Abstract
Pantothenate, commonly referred to as vitamin B5, is an essential molecule in the metabolism of living organisms and forms the core of coenzyme A. Unlike humans, some bacteria and plants are capable of de novo biosynthesis of pantothenate, making this pathway a potential target for drug development. Francisella tularensis subsp. tularensis Schu S4 is a zoonotic bacterial pathogen that is able to synthesize pantothenate but is lacking the known ketopantoate reductase (KPR) genes, panE and ilvC, found in the canonical Escherichia coli pathway. Described herein is a gene encoding a novel KPR, for which we propose the name panG (FTT1388), which is conserved in all sequenced Francisella species and is the sole KPR in Schu S4. Homologs of this KPR are present in other pathogenic bacteria such as Enterococcus faecalis, Coxiella burnetii, and Clostridium difficile. Both the homologous gene from E. faecalis V583 (EF1861) and E. coli panE functionally complemented Francisella novicida lacking any KPR. Furthermore, panG from F. novicida can complement an E. coli KPR double mutant. A Schu S4 ΔpanG strain is a pantothenate auxotroph and was genetically and chemically complemented with panG in trans or with the addition of pantolactone. There was no virulence defect in the Schu S4 ΔpanG strain compared to the wild type in a mouse model of pneumonic tularemia. In summary, we characterized the pantothenate pathway in Francisella novicida and F. tularensis and identified an unknown and previously uncharacterized KPR that can convert 2-dehydropantoate to pantoate, PanG.
INTRODUCTION
Coenzyme A (CoA) is a ubiquitous cofactor found in all domains of life and plays a central role in carbon and energy metabolism. Pantothenate forms the core of CoA; however, animals and some microorganisms lack the ability to synthesize pantothenate. The pantothenate biosynthetic pathway is present in some plants, fungi, bacteria, and archaea, making the pathway a strong candidate for the discovery of novel antibiotics (1). The pantothenate biosynthesis pathway was first genetically and chemically defined in Escherichia coli and Salmonella enterica serovar Typhimurium and is composed of four enzymes that use the precursors 2-oxoisovalerate and l-aspartic acid to make pantothenate (2, 3) (Fig. 1A). In one branch of the pathway, aspartate-1-decarboxylase, PanD, converts l-aspartate to β-alanine, and in a separate branch two enzymes, PanB, a ketopantoate hydroxymethyltransferase, and PanE, a ketopantoate reductase (KPR), convert 2-oxoisovalerate to pantoate (4). The acetohydroxy acid isomerase, IlvC, has also been shown to function as a KPR in E. coli and S. Typhimurium, converting 2-dehydropantoate to pantoate, similar to PanE (5, 6). In some species, e.g., Corynebacterium glutamicum, IlvC is the sole KPR (7). In the final step of the pantothenate synthesis pathway, pantoate and β-alanine are ligated by the pantothenate synthase, PanC, to form pantothenate (8). The published genomes of Francisella species contain panB, panC, and panD. No homolog of E. coli PanE exists in the sequenced Francisella genomes, and the most virulent strain of Francisella tularensis, strain Schu S4, contains a frameshift mutation in ilvC, suggesting a gap in the pantothenate biosynthetic pathway. Combining the known nutritional requirements of Francisella species with the sequenced genomes revealed that several strains require exogenous pantothenate for growth (9). Interestingly, the most virulent strain Schu S4 lacks a recognizable KPR and is a pantothenate prototroph, while the live vaccine strain (LVS) has a KPR but is clearly auxotrophic (10, 11). The genetic basis for differences in the requirement for pantothenate among Francisella strains is not known.
Fig 1.
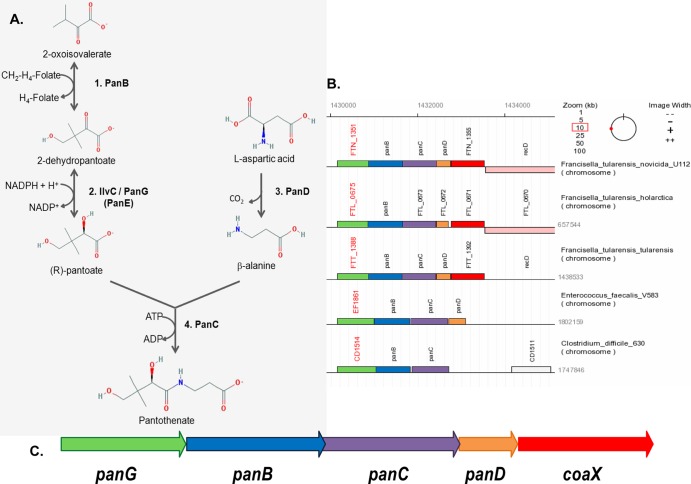
The biosynthetic pathway in Francisella species and the putative pantothenate operon. (A) The pantothenate biosynthetic pathway consists of two converging arms. PanB, the ketopantoate hydroxymethyltransferase, converts 2-oxoisovalerate with tetrahydrofolate to form 2-dehydropantoate. The substrate 2-dehydropantoate is then converted to (R)-pantoate by a number of enzymes, including IlvC and PanG, which are both capable of ketopantoate reductase (KPR) activity. On the other branch of the pathway, PanD, an aspartate-1-decarboxylase, converts l-aspartic acid to β-alanine. The pathway converges with PanC, the pantothenate synthase that ligates (R)-pantoate with β-alanine to form pantothenate. Molecules were made in PubChem Compound on NCBI, and the pathway was constructed using KEGG metabolic pathway 00770 as a reference (http://www.genome.jp/kegg-bin/show_pathway?FTN_00770). (B) The genomic organization of the putative pantothenate operon is conserved among sequenced Francisella strains containing the panGBCD genes and the pantothenate kinase gene coaX. (C) Synteny diagram of the putative operon in F. novicida, F. tularensis LVS, F. tularensis Schu S4, E. faecalis V583, and C. difficile 630 (35).
Francisella belongs to the Gammaproteobacteria and includes a number of species that share greater than 95% genomic similarity but differ in their ability to cause the disease tularemia in humans (12, 13). Of all the species, Francisella tularensis subsp. tularensis is the most virulent, exhibiting the highest morbidity and mortality while also having a very low infectious dose, less than 10 bacteria (11, 14). F. tularensis subsp. holarctica is also highly infectious for humans, but clinical disease associated with this bacterium is typically mild (15). The LVS was derived from an F. tularensis subsp. holarctica strain and is attenuated in humans. Francisella novicida is rarely associated with human disease. As a facultative intracellular pathogen, Francisella must acquire the nutrients needed to support growth while within host cells. Several genetic screens for replication-defective mutants have implicated biosynthetic pathways as contributing factors to the pathogenesis of Francisella species (16). Interruption in genes involved in purine, aromatic amino acid, and biotin biosynthesis results in attenuation of F. tularensis within a mouse model of tularemia (17–20). Collectively, these studies highlight the contribution of de novo biosynthetic pathways to the ability of F. tularensis to survive within the host environment. Using F. novicida, a genome-wide mutant screen identified two genes contained in the putative pantothenate operon that are required for pulmonary and systemic infections in mice. Inactivation of FTN_1351 (panG) or FTN_1355 (coaX) resulted in mutant strains impaired in their ability to proliferate in the organs of infected mice (21). Furthermore, transcriptional profiling of F. tularensis revealed a dramatic increase in expression of the F. tularensis homologs of panB, panC, and panD, as well as the homologs of FTN_1351 (panG) and FTN_1355 (coaX) in strains grown within bone marrow-derived macrophages. This suggests that pantothenate biosynthesis may play a role in the adaptation of Francisella to its intracellular niche (22). The requirement for de novo synthesis of pantothenate in virulence was demonstrated in a different intracellular pathogen, Mycobacterium tuberculosis, for which a double mutant in panC and panD was attenuated in both BALB/c and SCID mouse models of infection (23). Collectively the published data suggest that pantothenate biosynthesis may contribute to the virulence of Francisella, and the disparity in the requirement for pantothenate among Francisella strains led us to investigate the genetic basis for pantothenate biosynthesis in several species of Francisella.
MATERIALS AND METHODS
Bacterial strains and cell culture.
Escherichia coli strains (Table 1) were grown at 37°C in Luria-Bertani (LB) broth, on LB agar, or in M9 minimal medium supplemented with 1 μg/ml thiamine, 3.4 mM valine, 3 mM isoleucine, and 3 mM leucine. Unless otherwise noted, all F. tularensis strains (Table 1) were grown at 37°C on chocolate agar (25 g brain heart infusion [BHI] liter−1, 10 μg hemoglobin liter−1, 15 g agar liter−1) supplemented with 1% IsoVitalex (Becton-Dickson) or in Chamberlain's Defined Medium (CDM) (10). When necessary, hygromycin B (Hyg; Roche Applied Science) was used at 200 μg ml−1, sucrose was used at 5% for E. coli or 10% for F. tularensis (wt/vol), ampicillin (Amp; Roche Applied Science) was used at 100 μg ml−1, l-arabinose (Sigma-Aldrich) was used at 10 mM, and kanamycin (Km; Sigma-Aldrich) was used at 50 μg ml−1 for E. coli and 10 μg ml−1 for F. tularensis.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Source |
|---|---|---|
| Bacterial strains | ||
| E. coli DH10B | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara-leu) 7697 galU galK rpsL nupG λ− | Invitrogen |
| E. coli Top10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacΧ74 recA1 araD139 Δ(ara-leu) 7697 galU galK rpsL (Strr) endA1 nupG λ− | Invitrogen |
| E. coli panE::Kan JW0415-1 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) ΔpanE782::Kan λ−rph-Δ(rhaD-rhaB)568 hsdR514 | Keio Collection |
| E. coli ilvC::Kan JW3747-2 | Δ(araD-araB)567, ΔlacZ4787(::rrnB-3) λ−,rph-1, ΔilvC725::Kan Δ(rhaD-rhaB)568 hsdR514 | Keio Collection |
| F. novicida U112 | F. tularensis subsp. novicida U112 (taxid, 401614) | ATCC |
| F. holarctica LVS | F. tularensis subsp. holarctica LVS (taxid, 376619) | CDC |
| F. tularensis Schu S4 | F. tularensis subsp. tularensis SCHU S4 (taxid, 177416) | BEI Resources |
| E. faecalis | Strain V583 | Lance Thurlow |
| F. tularensis | ||
| panB::Tn strain | tnfn1_pw060328p05q156 T20 (ISFn2/FRT) (FTN_1352) | 24 |
| panC::Tn strain | tnfn1_pw060328p03q178 T20 (ISFn2/FRT) (FTN_1353) | 24 |
| panD::Tn strain | tnfn1_pw060323p04q175 <Kan-2> (FTN_1354) | 24 |
| panG::Tn strain | tnfn1_pw060420p03q142 T20 (ISFn2/FRT) (FTN_1351) | 24 |
| ilvC::Tn strain | tnfn1_pw060420p04q122 T20 (ISFn2/FRT) (FTN_1040) | 24 |
| FTN_1698::Tnflpa strain | tnfn1_pw06032p08q164 | This work |
| ilvC::Tnflpa panG::Tn strain | tnfn1_pw060420p04q122flp/tnfn1_pw060420p03q142 | This work |
| SKI01 | F. tularensis Schu S4 ΔpanG | This work |
| SKI02 | F. novicida U112 ilvC::Tnflp panG::Tn with pEDL70 | This work |
| SKI03 | F. novicida U112 ilvC::Tnflp panG::Tn with pEDL71 | This work |
| SKI04 | F. novicida U112 ilvC::Tnflp panG::Tn with pSKI01 | This work |
| SKI05 | F. tularensis Schu S4 ΔpanG with pSKI01 | This work |
| SKI06 | F. tularensis Schu S4 ΔpanG with pMP831 empty vector control | This work |
| SKI07 | F. novicida U112 ilvC::Tnflp panG::Tn with pMP831 empty vector control | This work |
| SKI08 | F. holarctica LVS with panD from F. novicida U112 | This work |
| SKI09 | E. coli ilvC::Flp panE::Kan (JW0415-1 and JW3747-2) | This work |
| SKI10 | E. coli ilvC::Flp panE::Kan (JW0415-1 and JW3747-2) with pSKI01 | This work |
| SKI11 | E. coli ilvC::Flp panE::Kan (JW0415-1 and JW3747-2) with pSKI04 | This work |
| Plasmids | ||
| pFFLP-hyg | Carries flp under Francisella groEL promoter for recombination of FRT sites, TempSens, Hygr | 25 |
| pKD46 | λ-Red recombineering plasmid, GenBank accession no. J02459.1, TempSens, Ampr | 26 |
| pFlp2 | Carries flp recombinase for recombination of FRT sites, Ampr, Sucs | 27 |
| pEDL70 | pMP822.panE, Hygr | This work |
| pEDL71 | pMP822.EF1861, Hygr | This work |
| pSKI01 | pMP831.panG FTN_1351, Hygr | This work |
| pSKI02 | pEDL50 with ΔpanG Schu S4 construct, Kmr, Sucs | This work |
| pSKI03 | pMP590 with panDU112 (FTN_1354), Kmr, Sucs | This work |
| pSKI04 | pMP822.ilvC, Hygr | This work |
| pMP590 | sacB suicide vector, Kmr, Sucs | 28 |
| pMP822 | E. coli-F. tularensis shuttle vector, Hygr, blaB promoter | 29 |
| pMP831 | E. coli-F. tularensis shuttle vector, Hygr | 29 |
| pEDL50 | Conjugative sacB suicide vector, Hygr, Sucs | 30 |
“flp” signifies a cured kanamycin cassette.
Growth assays.
Growth assays for F. tularensis and F. novicida were performed at 37°C while shaking in an Infinite M200 or M200 pro (Tecan) apparatus in 96-well microtiter plates with absorbance (optical density at 600 nm [OD600]) monitored every 15 min. Bacterial strains grown overnight on chocolate agar were resuspended in phosphate-buffered saline (PBS) to a Klett reading of 100 (approximately 1 × 109 CFU/ml). Resuspended cultures were diluted 1:20 into test media. The test medium consisted of CDM or of CDM without calcium pantothenate (Sigma-Aldrich). Genetic complementation in trans was evaluated in CDM lacking pantothenate. Chemical complementation was evaluated in CDM lacking pantothenate and supplemented with 100 μM pantolactone (Sigma-Aldrich), which can act in place of pantoate (31), or 100 μM β-alanine (Sigma-Aldrich).
Construction of a double mutant using an Flp/Frt recombination in F. novicida.
To create the ilvC::Tnflp panG::Tn mutant (“flp” signifies a cured Km cassette) we performed Flp-mediated recombination of ilvC::Tn bearing the T20 insertion by introducing pFFlp-hyg, a temperature-sensitive plasmid, by electroporation and isolated Km-sensitive colonies grown at 30°C (25). Removal of the Km cassette left an 80-bp insertion with the expected single FLP recombination target (FRT) site remaining. Once the Km resistance marker was excised, pFFlp-hyg was cured by growing the culture overnight at 37°C. The panG mutation was introduced by transformation of genomic DNA from the panG::Tn strain into the ilvC::Tnflp mutant and selecting for Km-resistant colonies, creating the double mutant (25). For genetic complementation, mutant strains were transformed with their respective plasmids (29). Plasmids were first transformed with the addition of 1 μl of TypeOne Restriction Inhibitor (Epicentre) into an FTN_1698::Tnflp restriction mutant strain (25).
Construction of the E. coli ilvC::Flp panE::Kan double mutant using FLP/FRT recombination and λ-Red recombineering.
Using λ-Red recombineering, the ilvC::flp panE::Kan double mutant (“flp” signifies a cured Km cassette) was created in E. coli by first introducing pFlp2 into ilvC::Kan (JW3747-2) by electroporation and isolating Km-sensitive colonies (26, 32). Removal of the kanamycin cassette left the signature single FRT site of FLP/FRT recombination, creating ilvC::Flp. Once the Km resistance marker was excised, pFlp2 was cured by growing the culture overnight and plating on LB with 5% sucrose and without NaCl, and the resulting colonies were screened for Amp sensitivity. Next, the λ-Red recombineering plasmid pKD46 was introduced into the ilvC::Flp strain by electroporation (27). When the absorbance of ilvC::Flp with pKD46 reached an OD600 of 0.1, 10 mM l-arabinose was added to induce expression of the λ-Red recombineering proteins: Gam, Bet, and Exo. The panE::Kan mutation was introduced by transformation into the ilvC::Flp mutant using PCR fragments generated from genomic DNA of panE::Kan (JV0415-1), and colonies were selected for Km resistance.
F. novicida CoA levels.
F. novicida was grown overnight on chocolate agar plates at 37°C and then resuspended to an OD600 of 0.2 in 50 ml of CDM lacking pantothenate and incubated for 5 h. The cells were pelleted at 10,000 × g for 5 min at 4°C and then resuspended in 0.5 ml of cold PBS and snap-frozen in liquid nitrogen. The cells were thawed and bead beaten 2 times for 30 s at 4°C using lysing matrix B tubes (MP Biomedicals). Cell debris was removed by centrifuging at 14,000 × g for 2 min. Protein concentration was determined by bicinchoninic acid (BCA) assay for each sample. To deproteinize the lysates, 100 μl of cold 1 N perchloric acid was added to each sample and centrifuged at 14,000 × g at 4°C for 2 min to pellet precipitated protein. To neutralize the supernatant, 30 μl of cold 3 M potassium bicarbonate was added to the samples. The concentrations of CoA were determined for each sample using the PicoProbe Acetyl-CoA assay kit (BioVision). The manufacturer's protocol was followed, except that the CoA quenching solution was not used, thereby allowing us to determine the total concentration of CoA in each sample.
Replacing LVS panD (panDLVS) with F. novicida panD (panDU112).
The putative panD (FTN_1354) allele was PCR amplified from F. novicida genomic DNA. The amplified DNA was subcloned into the TOPO pCRII vector (Invitrogen) for propagation and maintenance. The BamHI-NotI fragment containing the F. novicida panD allele was ligated into the sacB suicide vector pMP590 and electroporated into LVS. Allelic exchange was performed as previously described (28).
Deletion of FTT1388 (panG) in Schu S4.
The putative panG (FTT1388) allele plus 300 bp of DNA flanking each end was PCR amplified from F. tularensis Schu S4 genomic DNA. The amplified fragment was cut with BamHI and NotI and then ligated into the sacB suicide vector, pEDL50 (30). Using splice junction PCR, FTT1388 (panG) was eliminated from the plasmid, creating a product with AatII restriction sites on either end. The PCR product was digested with AatII and ligated, forming pSKI01. Conjugation and allelic exchange were then performed to introduce the clean in-frame deletion of panG into F. tularensis Schu S4 as previously described (30, 33).
Mouse infection.
Groups of 6-week-old C57BL/6 mice were anesthetized and inoculated intranasally with 50 CFU of Schu S4 or Schu S4 ΔpanG (FTT1388). Mice were euthanized on days 1 and 3 postinfection, and lungs, livers, and spleens were aseptically removed and homogenized into 2 ml of sterile PBS using a Biojector (BIOSPEC Products, Inc.). Bacterial CFU for each organ were enumerated by plating serial dilutions of tissue homogenates onto chocolate agar. The infection experiments were approved and performed according to the animal care and use guidelines established by IACUC.
RESULTS
Organization of the Francisella pantothenate biosynthesis pathway.
The advances in whole-genome sequencing have made identification of metabolic pathways through the use of sequence homology common. However, little experimental evidence is associated with these annotations, and gaps exist in many annotated metabolic pathways, including those of F. tularensis and F. novicida. Bioinformatics analysis suggests that the pantothenate biosynthesis pathway in F. novicida is much like that in E. coli, putatively using PanB, PanD, PanC, and IlvC to convert l-aspartate and 2-oxoisovalerate into pantothenate (Fig. 1A). Using F. novicida BLASTP (NCBI) generated E values of 7e10−69 for PanB, 6e10−58 for PanC, 3e10−20 for PanD, and 8e10−22 for IlvC relative to the characterized E. coli K-12 enzymes (34). Interestingly, no homolog of E. coli PanE was identified in any of the 36 sequenced Francisella genomes. In addition, the ilvC gene in F. tularensis Schu S4 contains a frameshift mutation that creates a premature stop codon after amino acid 74, suggesting a gap in the pantothenate biosynthetic pathway. LVS also has a truncation in the panD gene, shortening the encoded protein from 111 amino acids to 91 amino acids.
Unlike those of E. coli, the panB, panC, and panD genes of Francisella species are organized into a predicted operon (Fig. 1B and C), which is conserved in all the sequenced Francisella strains (35). In addition to panBCD, this putative operon contains additional predicted open reading frames (ORFs) both 5′ and 3′ of panBCD (Fig. 1C). There is an annotated ORF (panG) immediately 5′ of panBCD that encodes an uncharacterized conserved protein containing a domain of unknown function, DUF2520 (36). The ORF 3′ of panBCD encodes a putative type III pantothenate kinase (CoaX), which catalyzes the first dedicated step in CoA synthesis from pantothenate (37, 38). Francisella tularensis also contains another coaX homolog, FTT0112, which is not part of the putative operon.
The Francisella panBCD genes are required for growth in the absence of pantothenate.
We used the F. novicida comprehensive two-allele transposon mutant library to assess the functionality of individual components of the pantothenate biosynthesis pathway (24). The pantothenate biosynthetic pathway in F. novicida was characterized by complementing auxotrophic mutants with metabolic intermediates of pantothenate biosynthesis.
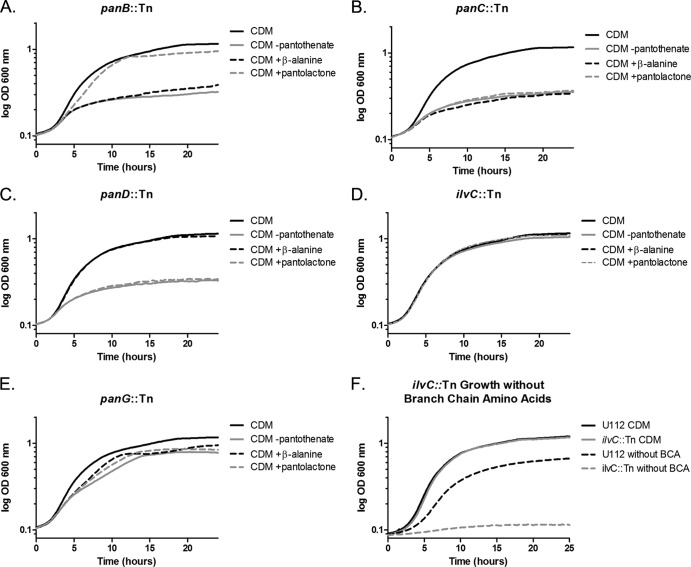
FTN_1352 (PanB) is a ketopantoate hydroxymethyl transferase that catalyzes the conversion of 2-oxoisovalerate to 2-dehydropantoate (Fig. 1A). Functional inactivation of panB results in mutant strains deficient in 2-dehydropantoate and loss of pantothenate synthesis. The panB::Tn mutant exhibited limited growth in CDM lacking pantothenate, and growth of this mutant was restored by supplementation with pantolactone (an alternative substrate to pantoate) (Fig. 2A), thereby bypassing the PanB/IlvC branch (31). Supplementation with β-alanine alone was not sufficient to support growth of this mutant (Fig. 2A), demonstrating that FTN_1352 functions in the PanB/IlvC branch of the pathway and is consistent with the annotation of FTN_1352 as panB.
Fig 2.
F. novicida functional complementation of panB::Tn, panC::Tn, panD::Tn, ilvC::Tn, and panG::Tn. Functional complementation of the pantothenate biosynthetic genes in F. novicida transposon mutant strains grown in 96-well microtiter plates with absorbance (OD600) monitored every 15 min in CDM lacking pantothenate (gray) either supplemented with β-alanine (black dotted line), pantolactone (gray dotted line), or calcium pantothenate (black). Shown are growth curves for panB::Tn (A), panC::Tn (B), panD::Tn (C), ilvC::Tn (D), and panG::Tn (E) mutants. (F) Growth of F. novicida U112 and ilvC::Tn in CDM with and without branch chain amino acids: F. novicida U112 in CDM (black), ilvC::Tn in CDM (gray), U112 in CDM without branch chain amino acids (black dotted line), and ilvC::Tn in CDM without branch chain amino acids (gray dotted line). Each growth curve was repeated a minimum of three times, and the graph represents the means of three replicate experiments.
FTN_1353 (PanC) encodes pantothenate synthase, which catalyzes the formation of pantothenate from l-pantoate and β-alanine (Fig. 1A). Inactivation of panC results in a strain that requires the addition of pantothenate for growth, and neither supplementation with pantolactone nor β-alanine rescued growth. This indicates that the FTN_1353 transposon mutation interrupts the pantothenate biosynthesis pathway downstream of both the PanD and PanB/IlvC branches, consistent with the phenotype of a pantothenate synthase mutant (Fig. 2B).
FTN_1354 (PanD) is an l-aspartate α-decarboxylase that forms β-alanine from l-aspartate (Fig. 1A), and inactivation of panD results in a strain that is auxotrophic for β-alanine. Consistent with this phenotype, the panD::Tn mutant exhibited growth defects in CDM lacking pantothenate and was rescued by the addition of β-alanine. Supplementation with pantolactone failed to restore growth of the panD::Tn mutant (Fig. 2C). These results demonstrate that inactivation of FTN_1354 results in a loss of β-alanine synthesis and are consistent with the prediction that FTN_1354 is a functional PanD (4).
FTN_1040 (IlvC) is known to function as an acetohydroxy acid isomerase essential for branch chain amino acid synthesis and can also function as a KPR in E. coli and S. Typhimurium (39). As the only recognized enzyme with KPR activity within the F. novicida genome, we were surprised to find that the ilvC::Tn transposon mutant in F. novicida did not require pantothenate for growth (Fig. 2D). This result suggested that there was another unrecognized enzyme with sufficient KPR activity to support pantothenate biosynthesis within F. novicida ilvC::Tn.
FTN_1351 (panG) is annotated as an uncharacterized conserved gene and is part of the putative pantothenate operon, so we tested the panG::Tn mutant's pantothenate growth requirement. Under each condition, the panG::Tn mutant grew, suggesting that either PanG may not be involved in pantothenate synthesis or it is functionally redundant with IlvC in KPR activity (Fig. 2E).
To determine if IlvC in F. novicida is a functional acetohydroxy acid isomerase, we grew wild-type F. novicida U112 and ilvC::Tn in CDM with and without branch chain amino acids (Fig. 2F). Only wild-type U112 could grow in CDM without branch chain amino acids, supporting our hypothesis that IlvC functions as an acetohydroxy acid isomerase in F. novicida.
New class of ketopantoate reductase found in a number or pathogenic organisms.
Ketopantoate reductases (KPR) have a dinucleotide-binding domain called a Rossmann-fold that consists of a βαβ pocket to accommodate the coenzyme NADPH (40). Within the Rossmann-fold is a consensus sequence, GXGXXG, which appears at the border between the first beta sheet and the alpha helix (41). In E. coli the PanE N-terminal Rossmann-fold domain and the C-terminal alpha-helical domain form a cleft, and at that cleft is a conserved essential residue, Lys176, that forms the hydrogen bond with C2 hydroxyl of pantoate (8). Pantoate also forms additional interactions with conserved residues Ser244, Asn98, and Asn180 (8). PanE and IlvC each have an N-terminal NADB_Rossmann superfamily domain (36). IlvC is an acetohydroxy acid isomerase, which can also function as a KPR and requires two cofactors, NADPH and Mg2+, for the reduction of 2-dehydropantoate (42).
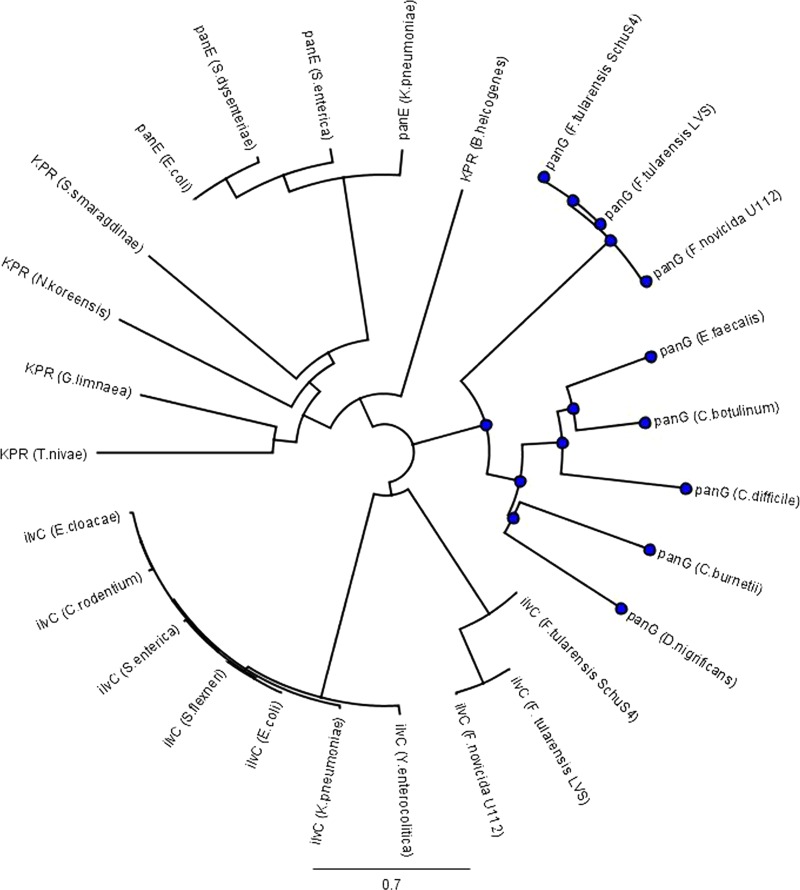
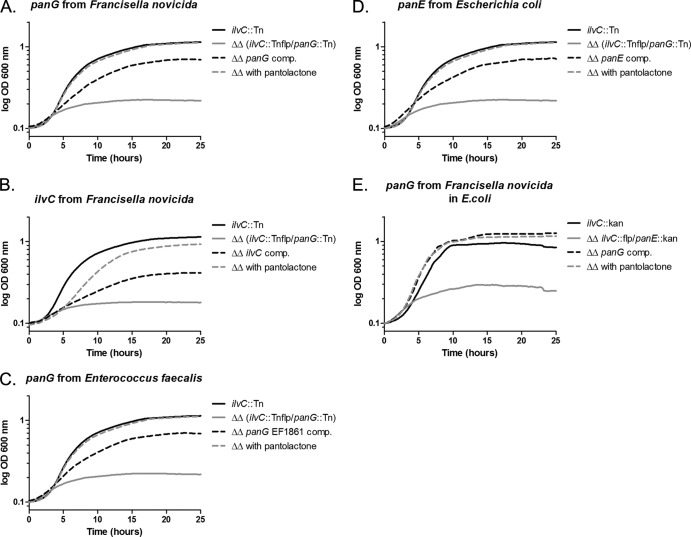
Bioinformatic analysis of PanG (FTN_1351) gave little insight into its function. The comparison of the sequence to PanE and other known KPR revealed no significant matches (5, 43). Phylogenetic analysis indicated that PanG is in a distinct group separate from annotated KPRs using the Jukes-Cantor genetic distance model (Fig. 3). There is no Rossmann domain identified in the C-terminal region of FTN_1351, and the GXGXXG motif is not present (41). The C-terminal region of FTN_1351 has a domain of unknown function, DUF2520 (E value of 1.5e10−6), that often accompanies an N-terminal Rossmann domain, suggesting that the protein could function as an uncharacterized KPR (36). We made a double mutation, ilvC::Tnflp panG::Tn, in F. novicida to determine if FTN_1351 protein could function as a KPR. The double mutant did not grow in medium lacking pantothenate (Fig. 4A). Growth of the double mutant was restored when complemented in trans with panG expressed under its native promoter, with ilvC driven by the Francisella blaB promoter or when the media was supplemented with pantolactone (Fig. 4A and B). This result confirms that IlvC is active in the F. novicida pantothenate metabolic pathway and that FTN_1351 is a novel KPR gene, which we named panG. Homologs of PanG are also found in other pathogenic bacteria, including Enterococcus faecalis, Coxiella burnetii, and Clostridium difficile. BLASTP analysis of F. tularensis Schu S4 PanG (FTT1388) revealed that this protein has significant homology with Enterococcus faecalis V583 (EF1861) (E value = 2e10−11), Coxiella burnetii (CBU1660) (E value = 2e10−7), and Clostridium difficile (CD630-15140) (E value = 8e10−6) (34). In E. faecalis and C. difficile, the panG genes are organized similarly in putative pantothenate operons (Fig. 1B). Expression of the E. faecalis V583 gene in F. novicida ilvC::Tnflp panG::Tn restored growth of this mutant strain to near-wild-type levels in CDM lacking pantothenate, confirming genetically that EF1861 is a functional KPR (Fig. 4C). PanE, an analog KPR from E. coli (EC 1.1.1.169), is not present in any sequenced genome of Francisella, and expression of this gene restored growth of F. novicida ilvC::Tnflp panG::Tn in medium lacking pantothenate (Fig. 4D). Expression of the F. novicida panG gene in the E. coli KPR ilvC::flp panE::kan double mutant restored growth in M9 minimal medium lacking pantothenate, confirming genetically that PanG is a functional KPR in E. coli (Fig. 4E).
Fig 3.
Phylogenetic tree of known ketopantoate reductase proteins and PanG. The phylogenetic tree of known KPR proteins and PanG was generated using Geneious Pro 5.5.6 Tree Builder with the cost matrix set to identity and Jukes-Cantor as the genetic distance model with no outgroups. All IlvC and PanE proteins are annotated on PubMed to be involved in pantothenate synthesis, while all PanG proteins are annotated as hypothetical proteins. Enterococcus faecalis V583 PanG is annotated as hypothetical protein EF1861, Clostridium difficile 630 PanG is annotated as hypothetical protein CD630-15140, Coxiella burnetii RSA 493 PanG is annotated as hypothetical protein CBU_1660, Clostridium botulinum BKT015925 PanG is annotated as hypothetical protein CbC4_0183, and Desulfotomaculum nigrificans DSM 574 PanG is annotated as hypothetical protein DUF2520.
Fig 4.
Genetic complementation of the F. novicida ilvC::Tnflp panG::Tn double mutant with F. novicida FTN_1351 panG, F. novicida ilvC, E. faecalis V583 (EF1861) panG, and E. coli panE and genetic complementation of the E. coli ilvC::Flp panE::Kan double mutant with F. novicida panG. Functional complementation experiments were carried out by growing Francisella in CDM lacking pantothenate in 96-well microtiter plates and measuring the absorbance (OD600) every 15 min for 30 h. Competent F. novicida isolates were transformed with DNA. (A) pSKI01 carrying F. novicida panG (FTN_1351) driven by its native promoter; (B) pSKI04 carrying ilvC from F. novicida driven by F. tularensis blaB promoter; (C) pEDL71 carrying panG from E. faecalis driven by F. tularensis blaB promoter; (D) pEDL70 carrying panE from E. coli driven by F. tularensis blaB promoter. ilvC::Tn mutant grown in CDM lacking pantothenate containing empty control vector pMP822/pMP831 (black), ilvC::Tnflp panG::Tn double mutant containing an empty control vector (gray), ilvC::Tnflp panG::Tn double mutant containing the respective complementing plasmid (black dotted line), and ilvC::Tnflp panG::Tn double mutant grown in CDM supplemented with pantolactone (gray dotted line). (E) E. coli ilvC::Flp panE::Kan double mutant complemented with pSKI01 carrying F. novicida panG driven by its native promoter. ilvC::Kan mutant grown in M9 medium lacking pantothenate containing an empty control vector (black), ilvC::Flp panE::Kan double mutant containing an empty control vector (gray), ilvC::Flp panE::Kan double mutant containing the respective complementing plasmid (black dotted line), and ilvC::Flp panE::Kan double mutant grown in M9 supplemented with pantolactone (gray dotted line). Each growth curve experiment was repeated three times, and the graph represents the mean of three replicate experiments.
Francisella novicida CoA levels.
Coenzyme A is made from pantothenate, cysteine, and adenosine and is an essential cofactor in the first step of the tricarboxylic acid (TCA) cycle. To determine the contribution of PanG and IlvC in the production of pantothenate and subsequent CoA synthesis, we measured the concentration of CoA after 5 h of pantothenate depletion in wild-type F. novicida and in the ilvC::Tn, panG::Tn, and ilvC::Tnflp panG::Tn mutants. CoA levels were not significantly different between the wild type and the ilvC::Tn mutant, suggesting that PanG can fulfill the requirement for KPR activity in F. novicida (Fig. 5). The CoA levels of the panG::Tn mutant strain were less than one-half of wild-type levels and similar to levels of the ilvC::Tnflp panG::Tn double mutant, suggesting that PanG is responsible for the majority of KPR activity in F. novicida (Fig. 5).
Fig 5.
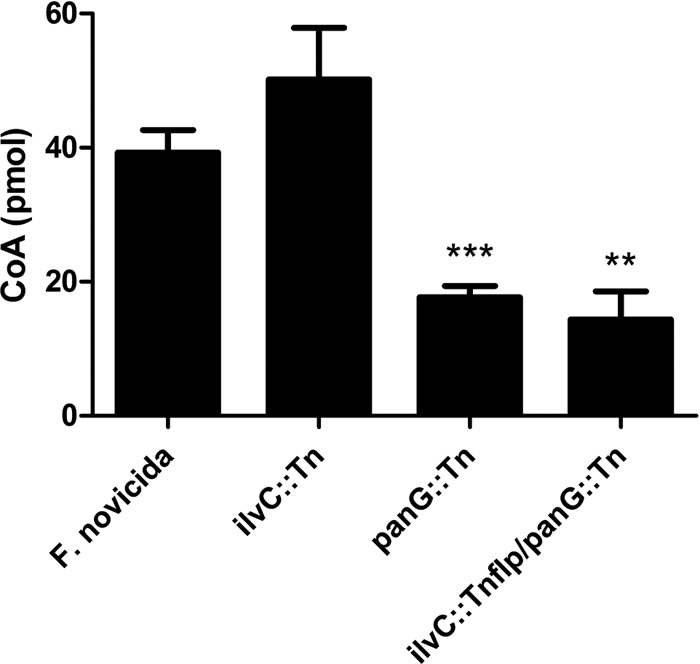
F. novicida CoA levels. CoA concentrations were measured from 50-ml cultures of F. novicida wild-type, ilvC::Tn, panG::Tn, and ilvC::Tnflp panG::Tn strains after 5 h of pantothenate depletion. All strains were grown to the same OD600 and were normalized to total protein. The CoA levels represented are the means ± standard deviations (SD) for three independent experiments. Statistical significance was determined by comparing the mutant values to those of wild-type F. novicida. ***, P < 0.0001; **, P < 0.001.
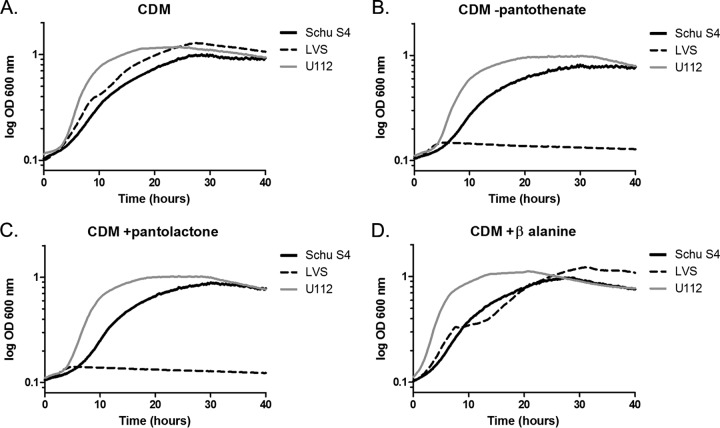
Francisella tularensis LVS is a β-alanine auxotroph.
Early studies revealed various nutritional requirements of Francisella strains for pantothenate that appeared to correlate with virulence in mice (9, 10). We assessed the requirement for pantothenate with three different strains of Francisella: F. tularensis Schu S4, F. tularensis LVS, and F. novicida U112. Each strain grew logarithmically in complete CDM (Fig. 6A to D). F. tularensis Schu S4 and F. novicida U112 grew logarithmically under each dropout condition, suggesting that both strains have complete pantothenate biosynthesis pathways (Fig. 6). However, F. tularensis LVS failed to grow in medium without pantothenate, indicating that LVS is a pantothenate auxotroph. This result is in agreement with the observations of Chamberlain (10). Additionally, we observed that growth of LVS could be restored by the addition of β-alanine, indicating that LVS lacks PanD activity (Fig. 6D). Comparison of the nucleotide sequences of the putative panGBCD genes among Francisella species revealed that F. tularensis LVS contains a base substitution in the annotated panD gene. This substitution creates a Q92 Ochre stop, resulting in a truncation of PanD by 20 amino acids. To determine if LVS β-alanine auxotrophy is due to a nonfunctional panD, we replaced panDLVS with the functional panDU112 via allelic exchange. As predicted, F. tularensis LVS harboring the panDU112 allele grew similarly in CDM with or without added pantothenate (Fig. 7). This demonstrates that the observed pantothenate auxotrophy of LVS results from a lack of PanD activity. This truncation in PanD is unique to LVS, so we questioned whether the lack of PanD activity contributed to the attenuated phenotype of this strain. However, repair of panD activity in F. tularensis LVS panDU112 did not confer a competitive advantage relative to F. tularensis LVS panDLVS in a mouse model of pneumonic tularemia (data not shown). Thus, the LVS panD mutant allele does not contribute to the attenuated phenotype of this strain.
Fig 6.
F. tularensis Schu S4, F. tularensis LVS, and F. novicida growth in pantothenate dropout media. Growth curves of F. tularensis subsp. tularensis Schu S4 (black), F. tularensis subsp. holarctica LVS (black dotted line), and F. novicida U112 (gray) were monitored in CDM (A), CDM lacking pantothenate (B), CDM lacking pantothenate supplemented with pantolactone (C), or CDM lacking pantothenate supplemented with β-alanine (D). Each strain was grown in triplicate in 96-well microtiter plates with absorbance (OD600) monitored every 15 min over 40 h. Each graph represents the mean of three replicate experiments.
Fig 7.
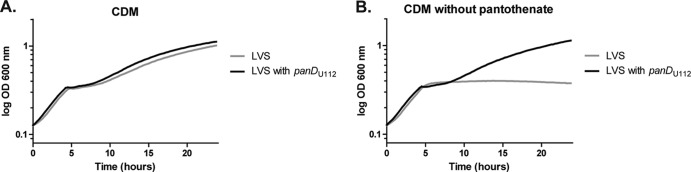
Repair of F. tularensis LVS β-alanine auxotrophy. Growth curves of LVS (gray) and LVS with the native panDLVS replaced with panDU112 from F. novicida U112 (black). Each strain was monitored in CDM (A) or CDM lacking pantothenate (B). Both strains were grown in 96-well microtiter plates, and the OD600 was determined every 15 min for 24 h. Each graph represents the mean OD600 of three replicate experiments.
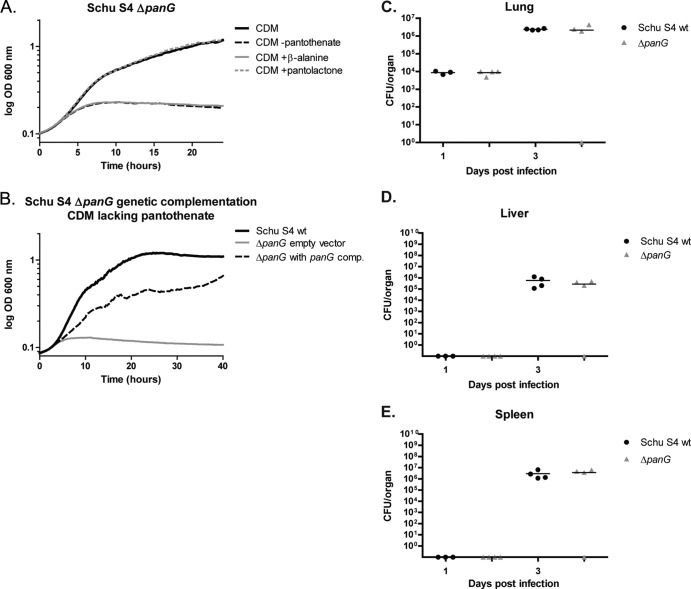
Francisella tularensis Schu S4 ΔpanG is a pantothenate auxotroph.
The ΔpanG (FTT1388) strain in Schu S4 requires pantothenate for growth and can be rescued with the addition of pantolactone in CDM lacking pantothenate or with panG expressed in trans. Schu S4 does not have a functional IlvC protein, at least in regard to its KPR activity, due to a frameshift mutation, making PanG the sole KPR. The ΔpanG strain can be complemented in trans with the panG gene from F. novicida driven by the F. novicida native promoter (Fig. 8B). Given that F. novicida panG (FTN_1351) was identified in a screen to be required for virulence in a mouse model of infection (21) and F. tularensis panG, panB, panC, panD, and coaX genes were highly upregulated within bone marrow-derived macrophages, pantothenate production and ultimately CoA synthesis may play a role in adaptation of Francisella to its intracellular niche (22). We wanted to determine if the ΔpanG mutation was important for infection in mice. With an intranasal inoculum of 50 CFU, we saw no difference in lung, liver, or spleen burdens when comparing ΔpanG to wild-type Schu S4 at 1 or 3 days postinoculation (Fig. 8C to E).
Fig 8.
F. tularensis subsp. tularensis Schu S4 ΔpanG growth and virulence phenotype. (A) Chemical complementation of Schu S4 ΔpanG grown in CDM (black), CDM lacking pantothenate (black dotted line), CDM lacking pantothenate supplemented with β-alanine (gray), and CDM lacking pantothenate supplemented with pantolactone (gray dotted line). (B) Genetic complementation of F. tularensis subsp. tularensis Schu S4 ΔpanG with F. novicida panG expressed by the native promoter in the shuttle vector pMP831. Growth curve of F. tularensis subsp. tularensis Schu S4 (black), F. tularensis subsp. tularensis Schu S4 ΔpanG with pMP831 (empty control vector) (gray), and F. tularensis subsp. tularensis Schu S4 ΔpanG complemented with pSKI01 (black dotted line). All growth curves were done in triplicate, monitoring absorbance (OD600) in a 96-well microtiter dish. Graphs represent the mean absorbance at OD600. (C to E) Recovery of Schu S4 or Schu S4 ΔpanG mutant in mice following intranasal inoculation. C57BL/6 mice were infected intranasally with either wild-type Schu S4 (black circles) or Schu S4 ΔpanG (gray triangles) at a lethal dose of 50 CFU. Mice were euthanized on days 1 and 3 postinfection, and lung burdens (C), liver burdens (D), and spleen burdens (E) were determined and graphed. Each symbol represents data from a single mouse. There were no significant differences in recovery of mutant versus wild-type organisms from any organ at any time point as determined by the nonparametric Mann-Whitney test.
DISCUSSION
Pantothenate forms the core of CoA and is a precursor to acyl carrier protein (ACP), making it essential in both energy and lipid metabolism. We characterized the genes that function in pantothenate biosynthesis in Francisella using genetic and chemical complementation approaches. These genes are organized into a putative operon, which also included a gene coding for a hypothetical protein, PanG (FTN_1351), which we discovered is a novel KPR responsible for converting 2-dehydropantoate to pantoate. PanG (FTN_1351) does not have the known conserved Rossmann-domain that is typically associated with KPRs. However, PanG does contain a C-terminal DUF2520, which usually accompanies an N-terminal Rossmann-like domain. PanG rescued growth of an E. coli KPR double mutant in the absence of pantothenate, and the panG homolog from E. faecalis V583 (EF1861) complemented KPR function in the F. novicida double mutant. EF1861 also contains a predicted C-terminal DUF2520 domain in addition to a predicted N-terminal Rossmann-like domain. The panG genetic data (FTN_1351 and EF1861) suggest that the DUF2520 domain is involved in KPR activity. Further biochemical characterization of PanG needs to be done to determine structural binding motifs and enzymatic activity. Normally, KPR proteins bind cofactors such as NAD or NADP; more work needs to be done to characterize what cofactor is required for PanG KPR activity. There is no GXGXXG motif in PanG, which is responsible for NADP binding in E. coli; however, amino acids 11 to 16 are GXGXXA, which is a sequence found in mitochondrial flavoenzymes that bind NADP (41). Using secondary-structure predictions of PanG, the GXGXXA consensus sequence starts right at the junction of a β-sheet and an α-helix that would be compatible with a KPR βαβ fold-forming sequence (44).
Functional complementation assays demonstrated that expression of panB, panC, and panD were each required for growth of F. novicida in medium devoid of pantothenate. In regard to KPR activity, only an ilvC::Tnflp panG::Tn double mutant of F. novicida created a pantothenate auxotroph, demonstrating that PanG is functionally redundant with the acetohydroxy acid isomerase, IlvC. Both the panG homolog from E. faecalis V583 and the known panE gene encoding a KPR from E. coli DH10B could functionally complement the F. novicida ilvC::Tnflp panG::Tn KPR double mutant. CoA levels were not significantly different between the wild type and the ilvC::Tn mutant after 5 h of pantothenate depletion, demonstrating that PanG can fulfill the requirement for KPR activity in F. novicida. CoA levels in both the panG::Tn mutant strain and the ilvC::Tnflp panG::Tn double mutant were less than one-half the concentration of the wild type, suggesting that PanG is responsible for the majority of KPR activity in F. novicida. We also found that the LVS strain is a β-alanine auxotroph resulting from a base substitution in panD causing an amino acid substitution that shortens the aspartate-1-decarboxylase. While the lack of PanD activity appears to be unique to the LVS strain, it does not contribute to the attenuation of this strain in mice. In addition, the Schu S4 frameshift in ilvC produced a nonfunctional protein in regard to KPR activity. We demonstrated that PanG is the sole KPR in Schu S4, since creating an in-frame panG deletion resulted in a pantothenate auxotroph. However, the Schu S4 ΔpanG strain did not have a demonstrable virulence defect in a mouse model of pneumonic tularemia. It is not clear what molecule this mutant acquired from the host or where it acquired it to satisfy its pantothenate requirement. Given that mice have a blood plasma pantothenate level of 20 μM while humans have only 2 to 4 μM, it is possible that an F. tularensis pantothenate synthesis mutant could be attenuated in humans but not mice (45). It is possible that mice have excess pantothenate in their bloodstream, considering their diet is supplemented with pantothenate in addition to that naturally acquired from their gut flora. E. coli can produce and secrete 15 times more pantothenate than required for CoA biosynthesis, and ruminants obtain sufficient quantities of pantothenate from their gut microorganisms (46, 47). Virtually all bacteria, including F. tularensis, can take up pantothenate, but F. tularensis does not have an annotated panF homolog responsible for sodium-cotransport of pantothenate (1). It appears that F. tularensis is able to obtain pantothenate, a CoA precursor (such as phosphopantetheine), or CoA from the host and is likely storing sufficient quantities of pantothenate to support logarithmic growth for several rounds of replication while inside cells, where pantothenate may be limited. Pantothenate is immediately phosphorylated once taken up by host cells and could affect the ability of F. tularensis to acquire pantothenate. Further work will be needed to determine how F. tularensis acquires pantothenate or other substituents of CoA from the host. It may be a valuable endeavor to look further down in the CoA pathway for potential drug targets for Francisella tularensis, starting with the putative type III pantothenate kinases (FTT1392 and FTT0112), which is the first committed step in CoA biosynthesis.
ACKNOWLEDGMENTS
This research was supported by NIH grant A1082870 to T.H.K.
We thank Colin Manoil and Larry Gallagher at the University of Washington for providing to us the 2-allele F. novicida mutant library and the pFFLP-hyg plasmid. We thank the E. coli Genetic Stock Center (Yale University) for the ilvC and panE deletion stains. We also thank Lance Thurlow (University of North Carolina at Chapel Hill) for E. faecalis V583 and Corey Quackenbush (University of North Carolina at Chapel Hill) for his technical assistance with the figures.
Footnotes
Published ahead of print 14 December 2012
REFERENCES
- 1. Leonardi R, Zhang YM, Rock CO, Jackowski S. 2005. Coenzyme A: back in action. Prog. Lipid Res. 44: 125– 153 [DOI] [PubMed] [Google Scholar]
- 2. Cronan JE, Jr, Littel KJ, Jackowski S. 1982. Genetic and biochemical analyses of pantothenate biosynthesis in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 149: 916– 922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manch JN. 1981. Mapping of a new pan mutation in Escherichia coli K-12. Can. J. Microbiol. 27: 1231– 1233 [DOI] [PubMed] [Google Scholar]
- 4. Cronan JE., Jr 1980. Beta-alanine synthesis in Escherichia coli. J. Bacteriol. 141: 1291– 1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elischewski F, Puhler A, Kalinowski J. 1999. Pantothenate production in Escherichia coli K12 by enhanced expression of the panE gene encoding ketopantoate reductase. J. Biotechnol. 75: 135– 146 [DOI] [PubMed] [Google Scholar]
- 6. Primerano DA, Burns RO. 1983. Role of acetohydroxy acid isomeroreductase in biosynthesis of pantothenic acid in Salmonella typhimurium. J. Bacteriol. 153: 259– 269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merkamm M, Chassagnole C, Lindley ND, Guyonvarch A. 2003. Ketopantoate reductase activity is only encoded by ilvC in Corynebacterium glutamicum. J. Biotechnol. 104: 253– 260 [DOI] [PubMed] [Google Scholar]
- 8. Ciulli A, Chirgadze DY, Smith AG, Blundell TL, Abell C. 2007. Crystal structure of Escherichia coli ketopantoate reductase in a ternary complex with NADP+ and pantoate bound: substrate recognition, conformational change, and cooperativity. J. Biol. Chem. 282: 8487– 8497 [DOI] [PubMed] [Google Scholar]
- 9. Nagle SC, Jr, Anderson RE, Gary ND. 1960. Chemically defined medium for the growth of Pasteurella tularensis. J. Bacteriol. 79: 566– 571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain RE. 1965. Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl. Microbiol. 13: 232– 235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsson P, Oyston PC, Chain P, Chu MC, Duffield M, Fuxelius HH, Garcia E, Halltorp G, Johansson D, Isherwood KE, Karp PD, Larsson E, Liu Y, Michell S, Prior J, Prior R, Malfatti S, Sjostedt A, Svensson K, Thompson N, Vergez L, Wagg JK, Wren BW, Lindler LE, Andersson SG, Forsman M, Titball RW. 2005. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat. Genet. 37: 153– 159 [DOI] [PubMed] [Google Scholar]
- 12. Champion MD, Zeng Q, Nix EB, Nano FE, Keim P, Kodira CD, Borowsky M, Young S, Koehrsen M, Engels R, Pearson M, Howarth C, Larson L, White J, Alvarado L, Forsman M, Bearden SW, Sjostedt A, Titball R, Michell SL, Birren B, Galagan J. 2009. Comparative genomic characterization of Francisella tularensis strains belonging to low and high virulence subspecies. PLoS Pathog. 5: e1000459 doi:10.1371/journal.ppat.1000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rohmer L, Fong C, Abmayr S, Wasnick M, Larson Freeman TJ, Radey M, Guina T, Svensson K, Hayden HS, Jacobs M, Gallagher LA, Manoil C, Ernst RK, Drees B, Buckley D, Haugen E, Bovee D, Zhou Y, Chang J, Levy R, Lim R, Gillett W, Guenthener D, Kang A, Shaffer SA, Taylor G, Chen J, Gallis B, D'Argenio DA, Forsman M, Olson MV, Goodlett DR, Kaul R, Miller SI, Brittnacher MJ. 2007. Comparison of Francisella tularensis genomes reveals evolutionary events associated with the emergence of human pathogenic strains. Genome Biol. 8:R102 doi:10.1186/gb-2007-8-6-r102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saslaw S, Eigelsbach HT, Wilson HE, Prior JA, Carhart S. 1961. Tularemia vaccine study. I. Intracutaneous challenge. Arch. Intern. Med. 107:689– 701 [DOI] [PubMed] [Google Scholar]
- 15. Sjostedt A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105: 1– 29 [DOI] [PubMed] [Google Scholar]
- 16. Pechous RD, McCarthy TR, Zahrt TC. 2009. Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol. Mol. Biol. Rev. 73: 684– 711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Napier BA, Meyer L, Bina JE, Miller MA, Sjostedt A, Weiss DS. 2012. Link between intraphagosomal biotin and rapid phagosomal escape in Francisella. Proc. Natl. Acad. Sci. U. S. A. 109: 18084– 18089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pechous R, Celli J, Penoske R, Hayes SF, Frank DW, Zahrt TC. 2006. Construction and characterization of an attenuated purine auxotroph in a Francisella tularensis live vaccine strain. Infect. Immun. 74: 4452– 4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pechous RD, McCarthy TR, Mohapatra NP, Soni S, Penoske RM, Salzman NH, Frank DW, Gunn JS, Zahrt TC. 2008. A Francisella tularensis Schu S4 purine auxotroph is highly attenuated in mice but offers limited protection against homologous intranasal challenge. PLoS One 3: e2487 doi:10.1371/journal.pone.0002487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su J, Yang J, Zhao D, Kawula TH, Banas JA, Zhang JR. 2007. Genome-wide identification of Francisella tularensis virulence determinants. Infect. Immun. 75:3089– 3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kraemer PS, Mitchell A, Pelletier MR, Gallagher LA, Wasnick M, Rohmer L, Brittnacher MJ, Manoil C, Skerett SJ, Salama NR. 2009. Genome-wide screen in Francisella novicida for genes required for pulmonary and systemic infection in mice. Infect. Immun. 77: 232– 244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wehrly TD, Chong A, Virtaneva K, Sturdevant DE, Child R, Edwards JA, Brouwer D, Nair V, Fischer ER, Wicke L, Curda AJ, Kupko JJ, III, Martens C, Crane DD, Bosio CM, Porcella SF, Celli J. 2009. Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell. Microbiol. 11: 1128– 1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sambandamurthy VK, Wang X, Chen B, Russell RG, Derrick S, Collins FM, Morris SL, Jacobs WR., Jr 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 8: 1171– 1174 [DOI] [PubMed] [Google Scholar]
- 24. Gallagher LA, Ramage E, Jacobs MA, Kaul R, Brittnacher M, Manoil C. 2007. A comprehensive transposon mutant library of Francisella novicida, a bioweapon surrogate. Proc. Natl. Acad. Sci. U. S. A. 104: 1009– 1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallagher LA, McKevitt M, Ramage ER, Manoil C. 2008. Genetic dissection of the Francisella novicida restriction barrier. J. Bacteriol. 190: 7830– 7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97: 6640– 6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212: 77– 86 [DOI] [PubMed] [Google Scholar]
- 28. LoVullo ED, Sherrill LA, Perez LL, Pavelka MS., Jr 2006. Genetic tools for highly pathogenic Francisella tularensis subsp. tularensis. Microbiology 152: 3425– 3435 [DOI] [PubMed] [Google Scholar]
- 29. LoVullo ED, Sherrill LA, Pavelka MS., Jr 2009. Improved shuttle vectors for Francisella tularensis genetics. FEMS Microbiol. Lett. 291: 95– 102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LoVullo ED, Miller CN, Pavelka MS, Jr, Kawula TH. 2012. TetR-based gene regulation systems for Francisella tularensis. Appl. Environ. Microbiol. 78: 6883– 6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stansly PG, Schlosser ME. 1945. The biological activity of pantolactone and pantoic acid. J. Biol. Chem. 161: 513– 515 [PubMed] [Google Scholar]
- 32. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2: 2006.0008 doi:10.1007/978-1-59745-321-9_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horzempa J, Carlson PE, Jr, O'Dee DM, Shanks RM, Nau GJ. 2008. Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis. BMC Microbiol. 8:172 doi:10.1186/1471-2180-8-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403– 410 [DOI] [PubMed] [Google Scholar]
- 35. Fong C, Rohmer L, Radey M, Wasnick M, Brittnacher MJ. 2008. PSAT: a web tool to compare genomic neighborhoods of multiple prokaryotic genomes. BMC Bioinform. 9: 170 doi:10.1186/1471-2105-9-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39:D225– D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brand LA, Strauss E. 2005. Characterization of a new pantothenate kinase isoform from Helicobacter pylori. J. Biol. Chem. 280: 20185– 20188 [DOI] [PubMed] [Google Scholar]
- 38. Yang K, Eyobo Y, Brand LA, Martynowski D, Tomchick D, Strauss E, Zhang H. 2006. Crystal structure of a type III pantothenate kinase: insight into the mechanism of an essential coenzyme A biosynthetic enzyme universally distributed in bacteria. J. Bacteriol. 188: 5532– 5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pledger WJ, Umbarger HE. 1973. Isoleucine and valine metabolism in Escherichia coli. XXII. A pleiotropic mutation affecting induction of isomeroreductase activity. J. Bacteriol. 114: 195– 207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lobley CM, Ciulli A, Whitney HM, Williams G, Smith AG, Abell C, Blundell TL. 2005. The crystal structure of Escherichia coli ketopantoate reductase with NADP+ bound. Biochemistry 44: 8930– 8939 [DOI] [PubMed] [Google Scholar]
- 41. Hanukoglu I, Gutfinger T. 1989. cDNA sequence of adrenodoxin reductase. Identification of NADP-binding sites in oxidoreductases. Eur. J. Biochem. 180: 479– 484 [DOI] [PubMed] [Google Scholar]
- 42. Shimizu S, Kataoka M, Chung MC, Yamada H. 1988. Ketopantoic acid reductase of Pseudomonas maltophilia 845. Purification, characterization, and role in pantothenate biosynthesis. J. Biol. Chem. 263: 12077– 12084 [PubMed] [Google Scholar]
- 43. Si D, Urano N, Shimizu S, Kataoka M. 2012. Cloning and overexpression of ketopantoic acid reductase gene from Stenotrophomonas maltophilia and its application to stereospecific production of D-pantoic acid. Appl. Microbiol. Biotechnol. 93: 1619– 1625 [DOI] [PubMed] [Google Scholar]
- 44. Cole C, Barber JD, Barton GJ. 2008. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36: W197– W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Webb ME, Smith AG, Abell C. 2004. Biosynthesis of pantothenate. Nat. Prod. Rep. 21: 695– 721 [DOI] [PubMed] [Google Scholar]
- 46. Finlayson HJ, Seeley RC. 1983. The synthesis and absorption of pantothenic acid in the gastrointestinal tract of the adult sheep. J. Sci. Food Agric. 34: 427– 432 [DOI] [PubMed] [Google Scholar]
- 47. Jackowski S, Rock CO. 1981. Regulation of coenzyme A biosynthesis. J. Bacteriol. 148: 926– 932 [DOI] [PMC free article] [PubMed] [Google Scholar]