Abstract
The chemotropic guidance cue netrin-1 mediates attraction of migrating axons during central nervous system development through the receptor Deleted in Colorectal Cancer (DCC). Downstream of netrin-1, activated Rho GTPases Rac1 and Cdc42 induce cytoskeletal rearrangements within the growth cone. The Rho guanine nucleotide exchange factor (GEF) Trio is essential for Rac1 activation downstream of netrin-1/DCC, but the molecular mechanisms governing Trio activity remain elusive. Here, we demonstrate that Trio is phosphorylated by Src family kinases in the embryonic rat cortex in response to netrin-1. In vitro, Trio was predominantly phosphorylated at Tyr2622 by the Src kinase Fyn. Though the phospho-null mutant TrioY2622F retained GEF activity toward Rac1, its expression impaired netrin-1-induced Rac1 activation and DCC-mediated neurite outgrowth in N1E-115 neuroblastoma cells. TrioY2622F impaired netrin-1-induced axonal extension in cultured cortical neurons and was unable to colocalize with DCC in growth cones, in contrast to wild-type Trio. Furthermore, depletion of Trio in cortical neurons reduced the level of cell surface DCC in growth cones, which could be restored by expression of wild-type Trio but not TrioY2622F. Together, these findings demonstrate that TrioY2622 phosphorylation is essential for the regulation of the DCC/Trio signaling complex in cortical neurons during netrin-1-mediated axon outgrowth.
INTRODUCTION
During central nervous system development, neurons extend axons across great distances toward their associated targets. The process of axon extension and pathfinding is mediated by the growth cone, a structure which emanates from the distal axon, composed of F-actin and microtubule networks (1, 2). The growth cone is able to sense and integrate a diverse range of extracellular guidance cues (secreted or membrane bound), which, in turn, trigger signaling pathways downstream of conserved receptors (3, 4). The netrin family of guidance cues is vital in neural development of both vertebrates and invertebrates (5). Netrin-1 has been shown to interact with at least four distinct families of transmembrane receptors: the Deleted in Colorectal Cancer (DCC) family, including DCC and its paralog neogenin, as well as DSCAM, the UNC-5 family, and, more recently, the amyloid precursor protein (6–11). Netrin-1 acts as a bifunctional guidance cue, promoting either attraction or repulsion of extending growth cones of various classes of neurons by signaling through its receptors and coreceptors (12). In the developing spinal cord and cerebral cortex of vertebrates, netrin-1 exerts its attractive functions through the receptor DCC (7, 13, 14). The significance of this pathway during neural development is underscored by the absence of spinal and cerebral commissures when expression of netrin-1 or DCC is disrupted (15, 16). Considerable efforts have been made to elucidate the molecular pathways downstream of the netrin-1/DCC interaction. Upon netrin-1 stimulation, DCC becomes highly phosphorylated on serine, threonine, and tyrosine residues (17). Phosphorylation of DCC at Tyr1418 by the Src kinase Fyn occurs downstream of netrin-1 stimulation and is required for axon outgrowth and guidance (17–20).
Rho GTPases are molecular switches that regulate multiple intracellular processes, including actin remodeling, by cycling between an inactive GDP-bound and active GTP-bound state (21, 22). During axon outgrowth and guidance, the recruitment and localized regulation of the Rho GTPases Rac1, Cdc42, and RhoA are fundamental for translating extracellular cues into cytoskeletal rearrangements within the growth cone (3, 23–26). Downstream of netrin-1/DCC, both Rac1 and Cdc42 become activated and drive axonal extension, while RhoA activation occurs most prominently during growth cone collapse (19, 27, 28). More recently, we have shown that RhoA/Rho kinase and Src kinases induce ezrin-radixin-moesin (ERM) protein phosphorylation, which is required to mediate netrin-1/DCC-induced cortical axon outgrowth (29). Rho GTPases are activated by guanine nucleotide exchange factors (GEFs), a family of proteins that stimulate GDP/GTP exchange (30). Recent studies have identified two mammalian GEFs mediating Rac1 activation downstream of netrin-1/DCC: DOCK180 and Trio (31, 32). Trio is the founding member of a family of GEFs which contain two Dbl homology/pleckstrin homology (DH-PH) GEF domains (GEFDs). Trio has activity toward both RhoG and Rac1 by its first GEFD (GEFD1), while the second GEFD (GEFD2) activates RhoA in vitro (33–36). Although its expression is ubiquitous, Trio is enriched in the brain, where it is present in 5 isoforms generated by alternative splicing (37). The genetic ablation of Trio in the mouse is lethal between embryonic day 15.5 (E15.5) and birth (38), with embryos presenting disorganization of neuronal projections in the developing spinal cord and brain (31). Although we have shown that Trio interacts in a signaling complex with DCC, the SH2/SH3 adaptor protein Nck-1, and p21-activated kinase (Pak1) in vitro (31), the mechanisms governing Trio localization and activity within the growth cone remain unknown. GEFs can be regulated by several molecular mechanisms, including phosphorylation, inter- and intramolecular interactions, and lipid binding (30). Here, we demonstrate that Trio is a substrate of Src kinases downstream of netrin-1/DCC in the embryonic rat cortex. Concomitantly, netrin-1 stimulation enhanced Trio interaction with DCC in the developing cortex. We show that Trio is phosphorylated by the Src kinase Fyn at Tyr2622, and phosphorylation of this site is potentiated by coexpression of DCC in cultured cells. Although point mutation at Tyr2622 did not affect the in vitro GEF activity of Trio, it impaired netrin-1-induced Rac1 activation. Expression of a phospho-null TrioY2622F mutant resulted in impaired DCC-mediated neurite outgrowth in N1E-115 neuroblastoma cells and inhibited axonal responsiveness to netrin-1 in cultured cortical neurons. Furthermore, TrioY2622F blocked netrin-1-mediated Trio/DCC interaction in the growth cone of cortical neurons, and depletion of Trio in cortical neurons reduced the level of cell surface DCC in growth cones, which could be restored by expression of wild-type Trio but not TrioY2622F. In addition, Trio participates in the dynamics of DCC surface localization in response to netrin-1. Together, these data suggest a novel regulatory mechanism wherein Trio, in addition to regulating Rac1, also modulates the function of DCC via its Tyr2622 phosphorylation site during netrin-1-induced axon extension.
MATERIALS AND METHODS
DNA constructs and antibodies.
pGEX-5X constructs encoding Trio protein fragments 1 to 8 were cloned using standard cloning procedures (cloning details can be obtained upon request). Fragments correspond to Trio amino acids as follows: fragment 1, 1 to 232; fragment 2, 1 to 702; fragment 2a, 1 to 485; fragment 2b, 464 to 699; fragment 3, 700 to 1157; fragment 4, 1157 to 1203; fragment 5, 1204 to 1701; fragment 6, 1848 to 2298; fragment 7, 2299 to 2627; and fragment 8, 2627 to 3038. Green fluorescent protein (GFP)-Trio single and double point mutants were derived from the wild-type form of GFP-Trio (39) using the QuikChange site-directed mutagenesis kit (Stratagene), according to the manufacturer's instructions. pRK5-Fyn and pRK5-DCC constructs have been described previously (17, 40). All constructs were verified by sequencing. The polyclonal anti-TrioMTP antibody was raised against a fragment encompassing residues 1581 to 1849 of the Trio-C isoform expressed as a glutathione S-transferase (GST) fusion protein in Escherichia coli (37). The antibody was affinity purified on Affi-Gel Sepharose (Bio-Rad) coupled to the same protein antigen. The resulting antibody preparation was then passed through an Affi-Gel Sepharose column coupled to the GST protein in order to retain the anti-GST antibodies contained in the preparation. The TrioMTP antibody immunoprecipitates and recognizes by Western blotting all Trio isoforms. Additional antibodies included anti-DCCINT (clone G97-449; BD Biosciences), anti-DCCEXT (clone AF5; Calbiochem), antiphosphotyrosine (clone 4G10) and antitubulin (Upstate), anti-GFP (Invitrogen), anti-Pak (C-12) and anti-Fyn (Santa Cruz), anti-pFAK (pY861) and anti-FAK (Invitrogen) anti-Rac1 (BD Transduction Laboratories), anti-pERK1/2 (pThr202/pThr204) and anti-ERK1/2 (Cell Signaling), and anti-rabbit antibody–Alexa Fluor 488 and anti-mouse antibody–Cy3 (Molecular Probes).
Cell culture and transfection.
HEK293, COS-7, and N1E-115 cells were cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM; Wisent Bioproducts) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin, and streptomycin (Invitrogen) under humidified conditions with 5% CO2. N1E-115 cells were plated on laminin-coated 100-mm dishes (25 μg/ml; BD Biosciences). For the neurite outgrowth assays, N1E-115 cells were plated in 35-mm dishes (1.25 × 106 cells/plate) containing glass coverslips coated with laminin. Cells were transfected with constructs as indicated below using linear polyethylenimine (PEI; PolySciences) at a 1:6 ratio (cDNA to PEI) as described previously (29).
Primary cortical neuron culture and electroporation.
Cortical neurons from E17 rat embryos were dissociated mechanically and electroporated with cDNA constructs encoding wild-type or mutant forms of GFP-Trio using the Amaxa rat neuron Nucleofector kit (Lonza). Postelectroporation, neurons were plated on poly-l-lysine (0.1 mg/ml; Sigma-Aldrich)-treated coverslips in 24-well plates at a density of 200,000 cells/well. Neurons were cultured in attachment medium (minimal essential medium [Invitrogen] supplemented with 1 mM sodium pyruvate [Invitrogen], 0.6% d-glucose [Sigma-Aldrich], and 10% horse serum). After 2 h, the medium was replaced with maintenance medium (Neurobasal-A medium [Invitrogen] supplemented with 2% B27 [Invitrogen] and 1% l-glutamine). At day in vitro 1 (DIV1), the neurons were treated for the times indicated below with purified recombinant chick myc–netrin-1 (500 ng/ml), which was produced and purified as described previously (16). Downregulation of endogenous Trio was achieved by electroporating dissociated E17 rat cortical neurons with 300 nM synthetic Trio small interfering RNAs (siRNAs) designed to target the 5′ untranslated region (UTR) of the rat Trio mRNA (siRNA 1, 5′-GUAAAUAUCAACCGCAUAAUU-3′; siRNA 2, 5′-GAACAUGAUUGACGAGCAUUU-3′; Dharmacon/Thermo Scientific) together with 2 μg of pmaxGFP vector (Lonza) used as a reporter. Only GFP-expressing neurons were assessed. The siRNA-resistant GFP-Trio point mutant plasmids were coelectroporated where indicated, and expression of the exogenous proteins was verified by Western blotting (see Fig. 5F).
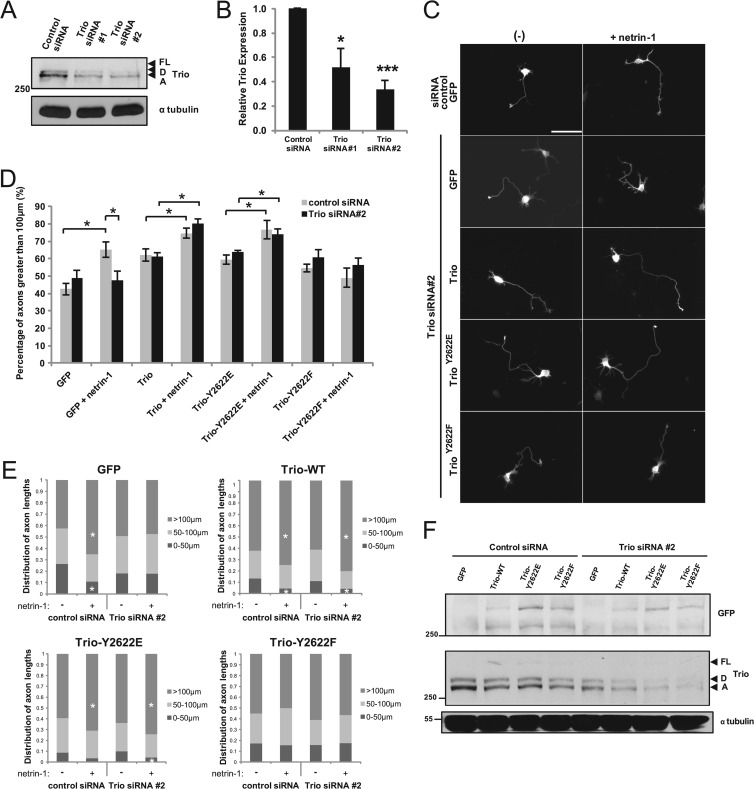
Fig 5.
TrioY2622 phosphorylation is required for netrin-1-mediated cortical axon outgrowth. (A) Endogenous Trio was silenced in E17 rat cortical neurons by expressing siRNA targeting the 5′ UTR of Trio mRNA, thereby knocking down all neural isoforms of Trio containing the Rac GEF domain. (B) Densitometric analysis of Trio-FL, Trio-D, and Trio-A from panel A. Error bars indicate SEMs (n = 4; *, P < 0.05; ***, P < 0.001). (C) Dissociated E17 rat cortical neurons were electroporated with Trio and siRNA constructs as indicated, in addition to a GFP-reporter construct. At DIV1, the neurons were stimulated with netrin-1 for 24 h before fixation. The axon lengths of GFP-positive neurons were calculated manually using MetaMorph software (>30 neurons/per condition, in duplicate). Scale bar, 55 μm. (D) The percentage of axons in panel C, measuring greater than 100 μm per condition. Error bars indicate SEMs (n = 5; *, P < 0.05). (E) Distribution of axon lengths of GFP-positive neurons in panel C (n = 5; *, P < 0.03). (F) Expression of Trio siRNA-resistant GFP-Trio cDNA constructs in cortical neuron cultures at DIV2. GFP-Trio was detected by Western blotting using an anti-GFP antibody. Subsequently, total Trio and tubulin levels were detected using appropriate antibodies. Trio isoforms: FL, full length; D, Trio-D; A, Trio-A; WT, wild type.
Cortical tissue culture.
Cortical tissues were dissected from E17 rat embryos. After light mechanical dissociation, tissues were transferred to a 4-well plate containing prewarmed maintenance medium and allowed equilibrate to 37°C. For pervanadate treatment, pervanadate (10 mM sodium orthovanadate and 10 mM hydrogen peroxide in phosphate-buffered saline [PBS]) was added to tissue cultures to a final concentration of 0.1 mM for 15 min prior to netrin-1 stimulation, and the total duration of pervanadate treatment was identical for each condition. Immediately following netrin-1 stimulation, samples were transferred on ice, collected, and processed as described.
GST-protein purification.
Recombinant proteins corresponding to Trio fragments 1 to 8 were expressed in E. coli BL21(DE3) cells, except for fragment 2, which was expressed in E. coli Rosetta, and were purified as described previously (41). Briefly, 1 liter of log-phase bacteria was induced with 1 mM isopropylthiogalactopyranoside (IPTG) and shaken overnight at 22°C. The bacteria were lysed in 20 ml of lysis buffer (20 mM Tris-HCl [pH 7.5], 0.1% Triton X-100, 1 mg/ml lysosyme, 1 mg/ml DNase I) and sonicated, and the lysates were centrifuged at 20,000 × g for 20 min at 4°C. The clarified lysate was mixed for 1 h at 4°C with glutathione-Sepharose 4B beads (GE Healthcare) equilibrated with 20 mM Tris-HCl (pH 7.5)–0.1% Triton X-100. The beads were washed four times with the same buffer and once with 50 mM Tris-HCl (pH 8.0), and the proteins were eluted in 20 mM Tris-HCl (pH 8.0) containing 10 mM reduced glutathione. Proteins were stored at −80°C in the presence of 30% glycerol.
In vitro kinase assays.
The GST-tagged fragments of Trio (1 μg) purified from E. coli were incubated in a 20-μl reaction mixture containing 50 mM Tris-HCl (pH 7.5), 1 μM dithiothreitol (DTT), 5 mM manganese chloride, 5 mM magnesium chloride, and 100 μM [γ-32P]ATP (PerkinElmer; 5 mCi/μmol; 10 μCi/reaction) in the presence of 50 ng recombinant active Fyn (Millipore). After incubation for 30 min at 30°C, the reactions were terminated by the addition of 10 μl of SDS sample buffer. Proteins were resolved by SDS-PAGE, and Coomassie blue-stained gels were dried and subjected to autoradiography.
Immunoprecipitation and Western blotting.
Cell lines expressing GFP-Trio constructs and cortical tissues were lysed in lysis buffer containing 20 mM HEPES (pH 7.5), 100 mM NaCl, 10% glycerol, 1% Triton X-100, 20 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 μM complete protease inhibitor cocktail (Roche Diagnostics). Protein lysates were subjected to centrifugation at 10,000 × g for 2 min at 4°C to remove insoluble materials. For assays using exogenous GFP-Trio, 1 mg of protein lysate was incubated for 3 h at 4°C with 20 μl of protein A-Sepharose beads and 2.5 μg of GFP antibody. In the case of primary cortical lysates, 2.5 mg of protein lysate was incubated for 1 h at 4°C with 15 μg of anti-TrioMTP antibody followed by a 2-h incubation with 40 μl of protein A-Sepharose beads (GE Healthcare). Beads were washed three times with ice-cold lysis buffer and heated to 95°C in SDS sample buffer. Protein samples were resolved by SDS-PAGE, transferred to nitrocellulose membranes for Western blotting with the appropriate antibodies, and visualized by enhanced chemiluminescence (ECL; PerkinElmer).
Rac1 activation assay.
Transfected HEK293 cells were serum starved overnight and then lysed in buffer containing 25 mM HEPES (pH 7.5), 1% NP-40, 10 mM MgCl2, 100 mM NaCl, 5% glycerol, 1 mM PMSF, and 1 μM protease inhibitor cocktail. Protein lysates were subjected to centrifugation at 10,000 × g for 2 min at 4°C to remove insoluble materials. Endogenous GTP-Rac1 was pulled down by incubating the protein lysates for 30 min at 4°C with the Cdc42/Rac interactive binding domain (CRIB) of mouse PAK3 (amino acids 73 to 146) fused to GST and coupled to glutathione-Sepharose beads (19, 42). The beads were washed twice with 25 mM HEPES (pH 7.5), 1% NP-40, 30 mM MgCl2, 40 mM NaCl, and 1 mM DTT and resuspended in SDS sample buffer. For the assays following netrin-1 stimulation, transfected COS-7 cells (which do not secrete endogenous netrin-1) were serum starved overnight before stimulation with netrin-1 (500 ng/ml), and the assays were performed using a Rac1 activation kit (Cytoskeleton) according to the manufacturer's instructions. In both cases, protein samples were resolved by SDS-PAGE and transferred onto nitrocellulose membranes for Western blotting using the anti-Rac1 antibody. The levels of GTP-bound proteins were assessed by densitometry using Quantity One software (Bio-Rad) and normalized to the total amount of GTPases detected in the total cell lysates.
Immunofluorescence, microscopy, and Pearson's correlation coefficient.
Transfected N1E-115 cells were fixed and permeabilized as described previously (19). Coimmunostaining was carried out with the primary antibodies indicated below and the respective Cy3- or Alexa Fluor 488-conjugated secondary antibodies. Cells were examined with an Axiovert 135 Carl Zeiss microscope using a 63× PLAN-Neofluar objective lens. Images were recorded with a digital camera (DVC Co.) and analyzed with Northern Eclipse software (Empix Imaging Inc.). Cortical neurons (DIV1 or 2) were fixed with 3.7% formaldehyde (Sigma-Aldrich) in 20% sucrose–PBS for 30 min at 37°C and permeabilized as described previously (29) unless otherwise indicated. Cortical neurons were visualized using an Olympus IX81 motorized inverted microscope using the 40× U PLAN Fluorite and 60× U PLAN S-APO oil objective lenses. Images were recorded with a CoolSnap 4K camera (Photometrics) and analyzed with the Metamorph software (Molecular Devices). For surface DCC detection, cortical neurons remained unpermeabilized, were blocked in 1% bovine serum albumin (BSA)–PBS, and were incubated with anti-DCCEXT in 1% BSA–PBS at 4°C overnight. Cy3-conjugated secondary antibodies were used to label surface DCC. Average pixel intensity of DCC fluorescence on growth cones and axonal surfaces was measured from acquired images by Metamorph software, using exclusive thresholding to eliminate background fluorescence. Coimmunostaining of cortical neurons was carried out as indicated below, and GFP-positive neurons were imaged on a Zeiss LSM510 laser-scanning confocal microscope with a PLAN-Apochromat 63×/1.4 oil objective lens and analyzed with Zen2009 software (Carl Zeiss Microscopy). Quantification of colocalization using Pearson's correlation coefficient (r) was performed using MetaMorph software, analyzing more than 10 growth cones per condition in at least 4 independent experiments. Student's unpaired t test was used for statistical analysis, and the data were presented as mean r values ± standard errors of the means (SEMs).
Neurite outgrowth analysis.
More than 100 transfected N1E-115 cells were analyzed for each condition. A neurite was defined as a process that measured at least one length of the cell body, and the proportion of transfected cells expressing a neurite was determined manually. To analyze axon outgrowth of primary cortical neurons, 30 electroporated (GFP-positive) cells were analyzed for each condition. Axonal lengths were measured manually from acquired images using MetaMorph software. Student's unpaired t test was used for statistical analysis, and the data were presented as mean cortical neuron axon lengths ± SEMs.
RESULTS
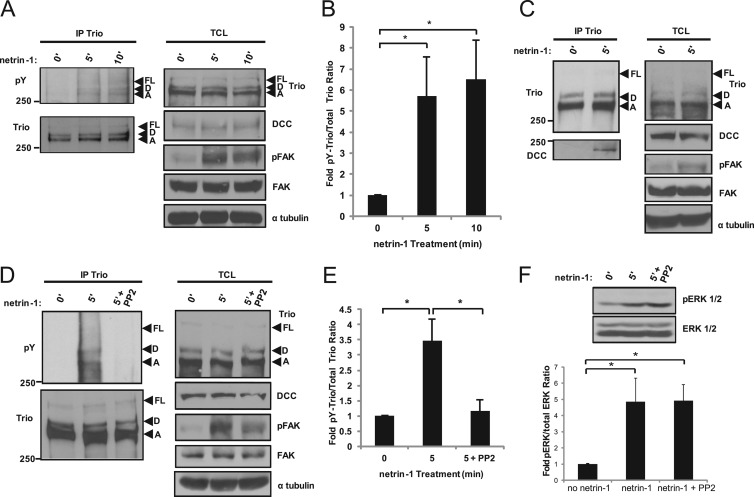
Netrin-1 induces Src kinase-dependent phosphorylation of Trio in the developing cortex.
To investigate whether endogenous Trio is tyrosine phosphorylated in response to netrin-1, embryonic E17 rat cortices were dissected and cultured in the presence of the tyrosine phosphatase inhibitor pervanadate prior to netrin-1 stimulation. After 5- and 10-min stimulations with netrin-1, the cortices were lysed and Trio was immunoprecipitated. Netrin-1 stimulation of cortical tissues induced a marked increase in tyrosine phosphorylation of full-length Trio and isoforms Trio-D and Trio-A at 5 min and 10 min, as shown by Western blotting with an antiphosphotyrosine antibody (Fig. 1A and B). Netrin-1 stimulation also induced FAK phosphorylation (Fig. 1A), which has been previously reported for commissural and cortical neurons, thereby serving as a positive control for a stimulated state in our model system (18). In addition to tyrosine phosphorylation of Trio, netrin-1 stimulation for 5 min resulted in an increased association of Trio with DCC in the embryonic cortex (Fig. 1C). Since the Src-kinase Fyn has been reported to be part of the signaling complex downstream of netrin-1/DCC (17), we sought to determine whether Src family kinases mediate tyrosine phosphorylation of Trio in response to netrin-1. To this end, we preincubated rat embryonic cortical tissue with the Src kinase inhibitor PP2 before stimulation with netrin-1 (Fig. 1D). The inhibition of Src kinases by PP2 resulted in a complete block in tyrosine phosphorylation of the three Trio isoforms induced by netrin-1 (Fig. 1D and E). In addition, Src kinase inhibition led to a reduction in FAK phosphorylation induced by netrin-1 (Fig. 1D), but not ERK1/2 phosphorylation (Fig. 1F). Therefore, netrin-1 induces tyrosine phosphorylation of Trio in a Src kinase-dependent manner and a concomitant association with DCC in the developing cortex.
Fig 1.
Netrin-1 induces Src kinase-dependent phosphorylation of Trio in the developing cortex. (A) Isolated E17 rat cortices were stimulated with netrin-1 for the indicated times. Trio isoforms were subjected to immunoprecipitation (IP), and phosphotyrosine (pY)-Trio and total Trio were detected by Western blotting using antiphosphotyrosine (4G10) and anti-Trio antibodies, respectively. Netrin-1 stimulation of DCC-induced signaling pathways was verified by FAK phosphorylation in total cell lysates (TCL). Trio isoforms: FL, full length; D, Trio-D; A, Trio-A. (B) Densitometric analysis of Trio-D and Trio-A from panel A. Error bars indicate SEMs (n = 5; *, P < 0.05). (C) Isolated E17 rat cortices were stimulated with netrin-1 and Trio isoforms were subjected to IP as for panel A, coimmunoprecipitated DCC was detected by Western blotting using an anti-DCC antibody, and TCL were immunoblotted as for panel A. (D) Isolated E17 rat cortices were stimulated with netrin-1 in the presence or absence of the Src family kinase inhibitor PP2 (10 μM). Trio was subjected to IP as for panel A, pY-Trio was detected by Western blotting, and TCL were immunoblotted as for panel A. (E) Densitometric analysis of Trio-D and Trio-A from D. Error bars indicate SEMs (n = 4; *, P < 0.05). (F) As for panel D, isolated E17 rat cortices were stimulated with netrin-1 in the absence or presence of the Src kinase inhibitor PP2 before lysis. ERK1/2 phosphorylation was detected by Western blotting. Densitometric analysis of levels of pERK1/2 and total ERK1/2 levels. Error bars indicate SEMs (n = 4; *, P < 0.05).
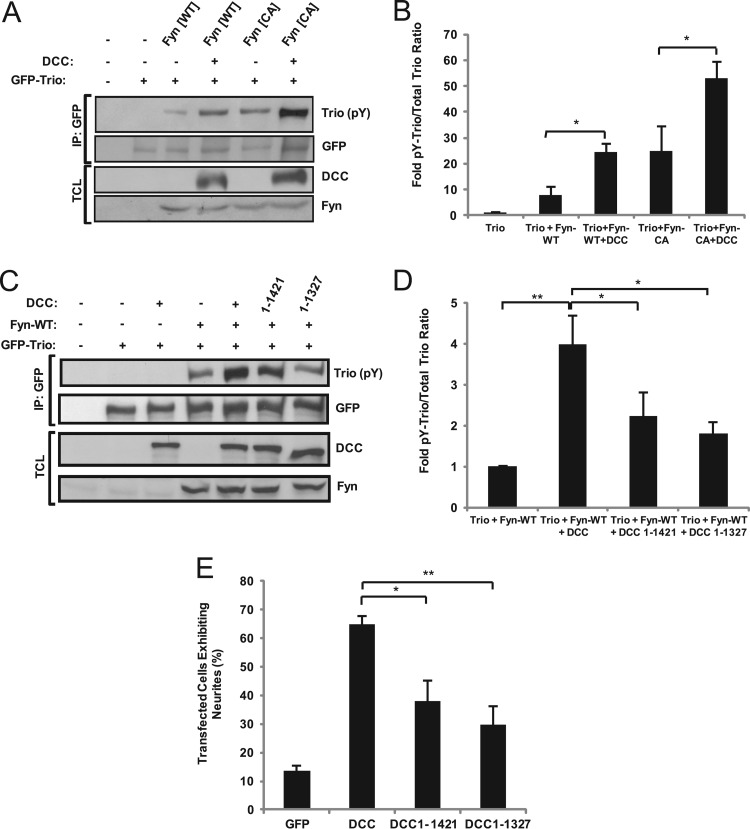
Phosphorylation of Trio downstream of the Src family kinase Fyn is enhanced by DCC in N1E-115 neuroblastoma cells.
We have previously demonstrated that phosphorylation of DCC at Tyr1418 by the tyrosine kinase Fyn is required for Rac1 activation and that Trio is required for this activation downstream of netrin-1 (17, 31). This led us to investigate whether Fyn could phosphorylate and thereby regulate Trio activity. To this end, we cultured N1E-115 neuroblastoma cells, which express netrin-1 but not DCC (19), and exogenously expressed Trio together with wild-type or constitutively active Fyn, with or without DCC. Trio tyrosine phosphorylation was observed in the presence of wild-type or active Fyn (Fig. 2A and B). Furthermore, coexpression of DCC with Trio and Fyn resulted in an additional increase in tyrosine phosphorylation levels of Trio induced by both wild-type and constitutively active Fyn (Fig. 2A and B). To investigate this increase in Trio phosphorylation induced by both Fyn and DCC, we coexpressed DCC mutants truncated in their intracellular domain, together with Fyn and Trio in N1E-115 cells. The removal of the C-terminal extremity of DCC, comprising the conserved P2 (residues 1327 to 1363) and P3 (residues 1421 to 1445) domains (43), significantly reduced the ability of DCC to enhance Fyn-induced phosphorylation of Trio (Fig. 2C and D). In correlation to this, when DCC mutant proteins lacking P2 and P3 domains were expressed in N1E-115 cells, their ability to induce neurite formation compared to the wild-type protein was significantly impaired (Fig. 2E). This result is consistent with previous reports showing that the P3 intracellular domain of DCC is required for attractive signaling of the growth cone in response to netrin-1 (44). Altogether, these data suggest that Trio is tyrosine phosphorylated downstream of Fyn and that an intact intracellular domain of DCC potentiates Fyn-induced tyrosine phosphorylation of Trio.
Fig 2.
DCC enhances Trio tyrosine phosphorylation by Fyn in N1E-115 neuroblastoma cells. (A) GFP-Trio was obtained by immunoprecipitation (IP) from N1E-115 neuroblastoma cells transfected with pEGFP-Trio, pRK5-Fyn (WT, wild type; CA, constitutively active), and pRK5-DCC. Phosphotyrosine (pY)-Trio and total Trio were detected by Western blotting using antiphosphotyrosine (4G10) and anti-GFP antibodies, respectively. TCL, total cell lysates. (B) Densitometric analysis of panel A. Error bars indicate SEMs (n = 4; *, P < 0.05). (C) GFP-Trio was obtained by IP from N1E-115 neuroblastoma cells transfected with pEGFP-Trio, pRK5-Fyn, and wild type (WT) or truncated forms of pRK5-DCC, as indicated. Western blotting was conducted as for panel A. TCL, total cell lysates. (D) Densitometric analysis of panel C. Error bars indicate SEMs (n = 4; *, P < 0.05; **, P < 0.005). (E) N1E-115 neuroblastoma cells were transfected with wild-type or truncated forms of pRK5-DCC, as indicated. Neurite outgrowth was assessed by fluorescence microscopy (n = 4; *, P < 0.05; **, P < 0.005).
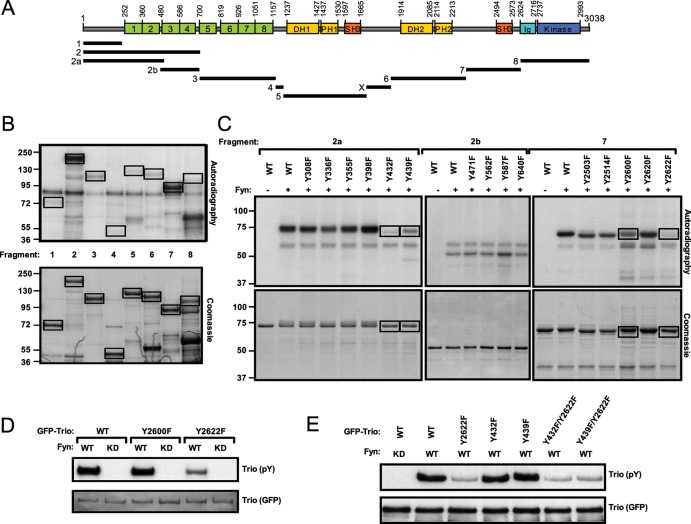
TrioY2622 is a major in vitro phosphorylation site of the tyrosine kinase Fyn.
To determine whether Trio is directly phosphorylated by Fyn, N-terminal GST-tagged protein fragments spanning the full length of Trio were employed in an in vitro kinase assay with recombinant Fyn (Fig. 3A). In this way, we identified two protein fragments of Trio to be highly phosphorylated by Fyn, one at the N terminus (fragment 2) and one at the C terminus (fragment 7) (Fig. 3B). Each of the five tyrosine residues in fragment 7 were replaced by a phenylalanine residue, and subsequent in vitro kinase assays revealed that mutation of Tyr2622 significantly reduced the overall phosphorylation of this fragment by Fyn, while mutation of adjacent Tyr2600 only slightly reduced phosphorylation (Fig. 3C, right side). When the amino acid substitution Tyr2622F was introduced into the full-length GFP-tagged Trio protein, tyrosine phosphorylation of Trio with Fyn coexpression was significantly reduced compared to that of the wild-type Trio protein in HEK293 cells, whereas the Tyr2600F substitution had no effect on Trio tyrosine phosphorylation (Fig. 3D). For fragment 2, two subfragments (fragments 2a and 2b) spanning the length of fragment 2 were generated, and each tyrosine residue contained within these fragments was replaced with a phenylalanine residue. Subsequent in vitro kinase assays revealed Tyr432 or Tyr439 to be Fyn phosphorylation sites (Fig. 3C, left side). However, when tyrosine-to-phenylalanine substitutions were introduced at these sites into full-length GFP-Trio, phosphorylation of Trio downstream of Fyn was not noticeably reduced in HEK293 cells (Fig. 3E). In addition, combined mutations of either Tyr432 or Tyr439 with Tyr2622 did not further reduce tyrosine phosphorylation of Trio compared to the single Tyr2622F substitution, indicating that the phosphorylation events observed on Tyr432 or Tyr439in vitro within fragment 2a do not occur in the context of full-length Trio expressed in cells. Therefore, these data show that Fyn phosphorylates Trio directly in vitro and that Tyr2622 is the major Fyn phosphorylation site.
Fig 3.
In vitro identification of TrioY2622 as the major Fyn phosphorylation site. (A) Schematic of Trio domain structure and GST-Trio fragment generation plan. Fragment X does not contain any tyrosine residue and was therefore not included in subsequent analyses. (B) In vitro kinase assay. Bacterially expressed GST-Trio fragments were incubated with purified, active Fyn and [γ-32P]ATP. Phosphorylation states of each fragment were determined by autoradiography (top) and compared to total input (Coomassie blue staining [bottom]). (C) Single amino acid substitutions of each tyrosine (Y) residue to phenylalanine (F) were introduced into fragments 2a, 2b, and 7, and a kinase assay was performed as for panel B. (D and E) Wild-type or point mutant forms of full-length GFP-Trio were cotransfected with either wild-type (WT) or kinase-dead (KD) Fyn in HEK293 cells as indicated. The GFP-Trio proteins were immunoprecipitated from cell lysates. pY-Trio and total GFP-Trio proteins were detected by Western blotting using an antiphosphotyrosine (4G10) antibody and an anti-GFP antibody, respectively.
Tyr2622 is not required for Trio intrinsic GEF activity toward Rac1 but participates in netrin-1-induced Rac1 activation and neurite outgrowth.
We have previously shown that the expression of DCC in N1E-115 cells induces neurite outgrowth in a netrin-1 and Trio-dependent manner through activation of Rac1 (19, 31). To determine the role of Trio phosphorylation by Fyn in its ability to induce neurite outgrowth in these cells, wild-type Trio, phospho-mimicking TrioY2622E, and phospho-null TrioY2622F were expressed (Fig. 4A and B). Both Trio and TrioY2622E stimulated neurite outgrowth of N1E-115 cells either alone or with DCC coexpression; in contrast, the ability of TrioY2622F to induce neurite outgrowth was reduced significantly compared to that of wild-type Trio (P < 5E−9) (Fig. 4A and B). Furthermore, TrioY2622F impaired the ability of DCC to induce neurite outgrowth in N1E-115 cells, acting as a dominant negative protein (P = 0.003) (Fig. 4A and B). These results suggest that phosphorylation of Trio at Tyr2622 participates in DCC-mediated neurite outgrowth.
Fig 4.
TrioY2622 participates in DCC-mediated neurite outgrowth and is required for netrin-1-mediated Rac1 activation. (A) N1E-115 cells were transfected with pEGFP-Trio, phosphomimetic point mutant pEGFP-TrioY2622E, or phospho-null point mutant pEGFP-TrioY2622F, with or without pRK5-DCC. Actin filaments were visualized by indirect immunofluorescence using phalloidin-tetramethyl rhodamine isocyanate (TRITC), and neurite outgrowth was assessed by fluorescence microscopy. Scale bar, 50 μm. (B) Quantification of transfected cells exhibiting neurites from panel A. Error bars indicate SEMs (n = 5; **, P < 0.005; ***, P < 0.0001). (C) GTP-loaded Rac1 was pulled down by GST-CRIB from protein lysates of HEK293 cells expressing the indicated GFP-Trio proteins. GTP-bound Rac1 (top) and total Rac1 (middle) were detected by Western blotting. (D) Densitometric ratio of GTP-bound Rac1 to total Rac1 normalized to GFP. Error bars indicate SEMs (n = 7). (E) COS-7 cells expressing the indicated GFP-Trio proteins and DCC were stimulated with netrin-1 for 5 min. GTP-loaded Rac1 was pulled down by GST-CRIB. GTP-bound Rac1 (top), total Rac1, and the indicated proteins were detected by Western blotting. (F) Densitometric ratio of GTP-bound Rac1 to total Rac1 normalized to wild-type Trio, unstimulated. Error bars indicate SEMs (n = 3; *, P < 0.02).
In order to determine if the reduction in neurite outgrowth by TrioY2622F was due to an impaired GEF activity toward Rac1, we performed Rac1 pulldown assays to assess the levels of activated, endogenous GTP-Rac1 trapped by specific binding to the CRIB domain of Pak fused to GST (31, 42). As expected, Trio expression in HEK293 cells increased Rac1-GTP levels (Fig. 4C and D), and we observed no significant difference in the abilities of the TrioY2622E and TrioY2622F mutant proteins to increase Rac1-GTP levels (Fig. 4C and D). We next investigated whether phosphorylation of Trio at Tyr2622 plays a role in netrin-1-induced Rac1 activation. For that purpose, we coexpressed wild-type, phospho-mimicking, and phospho-null Trio with DCC in COS-7 cells, which do not secrete netrin-1. In this context, expression of the phospho-null TrioY2622F completely inhibited netrin-1-mediated Rac1 activation after 5 min in comparison to the expression of wild-type Trio, while it did not affect netrin-1-induced ERK1/2 phosphorylation (Fig. 4E and F). Curiously, expression of phospho-mimicking TrioY2622E did not significantly restore netrin-1-induced Rac1 activation (Fig. 4E and F); however, it was not as inhibitory as the phospho-null TrioY2622F (Fig. 4F). This result suggests that replacing Tyr2622 with a negatively charged residue only is not sufficient to restore netrin-1-induced Rac1 activation. Together, these data demonstrate that TyrY2622 does not play a role in the intrinsic GEF activity of Trio toward Rac1 but is required for netrin-1 and DCC to mediate Rac1 activation and neurite outgrowth.
Phosphorylation of Trio at Tyr2622 is required for netrin-1-mediated cortical axon outgrowth.
Next, we explored the role of Trio tyrosine phosphorylation in netrin-1-mediated axon outgrowth of dissociated rat embryonic cortical neurons depleted of Trio expression. Endogenous Trio expression was downregulated in E17 rat cortical neurons by electroporation of synthetic siRNA targeting the 5′ UTR of Trio mRNA, leading to downregulation of Trio, Trio-A, and Trio-D isoforms (Fig. 5A and B). Consistent with our previous study on Trio−/− embryos (31), downregulation of Trio expression led to inhibition of netrin-1-induced axon outgrowth in cortical neurons (Fig. 5C), as illustrated by the percentage of axons greater than 100 μm (Fig. 5D) or by the distribution of axon lengths (Fig. 5E). Interestingly, reexpression of wild-type Trio or the phospho-mimicking TrioY2622E was able to restore netrin-1-induced axon outgrowth (Fig. 5C to E). Expression of the phospho-null TrioY2622F was, however, ineffective in rescuing netrin-1-mediated axon extension (Fig. 5C to E). Therefore, these data show that TrioY2622 phosphorylation is required for netrin-1 to mediate cortical axon extension. The finding that TrioY2622E was capable of recovering netrin-1-mediated axon outgrowth suggests that replacing the tyrosine with a negatively charged residue was sufficient to mimic the role of Tyr2622 in Trio function in this cellular context.
Phosphorylation of Trio at Tyr2622 is required for netrin-1-enhanced Trio/DCC interaction in the growth cone.
Since netrin-1 stimulation of cortical tissues enhances the interaction of Trio with DCC (Fig. 1C), we next investigated the role of Tyr2622 in this process. Wild-type Trio, phospho-mimicking TrioY2622E, and phospho-null TrioY2622F were expressed in isolated embryonic cortical neurons, and after a 5-min netrin-1 stimulation, the cell cultures were fixed and immunostained with antibodies against GFP and the intracellular domain of DCC (Fig. 6A). Netrin-1 promoted a significantly enhanced colocalization of DCC with Trio (r = 0.474; P = 0.031) but not with the phospho-null TrioY2622F mutant (r = 0.295; P = 0.996) in the growth cones, as determined by quantification of Pearson's correlation coefficient (r) (Fig. 6A and B). The phospho-mimicking amino acid substitution Y2622E slightly increased colocalization of Trio with DCC at the growth cone in response to netrin-1 (r = 0.434; P = 0.18), although this was not statistically significant (Fig. 6A and B).
Fig 6.
TrioY2622 phosphorylation is required for netrin-1-mediated Trio/DCC interaction. (A) Isolated E17 rat cortical neurons were electroporated with the indicated Trio constructs. At DIV1, the neurons were cultured with or without netrin-1 for 5 min before fixation. Expression of each protein was assessed by confocal microscopy (green, Trio constructs; red, DCC). Scale bar, 10 μm. Arrowheads, DCC/Trio colocalization, (B) The mean Pearson's correlation coefficient (r) of green (Trio) and red (DCC) channels within the growth cone was calculated using MetaMorph software. Error bars indicate SEMs (n = 4; *, P < 0.04). (C) GFP-Trio was immunoprecipitated (IP) from netrin-1-stimulated COS-7 cells expressing wild-type or the indicated point mutants of GFP-Trio and DCC. Coimmunoprecipitation of DCC was detected by Western blotting (n = 3). TCL, total cell lysates.
We next examined the contribution of TrioY2622 phosphorylation in the interaction with DCC biochemically. Wild-type and mutant GFP-Trio constructs were coexpressed with DCC in COS-7 cells prior to netrin-1 stimulation (Fig. 6C). After 5 min of netrin-1 stimulation, an increase in the amount of DCC coimmunoprecipitating with GFP-Trio was observed (Fig. 6C). Although TrioY2622F and TrioY2622E were still able to interact with DCC, no enhancement of DCC interaction was observed with either protein in response to netrin-1 stimulation (Fig. 6C). Together, these results are consistent with the observation that netrin-1 stimulation of cortical tissues enhances the interaction between wild-type Trio and DCC (Fig. 1C) and show that phosphorylation of Trio at Tyr2622 is required for this netrin-1-induced association in neurons (Fig. 6A and B).
Trio is required to maintain DCC surface expression in the growth cone of cortical neurons.
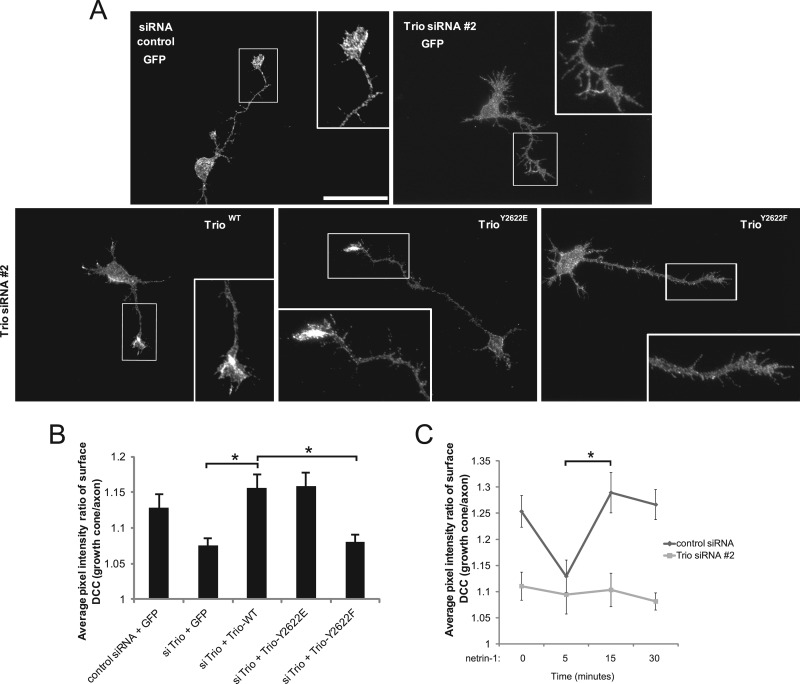
To determine if Trio influences DCC function by altering its localization, we assessed the surface expression of DCC in neurons depleted of endogenous Trio by immunostaining with antibodies against the extracellular domain of DCC under nonpermeabilizing conditions. When endogenous Trio expression was silenced, the intensity of cell surface DCC as detected by epifluorescence was reduced in the growth cones compared to that in cells expressing control siRNA (Fig. 7A and B). Intriguingly, reexpression of either siRNA-resistant wild-type Trio or TrioY2622E enhanced the average intensity of DCC at the growth cone surface, whereas TrioY2622F did not (Fig. 7A and B).
Fig 7.
Trio is required to maintain DCC surface expression at the growth cone. (A) Dissociated E17 rat cortical neurons were electroporated with the indicated Trio constructs and siRNA 2 in addition to a GFP-reporter construct. At DIV1, the neurons were fixed and the expression of surface DCC was assessed by staining with an antibody recognizing the extracellular domain of DCC under nonpermeabilizing conditions, and subsequent fluorescence microscopy (photomicrograph of DCC). Scale bar, 40 μm. (B) The average pixel intensity ratio of surface DCC expressed at the growth cones relative to axons of each GFP-expressing neuron was calculated using MetaMorph software (15 cells per condition). Error bars indicate SEMs (n = 4, 2 replicates each; *, P < 0.05). (C) Dissociated E17 rat cortical neurons were electroporated wtih either control siRNA or Trio siRNA 2 in addition to a GFP-reporter construct. At DIV1, the neurons were stimulated with netrin-1 (250 ng/ml) for the indicated times, fixed, and processed as for panel A. The average pixel intensity ratio of surface DCC expressed at the growth cones relative to axons of each GFP-expressing neuron was calculated using MetaMorph software (20 cells/condition). Error bars indicate SEMs (n = 3, 2 replicates each; *, P < 0.05).
We next investigated whether Trio participates in the dynamics of DCC surface localization in response to netrin-1. For that purpose, we performed a netrin-1 time course experiment with cortical neuron cultures and assessed surface DCC enrichment in the growth cones relative to axons of the same cells. When cortical neurons expressing control siRNA were stimulated with netrin-1 for 5 min, we observed a significant reduction in cell surface DCC intensity at the growth cones (Fig. 7C). At between 5 and 15 min of netrin-1 treatment, cell surface DCC was restored, and it was maintained until at least 30 min poststimulation. The observed increase in DCC surface intensity correlates well with the netrin-1-induced Trio tyrosine phosphorylation observed at 5 and 10 min in cortical tissues (Fig. 1A and B). Conversely, Trio-depleted neurons exhibited a reduced level of cell surface DCC intensity, and netrin-1-mediated DCC surface dynamics of these cells were abrogated throughout the time course (Fig. 7C). Taken together, these results suggest that Trio is required for a proper expression of DCC at the plasma membrane of growth cones and that phosphorylation at Tyr2622 on Trio plays an imperative role in this process.
DISCUSSION
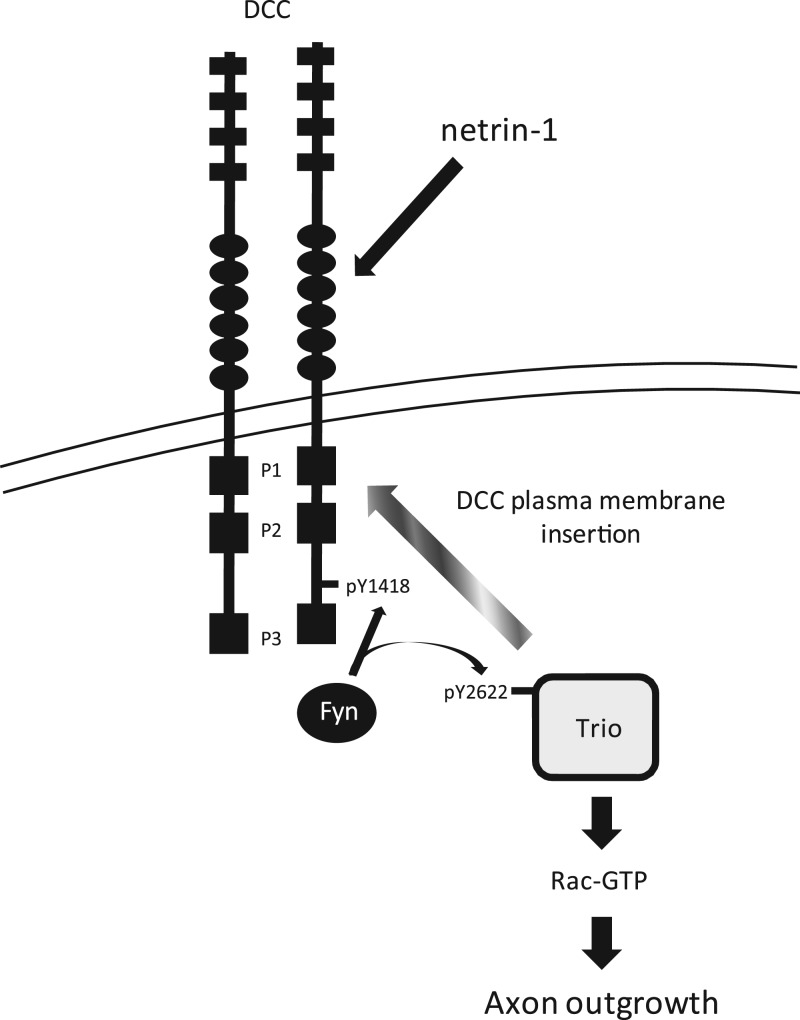
Previous studies have implicated the Rho GEF Trio as an important player during netrin-1-mediated axon outgrowth and guidance (31, 45–47). In this report, we provide evidence for a novel mechanism of regulation of Trio by tyrosine phosphorylation in response to the axon guidance cue netrin-1. We present biochemical and cellular evidence aligning the tyrosine phosphorylation of Trio by Src family kinases downstream of netrin-1/DCC in the mammalian embryonic cortex. We show that Trio is phosphorylated by the Src kinase Fyn at Tyr2622. Although this residue is not involved in the regulation of the intrinsic Trio GEF activity toward Rac1, it is important for netrin-1-mediated Rac1 activation and DCC-induced neurite outgrowth in N1E-115 neuroblastoma cells and for netrin-1-induced axon extension in cultured cortical neurons. Furthermore, phosphorylation of TrioY2622 is required for Trio/DCC interaction in response to netrin-1 and contributes to the proper localization of Trio and DCC at the growth cones of cultured cortical neurons stimulated with netrin-1. We also present evidence that Trio participates in maintaining DCC at the cell surface of neuronal growth cones and that phosphorylation of TrioY2622 is required for this process. In addition, Trio is also necessary for the dynamics of DCC surface localization in response to netrin-1. Therefore, we propose that TrioY2622 is essential for the proper assembly and stability of DCC/Trio signaling complexes at the cell surface of growth cones in order to mediate netrin-1-induced cortical axon outgrowth (Fig. 8).
Fig 8.
Model of Trio regulation during netrin-1/DCC signaling. Netrin-1/DCC engagement induces a Src-dependent phosphorylation of DCC at Tyr1418 (17) and Trio at Tyr2622. TrioY2622 is required for both netrin-1-induced activation of Rac1 and enhanced association with DCC. Phosphorylation of Trio at Tyr2622 participates in maintaining the level of surface DCC at the growth cone plasma membrane leading to axon outgrowth. Therefore, we propose that TrioY2622 is essential for the proper assembly and stability of the DCC/Trio signaling complex at the cell surface of growth cones in order to mediate netrin-1-induced cortical axon outgrowth.
Neural isoforms of Trio, which are generated by alternative splicing, have previously been reported by Portales-Casamar et al. (37). Trio-D, Trio-A, and Trio-C are highly expressed in both the embryonic and adult mammalian brains, with Trio-C being enriched in the cerebellum (37). Here, we show that Trio-D and Trio-A are highly expressed in the embryonic rat cortex compared to full-length Trio. Furthermore, we demonstrate that full-length Trio, Trio-D, and Trio-A are tyrosine phosphorylated upon netrin-1 stimulation in a Src kinase-dependent manner in the rat embryonic cortex. We show that Trio is predominantly phosphorylated by Fyn at Tyr2622, which is present in full-length Trio and Trio-D. Since the most abundant isoform (Trio-A), which lacks Tyr2622, is also highly tyrosine phosphorylated in response to netrin-1, we reason that additional Src kinase phosphorylation sites of Trio are likely induced by netrin-1 but are rendered inaccessible in the context of the full-length protein.
We demonstrate that the increased tyrosine phosphorylation of Trio by Fyn requires an intact C terminus of DCC. The phospho-null DCCY1418F mutant was still able to increase Trio phosphorylation by Fyn, suggesting that phosphorylation of DCC at Tyr1418 is not sufficient to potentiate Trio tyrosine phosphorylation by Fyn (data not shown). However, removal of the conserved P2 and P3 regions of DCC significantly reduced its ability to increase Fyn-mediated tyrosine phosphorylation of Trio. These results are in agreement with previous studies showing the importance of the carboxy tail of DCC during netrin-1-mediated axon growth and guidance (43). Since our previous results have shown that the interaction between DCC and Trio is indirect, possibly occurring through Pak1 and/or Nck1, an intact C-terminal domain of DCC is likely required for binding of these intermediate docking partners with Trio, thereby facilitating enhanced Src kinase-dependent phosphorylation of Trio (31).
Phosphorylation is a commonly used mechanism of regulation for both GEFs and GTPase-activating proteins (GAPs) in response to extracellular stimuli in various cellular systems (30). In the case of ephrin-Eph signaling, phosphorylation of GEFs and GAPs has been reported to play a predominant role in the regulation of axon guidance (48). For instance, tyrosine phosphorylation of ephexin-1, a GEF for RhoA, Cdc42, and Rac1, induces a shift in its exchange activity toward RhoA, thus promoting ephrin-A-mediated growth cone collapse (49). Vav2 and Tiam1 are also two GEFs that are regulated by phosphorylation during ephrin-Eph-mediated axon guidance (50, 51). Furthermore, the activity of the Rac-GAP α2-chimerin is stimulated by Src family kinase-dependent phosphorylation downstream of ephrin-A1/EphA4, resulting in growth cone collapse (52). In the case of Trio, its phosphorylation at Tyr2622 did not alter its intrinsic GEF activity toward Rac1. However, in the context of netrin-1 signaling, the phospho-null TrioY2622F mutant blocked netrin-1-induced Rac1 activation. Consequently, TrioY2622F expression also inhibited the ability of DCC to induce neurite outgrowth and was not able to rescue netrin-1-mediated axon outgrowth in cortical neurons depleted of Trio. In this report, we provide the molecular function of Trio tyrosine phosphorylation by showing that TrioY2622 is necessary for netrin-1 to enhance the interaction of Trio with DCC in coimmunoprecipitates and to increase colocalization of the Trio/DCC complex in the growth cones of rat embryonic cortical neurons. Moreover, when cortical neurons are depleted of Trio, we observed a significant decrease in cell surface DCC in growth cones, and the phospho-null TrioY2622F mutant was not able to rescue this effect. This raises the interesting possibility that Trio also acts upstream of the guidance receptor DCC to regulate its levels at the cell surface of the growth cones and that Tyr2622 is required for this function (Fig. 8). This proposed mechanism agrees well with previous studies with Caenorhabditis elegans that implicate the GEF activity of UNC-73 (Trio) and MIG-2 small GTPase (RhoG) as upstream regulators of the guidance receptors UNC-40 (DCC) and SAX-3 (robo) by affecting their membrane localization (46, 47, 53).
Our data indicate that the phospho-mimicking TrioY2622E partially restores Trio functions, showing that replacing a tyrosine with a negatively charged residue is not always sufficient to restore the function of a phosphotyrosine site. Since one notable consequence of protein tyrosine phosphorylation is the regulation of protein-protein interactions via binding to SH2 or PTB domains (54), we cannot exclude that phosphorylation of TrioY2622 induced by netrin-1 may create a binding site for potential PTB- or SH2-containing proteins that would mediate Trio regulation of netrin-1/DCC-induced signaling pathways. Alternatively, Tyr2622 may be a priming site that serves to promote a conformational change within Trio leading to specific protein-protein interactions and regulation of a Trio/DCC complex at the growth cone plasma membrane. In future studies, it will be of great interest to identify proteins interacting with Trio in a tyrosine phosphorylation-dependent manner that contribute to the regulation of cell surface DCC and, consequently, downstream signaling pathways. Our findings therefore provide the first demonstration of a mechanism of direct regulation of Trio in response to an extracellular stimulus, which echoes the importance of precise, localized regulation of Rho GEFs throughout biological systems.
ACKNOWLEDGMENTS
We thank Sylvie Fromont for her support with molecular biology techniques. We are grateful to Joseph Tcherkezian for providing cDNA constructs encoding DCC C-terminal deletion mutants. Also, we thank Min Fu from the McGill University Health Centre Imaging Facility for her assistance with the confocal microscopy.
This research was supported by Canadian Institute of Health research grant MOP-14701 and the Canada Foundation for Innovation-Leaders Opportunity Fund to N.L-V. and by Agence Nationale de la Recherche (ANR) grant 07-Neuro-006-01 to A.D. J.D. was supported by a Fonds de la Recherche en Santé du Québec (FRSQ) bourse de formation de Maîtrise, and N.L.-V. is an FRSQ Chercheur-National. J.B. was supported by a postdoctoral fellowship from Fondation de la Recherche Médicale.
Footnotes
Published ahead of print 10 December 2012
REFERENCES
- 1. Guan KL, Rao Y. 2003. Signalling mechanisms mediating neuronal responses to guidance cues. Nat. Rev. Neurosci. 4:941–956 [DOI] [PubMed] [Google Scholar]
- 2. Lowery LA, Van Vactor D. 2009. The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 10:332–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. 2003. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 26:509–563 [DOI] [PubMed] [Google Scholar]
- 4. Tessier-Lavigne M, Goodman CS. 1996. The molecular biology of axon guidance. Science 274:1123–1133 [DOI] [PubMed] [Google Scholar]
- 5. Barallobre MJ, Pascual M, Del Rio JA, Soriano E. 2005. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res. Brain Res. Rev. 49:22–47 [DOI] [PubMed] [Google Scholar]
- 6. Ackermann SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. 1997. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386:838–842 [DOI] [PubMed] [Google Scholar]
- 7. Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. 1996. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell 87:175–185 [DOI] [PubMed] [Google Scholar]
- 8. Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. 1997. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386:833–838 [DOI] [PubMed] [Google Scholar]
- 9. Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, Wu JY. 2009. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc. Natl. Acad. Sci. U. S. A. 106:2951–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. 2008. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell 133:1241–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rama N, Goldschneider D, Corset V, Lambert J, Pays L, Mehlen P. 2012. The amyloid precursor protein regulates netrin-1 mediated commissural axon outgrowth. J. Biol. Chem. [Epub ahead of print.] doi:10.1074/jbc.M111.324780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajasekharan S, Kennedy TE. 2009. The netrin protein family. Genome Biol. 10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kennedy TE, Serafini T, De la Torre JR, Tessier-Lavigne M. 1994. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78:425–435 [DOI] [PubMed] [Google Scholar]
- 14. Richards LJ, Koester SE, Tuttle R, O'Leary DDM. 1997. Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J. Neurosci. 17:2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. 1997. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386:796–804 [DOI] [PubMed] [Google Scholar]
- 16. Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. 1996. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87:1001–1014 [DOI] [PubMed] [Google Scholar]
- 17. Meriane M, Tcherkezian J, Webber CA, Danek EI, Triki I, McFarlane S, Bloch-Gallego E, Lamarche-Vane N. 2004. Phosphorylation of DCC by Fyn mediates netrin-1 signaling in growth cone guidance. J. Cell Biol. 167:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL. 2004. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat. Neurosci. 7:1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X, Saint-Cyr-Proulx E, Aktories K, Lamarche-Vane N. 2002. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J. Biol. Chem. 277:15207–15214 [DOI] [PubMed] [Google Scholar]
- 20. Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. 2004. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 7:1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jaffe AB, Hall A. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269 [DOI] [PubMed] [Google Scholar]
- 22. Rossman KL, Der CJ, Sondek J. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6:167–180 [DOI] [PubMed] [Google Scholar]
- 23. Antoine-Bertrand J, Villemure JF, Lamarche-Vane N. 2011. Implication of rho GTPases in neurodegenerative diseases. Curr. Drug Targets 12:1202–1215 [DOI] [PubMed] [Google Scholar]
- 24. Govek EE, Newey SE, Van Aelst L. 2005. The role of the Rho GTPases in neuronal development. Genes Dev. 19:1–49 [DOI] [PubMed] [Google Scholar]
- 25. Hall A, Lalli G. 2010. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb. Perspect. Biol. 2:a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Donnell M, Chance RK, Bashaw GJ. 2009. Axon growth and guidance: receptor regulation and signal transduction. Annu. Rev. Neurosci. 32:383–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moore SW, Correia JP, Lai Wing Sun K, Pool M, Fournier AE, Kennedy TE. 2008. Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin1. Development 135:2855–2864 [DOI] [PubMed] [Google Scholar]
- 28. Shekarabi M, Kennedy TE. 2002. The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell. Neurosci. 19:1–17 [DOI] [PubMed] [Google Scholar]
- 29. Antoine-Bertrand J, Ghogha A, Luangrath V, Bedford FK, Lamarche-Vane N. 2011. The activation of Ezrin-Radixin-Moesin (ERM) proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth. Mol. Biol. Cell 22:3734–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng Y. 2001. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 26:724–732 [DOI] [PubMed] [Google Scholar]
- 31. Briançon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, Fromont S, Piche C, Enslen H, Chebli K, Cloutier JF, Castellani V, Debant A, Lamarche-Vane N. 2008. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol. Cell. Biol. 28:2314–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li A, Gao X, Liu G, Xiong W, Wu J, Rao Y. 2008. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signalling. Nat. Neurosci. 11:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bateman J, Van Vactor D. 2001. The Trio family of guanine-nucleotide-exchange factors: regulators of axon guidance. J. Cell Sci. 114:1973–1980 [DOI] [PubMed] [Google Scholar]
- 34. Bellanger JM, Lazaro JB, Diriong S, Fernandez A, Lamb N, Debant A. 1998. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene 16:147–152 [DOI] [PubMed] [Google Scholar]
- 35. Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouviere C, Fort P. 2000. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J. Cell Sci. 113:729–739 [DOI] [PubMed] [Google Scholar]
- 36. Debant A, Serra-Pagès C, Seipel K, O'Brien S, Tang M, Park SH, Streuli M. 1996. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc. Natl. Acad. Sci. U. S. A. 93:5466–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Portales-Casamar E, Briancon-Marjollet A, Fromont S, Triboulet R, Debant A. 2006. Identification of novel neuronal isoforms of the Rho-GEF Trio. Biol. Cell 98:183–193 [DOI] [PubMed] [Google Scholar]
- 38. O'Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. 2000. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc. Natl. Acad. Sci. U. S. A. 97:12074–12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, Debant A. 2002. The human Rho-GEF Trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr. Biol. 12:307–312 [DOI] [PubMed] [Google Scholar]
- 40. Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. 2010. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell 141:632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neubrand VE, Thomas C, Schmidt S, Debant A, Schiavo G. 2010. Kidins220/ARMS regulates Rac1-dependent neurite outgrowth by direct interaction with the RhoGEF Trio. J. Cell Sci. 123:2111–2123 [DOI] [PubMed] [Google Scholar]
- 42. Picard M, Petrie RJ, Antoine-Bertrand J, Saint-Cyr-Proulx E, Villemure JF, Lamarche-Vane N. 2009. Spatial and temporal activation of the small GTPases RhoA and Rac1 by the netrin-1 receptor UNC5a during neurite outgrowth. Cell. Signal. 21:1961–1973 [DOI] [PubMed] [Google Scholar]
- 43. Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. 1999. A ligand-gated association between cytplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97:927–941 [DOI] [PubMed] [Google Scholar]
- 44. Stein E, Zou Y, Poo M, Tessier-Lavigne M. 2001. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science 291:1976–1982 [DOI] [PubMed] [Google Scholar]
- 45. Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. 2005. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Develpment 132:1983–1994 [DOI] [PubMed] [Google Scholar]
- 46. Levy-Strumpf N, Culotti JG. 2007. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat. Neurosci. 10:161–168 [DOI] [PubMed] [Google Scholar]
- 47. Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. 2007. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat. Neurosci. 10:169–176 [DOI] [PubMed] [Google Scholar]
- 48. Egea J, Klein R. 2007. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 17:230–238 [DOI] [PubMed] [Google Scholar]
- 49. Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O'Connell S, Cowan CW, Hu L, Goldberg JL, Debant A, Corfas G, Krull CE, Greenberg ME. 2005. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46:191–204 [DOI] [PubMed] [Google Scholar]
- 50. Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. 2005. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Cell 46:205–217 [DOI] [PubMed] [Google Scholar]
- 51. Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. 2007. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc. Natl. Acad. Sci. U. S. A. 104:7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shi L, Fu WY, Hung KW, Porchetta C, Hall C, Fu AKY, Ip NY. 2007. α2-Chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc. Natl. Acad. Sci. U. S. A. 104:16347–16352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Demarco RS, Struckhoff EC, Lundquist EA. 2012. The Rac GTP exchange factor TIAM-1 acts with Cdc-42 and the guidance receptor UNC-40/DCC in neuronal protrusion and axon guidance. PLoS Genet. 8(4):e1002665 doi:10.1371/journal.pgen.1002665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moran MF, Polakis P, McCormick F, Pawson T, Ellis C. 1991. Protein-tyrosine kinases regulate the phosphorylation, protein interactions, subcellular distribution, and activity of p21ras GTPase-activating protein. Mol. Cell. Biol. 11:1804–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]