUCTION
Streptococcus anginosus is a member of the anginosus group streptococci (AGS), which also include Streptococcus intermedius, S. constellatus subsp. constellatus, and S. constellatus subsp. pharyngis (1, 2). S. anginosus is an opportunistic pathogen and forms part of the normal flora in the human oral cavity, genitourinary tract, and gastrointestinal tract (3), and it is generally considered to have a relatively low pathogenic potential compared with other streptococci, in particular members of the pyogenic species group. However, S. anginosus is increasingly being recognized as being able to cause a wide range of purulent infections that commonly manifest as abscess formation, and its presence has also been detected in esophageal cancer (4–9). Despite the increased awareness of the clinical importance of S. anginosus, the molecular basis of pathogenicity of this species has not been determined. It has long been observed that some strains of S. anginosus give beta-hemolysis on blood agar, and it has been assumed that a beta-hemolytic reaction indicates production of a cytolytic factor(s) thought to be important in pathogenicity. To date, the only beta-hemolytic factor of AGS studied in detail is the toxin intermedilysin, a human-specific cholesterol (CHL)-dependent cytolysin (CDC) secreted from S. intermedius (10). Evidence to date indicates that the distribution of cdc gene homologues in AGS is limited to S. intermedius (11), and there are no reports describing other factors conferring beta-hemolytic capability on some strains of S. anginosus.
In the present study, identification of the factor for beta-hemolysis in strains of S. anginosus was conducted by a random gene knockout approach on the beta-hemolytic type strain of S. anginosus, NCTC10713. The gene(s) responsible for the production of the beta-hemolytic factor found in four nonhemolytic transformants shared high homology with the components of the sag operon, including sagA, present in pyogenic group streptococci, such as S. pyogenes, and encoding the cytolytic peptide streptolysin S (SLS). This is the first report on the distribution and characterization of the sag operon homologue in nonpyogenic, oral streptococci (i.e., S. anginosus). The specific gene structure of the sag operon homologue of S. anginosus (sagSA) and the contribution of sagSA to beta-hemolysis in beta-hemolytic S. anginosus are described in this article.
MATERIALS AND METHODS
Bacterial strains.
The type strain, NCTC10713, was used for molecular analyses of the SLS homologue of S. anginosus, and a further 125 strains of S. anginosus from human clinical sources were tested for epidemiological purposes. Initial confirmation of these strains as belonging to S. anginosus was by the PCR-based method of Takao et al. (12). The type strain of S. pyogenes GTC262 and a streptolysin O gene (slo)-deleted mutant of S. pyogenes strain NIH35 were also used for the comparison of hemolytic characteristics of SLS and SLS homologues. The latter was a kind gift from T. Sumitomo (Osaka University Graduate School of Dentistry). All strains were cultured in brain heart infusion (BHI) broth (Becton, Dickinson and Company) or on BHI agar plates in 5% CO2 (vol/vol) in air at 37°C. In order to observe beta-hemolysis, strains were spread onto or punctured into BHI agar plates containing 5% (vol/vol) human or horse blood and cultured as described above.
Transformation of S. anginosus by a competence-stimulating peptide.
Transformation of S. anginosus NCTC10713T was conducted using a competence-stimulating peptide (CSP) encoded by the comC gene of the comCDE system (13). An overnight culture of the target strain was incubated in BHI broth at 37°C in 5% (vol/vol) CO2 for 3 h in the presence of 100 μg/ml each of CSP and the DNA fragment to be incorporated into the genomic DNA of the target strain. Further incubation was continued at room temperature for 2 h to help facilitate transformation, and the transformation mixture was plated onto BHI agar plates containing appropriate antibiotics for the selection of transformants.
Screening of beta-hemolysis factors by random gene knockout.
In order to screen the factor(s) for beta-hemolysis of S. anginosus NCTC10713T, random gene knockout was conducted using plasmid pGhost9:ISS1 (14). The gene(s) encoding the beta-hemolytic factor was determined by plasmid rescue. Briefly, purified genomic DNA from candidate transformants which had lost their beta-hemolytic capability on BHI blood agar was digested with EcoRI and then self-ligated using DNA ligation Mighty mix (TaKaRa, Japan). Competent cells of Escherichia coli strain TG1 (14) were transformed with the self-ligated DNA according to a standard heating protocol and then plated onto LB agar containing 100 μg/ml erythromycin and incubated at 30°C. The genomic region disrupted by insertion with pGhost9:ISS1 was amplified by PCR using primers pGh9 02 Eco and 5′ ISS1(rev) Eco listed in Table 1 and the purified self-ligated DNA as the template. Each amplicon was purified, and DNA sequence analysis was carried out by BEX Co. Ltd. (Tokyo, Japan) using an ABI Prism 3130xl genetic analyzer (Applied Biosystems) DNA sequencer.
Table 1.
Primers used in this study
| No. | Name | Sequence (5′→3′)a | Target | Use |
|---|---|---|---|---|
| 1 | pGh9 02 Eco | GGTATACTACTGACAGCTTCC | pGhost9 ISS1 | Sequencing |
| 2 | 5′ ISS1(rev) Eco | CGTAGATAATAACCAACAGCG | pGhost9 ISS1 | Sequencing |
| 3 | up-sagA2-inv-Bw | TTGAGTTGTTTCTGCCACGC | sagA2 | Sequencing |
| 4 | sagB-inv-Bw | AGTCCTTCAACCTGTCTGGC | sagB | Sequencing |
| 5 | sagC-Fw1 | ATGAAATATCAATTAAATAG | sagC | Sequencing |
| 6 | sagC-inv-Bw | ATTGTTCGCCCAGCCTCTGC | sagC | Sequencing |
| 7 | sagC-Fw2 | CACAAGGTGCGCTGGCAAAA | sagC | Sequencing |
| 8 | sagC-Fw3 | CCCGTGAAATGGTAAAAGAC | sagC | Sequencing |
| 9 | sagD-inv-Bw | AAAATCCCTGTCCGATTGCC | sagD | Sequencing |
| 10 | sagD-Fw | CCAATTGATATGACCATCGG | sagD | Sequencing |
| 11 | sagE-Fw | TATTGATGTTGTGCTGACGC | sagE | Sequencing |
| 12 | sagF-Fw | TTTGGTGTATGGTAGCCACC | sagF | Sequencing |
| 13 | sagH-Bw | GCTCCTTCAAGCTGATAGCG | sagH | Sequencing |
| 14 | sagH-Bw2 | CCTAAGCCTAGATTAACGAG | sagH | Sequencing |
| 15 | sagI-down-Bw1 | CAGCTGGCTGCTTTGTTGGC | sagI | Sequencing |
| 16 | sagI-inv-Fw | AAGGGAGTTTATATGCTTCC | sagI-down | Sequencing |
| 17 | sagI-down-Bw2 | GGCACTATTAAAAATAGCAT | sagI-down | Sequencing |
| 18 | sagI-down-Bw3 | GCTACAGACTGTTGACGAAC | sagI-down | Sequencing |
| 19 | sagA1-Fw | ATGTTAAAATTTTCTTCAAACG | sagA1 | Detection |
| 20 | sagA1-Bw | TTATTTTGTAGGTGCTACGG | sagA1 | Detection |
| 21 | sagA2-Fw | ATGCTTAAATTAGATTCACATATTATGG | sagA2 | Detection |
| 22 | sagA2-Bw | TTAAGGTTTAATGTTTGTTGAACC | sagA2 | Detection |
| 23 | up-operon-Fw1 | GACGAGCAGATGTAGATGCC | up-operon | Mutant construction |
| 24 | up-sagA2-fus-Fw | ATGAAAAATTATGAGAATGC | up-sagA2 | Mutant construction |
| 25 | erm-Fw(BamHI) | AATGGATCCCCCGATAGCTTCCGCTATTG | erm | Mutant construction |
| 26 | erm-Bw(PstI) | GTACTGCAGCTAATAATTTATCTACATTCC | erm | Mutant construction |
| 27 | erm-Fw(SalI) | CGCGTCGACCCCGATAGCTTCCGCTATTG | erm | Mutant construction |
| 28 | up-operon-Fw2 | TGTCGCACGGAACCATTCGC | up-operon | Mutant construction |
| 29 | up-operon-Bw(PstI) | GCGCTGCAGGCGCGTTCTTATAGCATTTG | up-operon | Mutant construction |
| 30 | up-operon-Fw(SalI) | CGCGTCGACGATGGTTATATTTGTGAAATAGG | up-operon | Mutant construction |
| 31 | up-operon-Bw | GCGTAAACATTTTCAAACTAC | up-operon | Mutant construction |
| 32 | up-sagA2-Fw(BamHI) | CAGGATCCTCATGGATTGCTAGC | up-sagA2 | Mutant construction |
| 33 | up-sagB-Fw | AATTTTGTTTATAGGAATTAG | up-sagB | Mutant construction |
| 34 | up-sagB-fus-Bw | GCATTCTCATAATTTTTCATC | up-sagB | Mutant construction |
| 35 | sagA1-comp-Fw1(EcoRI) | GGGAATTCGGATTTGATAGTAATGTACG | up-sagA1 | Complementation |
| 36 | sagA1-comp-Fw2(EcoRI) | GGGAATTCAGTGTAAAGATTAGCTAGCC | up-sagA1 | Complementation |
| 37 | sagA1-comp-Bw(PstI) | GCGCTGCAGGCTTCATCCAAAGAATCGTC | sagA1-down | Complementation |
| 38 | sagA2-comp-Fw(EcoRI) | GGGAATTCGTTCATGATGAGTTAAAAAC | up-sagA2 | Complementation |
| 39 | sagA2-comp-Bw(PstI) | GCGCTGCAGAGATGAATCGATGCTTTACC | sagA2-down | Complementation |
| 40 | sagB-S1-Fw | AGCTTGTTGCTCAGCATCTCATGCC | sagB | 5′-RACE |
| 41 | sagB-A1-Bw | GATGATACTCTGAGAAAGAGAAGTGGTTGG | sagB | 5′-RACE |
| 42 | sagB-S2-Fw | TTCTGAATTTTTCACAGATTCAGCTGTAGC | sagB | 5′-RACE |
| 43 | sagB-A2-Bw | GCTTCGTCTCTTGTCAGGTGAGAGG | sagB | 5′-RACE |
| 44 | sagB-RT-Bw(phospho) | Phospho-AGACAGAGAAGCTGC | sagB | 5′-RACE |
| 45 | M13 Forward primer | GTAAAACGACGGCCAGT | pUC18 | Sequencing |
| 46 | M13 Reverse primer | CAGGAAACAGCTATGAC | pUC18 | Sequencing |
Restriction sites are underlined.
Sequencing of the gene(s) encoding the beta-hemolytic factor(s) was carried out by primer walking based on the sequence information obtained by the method mentioned above. The primers used for primer walking are listed in Table 1 (primers 1 to 18). For the preparation of templates for sequencing, PCR was conducted using PrimeSTAR DNA polymerase (TaKaRa) and the genomic DNA prepared from S. anginosus NCTC10713T as the template. The DNA sequencing of the amplicon was carried out as described above.
Phylogenetic analysis of beta-hemolytic factors.
A phylogenetic tree of SagA homologues was constructed by Njplot (15) using microcin B17 as an out-group. The amino acid sequences (accession numbers in parentheses) of SagA and its homologues from the following organisms were used for this phylogenetic analysis: Streptococcus pyogenes MGAS5005 (CP000017), Streptococcus dysgalactiae subsp. equisimilis GGS_124 (AP010935), Streptococcus equi subsp. equi 4047 (NC_012471), S. equi subsp. zooepidemicus MGCS10565 (NC_011134), S. equi subsp. zooepidemicus H70 (NC_012470), Streptococcus iniae 9117 (AF465842), Streptococcus pseudoporcinus SPIN20026 (NZ_AENS01000034), Streptococcus constellatus subsp. pharyngis SK1060 (NZ_AFUP01000001), Streptococcus anginosus NCTC10713T (SagA1 and SagA2; JN619420), S. anginosus R84/4972 (SagA1 and SagA2; JN619421), Clostridium botulinum Loch Maree (clostridiolysin S; CP000962), C. botulinum ATCC 3502 (AM412317), C. botulinum 657 (clostridiolysin S; NC_012658), Listeria monocytogenes F2365 (listeriolysin S; NC_002973), Staphylococcus aureus RF122 (staphylysin S; AJ938182) (16), and Escherichia coli MC4100 (microcin B17; M15469). Prior to Njplot analysis, the amino acid sequences described above were aligned by ClustalX (17).
PCR amplification of the genes encoding beta-hemolytic factors.
Each entire coding region for the beta-hemolytic factor was amplified by PCR with the primer sets sagA1-Fw and sagA1-Bw for sagA1, sagA2-Fw and sagA2-Bw for sagA2, sagA1-Fw and sagA2-Bw for the region containing sagA1 and sagA2, and sagA1-Fw and sagB-A2-Bw for the region from sagA1 to sagB and with the template genomic DNA prepared by the method described previously (18). The reaction mixture (total volume, 10 μl), containing 0.25 U of GoTaq DNA polymerase (Promega), 0.2 mM deoxynucleoside triphosphates (dNTPs) (Promega), 1.5 mM MgCl2 (Promega), 0.25 μM primers, and genomic DNA, was pretreated for 2 min at 94°C. Subsequently, the mixture was amplified (30 cycles) using the following parameters: 98°C for 30 s, 55°C for 30 s, and 72°C for 10 s, before a final heating step at 72°C for 5 min. The amplification of target genes was confirmed by agarose gel electrophoresis using TBE buffer system (89 mM Tris, 89 mM borate, 2 mM EDTA [pH 8.0]).
Measurement of cytolytic activity for culture supernatant.
The culture supernatants of tested strains were prepared by centrifugation (9,000 × g, 5 min) of mid- to late-logarithmic-phase cultures in BHI (optical density at 660 nm [OD660] of 1.0) incubated at 37°C in 5% (vol/vol) CO2. Human erythrocytes washed with phosphate-buffered saline (PBS) were incubated with serially diluted culture supernatant with PBS at 37°C for 1 h and then centrifuged (750 × g, 5 min). Subsequently, the precipitated intact erythrocytes were washed twice with PBS (750 × g, 5 min) and resuspended in sterilized deionized water. The OD540 of each suspension was measured in order to calculate the hemolytic activity according to the method previously reported (10). To assay susceptibility to inhibitors, prior to the interaction with erythrocytes the culture supernatant was preincubated for 5 min at room temperature with (i) lecithin at a final concentration of 0.02% (wt/vol), (ii) cholesterol at a final concentration of 1 μM, or (iii) both.
Construction of deletion mutants for the genes encoding the beta-hemolytic factor.
The deletion mutants for sagA1 and/or sagA2 were constructed by double-crossing-over homologous recombination with an erythromycin resistance marker for selection. The fragments for transformation were generated by PCR amplification using PrimeSTAR DNA polymerase (TaKaRa). A PCR primer set with individual restriction sites at each 5′ end was used in amplification of the erythromycin resistance gene cassette (erm) in order to secure effective ligation and the desired direction (Table 1).
In order to construct several mutants used in this study, a strain with erm and without sagA1 and sagA2 (template mutant) was first constructed as follows. Each component of the insert fragment was amplified by PCR using the following primer sets: (i) up-sagA2-fus-Fw and sagC-inv-Bw for the amplification of the region from sagB to sagC, (ii) up-sagA2-Fw(BamHI) and up-sagB-fus-Bw for the promoter region of sagA2, (iii) up-operon-Fw1 and up-operon-Bw(PstI) for the upstream region of sagA1, and (iv) erm-Fw(BamHI) and erm-Bw(PstI) for the erythromycin-resistant gene cassette. Amplicons were purified using a QIAEX II gel extraction kit (Qiagen). The purified fragments of sagB to sagC and the sagA2 promoter region were used as a template for fusion PCR carried out using Ex Taq DNA polymerase (TaKaRa) with the following reaction conditions: denaturation for 2 min at 94°C, followed by 30 cycles of 30 s at 98°C, 30 s at 55°C, and 10 s at 72°C before a final heating at 72°C for 5 min. The fusion PCR product, the fragment upstream of sagA1, and the erm fragment, were digested by restriction enzymes (PstI for upstream of sagA1, BamHI for the fusion PCR product, and both PstI and BamHI for erm), purified, and then ligated using DNA ligation Mighty mix (TaKaRa). The ligated mixture was used as the template in nested PCR with primer set up-operon-Fw2 and sagB-inv-Bw (Table 1), and the purified DNA fragment was used for transformation by CSP as described above. The clone with the desired mutation in the genomic DNA was screened for growth in the presence of 1 μg/ml erythromycin and the formation of a zone of beta-hemolysis on blood agar. The deletion of the target gene was confirmed by DNA sequencing of the amplified fragment of the target region with PrimeSTAR DNA polymerase (TaKaRa). The genomic DNA of the constructed template mutant was used as the template for PCR amplification of the erm gene fragment to generate the sagA1 deletion mutant, the sagA2 deletion mutant, and the control mutant with the erm gene upstream of sagA1.
Next, in order to prepare the remaining fragment for construction of these mutants, PCR amplification was conducted using the genomic DNA of S. anginosus NCTC10713T as a template using primers up-sagA2-Fw(BamHI) and sagC-inv-Bw for construction of the sagA1 deletion mutant, up-operon-Fw(SalI), sagA1-Bw, up-sagB-Fw, and sagC-inv-Bw for construction of the sagA2 deletion mutant, and up-operon-Fw(SalI) and sagC-inv-Bw for construction of the control mutant. The prepared components were digested with BamHI (for the sagA1 deletion mutant) or SalI (for the sagA2 deletion mutant and control strain) and then ligated, and the mixture was used as the PCR template with the primer set up-operon-Fw2 and sagB-inv-Bw (Table 1). The purified amplicon was used for transformation.
In order to use a host strain for complementation experiments of sagA1 and sagA2 described below, another double-deletion mutant for sagA1 and sagA2 was constructed as follows. Each fragment required was amplified by PCR using the following primer sets and templates: primer set up-operon-Fw1 and up-operon-Bw and the genomic DNA prepared from the control mutant for amplification of the region containing the erm gene and primer set up-sagB-Fw and sagC-inv-Bw and the genomic DNA prepared from S. anginosus NCTC10713T for the amplification of the region containing sagB to sagC. The purified fragments were phosphorylated and ligated using DNA ligation Mighty mix (TaKaRa), and nested PCR was conducted using primer set up-operon-Fw2 and sagB-inv-Bw to prepare the fragment for transformation.
The purified DNA fragments prepared by nested PCR were used for transformation with CSP. The clone with the desired mutation in the genomic DNA was also screened by the method described above. The construction of the mutants was also confirmed by DNA sequencing of the amplified fragment of the target region using PrimeSTAR DNA polymerase (TaKaRa).
Complementation experiment of beta-hemolysis in the sagA1 and sagA2 double-deletion mutant.
The plasmids for complementation experiments were constructed as follows. DNA fragments for insertion were amplified by PCR with primer sets (i) sagA1-comp-Fw1(EcoRI) and sagA2-comp-Bw(PstI) for amplification of the region of sagA1 plus sagA2 with three potential prokaryotic promoters for sagA1, (ii) sagA1-comp-Fw1(EcoRI) and sagA1-comp-Bw(PstI) for amplification of the region of sagA1 with three potential prokaryotic promoters for sagA1, and (iii) sagA2-comp-Fw(EcoRI) and sagA2-comp-Bw(PstI) for amplification of the region of sagA2 with their own promoter, using genomic DNA from S. anginosus NCTC10713T as the template. Other DNA fragments were also amplified with primer sets (iv) sagA1-comp-Fw2(EcoRI) and sagA2-comp-Bw(PstI) for amplification of the region of sagA1 plus sagA2 with the latter two of the three potential prokaryotic promoter sequences for sagA1 and with (v) sagA1-comp-Fw2(EcoRI) and sagA1-comp-Bw(PstI) for amplification of the region of sagA1 with the latter two of the three potential prokaryotic promoter sequences for sagA1. These amplicons were digested with both EcoRI and PstI, ligated into pSETN1 (19) digested with the same restriction enzymes. Competent cells of Escherichia coli DH5α Z1 (20) were transformed with these plasmids according to the standard heating method and then plated on LB agar containing 40 μg/ml chloramphenicol and incubated at 37°C. After sequence confirmation, the constructed plasmids were used for complementation experiments. Each plasmid for complementation was introduced into the sagA1 and sagA2 double-deletion mutant by the CSP described above and plated onto BHI agar containing 3 μg/ml chloramphenicol and 1 μg/ml erythromycin. The complementation of target genes was evaluated by the formation of beta-hemolysis on blood agar.
Investigation of transcription of the sag operon homologue of S. anginosus.
The investigation of transcription for sagSA was conducted by 5′ rapid amplification of cDNA ends (5′-RACE). Briefly, the total RNA was prepared from the early-log-phase (8 h) culture of S. anginosus NCTC10713T by NucleoSpin RNA II (Macherey-Nagel) according to the manual appended to the kit with a modification for the cell lysis condition using lysozyme and mutanolysin with an RNase inhibitor. 5′-RACE was conducted with the 5′-Full RACE Core set (TaKaRa) according to the manual appended to the kit using primer sagB-RT-Bw(phospho) for reverse transcription. The PCR enzyme used was Ex Taq DNA polymerase (TaKaRa), the primer set for the first PCR was sagB-S1-Fw and sagB-A1-Bw, and that for the second PCR was sagB-S2-Fw and sagB-A2-Bw (Table 1).
In order to determine the start point of each sagSA transcript, sequencing of the products obtained in 5′-RACE analysis was carried out as follows. The 5′-RACE product fractions were purified from the agarose fragments (two fractions: upper region from around 1,000 bp to 800 bp and the lower region from around 500 bp to 200 bp) after agarose gel electrophoresis and phosphorylated by T4 polynucleotide kinase (Roche) at 37°C for 1 h. Plasmid pUC18 was cut by SmaI, and both resultant blunt ends were dephosphorylated using shrimp alkaline phosphatase (Roche). Subsequently, the purified dephosphorylated pUC18 and each phosphorylated 5′-RACE product fraction were ligated using DNA ligation Mighty mix (TaKaRa). The competent cells of E. coli JM109 were transformed by each recombinant plasmid and screened on an LB plate containing 50 to 100 μg/ml of ampicillin, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and 0.004% (wt/vol) 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal). After an overnight incubation at 37°C, the plasmids were purified from white colonies, and the size of each cloned fragment was confirmed by double digestion with EcoRI and SalI. Finally, DNA sequence analysis of each insert was carried out using sequence primers, M13 Forward primer and M13 Reverse primer (Table 1).
Nucleotide sequence accession numbers.
Nucleotide sequence data have been submitted to the DDBJ/EMBL/GenBank databases under accession no. JN619420 for the sag operon homologue of S. anginosus NCTC10713T and JN619421 and JN619422 for the regions around sagA1 and sagA2 of S. anginosus R84/4972 and R87/1657, respectively.
RESULTS
Screening and identification of the gene encoding the beta-hemolysis factor of S. anginosus.
Screening of the gene encoding the beta-hemolytic factor was carried out by random gene knockout with pGhost9:ISS1 in S. anginosus strain NCTC10713T. Previously, successful random gene knockout with this vector system was reported in Streptococcus agalactiae for screening the genetic locus essential for the production of hemolysin (21). Four nonhemolytic clones were detected and revealed that the genes with insertion of pGhost9:ISS1 were homologues of sagB, sagE, and sagI (2 clones), corresponding to genes coding for components of the sag operon of pyogenic group streptococci (Fig. 1).
Fig 1.
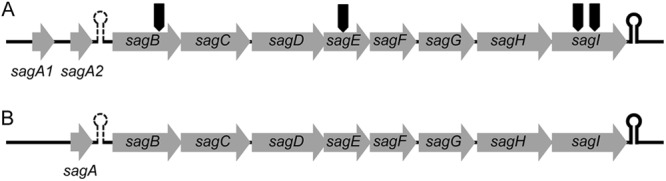
Genetic structure of sagSA (A) and the “typical” sag operon from other pyogenic group streptococci (B). sagSA is characterized by the presence of twin sagA homologues (sagA1 and sagA2). The arrows in panel A indicate the insertion sites of pGhost9:ISS1 in four nonhemolytic transformants obtained in random gene knockout experiments.
The entire DNA sequence of the sagSA was determined by primer walking using the primers listed in Table 1 and based on the partial DNA sequence information of the sagE and sagI genes. In comparison to the nucleotide sequencing of the shotgun sequence of S. anginosus SK52 (NCTC10713T) in the NCBI database (BioProject PRJNA64677), our sequence data in the present study differed by 2 nucleotides in the 9210 base sequence of the sagSA: one located in the sagB homologue and the other located in the sagI homologue. The nucleotide difference in sagI leads to a silent mutation. However, the nucleotide difference in the ORF of sagB is important because 1 base deletion is found in the BioProject database, and this nucleotide deletion results in a frameshift mutation. If the BioProject data are correct, this mutation results in a truncation of the sagB homologue to make a short product of 16 amino acid residues, whereas based on our sequencing results, SagB is predicted to code for 316 amino acid residues (i.e., the same number as SagB of S. pyogenes). This suggests that the data registered in the BioProject database for the sag operon homologue from S. anginosus SK52 should be corrected.
It was determined that sagSA consisted of functional genes homologous to the components of the sag operon of S. pyogenes by gene analysis using GENETYX software. The arrangement of the genes from sagB to sagI of sagSA was very similar to that of S. pyogenes (Fig. 1). However, a significant difference was observed around the sagA gene. Interestingly, two sagA homologues existed in tandem at the upstream region of the sagB gene in sagSA. No such tandem structure was found in the sag operons of pyogenic group streptococci with a single sagA gene (Fig. 1). As shown in Fig. 1A, the two sagA homologues in S. anginosus are designated sagA1 and sagA2, respectively.
A phylogenetic analysis for the transcriptional products of sagA1 and sagA2 was carried out (Fig. 2). The phylogenetic tree shows that SagA homologues of S. anginosus (SagA1 and SagA2) were clustered separately from a pyogenic streptococcal SagA (Fig. 2). Recently, whole-genome shotgun sequencing of S. constellatus subsp. pharyngis strain SK1060 (BioProject PRJNA67177) was reported, and it was revealed that this subspecies also has a homologue of the sag operon: SagA deduced from the sagA gene in this homologue was more similar to SagA1 than was SagA2.
Fig 2.
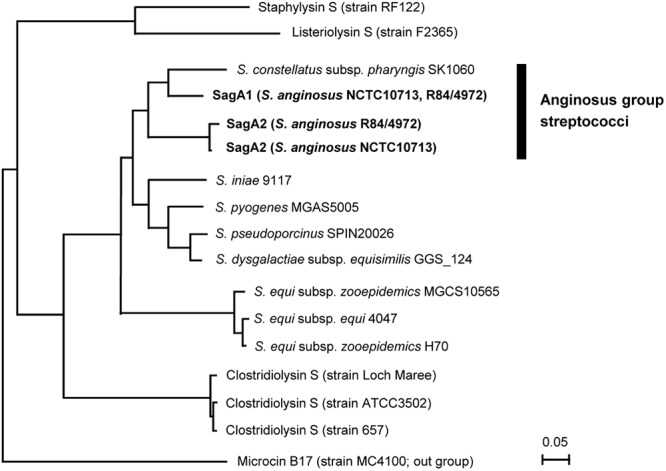
Phylogenetic tree of SagA of streptococci and their homologues in other bacterial genera. The amino acid sequences were aligned by ClustalX, and the phylogenetic tree was constructed by Njplot using microcin B17 as an out-group. SagA1 and SagA2 from S. anginosus strains are indicated in boldface.
The DNA sequence of the genes making up sagSA was compared with their homologues in the sag operons found in other pyogenic group streptococci, including S. pyogenes MGAS5005 (GenBank accession no. CP000017), S. dysgalactiae subsp. equisimilis GGS_124 (GenBank accession no. AP010935), S. equi subsp. zooepidemicus MGCS10565 (GenBank accession no. CP001129), and S. iniae strain 9117 (GenBank accession no. AF465842). The sequence identity of each gene is listed in Table 2. The components of sagSA, sagA encoding the cytolytic peptide protoxin (22), sagB, sagC, and sagD contributing to heterocycle formation of protoxin (23), and sagG, sagH, and sagI contributing to extracellular transport of the mature toxin (22) shared higher DNA sequence identity (>66%) with those of the pyogenic group species sag operon, although the sequence homologies of sagE and sagF were found to be relatively low (Table 2). These DNA homology results indicate that the product of each component of sagSA may have a similar function to the corresponding product derived from sag operons in pyogenic group species (sagBCD to heterocycle formation and sagGHI to extracellular transport) and that sagA1 and sagA2 will be processed and exported to the extracellular milieu to function in the same way as SLS.
Table 2.
Sequence homology of the genes composing sagSA of strain NCTC10713T against the corresponding genes of other streptococcal sag operons
| Speciesa | % identityb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| sagA1c | sagA2c | sagB | sagC | sagD | sagE | sagF | sagG | sagH | sagI | |
| S. pyogenes | 69 | 70 | 69 | 69 | 74 | 56 | 53 | 71 | 70 | 67 |
| S. dysgalactiae subsp. equisimilis | 71 | 68 | 71 | 69 | 75 | 57 | 57 | 72 | 71 | 68 |
| S. equi subsp. zooepidemicus | 73 | 67 | 71 | 68 | 74 | 60 | 57 | 69 | 73 | 68 |
| S. iniae | 70 | 68 | 71 | 70 | 75 | 62 | 55 | 70 | 72 | 66 |
Shown are results from S. pyogenes MGAS5005, S. dysgalactiae subsp. equisimilis GGS_124, S. equi subsp. zooepidemicus MGCS10565, and S. iniae 9117.
The results represent the percentage of identity of each gene in the sagSA of strain NCTC10713T versus the corresponding gene in other streptococcal sag operons.
Both sagA1 and sagA2 genes were compared to the sagA genes of other streptococci.
Structural comparison of sagA and the translation product in S. anginosus and S. pyogenes.
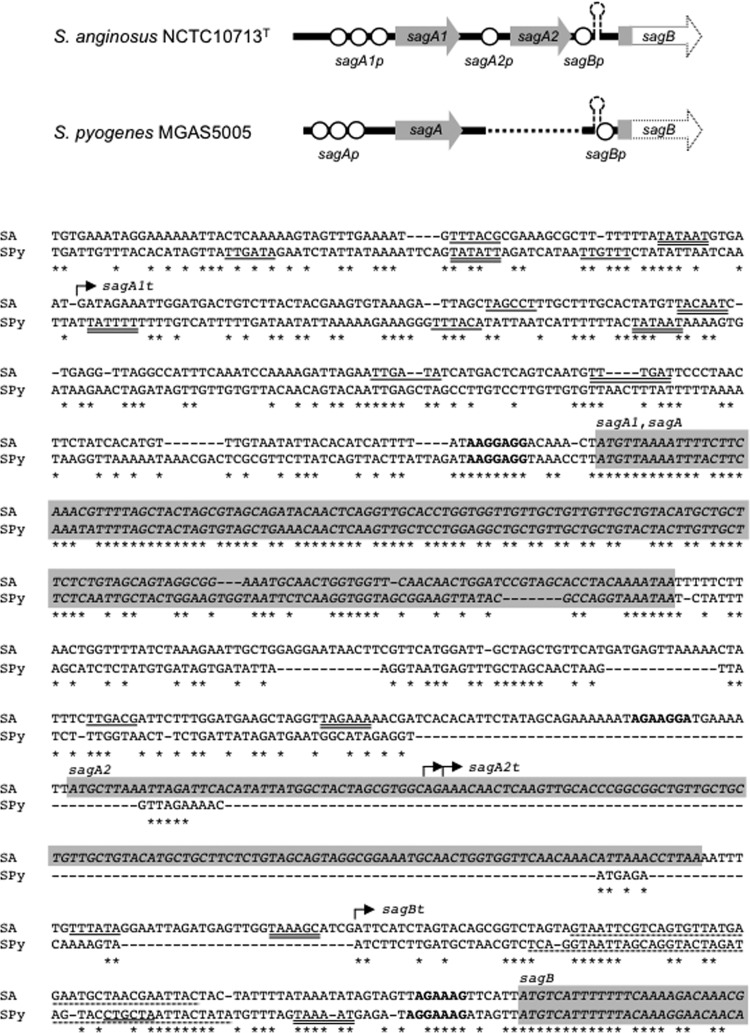
DNA sequence analysis of the region around sagA1 and sagA2 in S. anginosus revealed that sagA1 and sagA2 had three prokaryotic promoter sequences and one potential prokaryotic promoter sequence, respectively, with an additional promoter located between sagA2 and sagB for the sagB gene and other downstream genes in the operon (Fig. 3). The sag operon of S. pyogenes also had the potential prokaryotic promoter sequences upstream of sagA, although the positions of these promoters differed from those of the promoters for sagSA (Fig. 3). Interestingly, a terminator-like structure was found upstream of sagB in both S. anginosus and S. pyogenes. However, in S. anginosus the terminator-like structure was located between the sagB promoter and its Shine-Dalgarno (SD) sequence, in contrast to S. pyogenes, where it partially overlapped the potential promoter sequence of sagB (Fig. 3). Alignment of the regions upstream of sagB for S. anginosus and S. pyogenes showed that the position of sagA in the latter species corresponded to the position of sagA1 in S. anginosus and not to that of sagA2 (Fig. 3). Overall, the region within the operons from sagB to sagI exhibited a much higher degree of conservation between S. anginosus and pyogenic group streptococci than was observed in the region around sagA. Consequently, the alignment of the deduced amino acid sequences of sagA1 and sagA2 products, SagA1 and SagA2 of S. anginosus NCTC10713T (Fig. 4A), shows that the primary structures of SagA1 and SagA2 are highly conserved, although several amino acid deletions and substitutions were observed, especially in the C-terminal region of SagA2. Moreover, the alignment of SagA1, SagA2, and SLS/SagA of S. pyogenes revealed sequence conservation between the peptides overall (Fig. 4B), but with considerable diversity in the C-terminal region, including a notable deletion of a single amino acid (corresponding to S39 in SLS/SagA of S. pyogenes) observed in both SagA1 and SagA2 of S. anginosus (Fig. 4B). The amino acids potentially contributing to heterocycle formation are also shown in Fig. 4C, and the number and location of candidate amino acids concerned with oxazoline/thiazoline formation were observed to vary between SagA1 and SagA2 of S. anginosus and SagA of S. pyogenes.
Fig 3.
Genetic structure of the promoter regions of sagA1 to sagB in S. anginosus and sagA to sagB in S. pyogenes. The typical prokaryotic promoters predicted from their DNA sequences are shown with suffix “p” in the scheme at the top. The nucleotide sequence alignments of the region from the promoter of sagA1 to the head part of sagB ORF of S. anginosus NCTC10713T (SA) and from the promoter of sagA to the head part of sagB of S. pyogenes MGAS5005 (SPy) are also shown. The deduced −35 and −10 regions are single and double underlined, respectively. The potential promoters with a score value above 57.00 predicted in the analyses using the program “Search for Promoter Sequence” within GENETYX software are shown. Moreover, a deduced promoter for sagB of S. anginosus is also shown, although the score value in this case was 53.25. Rho-independent terminators are indicated by a broken line, and SD sequences shown in bold. The ORF of each gene is shown italicized with a shaded background. The start point of each transcript of sagSA revealed by 5′-RACE analysis (sagA1t, sagA2t, and sagBt) is indicated by an arrow.
Fig 4.
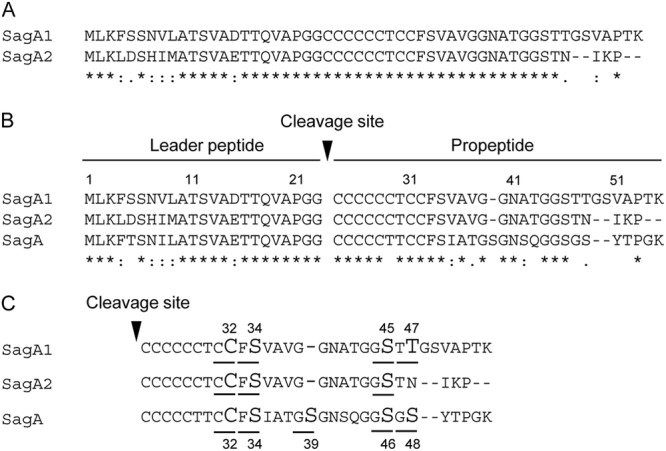
Alignments of amino acid sequences of SagA and homologues. (A) Identity of SagA1 with SagA2 of S. anginosus NCTC10713T. (B) Alignment of the amino acid sequences of SagA homologues: SagA1 and SagA2 of S. anginosus NCTC10713T and typical SagA of S. pyogenes MGAS5005. The alignment analyses were conducted using ClustalX. The symbols indicating identity/homology of primary sequence are as follows: *, identical amino acid, “:,” amino acid highly conserved; “.,” amino acid somewhat similar. The amino acids deduced to contribute to heterocycle formation in SagA1, SagA2, and SagA are underlined, and the amino acids predicted to be indispensable residues for heterocycle formation according to the analysis in SagA are shown in large font (C).
Transcription of the sag operon of S. anginosus.
The transcription of sagSA was confirmed with particular regard to the function of the promoter(s) in the operon. Since sagSA had several predicted promoters (shown in Fig. 3), 5′-RACE analysis was carried out. Partial cDNAs covering from the 5′ end to midway through the sagB ORF of each transcript were amplified by PCR. The result shows that two major bands were amplified: an upper band of approximately 1,000 bp and a lower band of below 500 bp (Fig. 5A). The length of both amplicons (shown in Fig. 5A) corresponded well with the sizes predicted from the sequence information of sagSA, i.e., with larger and smaller amplicons transcribed from the sagA1 promoter(s) and from the sagA2 promoter, respectively. A small band of approximately 250 bp in size, predicted as the transcript from the sagB promoter, was detected as a faint band in this analysis.
Fig 5.
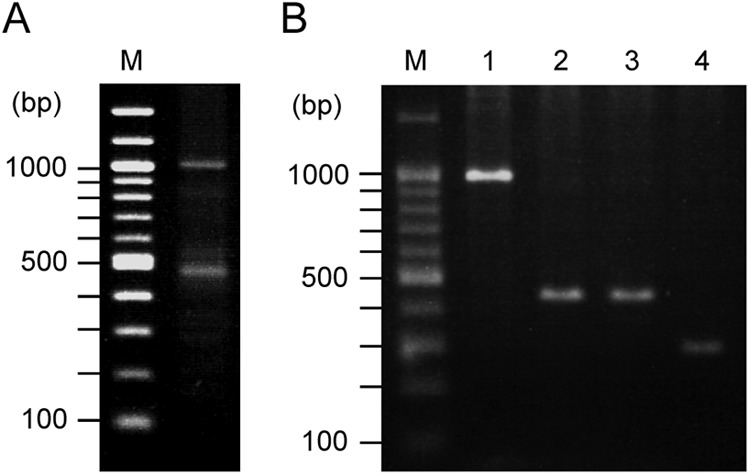
(A) 5′-RACE analysis of sagSA. Total RNA from early-logarithmic-phase cells was prepared and used for 5′-RACE analysis. (A) The resulting DNA fragments containing the 5′ terminus of cDNA from mRNA sequence(s) of sagSA were amplified by nested PCR and analyzed by agarose-gel electrophoresis. M, hundred-base-pair ladder marker. (B) Cloned 5′-RACE products for sequencing. The 5′-RACE product cloned in the SmaI site of pUC18 was obtained by double digestion with EcoRI and SalI and separated by agarose gel electrophoresis. M, hundred-base-pair ladder size marker. Lanes 1 to 4 show the 5′-end part of cDNA deduced to be the transcripts from sagA1p (lane 1), sagA2p (lanes 2 and 3), and sagBp (lane 4).
Subsequently, the start points of the sagSA transcripts were also investigated after cloning of the 5′-RACE products. Although the transcript induced by the sagB promoter was relatively faint in Fig. 5A, we succeeded in cloning the cDNAs with the 5′-end sequence information of the transcripts from three gene promoters (Fig. 5B). Sequencing of the cDNA clones revealed that the promoters for sagA1, sagA2, and sagB were all functional in vivo, and the start points of sagSA transcription are shown in Fig. 3. Among the three potential prokaryotic promoters for sagA1, it was revealed that the first promoter (−35 sequence, TTTACG; −10 sequence, TATAAT; score, 67.46) was preferentially used for the transcription from sagA1 onward. The promoter for sagA2 (−35 sequence, TTGACG; −10 sequence, TAGAAA; score, 60.36) was also functional; however, the cloned transcript cDNAs were partially deleted in their 5′-terminal region, including midway through the sagA2 gene with their SD sequence. This fact suggests the transcript induced by this promoter may be unstable, and the intracellular level of intact transcript may be low. Interestingly, the predicted promoter for sagB (−35 sequence, TTTATA; −10 sequence, TAAAGC; score, 53.25) also functioned in vivo. Nevertheless the latter was difficult to detect, as shown in Fig. 5A, perhaps because of low transcription frequency.
Characterization of beta-hemolysis induced by S. anginosus.
As a result of the random gene knockout experiment indicating that sagSA is responsible for beta-hemolysis in S. anginosus, the hemolytic activity was characterized. It was reported that the hemolytic activity of SLS secreted by S. pyogenes, which is a typical mature hemolysin product of the sag operon, is inhibited by the membrane lipid component lecithin (24). Therefore, the hemolytic activity shown by the culture supernatant of S. anginosus NCTC10713T was investigated in the absence or presence of lecithin. It was observed that the hemolysis induced by S. anginosus NCTC10713T culture supernatant decreased markedly in the presence of lecithin (Fig. 6A), suggesting that the SLS-like peptide secreted from S. anginosus NCTC10713T has similar biochemical properties and biological function to the SLSs of pyogenic group streptococci, such as S. pyogenes. On the other hand, obvious inhibition of the hemolytic activity in the presence of lecithin was not shown for culture supernatant of S. pyogenes GTC262T (Fig. 6A). However, the hemolytic activity of culture supernatant of S. pyogenes GTC262T was significantly inhibited in the presence of CHL and completely inhibited by the combination of CHL and lecithin. These results suggest that a large part of the hemolysis by S. pyogenes GTC262T depends on the hemolysin with susceptibility to CHL (i.e., SLO).
Fig 6.
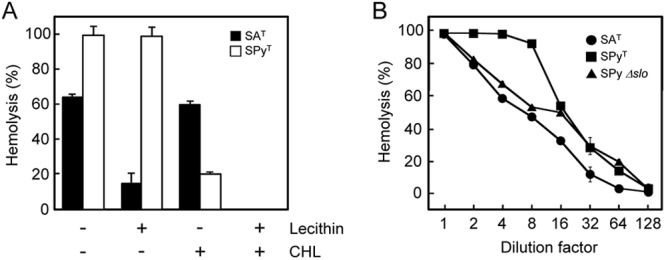
(A) Effect of lecithin and cholesterol on hemolysis induced by the hemolysin secreted by S. anginosus and S. pyogenes type strains. Culture supernatants were preincubated with 0.02% (wt/vol) lecithin or 1 μM cholesterol (CHL) and then incubated with human erythrocytes. Control reaction mixtures contained no lecithin or cholesterol. All results are shown as mean values (n = 3) with standard deviations (SD). (B) Dose dependency of hemolysis by culture supernatant of the S. anginosus type strain, S. pyogenes type strain, and the S. pyogenes slo-deleted mutant (Δslo). The supernatants from mid- to late-logarithmic-phase culture (OD660 of 1.0) of the S. anginosus type strain (circles), S. pyogenes type strain (squares), and the S. pyogenes Δslo mutant (triangle) were 2-fold serially diluted and then incubated with human erythrocytes. All results are shown as mean values (n = 2) with differences.
The dose dependency of hemolytic activity shown by culture supernatants of S. anginosus NCTC10713T and S. pyogenes GTC262T was also investigated. Similarly to the result shown in Fig. 6A, the hemolytic activity of S. pyogenes GTC262T was higher than that of S. anginosus NCTC10713T (Fig. 6B). In order to exclude the involvement of SLO, a Δslo mutant of S. pyogenes was also investigated. It was revealed that the dose dependency of hemolytic activity of S. anginosus NCTC10713T was nearly the same as that of the Δslo mutant of S. pyogenes secreting only SLS. From the data shown in Fig. 6A and B, we conclude that hemolysis by beta-hemolytic S. anginosus NCTC10713T is induced by a similar hemolysin to the SLS of S. pyogenes.
Contribution of the sagA1 and sagA2 genes to beta-hemolysis of S. anginosus.
The correlation between the presence of the sagSA operon and beta-hemolysis was also investigated genotypically. One hundred twenty-six strains of S. anginosus were assayed for possession of the sagA1 and sagA2 genes by PCR amplification, and it was revealed that, without exception, all beta-hemolytic S. anginosus strains possessed both sagA1 and sagA2 genes, and all nonhemolytic strains had neither sagA1 nor sagA2 genes (Table 3). The results of testing of 3 representative strains of beta-hemolytic S. anginosus (including NCTC10713T) and 4 nonhemolytic S. anginosus strains are shown in Fig. 7. All beta-hemolytic S. anginosus strains had the characteristic tandem organization of sagA1 and sagA2 upstream of sagB seen with NCTC10713T (Fig. 1 and 3).
Table 3.
Detection of the sagA1 and sagA2 genes by PCR amplification in S. anginosus clinical isolates
| Strain type | Positivity of PCR amplification (no. of positive strains/total) |
||
|---|---|---|---|
| Only sagA1 | Only sagA2 | sagA1 and sagA2 | |
| Beta-hemolytic | 0/24 | 0/24 | 24/24 |
| Nonhemolytic | 0/102 | 0/102 | 0/102 |
Fig 7.
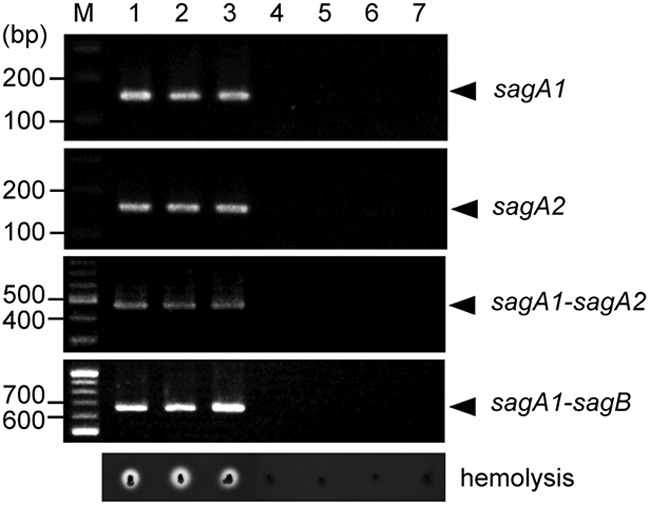
Relationship between the presence of the sagA1 and sagA2 genes and beta-hemolysis. Typical results of PCR amplification obtained in three beta-hemolytic strains and four nonhemolytic strains of S. anginosus are shown. Beta-hemolysis on blood agar plates for these strains is also indicated.
In order to investigate the contribution of both sagA1 and sagA2 expression to beta-hemolysis, targeted gene deletion was conducted in S. anginosus NCTC10713T. Figure 8 shows a schematic representation of the genetic structures of the deletion mutants constructed. The mutated strains were a sagA1 sagA2 double-deletion mutant (Fig. 8B), a sagA2 single-deletion mutant (Fig. 8C), and a sagA1 single-deletion mutant (Fig. 8D). Moreover, a mutant with only an erythromycin-resistant gene cassette inserted just upstream of the region of sagSA was constructed as the control strain for these deletion mutants (Fig. 8E). The hemolytic phenotype of each mutant was investigated on human blood agar plates by stab inoculation (Fig. 8, right-hand side). The double-deletion mutant showed a complete loss of beta-hemolytic activity, whereas both sagA2 and sagA1 single-deletion mutants still gave beta-hemolysis, demonstrating that sagA1 and sagA2 each contributed to the beta-hemolytic phenotype. It was noted that the beta-hemolytic zone caused by the sagA1 single-deletion mutant was smaller than that caused by the parent strain (Fig. 8).
Fig 8.
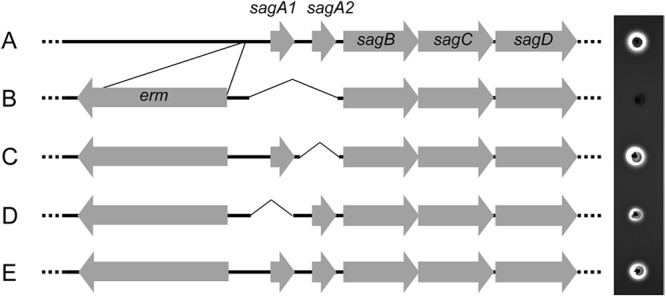
Relationship between the presence of sagA homologues and beta-hemolysis from gene deletion experiments. Schemes of the genetic structure of parent strain S. anginosus NCTC10713T (A), the sagA1 sagA2 double-deletion mutant (B), the sagA2 single-deletion mutant (C), the sagA1 single-deletion mutant (D), and a control mutant containing an erythromycin resistance gene cassette in the reverse direction just upstream of the intact sagSA (E) are shown together with their beta-hemolytic activity on human blood agar plates.
Subsequently, complementation experiments of the sagA1 and sagA2 genes using their own promoters were also carried out, as shown in Fig. 9. The hemolytic activity of the complemented strain with both sagA1 and sagA2 genes was successfully restored to virtually the original levels of the control and parent strains (Fig. 9B). Moreover, complementation with either the sagA1 or sagA2 gene also gave beta-hemolytic activity, indicating that both sagA1 and sagA2 genes can function individually to confer beta-hemolytic activity on S. anginosus (Fig. 9D and F). Similarly to the sagA1 single-deletion mutant (Fig. 8), the beta-hemolysis observed in the sagA2-complemented strain was, again, weaker than that of the control strain (Fig. 9F). Interestingly, the promoter region for the sagA1 gene lacking the first promoter preferentially used in vivo also induced transcription of both sagA1 and sagA2 or sagA1 (Fig. 9C and E).
Fig 9.
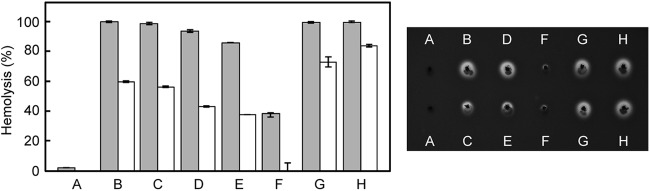
Complementation of sagA homologues in the sagA1 sagA2 double-deletion mutant. (Right) Hemolysis on human blood agar after 1 day of cultivation. (A) The sagA1 sagA2 double-deletion mutant. (B to F) Complementation of strains with both sagA1 and sagA2 transcribed by the intact promoter of sagA1 with three potential promoters (Fig. 3) (B), by the truncated promoter of sagA1 with the second and the last potential promoters (see Fig. 3) (C), with only sagA1 transcribed by the intact promoter of sagA1 (D) or by the truncated promoter of sagA1 (E), or with only sagA2 transcribed by its own promoter (F). Hemolytic patterns of a control mutant with an erythromycin-resistance gene cassette just upstream of intact sagSA (G) and the parent strain (H) are also shown. (Left) Graph showing the hemolytic activity of the culture supernatant for each strain. Gray bars indicate the hemolytic activity of the original culture supernatant, and white bars indicate the hemolytic activity of culture supernatant diluted 3-fold with PBS. Results are shown as mean values (n = 2) with the differences between duplicates indicated.
DISCUSSION
Beta-hemolysis on blood agar plates is of significance to the medical microbiologist, being taken as an indication of the production of cytolytic factor(s) such as cytolysins. A significant proportion of clinical isolates of S. anginosus, including the type strain, NCTC10713, produce beta-hemolysis on blood-agar plates. However, the factor causing beta-hemolysis in this species has not previously been identified and characterized in detail.
In this study, we investigated beta-hemolysis in S. anginosus NCTC10713T and demonstrated that the translational products of the sagA genes in sagSA are responsible for this phenotype. The sag operon is a gene cluster composed of 9 genes and is present in the pyogenic group streptococci (22). In S. pyogenes, the effector molecule, streptolysin S (SLS), functions as the beta-hemolysin and is the product of the sagA gene within the sag operon. The other genes in the operon contribute to the maturation (sagB, sagC, and sagD) and secretion (sagG, sagH, and sagI) of SLS (22, 23). The genetic structure of the sag operon is highly conserved in the pyogenic group species (25, 26). Recently, the presence of sag operon homologues has been reported in other Gram-positive human pathogens: for example, Clostridium spp. (27), Listeria monocytogenes (28), and Staphylococcus aureus (29). The products of these operons, SLS-like peptides, are now categorized in a family named the thiazole/oxazole-modified microcins (TOMMs) (16). The typical operon structure encoding the TOMMs in Streptococcus spp. has been shown to be composed of 9 genes (Fig. 1B). However, it was demonstrated that the sagSA operon in S. anginosus was composed of 10 genes, including two sagA homologues (designated sagA1 and sagA2) and conserved sagB to sagI genes (Fig. 1A). This operon structure is specific for S. anginosus, and it has not been observed in other bacterial sag operons. Interestingly, our investigation clearly shows that only beta-hemolytic strains of S. anginosus possess sagSA and that the presence of sagSA in S. anginosus is not universal, as is the case in S. pyogenes and other pyogenic group streptococci, which all possess the sag operon. The DNA sequence of sagA in pyogenic group streptococci showed higher homology with sagA1 than with sagA2 of S. anginosus (Fig. 3). The reason why S. anginosus has two sagA homologues is unclear; however, the tandem arrangement of sagA1 and sagA2 in sagSA may be due to gene duplication of sagA in an “S. pyogenes-type” sag operon with a single sagA mutation and subsequent mutations in sagA2. Alternatively the previous loss of sagA2 from an original sagSA operon might have resulted in the operon arrangement observed in S. pyogenes.
In the gene deletion experiments, the sagA1 single-deletion mutant showed weaker beta-hemolysis than its parent strain and the sagA2 deletion strain (Fig. 8D). A similar tendency was observed from the complementation experiments: the sagA2-complemented strain also showed weaker restored beta-hemolysis than that of the sagA1-complementated strain (Fig. 9F). These results suggested that the contribution to beta-hemolysis by S. anginosus of SagA1 was stronger than that of SagA2. We hypothesize that the weaker beta-hemolysis observed in the sagA1 single-deletion strain than was observed in the sagA2 single-deletion strain is due to transcript stability, as indicated by the results of the 5′-RACE analysis (Fig. 3 and 5); the amount of intact/functional mRNA for SagA2 would be lower than that for SagA1, resulting in less hemolytic activity by the former. Because it is well known that the immunogenicity of SLS is quite low and the antibody usable for the detection of SLS is difficult to raise (30), we have not yet succeeded in quantifying SagA1 and SagA2 individually in order to obtain a definite conclusion to this question.
In the complementation experiments, the beta-hemolytic activity of the sagA2-complemented strain was reduced and was not restored to the level of the sagA1 single-deletion mutant (Fig. 9F). DNA sequence analysis of sagSA demonstrated not only a deduced promoter sequence upstream of the sagB ORF but also the presence of a terminator-like structure between the promoter and SD sequence (Fig. 3). This terminator-like structure did not stop the transcription induced by the promoters of the sagA1 and sagA2 genes, as shown in Fig. 5A. Moreover, the transcription induced by the sagB promoter occurred as shown in Fig. 3 and 5B, and resulted in the maturation and transport of SagA homologues complemented in trans, as shown in Fig. 9, although the transcript induced by the sagB promoter was faintly detectable in 5′-RACE analysis, as shown in Fig. 5A. The terminator-like structure could not prevent mRNA elongation from sagA1 and sagA2; however, this may cause reduced function of the sagB promoter at the initial step in transcription, and the components for maturation and transport of SagA1 and SagA2 (i.e., SagB to SagI) may not be produced efficiently in the sagA1 and sagA2 double-deletion mutant. Therefore, the low level of SagB to SagI and the low stability of transcriptional product from the sagA2 promoter mentioned above, working together, would induce a reduced beta-hemolysis phenotype in the sagA2-complemented strain.
Interestingly, the data also suggested that a certain essential difference(s) might exist between the molecular characters of SagA1 and SagA2 of S. anginosus and the typical SagA of pyogenic group streptococci. This derives from the fact that a critical/important amino acid (S39) for the heterocycle formation and the cytolytic activity of S. pyogenes SagA is deleted in both S. anginosus SagA1 and SagA2 (Fig. 4C). Moreover, the numbers of heterocycles deduced in SagA1 and SagA2 may differ from that of SagA of S. pyogenes. These differences in amino acid sequence and predictable heterocycle formation are more significant than the intraoperon difference in SagA homologues of S. anginosus NCTC10713T (i.e., between SagA1 and SagA2) and suggest that mature SagA homologues of S. anginosus NCTC10713T may differ in the structure essential for cytotoxicity and/or the maturation detail, compared to SagA in S. pyogenes. Furthermore, the structural differences between SagA1 and SagA2 suggest potential differences in their interaction with other molecules (e.g., enzymes) responsible for their maturation and/or with target molecules, such as membrane phospholipids affecting maturation efficiency and specific activity. Therefore, the structure-activity relationship of each SagA homologue is of great interest.
In the present study, the novel factors responsible for the beta-hemolysis induced by S. anginosus have been revealed and their properties investigated. In contrast to a typical sag operon of pyogenic group streptococci, the presence of twin sagA genes (sagA1 and sagA2) is a significant characteristic of beta-hemolytic S. anginosus. The data obtained here demonstrate the twin sagA homologues to be fundamental units for beta-hemolysis within S. anginosus and suggest that SagA1 and SagA2 in S. anginosus might have distinctive characteristics compared to SagA in pyogenic group streptococci. Thus, the elucidation of the mechanisms of transcription, expression, and maturation of SagA1 and SagA2 may be important for understanding the pathogenicity of beta-hemolytic S. anginosus. Further investigation is currently proceeding focused on comparing the properties of SagA1 and SagA2 of beta-hemolytic S. anginosus and pyogenic group streptococcal SagA (SLS).
ACKNOWLEDGMENT
We are grateful to T. Sumitomo, Department of Oral and Molecular Microbiology, Osaka University Graduate School of Dentistry, for the kind gift of a Δslo mutant of S. pyogenes strain NIH35.
Footnotes
Published ahead of print 4 January 2013
REFERENCES
- 1. Whiley RA, Beighton D. 1991. Emended descriptions and recognition of Streptococcus constellatus, Streptococcus intermedius, and Streptococcus anginosus as distinct species. Int. J. Syst. Bacteriol. 41:1–5 [DOI] [PubMed] [Google Scholar]
- 2. Whiley RA, Hall LMC, Hardie JM, Beighton D. 1999. A study of small-colony, β-hemolytic, Lancefield group C streptococci within the anginosus group: description of Streptococcus constellatus subsp. pharynges subsp. nov., associated with the human throat and pharyngitis. Int. J. Syst. Bacteriol. 49:1443–1449 [DOI] [PubMed] [Google Scholar]
- 3. Whiley RA, Beighton D, Winstanley TG, Fraser HY, Hardie JM. 1992. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J. Clin. Microbiol. 30:243–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruoff KL. 1988. Streptococcus anginosus (“Streptococcus milleri”): the unrecognized pathogen. Clin. Microbiol. Rev. 1:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarridge JE, III, Attorri S, Musher DM, Hebert J, Dunber S. 2001. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin. Infect. Dis. 32:1511–1515 [DOI] [PubMed] [Google Scholar]
- 6. Morita E, Narikiyo M, Yano A, Nishimura E, Igaki H, Sasaki H, Terada M, Harada N, Kawabe R. 2003. Different frequencies of Streptococcus anginosus infection in oral cancer and esophageal cancer. Cancer Sci. 94:492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narikiyo M, Tanabe C, Yamada Y, Igaki H, Tachimori Y, Kato H, Muto M, Montesano R, Sakamoto H, Nakajima Y, Sasaki H. 2004. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 95:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sasaki M, Yamaura C, Ohara-Nemoto Y, Tajika S, Kodama Y, Ohya T, Harada R, Kimura S. 2005. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 11:151–156 [DOI] [PubMed] [Google Scholar]
- 9. McKenzie TJ, Lillegard JB, Grotz TE, Moir CR, Ishitani MB. 2010. Pyogenic liver abscess secondary to Streptococcus anginosus in an adolescent. J. Pediatr. Surg. 45:E15–E17 [DOI] [PubMed] [Google Scholar]
- 10. Nagamune H, Ohnishi C, Katsuura A, Fushitani K, Whiley RA, Tsuji A, Matsuda Y. 1996. Intermedilysin, a novel cytotoxin specific for human cells secreted by Streptococcus intermedius UNS46 isolated from a human liver abscess. Infect. Immun. 64:3093–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagamune H, Whiley RA, Goto T, Inai Y, Maeda T, Hardie JM, Kourai H. 2000. Distribution of the intermedilysin gene among the anginosus group streptococci and correlation between intermedilysin production and deep-seated infection with Streptococcus intermedius. J. Clin. Microbiol. 38:220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takao A, Nagamune H, Maeda N. 2004. Identification of the anginosus group within the genus Streptococcus using polymerase chain reaction. FEMS Microbiol. Lett. 233:83–89 [DOI] [PubMed] [Google Scholar]
- 13. Havarstein LS, Hakenbeck R, Gaustad P. 1997. Natural competence in the genus Streptococcus: evidence that streptococci can change phenotype by interspecies recombinational exchanges. J. Bacteriol. 179:6589–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maguin E, Prevost H, Ehrlich SD, Gruss A. 1996. Efficient insertional mutagenesis in lactococci and other Gram-positive bacteria. J. Bacteriol. 178:931–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perrière G, Gouy M. 1996. WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie 78:364–369 [DOI] [PubMed] [Google Scholar]
- 16. Molloy EM, Cotter PD, Mitchell DA, Ross RP. 2011. Streptolysin S-like virulence factors: the continuing sagA. Nat. Rev. Microbiol. 9:670–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 18. Goto T, Nagamune H, Miyazaki A, Kawamura Y, Ohnishi O, Hattori K, Ohkura K, Miyamoto K, Akimoto S, Ezaki T, Hirota K, Miyake Y, Maeda T, Kourai H. 2002. Rapid identification of Streptococcus intermedius by PCR with the ily gene as a species marker gene. J. Med. Microbiol. 51:178–186 [DOI] [PubMed] [Google Scholar]
- 19. Tomoyasu T, Tabata A, Imaki H, Tsuruno K, Miyazaki A, Sonomoto K, Whiley RA, Nagamune H. 2012. Role of Streptococcus intermedius DnaK chaperone system in stress tolerance and pathogenicity. Cell Stress Chaperones 17:41–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lutz R, Bujard H. 1997. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 25:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spellerberg B, Pohl B, Haase G, Martin S, Weber-Heynemann J, Lutticken R. 1999. Identification of genetic determinants for the hemolytic activity of Streptococcus agalactiae by ISS1 transposition. J. Bacteriol. 181:3212–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nizet V, Beall B, Bast DJ, Datta V, Kilburn L, Low DE, De Azavedo JCS. 2000. Genetic locus for streptolysin S produced by group A Streptococcus. Infect. Immun. 68:4245–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitchell DA, Lee SW, Pence MA, Markley AL, Limm JD, Nizet V, Dixon JE. 2009. Structural and functional dissection of the heterocyclic peptide cytotoxin streptolysin S. J. Biol. Chem. 284:13004–13012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koyama J. 1965. Kinetic study on streptolysin S. J. Biochem. 57:103–108 [DOI] [PubMed] [Google Scholar]
- 25. Fuller JD, Camus AC, Duncan CL, Nizet V, Bast DJ, Thune RL, Low DE, De Azavedo JC. 2002. Identification of a streptolysin S-associated gene cluster and its role in the pathogenesis of Streptococcus iniae disease. Infect. Immun. 70:5730–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Humar D, Datta V, Bast DJ, Beall B, De Azavedo JC, Nizet V. 2002. Streptolysin S and necrotizing infections produced by group G streptococcus. Lancet 359:124–129 [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez DJ, Lee SW, Hensler ME, Markley AL, Dahesh S, Mitchell DA, Bandeira N, Nizet V, Dixon JE, Dorrestein PC. 2010. Clostridiolysin S, a post-translationally modified biotoxin from Clostridium botulinum. J. Biol. Chem. 285:28220–28228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cotter PD, Draper LA, Lawton EM, Daly KM, Groeger DS, Casey PG, Ross RP, Hill C. 2008. Listeriolysin S, a novel peptide haemolysin associated with a subset of lineage I Listeria monocytogenes. PLoS Pathog. 4:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee SW, Mitchell DA, Markley AL, Hensler ME, Gonzalez D, Wohlrab A, Dorrestein PC, Nizet V, Dixon JE. 2008. Discovery of a widely distributed toxin biosynthetic gene cluster. Proc. Natl. Acad. Sci. U. S. A. 105:5879–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dale JB, Chiang EY, Hasty DL, Courtney HS. 2002. Antibodies against a synthetic peptide of SagA neutralize the cytolytic activity of streptolysin S from group A streptococci. Infect. Immun. 70:2166–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]