Abstract
Recombinant vesicular stomatitis virus (VSV) is a promising therapeutic vaccine platform. Using a transgenic mouse model of chronic hepatitis B virus (HBV) infection, we evaluated the therapeutic potential of a VSV vector expressing the HBV middle surface envelope glycoprotein (MS). VSV-MS immunization generated HBV-specific CD8 T cell and antibody responses in transgenic mice that express low HBV antigen levels. These findings support the further development of VSV as a therapeutic vaccine vector for chronic HBV.
TEXT
Despite the successful implementation of universal vaccination policies, approximately 2 billion people have been infected with hepatitis B virus (HBV), resulting in the establishment of chronic infection in more than 240 million individuals worldwide (1). Current therapeutic options for chronic HBV infection include nucleot(s)ide analogues and alpha interferon (IFN-α), but each has a number of disadvantages. IFN-α treatment is effective only in a proportion of patients and is associated with significant side effects (2), while long-term treatment with nucleot(s)ide analogues rarely cures the virus and is limited by drug-resistant mutants (3). If left untreated, chronic HBV infection can result in liver cirrhosis and hepatocellular carcinoma (4). Due to the limitations of current treatment options and the risk of severe liver disease associated with chronic infection, there remains a need to develop new therapies for HBV. Host immune dysfunction, characterized by weak and ineffective T cell responses to the virus, is a key feature of chronic HBV (5). Immunomodulatory therapies such as therapeutic vaccination that are aimed at generating HBV-specific T cells with effector functions capable of eliminating the virus may provide highly efficacious treatment options for chronic HBV patients.
Vesicular stomatitis virus (VSV)-based vaccine vectors generate potent HBV-specific cellular and humoral immune responses following a single dose in antigen-naïve mice (6). Additionally, in certain regimens, VSV-based vaccines generate more robust and polyfunctional CD8 T cells than DNA vaccines and other potential viral vaccine platforms (6), a finding which may be attributed to the cytopathic effects associated with VSV infection (7). The ability of VSV to generate potent CD8 T cell responses may make it better suited as a therapeutic strategy for chronic HBV infection than other immunization methods, as recovery from acute HBV infection is associated with strong, multispecific T cell responses to HBV antigens (8). Furthermore, immunization with VSV has been demonstrated to be effective as a therapeutic strategy for cervical carcinoma and other papillomavirus-associated cancers in animal models, an effect that was shown to be CD8 T cell dependent (9, 10). Therefore, we sought to determine the potential of recombinant VSV expressing the HBV middle envelope surface glycoprotein (VSV-MS) as a therapeutic vaccine for chronic HBV infection.
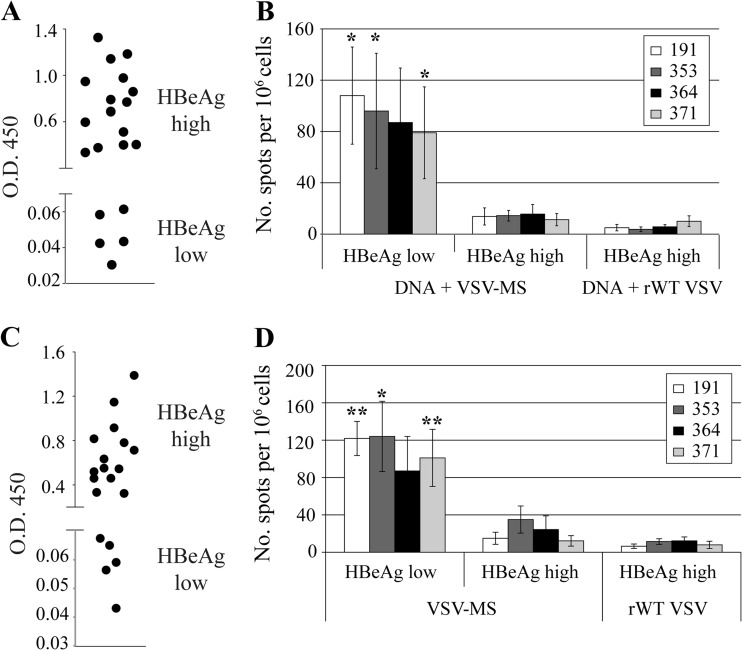
We utilized 1.3.32 HBV transgenic (Tg) mice to examine if VSV-MS immunization can generate functional immune responses in the context of HBV replication. HBV 1.3 transgenic mice encode the full HBV genome and mimic chronic HBV infection in humans, as they are immunologically tolerant to the viral antigens (11). Therapeutic immunization for chronic HBV in humans would likely require concurrent administration of antiviral drugs to lower viral antigen levels, thus limiting immunopathology and restoring the effector functions of T cells that have become exhausted in the presence of large amounts of viral antigen. To model the reduction in antigenemia that would accompany antiviral therapy, HBV 1.3 transgenic mice expressing either low or high HBV e antigen levels (HBeAglow or HBeAghigh, respectively) were immunized. Mice designated HBeAghigh expressed HBeAg levels equivalent to an optical density at 450 nm (OD450) of >0.2, as measured by an enzyme-linked immunosorbent assay (ELISA) using a 1:50 dilution of serum, while mice assigned to the HBeAglow group measured levels equivalent to an OD450 of <0.1 (Fig. 1A and C). To obtain better separation of antigen expression levels between the two experimental groups, animals with serum HBeAg ELISA OD450 values between 0.1 and 0.2 were not used.
Fig 1.
HBV-specific CD8 T cell responses are elicited following intranasal immunization with VSV-MS. (A) HBV 1.3 transgenic mice (n = 21) were screened for HBeAg expression and separated into HBeAghigh (n = 16) or HBeAglow (n = 5) immunization groups. (B) Mice were primed with 50 μg pCMV-S2/S plasmid DNA intramuscularly and 3 weeks later were intranasally infected with 1 × 106 PFU VSV-MS (n = 13) or rWT VSV (n = 8). Seven days postboost, splenocytes were harvested and analyzed using an IFN-γ ELISPOT assay. The number of cells responding to stimulation is presented as a quantification of the number of spot-forming cells (SFC)/106 cells. (C) HBV 1.3 transgenic mice (n = 19) were again screened for HBeAg expression and separated into HBeAghigh (n = 14) or HBeAglow (n = 5) immunization groups. (D) Mice were intranasally infected with 1 × 106 PFU VSV-MS (n = 12) or rWT VSV (n = 7). Seven days postimmunization, splenocytes were harvested and analyzed as in panel B. The number of SFC/106 cells is significantly (by Student's t test) higher following stimulation with the indicated peptides (**, <0.001; *, <0.05) in the HBeAglow mice than in the HBeAghigh mice. All values presented are the averages of each group, and error bars show the standard errors of the mean.
We first tested VSV-MS using a prime-boost immunization protocol, as this had the potential to generate the greatest number of HBV-specific T cells. Female 8- to 12-week-old HBV.CB6F1bxd mice were primed by intramuscular injection with a plasmid (pCMV-S2S) expressing the middle envelope protein of HBV (ayw subtype) (12). Three weeks later, mice were boosted with VSV-MS or empty recombinant wild-type VSV (rWT VSV) delivered intranasally. To compare the HBV-specific CD8 T cell responses following immunization, IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assays were conducted. At 7 days postboost, splenocytes were isolated from immunized mice and the CD8 T cell response was analyzed following an overnight stimulation with a VSV N protein peptide (RGYVYQGL), two peptides corresponding to known HBV surface antigen (HBs)-specific, H-2d-restricted epitopes (HBs 191-202 [IPQSLDSWWTSL] and HBs 364-372 [WGPSLYSIL]), or two peptides corresponding to known HBs-specific, H-2b-restricted epitopes (HBs 353-360 [VWLSVIWM] and HBs 371-378 [ILSPFLPL]) (13–16). While HBs-specific responses were readily detected in HBeAglow transgenic mice following pCMV-S2S and VSV-MS immunization, HBs-specific responses were at or below the limit of detection in HBeAghigh transgenic mice (Fig. 1B).
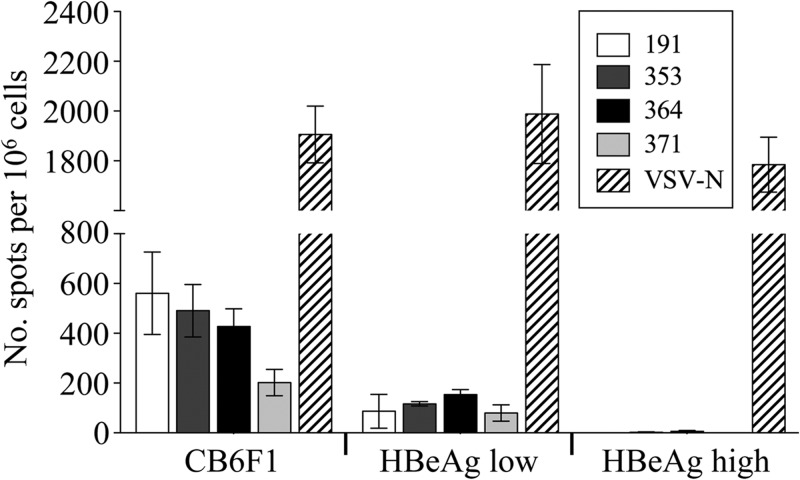
In order to distinguish the contribution of VSV-MS to the T cell response generated by the DNA prime-VSV boost regimen, a second set of HBV transgenic mice was immunized with a single intranasal inoculation of rWT VSV or VSV-MS. Consistent with results obtained following a prime-boost, HBs-specific responses were detected in HBeAglow transgenic mice immunized with VSV-MS alone (no DNA prime) (Fig. 1D). These responses were similar in magnitude to those generated by prime-boost and indicate that VSV, but not DNA, can activate a T cell response in low-expressing mice. Similar to intranasal infection, HBs-specific responses were also observed following a single intramuscular immunization of HBeAglow, but not HBeAghigh, mice with VSV-MS (Fig. 2).
Fig 2.
HBV-specific CD8 T cell responses are elicited following intramuscular immunization with VSV-MS. Nontransgenic CB6F1 (n = 5) or HBV transgenic HBeAghigh (n = 4) or HBeAglow (n = 3) mice were intramuscularly infected with 1 × 106 PFU of VSV-MS. Seven days postinfection, splenocytes were harvested and analyzed using an IFN-γ ELISPOT assay. The number of cells responding to stimulation with the HBV MS 191, 353, 364, or 371 peptide or a control VSV N protein peptide is presented as a quantification of the number of SFC/106 cells. Values presented are the averages of each group, and error bars show the standard errors of the mean.
The HBs-specific T cell response generated in immunized low-expressing mice was consistently 5-fold lower in magnitude than the responses we observed in nontransgenic mice (Fig. 2) (6). This indicates that the low antigen expressers retained some degree of immunotolerance to the HBV proteins despite only low levels of antigen expression, although they are not fully tolerant like the high-expressing mice. Furthermore, the responses observed in HBeAglow mice appeared to be generated through de novo activation of HBs-specific T cells that have escaped negative selection rather than through expansion of preexisting activated HBs-specific T cells, as we were unable to identify HBs-specific T cells in these mice by major histocompatibility complex (MHC) class I tetramer staining prior to immunization (data not shown).
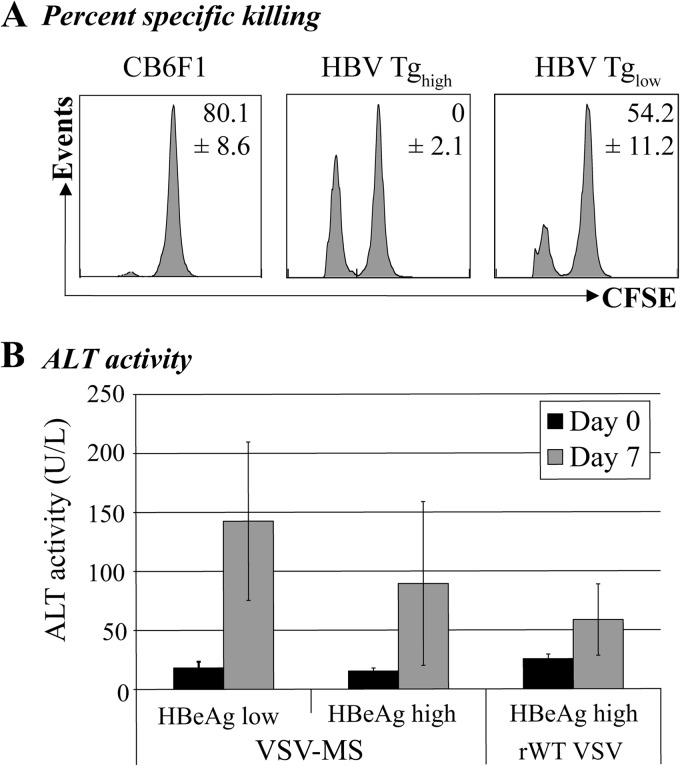
To determine whether the HBs-specific CD8 T cells generated by VSV-MS immunization in the HBeAglow mice maintain their cytolytic function, we conducted an in vivo killing assay. Splenocytes from CB6F1 mice were pulsed with 10 μM HBs 191-202 peptide or were left unpulsed and were then labeled with either a low (0.5 μM) or high (5 μM) concentration of carboxyfluorescein diacetate succinimidyl ester (CFSE), respectively. Ten million CFSE-labeled cells (5 × 106 cells of each population) were injected intravenously into VSV-MS-immunized CB6F1, HBeAghigh, and HBeAglow mice. Four hours posttransfer, splenocytes were prepared and analyzed by flow cytometry to determine the percent specific lysis. Though there was no detectable in vivo killing activity in the immunized HBeAghigh transgenic mice, there was significant killing activity in the immunized HBeAglow mice (Fig. 3A). Despite the fact that this activity was reduced compared to that of HBV-naïve mice, a corresponding specific elevation in serum alanine aminotransferase (ALT) activity measured in HBeAglow mice following VSV-MS immunization indicates that this activity is sufficient to kill at least a fraction of HBV-expressing hepatocytes (Fig. 3B).
Fig 3.
HBV-specific T cells generated in HBeAglow mice maintain cytolytic function. (A) HBV 1.3 transgenic recipient mice (n = 10) were screened for HBeAg expression and separated into HBeAghigh (n = 6) or HBeAglow (n = 4) immunization groups. Recipient HBV transgenic or CB6F1 mice (n = 5) were primed with pCMV-S2/S and boosted with VSV-MS 3 weeks later. Target splenocytes harvested from CB6F1 mice were left untreated or pulsed with HBV peptide (HBs epitope 191). HBV peptide-pulsed cells were labeled with a low concentration of CFSE (0.5 μM), while untreated cells were labeled with a high concentration of CFSE (5 μM). Target splenocytes were mixed, and 1 × 107 cells were transferred (via intravenous injection) into the recipient, immunized mice 7 days postboost. Percent specific killing was calculated following flow cytometric analysis of remaining target splenocytes harvested from recipient mice 4 h posttransfer. (B) HBeAghigh (n = 16) or HBeAglow (n = 5) mice were primed with pCMV-S2/S and 3 weeks later boosted with VSV-MS (n = 13) or rWT VSV (n = 5). Seven days postboost, mice were bled and serum alanine aminotransferase (ALT) activity was measured using Infinity ALT reagent. All values presented are the averages of each group, and error bars show the standard errors of the mean.
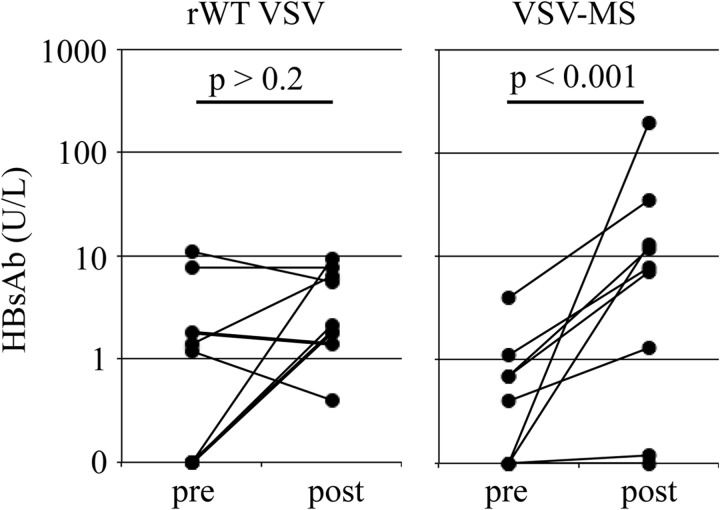
We next determined whether immunization with VSV-MS elicits an HBs-specific antibody (Ab) response in the transgenic mice. HBV transgenic mice (both HBeAghigh and HBeAglow) were immunized by intranasal administration of 1 × 106 PFU of rWT VSV or VSV-MS, and the HBs-specific antibody response was measured by quantitative ELISA 30 days later. In contrast to infection with rWT VSV, immunization with VSV-MS elicited a significant increase in HBs-specific antibody titers (Fig. 4). Unlike the CD8 T cell response, however, the Ab response did not correlate with viral antigen expression levels (data not shown).
Fig 4.
VSV-MS immunization generates HBs-specific antibodies in HBV transgenic mice. Serum HBs-specific antibody titers in HBV transgenic mice prior to (pre) or 30 days after (post) intranasal immunization with 1 × 106 PFU of rWT VSV (n = 9) or VSV-MS (n = 9). Preimmunization antibody levels were not significantly different between the two groups (P > 0.05; Wilcoxon rank sum test). Each line connects the pre- and postimmunization values for a single animal. Statistical significance between pre- and postimmunization antibody titers was determined using the Wilcoxon signed-rank test.
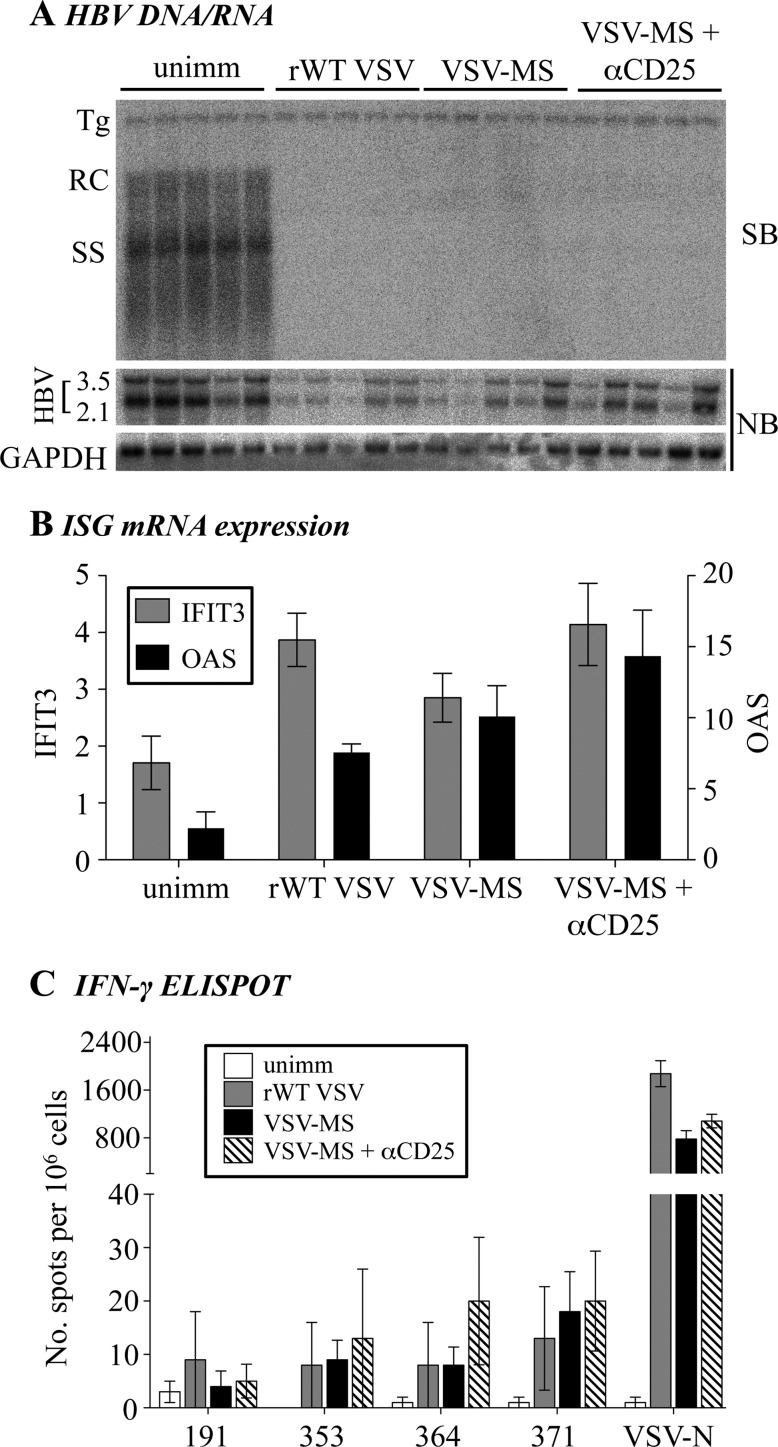
A previous study has shown that the HBs-specific T cell response generated by an HBV MS plasmid DNA expression vector is enhanced by administration of αCD25 monoclonal Ab to deplete suppressive regulatory T cells (Treg) (17). We also found that administration of αCD25 antibody modestly increases (approximately 1.5-fold) the HBs-specific CD8 T cell response in VSV-MS immunized normal mice (data not shown). Therefore, we examined whether administration of αCD25 to transgenic mice would restore the HBs-specific T cell response in HBeAghigh animals. As previously demonstrated for other viruses, such as adenovirus and murine cytomegalovirus (18), infection of HBV Tg mice with VSV results in an antigen-nonspecific downregulation of HBV DNA and RNA expression in the liver, likely due to systemic activation of the IFN-α/β antiviral response (Fig. 5A). Consistent with this mechanism, we also found increased IFN-stimulated gene expression in the liver following VSV infection (Fig. 5B) (2′,5′-OAS and IFIT3 were analyzed as representative IFN-stimulated genes). Administration of αCD25 antibody, however, did not improve the HBs-specific T cell response in HBeAghigh mice (Fig. 5C).
Fig 5.
Treg cell depletion does not improve the CD8 T cell response in HBeAghigh mice. HBeAghigh HBV Tg mice (n = 5 per group) were immunized intranasally with 1 × 106 PFU of rWT VSV or VSV-MS. αCD25 monoclonal Ab (500 μg) was also delivered intraperitoneally to one group of animals 24 h prior to VSV-MS infection, and mice were euthanized 7 days postinfection. (A) Total hepatic DNA was analyzed for HBV replication by Southern blotting (SB). Bands corresponding to integrated transgene (Tg), relaxed-circular (RC), and single-stranded (SS) HBV DNA replication intermediates are indicated. Northern blotting (NB) was used for analysis of HBV 3.5-kb and 2.1-kb mRNA expression, normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B) Total hepatic RNA was analyzed for expression of the representative IFN-stimulated genes (ISG) IFIT3 and 2′,5′-OAS, as measured by quantitative reverse transcription-PCR (RT-PCR). Results are displayed as fold differences relative to one uninfected mouse and are normalized to GAPDH expression. (C) Splenocytes were harvested, and the CD8 T cell response to the four HBs peptides (191, 353, 364, and 371) and the VSV N peptide were analyzed by an IFN-γ ELISPOT assay. unimm, unimmunized.
These findings not only support the concept that VSV may be an effective therapeutic vaccine vector for chronic HBV due to its ability to generate robust CD8 T cell responses, they also indicate that therapeutic immunization in the context of lowered viral replication may have the most potential for success. In addition to the results presented here, a growing body of evidence using experimental models also supports this notion. Antiviral treatment has been used in conjunction with therapeutic vaccination in other animal models of chronic hepatitis, including duck and woodchuck, resulting in sustained therapeutic effects (19–21). A lentivector expressing an HBs-immunoglobulin Fc fusion antigen has also been demonstrated to induce HBV-specific immune responses in a transgenic mouse model expressing low levels of hepatitis B surface antigen (22). Despite some success in animal models, clinical trials combining antiviral therapy with recombinant protein vaccination in chronic HBV patients have been ineffective in viral clearance and control (23, 24). This highlights the complexities of the host-viral interactions at play during chronic HBV infection and underscores the need for new immunotherapeutic strategies that can overcome these mechanisms of persistence. Novel vaccination platforms, such as VSV, may prove effective in this pursuit and provide an effective alternative strategy for the treatment of chronic HBV.
ACKNOWLEDGMENTS
This work was supported by grant CA131133 from the National Institutes of Health. Xin Wei was supported by a training grant from the China Scholarship Council (2011659504).
Footnotes
Published ahead of print 26 December 2012
REFERENCES
- 1. World Health Organization 2012. Hepatitis B fact sheet. Fact sheet no. 204. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs204/en/ [Google Scholar]
- 2. Perrillo R. 2009. Benefits and risks of interferon therapy for hepatitis B. Hepatology 49:S103–S111 [DOI] [PubMed] [Google Scholar]
- 3. Papatheodoridis GV, Manolakopoulos S, Archimandritis AJ. 2008. Current treatment indications and strategies in chronic hepatitis B virus infection. World J. Gastroenterol. 14:6902–6910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen C, Holmberg SD, McMahon BJ, Block JM, Brosgart CL, Gish RG, London WT, Block TM. 2011. Is chronic hepatitis B being undertreated in the United States? J. Viral Hepat. 18:377–383 [DOI] [PubMed] [Google Scholar]
- 5. Chisari FV, Ferrari C. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29–60 [DOI] [PubMed] [Google Scholar]
- 6. Cobleigh MA, Buonocore L, Uprichard SL, Rose JK, Robek MD. 2010. A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose. J. Virol. 84:7513–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cobleigh MA, Bradfield C, Liu Y, Mehta A, Robek MD. 2012. The immune response to a vesicular stomatitis virus vaccine vector is independent of particulate antigen secretion and protein turnover rate. J. Virol. 86:4253–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bertoletti A, Gehring AJ. 2006. The immune response during hepatitis B virus infection. J. Gen. Virol. 87:1439–1449 [DOI] [PubMed] [Google Scholar]
- 9. Brandsma JL, Shylankevich M, Su Y, Roberts A, Rose JK, Zelterman D, Buonocore L. 2007. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J. Virol. 81:5749–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liao JB, Publicover J, Rose JK, DiMaio D. 2008. Single-dose, therapeutic vaccination of mice with vesicular stomatitis virus expressing human papillomavirus type 16 E7 protein. Clin. Vaccine Immunol. 15:817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guidotti LG, Matzke B, Schaller H, Chisari FV. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158–6169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis HL, Schirmbeck R, Reimann J, Whalen RG. 1995. DNA-mediated immunization in mice induces a potent MHC class I-restricted cytotoxic T lymphocyte response to the hepatitis B envelope protein. Hum. Gene Ther. 6:1447–1456 [DOI] [PubMed] [Google Scholar]
- 13. Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J. Exp. Med. 178:1541–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schirmbeck R, Bohm W, Fissolo N, Melber K, Reimann J. 2003. Different immunogenicity of H-2 Kb-restricted epitopes in natural variants of the hepatitis B surface antigen. Eur. J. Immunol. 33:2429–2438 [DOI] [PubMed] [Google Scholar]
- 15. Schirmbeck R, Dikopoulos N, Kwissa M, Leithauser F, Lamberth K, Buus S, Melber K, Reimann J. 2003. Breaking tolerance in hepatitis B surface antigen (HBsAg) transgenic mice by vaccination with cross-reactive, natural HBsAg variants. Eur. J. Immunol. 33:3342–3352 [DOI] [PubMed] [Google Scholar]
- 16. Sette AD, Oseroff C, Sidney J, Alexander J, Chesnut RW, Kakimi K, Guidotti LG, Chisari FV. 2001. Overcoming T cell tolerance to the hepatitis B virus surface antigen in hepatitis B virus-transgenic mice. J. Immunol. 166:1389–1397 [DOI] [PubMed] [Google Scholar]
- 17. Furuichi Y, Tokuyama H, Ueha S, Kurachi M, Moriyasu F, Kakimi K. 2005. Depletion of CD25+CD4+ T cells (Tregs) enhances the HBV-specific CD8+ T cell response primed by DNA immunization. World J. Gastroenterol. 11:3772–3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cavanaugh VJ, Guidotti LG, Chisari FV. 1998. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J. Virol. 72:2630–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Guerhier F, Thermet A, Guerret S, Chevallier M, Jamard C, Gibbs CS, Trepo C, Cova L, Zoulim F. 2003. Antiviral effect of adefovir in combination with a DNA vaccine in the duck hepatitis B virus infection model. J. Hepatol. 38:328–334 [DOI] [PubMed] [Google Scholar]
- 20. Menne S, Roneker CA, Korba BE, Gerin JL, Tennant BC, Cote PJ. 2002. Immunization with surface antigen vaccine alone and after treatment with 1-(2-fluoro-5-methyl-beta-l-arabinofuranosyl)-uracil (L-FMAU) breaks humoral and cell-mediated immune tolerance in chronic woodchuck hepatitis virus infection. J. Virol. 76:5305–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menne S, Roneker CA, Tennant BC, Korba BE, Gerin JL, Cote PJ. 2002. Immunogenic effects of woodchuck hepatitis virus surface antigen vaccine in combination with antiviral therapy: breaking of humoral and cellular immune tolerance in chronic woodchuck hepatitis virus infection. Intervirology 45:237–250 [DOI] [PubMed] [Google Scholar]
- 22. Hong Y, Peng Y, Mi M, Xiao H, Munn DH, Wang GQ, He Y. 2011. Lentivector expressing HBsAg and immunoglobulin Fc fusion antigen induces potent immune responses and results in seroconversion in HBsAg transgenic mice. Vaccine 29:3909–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoa PT, Huy NT, Thu LT, Nga CN, Nakao K, Eguchi K, Chi NH, Hoang BH, Hirayama K. 2009. Randomized controlled study investigating viral suppression and serological response following pre-S1/pre-S2/S vaccine therapy combined with lamivudine treatment in HBeAg-positive patients with chronic hepatitis B. Antimicrob. Agents Chemother. 53:5134–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vandepapeliere P, Lau GK, Leroux-Roels G, Horsmans Y, Gane E, Tawandee T, Merican MI, Win KM, Trepo C, Cooksley G, Wettendorff M, Ferrari C. 2007. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 25:8585–8597 [DOI] [PubMed] [Google Scholar]